Review Article - Diabetes Management (2016) Volume 6, Issue 6
Recent advance in diabetes therapy: pancreatic beta cell regeneration approaches
- Corresponding Author:
- Tesemma Sileshi Chala
Department of Pharmacy, College of Medicine and Health Sciences, Ambo University, Ambo, Ethiopia
E-mail: tesemmasileshi@gmail.com
Abstract
Diabetes mellitus (DM) is a chronic metabolic disorder characterized by elevated blood glucose. According to WHO, more than 400 million people live with diabetes and the number is expected to rise to 592 million by 2035. Improved glycemic control decreases the risk of development of microvascular and macrovascular complication and disease progression. The currently available treatments options are either associated with an increased incidence of adverse effects or fail to prevent progression of diabetes-associated complication. As core pathologic condition in diabetes is either β-cell destruction or insufficiency, recent research focuses mainly on improving glucose homeostasis by regeneration of this key cell type. These cellular based therapeutic approaches are focused on the transplantation of the islet cells or β-cell, stimulation of human β-cell proliferation, in vivo β-cell regeneration and protect β-cells from apoptosis. However, none of these approaches are in clinical use due to a shortage of donor; the inefficient technique of β-cell generation and β-cell in adult humans does not grow sufficiently. This review focuses on current progress in β-cells regeneration approaches for the treatment of diabetes mellitus.
Keywords
diabetes, beta cell, hesc, ipsc, mscs, reprogramming
Introduction
Diabetes mellitus (DM) is a serious metabolic disorder characterized by elevated blood glucose level resulting from the absence of insulin secretion, or insufficiency of insulin action. The raised blood glucose level if not managed appropriately results in a damage and failure of organs, such as the eyes, kidneys, nerves, heart, and blood vessels [1]. The number of people in the world with diabetes has increased dramatically over recent years. According to the latest WHO report more than 400 million people live with diabetes [2]. This number may rise to 591.9 million in 2035. The expected increase in the number of adults with diabetes is as follows; in low-income countries (108%), followed by lower middle-income countries (60%), upper middle-income countries (51%), and high-income countries (28%) [3].
Diabetes is classified into the following general categories: Type 1 diabetes (T1DM), Type 2 diabetes (T2DM), Gestational diabetes mellitus (GDM) and other specific types of diabetes. T1DM is characterized by complete destruction of β cells due to an autoimmune attack on β cells, which leads to absolute insulin deficiency. T2DM, which accounts for 90–95% of those with diabetes, is a disorder characterized by insulin resistance and relatively insulin deficiency. As the diseases progress β-cell function continues to decline and insulin production decreases. GDM is diabetes diagnosed in the second or third trimester of pregnancy that is not clearly either T1DM or type T2DM [1,4]. Other specific types of diabetes occur due to various causes such as genetic defects of the β-Cell, diseases of the exocrine pancreas (such as cystic fibrosis), drug or chemical induced diabetes (such as with glucocorticoid use, in the treatment of HIV/AIDS), genetic defects in insulin action, and infections [4,5]. Loss of β-cell number and function underlies much of the pathology of diabetes [6,7].
Several approaches are presently available to reduce the hyperglycemia in DM. Today, more than 11 classes of diabetes drugs are available. As described above the main future of T1DM is absolute loss of insulin and the mainstay of therapy is, therefore, insulin replacement. For T2DM treatment Metformin, sulfonylureas (SU) and thiazolidinediones (TZD) are the most widely used classes of drugs. Dipeptidyl peptidase-IV (DPP-4) inhibitors, glucagon-like polypeptide-1 (GLP-1) agonists are recently included in the therapy of DM [6,8]. In addition, several miscellaneous drugs have been approved for clinical uses in DM management. Despite this increase in a number of drugs, DM continues to be associated with blindness, kidney failure, cardiovascular complication and nerve damage [9].
To overcome the shortcoming of current diabetes therapy, restoring the functionality of β cell either by islet transplantation or by differentiated stem cells has attracted the attention of many researchers and several articles have been published in recent years [10-15]. These can be achieved by the (i) proliferation of existing β-cells, (ii) neogenesis from non-beta pancreatic cell precursors (iii) reprogramming of non-pancreatic cells [14,15]. Although these approaches seem feasible, many challenges remain, including autoimmunity reaction, and the need for the use of toxic immunosuppressive drugs and β-cells for transplantation due to lack of efficient technique for β cell differentiation. These areas of research are presently at its infancy stage and this review primarily explore progress has been made in β cells regeneration as an option for diabetes therapy.
Pancreas development and cell differentiation
The pancreas consists mainly of two organs in one: the exocrine, which produces digestive enzymes, and the endocrine [16] which constitutes just 1-2% of the adult pancreas, and includes insulinproducing β-cells, glucagon-producing β-cells, somatostatin-producing β-cells, ghrelin-producing β-cells and pancreatic polypeptide-producing PPcells [17]. These endocrine cells secrete hormones into the bloodstream to regulate nutrient metabolism and glucose homeostasis [18].
Pancreas organogenesis begins at about embryonic day 9.0 in mice and 4 weeks into human gestation and it consist of (i) formation of the endoderm, (ii) specification of the pancreatic primordium, (iii) differentiation of exocrine and endocrine cell types, and (iv) specification of endocrine cells lineage [19-21].
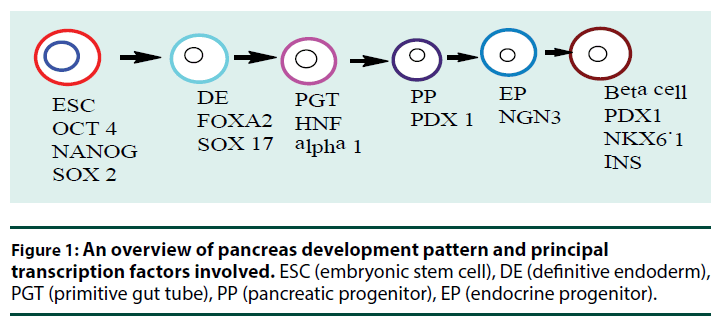
Pancreatic development begins with gastrulation when blastula reorganizes into a three-layered structure composed of the ectoderm, mesoderm, and endoderm germ layers. Through the action of transforming growth factor-β (TGF-β) such as Nodal each layer gives rise to specific organs in the developing embryo and the pancreas develop from endoderm germ layers. Nodal proteins play an evolutionarily conserved role in initiating gastrulation and in driving endoderm formation [22,23]. Wnt signaling is critical for gastrulation and mouse embryos lacking Wnt3 fail to gastrulate [24]. The definitive endoderm (DE) formed by the action of Nodal is marked by expression of different receptors such as forkhead box A2 (FOXA2), SRY (sex determining region Y)-box 17 (SOX17) [25]. During early pancreatic development, the FOXA2 transcription factor is consistently expressed from week 4 forward in human and act as a pioneer factor to regulate Pdx1 (pancreatic and duodenal homeobox 1) expression in mice [26,27]. Sox17 and FoxA2 are essential for the generation of endodermal tissue and the gut tube that derives from it [28]. The primitive gut tube becomes re-patterned along the anterior–posterior and dorsal–ventral axes into broad foregut, midgut, and hindgut domain. The pancreas originates from foregut which also gives rise to the esophagus, trachea, stomach, lungs, thyroid, liver, and biliary system (pancreatic organogenesis sequence and involved transcription factors are summarized in Figure 1 [21,29-31].
▪ Pancreatic endoderm formation from definitive endoderm
The pancreas originates from two separate domains of the foregut endoderm, one dorsal, and one ventral which form two separate buds; dorsal pancreatic bud and ventral pancreatic bud respectively. The dorsal pancreatic bud first receives inductive signals from the mesoderm during gastrulation, then permissive signals from the nearby notochord, dorsal aorta, and finally proliferative signals from the pancreas mesenchyme [17]. For example, Fibroblast growth factor (FGF) and (TGF-β) signaling from the notochord suppress the expression of sonic hedgehog (Shh) in the presumptive dorsal pancreatic epithelium, resulting in dorsal pancreatic bud formation [32]. Whereas inhibition of Shh is required for early pancreas specification, Hedgehog (Hh) activities are required later in the expansion of the pancreatic epithelium, and in the regulation of insulin gene expression in the mature β-cells [33]. Similarly, the ventral pancreas is patterned by distinct sets of signals from the lateral plate mesoderm and cardiac mesoderm such as FGF and bone morphogenic proteins (BMP) signal [17,34,35]. FGF’s from the cardiogenic mesenchyme inhibit ventral pancreas development in favor of liver development [36]. Generally, these signals activate transcription factors specific to each organ domain. For details of transcription factors mediating pancreatic organogenesis and maturation, one could refer to a review by Dassaye et al. [37].
Once the ventral and dorsal prepancreatic domains are specified, several transcription factors become specifically expressed in the prospective pancreatic regions. Among the earliest transcription factors that mark this region are duodenal homeobox factor 1 (Pdx1), and Pancreas transcription factor 1A (Ptf1A) [38,39]. These transcription factors (TFs) perform multiples roles at different stages of pancreas formation [40] and mutation in the gene encoding for them resulted in pancreatic agenesis and permanent neonatal diabetes mellitus [41,42]. Pdx1 is required during both early pancreas organogenesis and in maintaining adult β–cell function and identity [43]. PDx1 is broadly expressed in human at around 4 weeks but earlier in mice with a high level of expression being restricted later to endocrine cells just before birth in the pancreas [44,45]. Ptf1A is also essential for the commitment and proliferation of pancreatic progenitors as a recent study identified mutations in the human Ptf1A enhancer resulting in pancreatic agenesis [42].
After specification, pancreatic buds rapidly grow into the surrounding mesenchyme, but not yet acquired the macroscopic structure of the mature organ, nor have they formed the differentiated cell types necessary for pancreatic function. Instead, they are almost entirely composed of multipotent progenitor cells (MPCs). The surrounding mesenchyme produces proliferative signals such as fibroblast growth factor 10 (FGF- 10) [46,47]. FGF10 has been shown to regulate Ptf1A and Sox9 expression in the epithelium which explains why FGF10 deletion results in a phenotype similar to that observed in either Ptf1a- or Sox9-deficient pancreas [48]. This MPC is characterized by the expression of Pdx1, Ptf1A, Gata4, Sox9, and Foxa2 and the interaction between them reinforce the stable pancreatic fate [49,50]. Sox9 is essential to maintain MPC proliferation, survival and undifferentiated character [39]. Gata4/Gata6 mutant mice exhibit severe pancreas agenesis and Pdx1 expression is reduced in multipotent pancreatic progenitors suggesting the role of these TFs in pancreatic organogenesis [51]. As a result of the action of these TFs developing organ continues to grow rapidly and the pancreatic epithelium undergoes dynamic structural changes, resulting in multiple protrusions that bud from the edges. In addition to the changes in organ shape, the formerly multipotent MPCs have taken the first step toward lineage commitment [52].
▪ Onset of exocrine and endocrine development
When the pancreatic epithelium undergoes a significant process of expansion, branching, and differentiation, MPCs segregate into spatially distinct ‘tip’ and ‘trunk’ domains that generate acinar progenitor cells, and bipotent (ductal/ endocrine) progenitor cells, respectively. The pro-acinar tip cells and trunk cells separation are mediated by the action of TFs [53,54]. Ptf1A is expressed exclusively in the tip cells whereas Sox9, hepatocyte nuclear factor (Hnf1β), and Nkx6.1 are expressed in the trunk domain. PTF1A and the NKX6.1/6.2 factors mutually antagonize each other to specify an exocrine or ductal/endocrine fate. Nkx6.1/6.2 promotes trunk identity and segregate to trunk cells, and Ptf1A has an equivalent role in the tip compartment [51].
Acinar cell development and expansion is guided by the temporal activities of embryonic signaling pathways, including Notch and Wnt signaling [55,56]. Notch signaling assumes a central role in the patterning of MPC into proendocrine and pro-acinar progenitor lineages [57]. Activation of Notch signaling in MPCs promotes a ductal/endocrine progenitor fate. Conversely, Notch signaling attenuation in the early pancreatic epithelium results in an increase in proacinar tip cells and diminished expression of trunk-specific TFs such as Nkx6.1 and Sox9 [55]. Canonical Wnt signaling, mediated by β-catenin, is essential for the development of pancreatic acinar cells; sustained activation of the pathway promotes acinar cell proliferation [56].
Whereas tip cells are fated to become acinar cells which secrete a digestive enzyme, the trunk domain is bipotential and produces ductal and endocrine cells. In contrast to acinar cells, which remain at the distal tips, endocrine and duct progenitors segregate from their acinar counterparts and form a 3-D network of tubules lined by a single layer of polarized epithelial cells [58,59]. Then the decision between endocrine and duct lineage involves the regulation of Neurogenin3 (Ngn3) levels. A subset of cells in the progenitor cords that express TF Ngn3 marks the onset of endocrine cell differentiation while a trunk epithelial cells that do not activate Ngn3 eventually contribute to the ductal tree [60,61]. Concomitantly, Ngn3 a transcription factor that is required for endocrine cell development is transiently expressed. Its up-regulation results in separation of endocrine cells from the duct lineage.
Endocrine differentiation
Lineage tracing study has revealed that islet endocrine cell types derive from Ngn3-positive cells. According to Johansson et al Ngn3+ progenitor cells first differentiated into β-cells, then β-and β-cells, and finally PP-cells in mouse [62]. But β-cell is detected in human first and followed by β-cells, β-cells, and PPcells respectively [29]. During differentiation of Ngn3+ endocrine precursors into hormoneproducing cells, dynamic changes in gene expression occur. This results in activation of Ngn3-dependent transcription factors (Pax4, Arx, Rfx6, NeuroD1, Pax6, Nkx2.2, and Nkx6.1) which allow endocrine precursor cells to move toward a single hormone producing cell fate [50,63].
The transcription factors Pax4, Pdx1, Nkx2-2 and Nkx6.1 are involved in β-cell specification [37]. Several studies have shown the significant role of these TFs. In particular, forced expression of Pax4, Pdx1, or Nkx6.1 in endocrine precursors favors the β-cell fate at the expense of β-cells [64,65]. Pdx1 deficient mice have shown to be glucose intolerant [66] and Pdx1 deleted β cell is not functional [67,68]. Furthermore, Pdx1-deleted β cell rapidly acquired structural and physiological features of β cell, indicating that a robust cellular reprogramming had occurred [43]. Similarly, mice lacking Nkx6.1 display a diminished number of β-cells while other endocrine cell development unchanged [69]. Nkx2.2 null mice also displayed severe hyperglycemia died from it. There are also a decreased numbers of β-cells and PP-cells [70]. Pax4 mutant mice also display a phenotype similar to Nkx2.2 mice and die a few days postpartum [71,72]. Another factor expressed in the β cell is NEUROD. Deletion of Neurod1 resulted in severe glucose intolerance and impaired insulin secretion [73]. Another TF required for β cell development is MafA and MafB. MafB is produced during development and found to regulate potential effectors of glucose sensing, hormone processing, vesicle formation, and insulin secretion whereas MafA is required to sustain expression in adults [74]. MafA mutant mice have no change in endocrine development [75]. Arx is a key factor in β-cell specification and appears to play a key role in this process. Deficiency in Arx leads to a complete loss of β-cells, concomitant with an increase in β- and β-cells [76]. Both Pax4 and Nkx6.1 are direct transcriptional repressors of Arx [64,72].
Β-Cell regeneration approaches for diabetic therapy
As described above both T1DM and T2DM main feature is a defect in either β cells mass or function [6,7]. T1DM results from the complete destruction of pancreatic β-cells by an autoimmune mechanism [4] whereas type 2 diabetes involves impaired β-cell function or insulin resistance at the target cell. An autopsy study found a reduction of more than 40% of β-cells in type 2 diabetic patients, which demonstrates that β-cell loss may also contribute to insulin deficiency in T2DM [77]. As a result, recent researches focus on improving blood glucose regulation by preserving, expanding, or replacing and then improving the function of the β cell in a diabetic patient.
This method for the treatment of diabetes is focused mainly on the transplantation of the pancreas or islet cells and stimulation of human β-cell proliferation to reconstitute the insulin secreting functional β cells. However, these approaches are hampered by several factors such as the need for the use of toxic immunosuppressive agents, and dormant nature of β cells [78]. Other approaches are to protect β-cells from apoptosis [6] and to reprogramme [79] or transdifferentiate other cell types to become insulin-producing cells [80]. Among this approach here recent developments in β-cell regeneration is presented.
Several approaches have been designed to produce insulin-producing β-cell. These include producing β-cell from: (1) Embryonic Stem Cells (ESCs) [81], (2) Induced Pluripotent Stem Cells (IPSCs) [82], (3) Adult Mesenchymal Stem Cells (MSCs) [83], (4) Reprogramming/ transdifferentiation of various none β-cell types, and (5) Induction of replication of existing β cells [79,84].
▪ Embryonic pluripotent stem cells
Embryonic Stem Cells (ESC) are immortal pluripotent cell lines that display a unique capacity to differentiate into somatic cell types of all 3 germ layers: endoderm, ectoderm, and mesoderm [85]. Advance in techniques allowing for isolation and subsequent differentiation of ESCs into specific cell types has raised hope that successful differentiation of ESCs into functional β cells may provide a therapeutically relevant strategy to replace β-cell loss in diabetic patient. The first β-like cells from mouse ESCs was reported in 2001 by Lumelsky et al. [86]. Their method was based on the production of a highly enriched population of nestin-positive cells from embryoid bodies. Another group later reported that human ESCs also differentiated spontaneously to generate insulin-expressing cells [87]. Although these early results provided important evidence for generation of insulinproducing cells, the lack of a sufficient number of insulin-producing cells and low insulin content of generated cells were the limitation to proceed for further clinical studies.
The positive results of these early attempts and advance in the understanding of embryonic β cell development focused researchers on driving ESC differentiation using a variety of protocols. A team from a biotechnology company, Viacyte, Inc. developed a method resembling in vivo pancreatic organogenesis and drove ESCs toward an endocrine phenotype in vitro and obtained a cell with insulin content approaching that of adult islets [88]. However, the generated insulin-producing cells responded to glucose minimally, failed expression of key β-cell transcriptional factors such as NKX6-1 and abnormal polyhormonal phenotypes [89]. The combination of key TFs involved in β-cell development, such as Pdx1, MafA, Ngn3, and NeuroD, improved the efficiency of mouse ESC differentiation into insulin-producing cells [90]. Pagliuca et al recently differentiated pancreatic progenitor to glucose responsive β cells utilizing several new small molecules. These β cells were robustly responsive to glucose challenges with levels of secreted human insulin similar to primary islets [91]. Interestingly, their protocol also describes a novel method of scaling in vitro β cell production for the potential therapeutic application.
In the absence of producing mature β-cells in vitro, other groups sought to use the in vivo environment to induce maturation. For instance, implantation of pancreatic progenitor cells into streptozocin-induced diabetic mice was shown to ameliorate diabetic hyperglycemia and results suggest that the human ESC-derived cells have the capacity to become glucose-responsive β-like cells [92]. Viacyte, Inc has recently started clinical trials to test the efficacy of encapsulated and implanted pancreatic progenitors for the treatment of T1DM [93].
▪ Induced pluripotent stem cells
Induced pluripotent stem cells (iPSCs) are another potential source of β cells generation. The β-cell differentiation potential of iPSCs has been shown in vitro [94] and in vivo [95]. Several protocols have been reported for generation of for iPSCs. Among these methods are retroviral transductions of reprogramming factors to human fibroblasts lines [96], by transient transfection of plasmid DNA encoding reprogramming factors [97] and using a dox-inducible lentiviral system carrying four Yamanaka factors [98] are few to mention. iPSC generation was carried out either by a retroviral gene delivery system or using mRNA for reprogramming [99,100]. iPSCs generated by transduction with retroviruses would unavoidably result in viral genome integration into a host cell which may alter the expression of oncogenes and leads to tumorigenesis, and the efficiency of using mRNA has not been proved [101]. Recently reprogramming with non-synthetic mRNA was reported and we promise for safe generation of iPSCs of human origin by this protocol [102].
Another alternative for generation of clinically applicable human iPSCs requires the use of technologies such as nanoparticle-mediated technology to generate integration-free iPSCs, and use of small molecules such as valproic acid (an HDAC (histone deacetylate) inhibitor), butyrate (an HDAC inhibitor), CHIR99021 (a GSK3β (glycogen synthase kinase 3β) inhibitor), forskolin (coleonol; an adenylyl cyclase activator), tranylcypromine (a monoamine oxidase inhibitor), and TTNPB (arotinoid acid; an analog of retinoic acid (RA)) were reported [103].
Despite the safety issue, iPSCs opened the possibility of autologous cell therapy, thus avoiding the complications of immune rejection and also circumventing some ethical issues with harvesting human ESC. Moreover, the iPSC approach has been used to generate disease-specific IPSCs and insulin-producing cells from patients with diabetes. Thus, iPSCs provide an additional advantage of generating patient-specific cell therapy [104]. Indeed induced pluripotent stem cells may be a promising source of β cell for diabetes therapy. For example, iPSCs have been converted to islet-like clusters which produced insulin and responded to glucose [105].
▪ Mesenchymal stem cells
Mesenchymal Stem Cells (MSCs) are multipotent progenitor cells that were first described in 1967 by Friedenstein et al., who from bone marrow isolated adherent, fibroblast-like clonogenic cells called colonyforming unit-fibroblasts (CFU-F). Although MSCs originally identified in the bone marrow, they are found in almost all tissues. But MSCs from the bone marrow, adipose tissue, the umbilical cord, fetal liver, muscle, and lung can be isolated easily [106,107]. MSCs are a stem cell with self-renewal potential [107]. MSCs have well-characterized immunomodulatory effect and tissue regenerative potential. The combination of immunomodulatory activity, their low immunogenicity, and tissue regenerative potential remains a significant scientific and clinical interest. MSCs are under investigation for the treatment of many diseases including diabetes, myocardial infarction, liver cirrhosis, immunity disorder, and others [107]. The potential uses of MSCs in diabetes are a systemic injection of MSCs to suppress autoreactive T cells [108]; co-transplantation with islet allografts to enhance islet engraftment in an immuno-privileged microenvironment; and differentiation of MSCs into insulinproducing cells [109-112]. MSC has also a potential benefit in the treatment of diabetes associated complication [110].
Several animal model studies and clinical trials have proven the potential use of MSCs in diabetes therapy [111,113-116]. For example, MSC derived from different sources such as dental pulp stem cell, placenta-derived MSCs, amnion derived MSCs, umbilical cord blood MSCs, Wharton’s jelly MSCs, were differentiated into glucose responsive insulin producing cells [117,118]. Similarly, studies aimed at the use of MSC for the management of both macrovascular complications and microvascular complications were carried out in a different setting and showed inspiring results [119].
MSCs offer a significant advantage compared to other stem cells such as ESC or iPSCs. Advantages of MSCs are as follows: (1) The high proliferation and multiple differentiation potentials of MSCs; (2) MSCs can minimize the immune rejection reaction; (3) MSCs can avoid the growth of tumors; (4) MSCs can also avoid social and ethical problems [120].
Although it is known which MSCs can be used for generation of β-cells, amongst these which will be commercially viable and economic source in terms of yield of deriving functional islets is a need to be identified. In addition, the long term stability of the MSC derived insulin-producing cells has not been reported either in vitro or in vivo.
Reprogramming/transdifferentiation of various none β-cell types
Another approach to differentiating β-cells in a stepwise fashion from pluripotent stem cells is the reprogramming of terminally differentiated cell type. Yamanaka and colleagues were demonstrated that terminally differentiated cells could be reprogrammed to pluripotent stem cells by the forced expression of transcription factors [121]. Early examples are insulin-producing liver cells have been generated by adenoviral delivery of NeuroD ± Betacellulin or a combination of PDx-1 and Neuro D or N3 into mice [122]. More recently, Zhou et al. demonstrated that mouse pancreatic exocrine cells forced to express the combination of PDx1, MafA, and Ngn3 or Neuro D can be converted into insulinproducing cells with an efficiency approaching 25% of infected cells [123]. Similarly other cell types have been successfully reprogrammed into β-cells.
▪ Acinar to β-cell reprogramming
The acinar cell has been shown to transdifferentiate into β-cells in vivo and invitro with the generation of duct cells as an intermediate step [10]. One example of this reprogramming-based approach was the conversion of mouse acinar cells to β-cells in vivo via viral expression of particular genes [124]. Another is co-transplantation of fetal pancreatic cells and acinar cells into immunodeficient mice gave rise to endocrine cells [125]. Furthermore, forced expression of three key transcription factors, Pdx1, Ngn3, and MafA, induced acinar to β-cell conversion and rescued hyperglycemia in streptozotocininduced diabetic animals [126,127]. These study reports suggest that β-cells can be derived from the transdifferentiation of acinar cells which support the possible reprogramming of acinar cell to fully functional insulinproducing cells.
▪ α-cell to β-cell reprogramming
α and β cells have closer lineage relationship and express many of the same transcription factors. This close lineage relationship among them may provide an opportunity for efficient transdifferentiation among these cell types. Furthermore, many genes that are part of the β cell signature were bivalently marked in differentiated α cells [128]. As a result, α cell reprogramming could be another attractive option for β cell regeneration. Indeed, Formina and co-workers have identified a small molecule with a limited ability to induce insulin expression by human α-like cells [129].
As described above in pancreas development several TF play a critical role in differentiation of pancreatic progenitor to specific pancreatic cells. For example, Arx promotes the fate of α cell, whereas Pax4 induces β cell lineages at last stage cell differentiation [65,66]. Pdx1which is a marker for pancreas has been used for reprogramming α cells in many studies. For example, Yang et al. found that enforced Pdx1 expression from the Ngn3+ endocrine commitment point onward during the embryonic period resulted in increased β cell allocation accompanied with reduced α cell numbers [130]. Pax4 promotes alphacell- neogenesis and the acquisition of beta-cell features, and the resulting beta-like cells being capable of counteracting chemically induced diabetes [131]. Interestingly, in STZ-induced β cell loss, Pax4-induced β cell conversion to α cells and progressive normalization of hyperglycemia was observed [132,133]. Recently, Sangon et al. reported Hnf4β expression by α cells suppressed glucagon expression, induced key beta cell-specific markers (insulin, C-peptide, glucokinase, GLUT2 and Pax4) and enabled the cells to secrete insulin in a glucose-regulated manner [134].
▪ Induction of replication of existing β cells
During adult life β-cell turnover is very slow and this maintains β-cell mass (79). For example in mice β cells replication decline from 20% per day in mouse pups to over 10% at weaning, and to 2-5% in young adults and 0.07% in one-yearold mice [35]. However, under the conditions of hyperglycemia the older mice β cell proliferate more, which suggests that adult β cells have the ability to replicate [135]. The intracellular mediator of this compensatory mechanism is recently identified to be activation of protein kinase C β (PKCβ). PKCβ activation is a key for early compensatory beta cell replication in insulin resistance by regulating the downstream signals mTOR and cyclin-D2 [136].
Several studies have shown proliferation in primary β-cells following a variety of genetic or pharmacologic interventions. For example, cdk6 have supported human β-cell proliferation and function in vivo (138); aminopyrazine compounds stimulated robust β-cell proliferation in adult primary islets and Aminopyrazinetreated human islets retained functionality in vitro and after transplantation into diabetic mice [137,138]; Harmine has been demonstrated to stimulate an increase in proliferation of adult primary human β cells [139]. The concept of inducing rodent or human β cells to replicate seems applicable but challenges of oncogenicity as a result of manipulating specific mitogenic or regenerative pathways and autoimmunity remains a critical issue as without controlling or protecting against autoimmunity new β cells loss will occur.
▪ Transdifferentiation of other cells to β cells
Cellular reprogramming of other cells also has been proven to be a potential source of new β cells. Hepatocytes, biliary cells, gastrointestinal cells, neural cells, keratinocytes and pancreatic ductal cells were transdifferentiated by different technique to β cells. The majority of the generated β cells were glucose responsive. For a recent review of these areas see the work of Wei and Hong [140].
Conclusion and Future Perspectives
Although more treatment options are available for diabetes patients today than ever before, no therapy in use can consistently halt, reverse, or cure both type 1 and type 2 diabetes. Moreover, current diabetes treatments have significant limitation such as limited efficacy, unwanted side effects, inconvenient dosing and do not eliminate long-term complications associated with the disease. As a result, alternative therapeutic strategies are being investigated.
Recently, there has been a significant advance in our understanding of pancreatic development. Now it seems possible to derive insulinproducing cells from stem cells. However, there is a need for further development of methods for differentiation and selection of completely functional β cells. Furthermore, the key issues like safety concerns, transplantation issues and immunological reaction, and ethical issues of ESCs limit their advance to clinical trials. Nevertheless, the scientific advances enabled us to produce insulin-secreting cells hopefully in the future years may come up with the solutions to use stem cells as a source of β cells to cure diabetes.
Funding
The author(s) received no financial support for the preparation of this review.
References
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 37(1), 81-90 (2014).
- WHO. Global report on diabetes (2016).
- Guariguata L, Whiting DR, Hambleton I et al. Global estimates of diabetes prevalence for 2013 and projections for 2035 for the IDF Diabetes Atlas. Diabetes. Res. Clin. Pract. 103, 137-149 (2014).
- American Diabetes Association. Classification and Diagnosis of Diabetes. Diabetes Care 39(1), 13-22 (2016).
- American Diabetes Association Standards of Medical Care in Diabetes-2016. A Bridged for Primary Care Providers Clinical Diabetes. 34(1), 3-21 (2016).
- Vetere A, Choudhary A, Burns SM, Wagner BK. Targeting the pancreatic β-cell to treat diabetes. Nat. Rev. Drug. 13, 278-289 (2014).
- Philippe A, Halban, Kenneth S, et al. Beta-Cell Failure in Type 2 Diabetes: Postulated Mechanisms and Prospects for Prevention and Treatment. Diabetes Care 37, 1751-1758 (2014).
- Lisa S, Benjamin M, Joseph P et al. The Ideal Diabetes Therapy: What Will It Look Like? How Close Are We? Clin. Diabetes. 30(2), 44-53 (2012).
- Fowler MJ. Microvascular and Macrovascular Complications of Diabetes. 26(2), 77-82 (2008).
- Hyo-Sup Kim, Moon-Kyu Lee. β-Cell regeneration through the transdifferentiation of pancreatic cells: Pancreatic progenitor cells in the pancreas. J. Diabetes. Investig. 7, 286-296 (2016).
- Wang P, Fiaschi-Taesch NM, Vasavada RC et al. Diabetes mellitus-advances and challenges in human β-cell proliferation. Nat. Rev. Endocrinol. 11(4), 201-12 (2015).
- Couri CE, Voltarelli JC. Stem cell-based therapies and immunomodulatory approaches in newly diagnosed type 1 diabetes. Curr. Stem. Cell. Res. Ther. 6, 10-15 (2011).
- Aly H, Rohatgi N, Marshall CA et al. A Novel Strategy to Increase the Proliferative Potential of Adult Human β-Cells While Maintaining Their Differentiated Phenotype. PLoS ONE 8(6), e66131 (2013).
- Domı´nguez-Bendala J, Inverardi L, Ricordi C et al. Regeneration of pancreatic beta-cell mass for the treatment of diabetes. 12(6), 731-741 ( 2012).
- Gianani R. Beta cell regeneration in human pancreas. 33, 23-27 (2011).
- Kofent J, Spagnoli FM. Xenopus as a model system for studying pancreatic development anddiabetes. Semin. Cell. Dev. Biol. 51, 106-116 (2016).
- Pan FC, Wright C. Pancreas Organogenesis: From Bud to Plexus to Gland. Dev. Dynam. 240, 530-565 (2011).
- Quesada I, Tudur E, Ripoll C, Nadal A. Physiology of the pancreatic α-cell and glucagon secretion: role in glucose homeostasis and diabetes. J. Endocrinol. 199, 5-19 (2008).
- Edlun H. Pancreatic Organogenesis Developmental Mechanisms and Implications for Therapy. Nat. Genet. 3, 524-532 (2002).
- Piper K, Brickwood S, Turnpenny LW et al. Beta cell differentiation during early human pancreas development. J. Endocrinol. 181, 11-23 (2004).
- Sukanya L, Elizabeth AO, Norann AZ. Primary Cilia in Pancreatic Development and Disease. Birth. Defects. Res. 102, 139-158 (2014).
- Zorn AM, Wells JM. Molecular basis of vertebrate endoderm development. Int. Rev. Cytol. 259, 49-111 (2007).
- Gittes GK. Developmental biology of the pancreas:A comprehensive review. Dev. Biol. 326, 4-35 (2009).
- Liu P, Wakamiya M, Shea MJ et al. Requirement for Wnt3 in vertebrate axis formation. Nat. Genet. 22, 361-365 (1999).
- Talitha VM, Mark OH. Maturation of Stem Cell-Derived Beta-cells Guided by the Expression of Urocortin 3. Rev. Diabet. Stud. 11(1), 115-132 (2014).
- Jeon J, Correa-Medina M, Ricordi C, Edlund H, Diez JA. Endocrine cell clustering during human pancreas development. J. Histochem. Cytochem. 57, 811-824 (2009).
- Gao N, LeLay J, Vatamaniuk M et al. Dynamic regulation of Pdx1 enhancers by Foxa1 and Foxa2 is essential for pancreas development. Genes. Dev. 22, 3435- 3448 (2008).
- Felicia W, Douglas A. How to make a functional β-cell? Development 140, 2472-2483 (2013).
- Aaron M, James M. Vertebrate Endoderm Development and Organ Formation. Annu. Rev. Cell. Dev. Biol. 25, 221-251 (2009).
- Jacqueline FO, Claire JS. Endocrine pancreatic development: impact of obesity and diet. Front. Physiol. 4, 70 (2013).
- Jaramillo M, Mathew S, Task K, Barner S, Banerjee I. Potential for Pancreatic Maturation of Differentiating Human Embryonic Stem Cells is Sensitive to the Specific Pathway of Definitive Endoderm Commitment. PLoS ONE 9(4), e94307 (2014).
- Jergensen MC, Ahnfelt-Renne J, Hald J et al. An illustrated review of early pancreas development in the mouse. Endocr. Rev. 28, 685-705 (2007).
- Lau J, Hebrok M. Hedgehog signalling in pancreas epithelium regulates embryonic organ formation and adult beta-cell function. Diabetes 59, 1211-1221 (2010).
- Hebrok M, Kim SK, Melton DA. Notochord repression of endodermal Sonic hedgehog permits pancreas development. Genes. Dev. 12, 1705-1713 (1998).
- Márquez-Aguirre AL, Canales-Aguirre AA, Padilla-Camberos E, Esquivel-Solis H, Díaz-Martínez NE. Development of the endocrine pancreas and novel strategies for β-cell mass restoration and diabetes therapy. Braz. J. Med. Biol. Res. 48(9), 765-776 (2015).
- Deutsch G, Jung J, Zheng M, Lora J, Zaret KS. A bipotential precursor population for pancreas and liver within the embryonic endoderm. Development 128, 871-881 (2001).
- Dassaye R, Naidoo S, Cerf ME. Transcription factor regulation of pancreatic organogenesis, differentiation and maturation. Islets 8(1), 13-34 (2016).
- Seymour PA. Sox9: A Master Regulator of the Pancreatic Program. Rev. Diabet. Stud. 11(1), 51-83 (2014).
- Seymour PA, Freude KK, Tran MN et al. SOX9 is required for maintenance of the pancreatic progenitor cell pool. Proc. Natl. Acad. Sci. 104, 1865-1870 (2007).
- Murtaugh CL. Pancreas and beta-cell development: from the actual to the possible. Development. 134, 427-438 (2007).
- Eifes S, Chudasama KK, Molnes J et al. A novel GATA6 mutation in a child with congenital heart malformation and neonatal diabetes. Clin. Case. Rep. 1, 86-90 (2013).
- Weedon MN, Cebola I, Patch AM et al. Recessive mutations in a distal PTF1A enhancer cause isolated pancreatic agenesis. Nat. Genet. 46, 61-64 (2014).
- Gao T, McKenna B, Li C et al. Pdx1 maintains β cell identity and function by repressing α cell program. Cell. Metab. 19, 259-271 (2014).
- Jennings RE, Berry AA, Kirkwood-Wilson R et al. Development of the human pancreas from foregut to endocrine commitment. Diabetes. 62, 3514-3522 (2013).
- Stoffers DA, Heller RS, Miller CP, Habener JF. Developmental expression of the homeodomain protein IDX-1 in mice transgenic for an IDX-1 promoter/lacZ transcriptional reporter. Endocrinology 140(11), 5374-5381 (1999).
- Ye F, Duvillie B, Scharfmann R. Fibroblast growth factors 7 and 10 are expressed in the human embryonic pancreatic mesenchyme and promote the proliferation of embryonic pancreatic epithelial cells. Diabetologia 48, 277-281 (2005).
- Bonfanti P, Nobecourt E, Oshima M et al. Ex vivo expansion and differentiation of human and mouse fetal pancreatic progenitors are modulated by epidermal growth factor. Stem. Cells. Dev. 24, 1766-1778 (2015).
- Seymour PA, Shih HP, Patel NA et al. A Sox9/Fgf feed-forward loop maintains pancreatic organ identity. Development 139, 3363-3372 (2012).
- Zhou Q, Law AC, Rajagopal J et al. A multipotent progenitor domain guides pancreatic organogenesis. Dev. Cell. 13, 103-114 (2007).
- Rieck S, Bankaitis ED, Wright CV. Lineage determinants in early endocrine development. Semin. Cell. Dev. Biol. 23, 673- 684 (2012).
- Carrasco M, Delgado I, Soria B, Martin F, Rojas A. GATA4 and GATA6 control mouse pancreas organogenesis. J. Clin. Investig. 122(10), 3504-3515 (2012).
- Shih HP, Wang A, Sander M. Pancreas Organogenesis: From Lineage Determination to Morphogenesis. Annu. Rev. Cell. Dev. Biol.29, 81-105 (2013).
- Schaffer AE, Freude KK, Nelson SB et al. Nkx6 transcription factors and Ptf1a function as antagonistic lineage determinants in multipotent pancreatic progenitors. Dev. Cell. 18, 1022-1029 (2010).
- Kopp JL, Dubois CL, Schaffer AE et al. Sox9+ ductal cells are multipotent progenitors throughout development but do not produce new endocrine cells in the normal or injured adult pancreas. Development 138(4), 653-665 (2011).
- Solomon A, Jan J. Notch signaling in the pancreas: patterning and cell fate specification. WIREs. Dev. Biol. 2, 531-544 (2013).
- Murtaugh C. The what, where, when and how of Wnt/β-catenin signaling in pancreas development. Organogenesis 4(2), 81-86 (2008).
- Afelik S, Qu X, Hasrouni E et al. Notch-mediated patterning and cell fate allocation of pancreatic progenitor cells. Development 39(10), 53-1744 (2012).
- Zhou Q, Law AC, Rajagopal J et al. A multipotent progenitor domain guides pancreatic organogenesis. Dev. Cell. 13, 14-103 (2007).
- Villasenor A, Chong DC, Henkemeyer M, Cleaver O. Epithelial dynamics of pancreatic branching morphogenesis. Development 137, 305-4295 (2010).
- Magenheim J, Klein AM, Stanger BZ et al. Ngn3 (+) endocrine progenitor cells control the fate and morphogenesis of pancreatic ductal epithelium. Dev. Biol. 359, 26-36 (2011).
- Wang S, Yan J, Anderson DA et al. Neurog3 gene dosage regulates allocation of endocrine and exocrine cell fates in the developing mouse pancreas. Dev. Biol. 339, 26-37 (2010).
- Johansson KA, Dursun U, Jordan N et al. Temporal control of neurogenin3 activity in pancreas progenitors reveals competence windows for the generation of different endocrine cell types. Dev. Cell. 12, 65-457 (2007).
- Gouzi M, Kim YH, Katsumoto K, Johansson K, Grapin-Botton A. Neurogenin3 initiates stepwise delamination of differentiating endocrine cells during pancreas development. Dev. Dyn. 240, 589-604 (2011).
- Schaffer AE, Taylor BL, Benthuysen JR et al. Nkx6.1 controls a gene regulatory network required for establishing and maintaining pancreatic beta cell identity. PLoS. Genet. 9, e1003274 (2013).
- Yang YP, Thorel F, Boyer DF, Herrera PL, Wright CV. Context-specific α-to-β-cell reprogramming by forced Pdx1 expression. Genes. Dev. 25, 85-1680 (2011).
- Brissova M, Shiota M, Nicholson WE et al. Reduction in pancreatic transcription factor PDX- 1 impairs glucose-stimulated insulin secretion. J. Biol. Chem. 277, 11225-11232 (2002).
- Gannon M, Ables ET, Crawford L et al. Pdx-1 function is specifically required in embryonic β cells to generate appropriate numbers of endocrine cell types and maintain glucose homeostasis. Dev. Biol. 314, 406-417 (2008).
- Fujimoto K, Polonsky KS. Pdx1 and other factors that regulate pancreatic β-cell survival. Diabetes. Obes. Metab. 11(4), 30-37 (2009).
- Sander M, Sussel L, Conners J et al. M. Homeobox gene Nkx6.1 lies downstream of Nkx2.2 in the major pathway of β-cell formation in the pancreas. Development 127, 5533-5540 (2000).
- Sussel L, Kalamaras J, Hartigan-O'Connor DJ, et al. Mice lacking the homeodomain transcription factor Nkx2.2 have diabetes due to arrested differentiation of pancreatic β cells. Development. 125, 2213-2221 (1998).
- Prado CL, Pugh-Bernard AE, Elghazi L, Sosa-Pineda B, Sussel L. Ghrelin cells replace insulin-producing beta cells in two mouse models of pancreas development. Proc. Natl. Acad. Sci. USA. 101(9), 2924-2929 (2004).
- Tiziana N, Fabio A, Monica C et al. Pax4 acts as a key player in pancreas development and plasticity. Semin. Cell. Dev. Biol. 44, 107-114 (2015).
- Gu C, Stein GH, Pan N et al. Pancreatic β cells require NeuroD to achieve and maintain functional maturity. Cell. Metab. 11, 298-310 (2010).
- Artner I, Hang Y, Mazur M et al. MafA and MafB Regulate Genes Critical to β-Cells in a Unique Temporal Manner. Diabetes 59(10), 2530-2539 (2010).
- Zhang C, Moriguchi T, Kajihara M et al. MafA is a key regulator of glucose stimulated insulin secretion. Mol. Cell. Biol. 25, 76-4969 (2005).
- Collombat P, Mansouri A, Hecksher-Sorensen J et al. Opposing actions of Arx and Pax4 in endocrine pancreas development. Genes. Dev. 17(20), 2591-2603 (2003).
- Rahier J, Guiot Y, Goebbels RM, Sempoux C, Henquin JC. Pancreatic Beta-Cell Mass in European Subjects with Type 2 Diabetes. Diabetes. Obes. Metab. 10, 32-42 (2008).
- Meier JJ, Butler AE, Saisho Y et al. Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes 57, 1584-1594 (2008).
- Orlando G, Gianello P, Salvatori M et al. Cell Replacement Strategies Aimed at Reconstitution of the β-Cell Compartment in Type 1 Diabetes. Diabetes 63, 144-1433 (2014).
- Limbert C, Paeth G, Jakob F, Seufert J. Beta-cell replacement and regeneration Strategies of cell-based therapy for type 1 diabetes mellitus. Diabetes. Res. Clin. Pract. 79(3), 389-399 (2008).
- Hoof DV, D'Amour KV, Michael S. German Derivation of insulin-producing cells from human embryonic stem cells. Stem. Cell. Res. 3(2-3), 73-87 (2009).
- Kumar SS, Alarfaj AA, Munusamy MA et al. Recent Developments in β-Cell Differentiation of Pluripotent Stem Cells Induced by Small and Large Molecules. Int. J. Mol. Sci. 15, 23418-23447 (2014).
- Caoa X, Zhi-Bo Hanb, Zhaoc H, Liu Q. Transplantation of mesenchymal stem cells recruits trophic macrophages to induce pancreatic beta cell regeneration in diabetic mice. Int. J. Biochem. Cell. Biol. 53, 9-372 (2014).
- Shena J, Chenga Y, Hanb Q, Mua Y, Hanb W. Generating insulin-producing cells for diabetic therapy: Existing strategies and new development. Ageing. Res. Rev. 12, 469- 478 (2013).
- Biswas A, Hutchins R. Embryonic Stem Cells. Stem. Cells. Dev. 16(2), 213-222 (2007).
- Lumelsky N, Blondel O, Laeng P et al. Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science 292, 1389-1394 (2001).
- Assady S, Maor G, Amit M et al. Insulin production by human embryonic stem cells. Diabetes 50, 7-1691 (2001).
- D’Amour KA, Bang AG, Eliazer S et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat. Biotechnol. 24, 1392-1401 (2006).
- Hrvatin S, O’Donnell CW, Deng F et al. Differentiated human stem cells resemble fetal, not adult, β cells. Proc. Natl. Acad. Sci. U S A. 111(8), 3038-3043 (2014).
- Xu H, Tsang KS, Chan JC et al. The Combined Expression of Pdx1 and MafA with Either Ngn3 or NeuroD Improves the Differentiation Efficiency of Mouse Embryonic Stem Cells into Insulin-Producing Cells. Cell. Transplant. 22, 147-158 (2013).
- Pagliuca FW, Millman JR, Gürtler M et al. Generation of functional human pancreatic β cells in vitro. Cell 159,428-439 (2014).
- Rezania A, Bruin JE, Riedel MJ et al. Maturation of human embryonic stem cell-derived pancreatic progenitors into functional islets capable of treating pre-existing diabetes in mice. Diabetes 61, 2016-2029 (2012).
- https://clinicaltrials.gov/show/NCT02239354 accessed on 03/2/2016
- Maehr R, Chen S, Snitow M et al. Generation of pluripotent stem cells from patients with type 1 diabetes. Proc. Natl. Acad. Sci. U S A. 106, 15768 -15773 (2009).
- Zhu FF, Zhang PB, Zhang DH et al. Generation of pancreatic insulin-producing cells from rhesus monkey induced pluripotent stem cells. Diabetologia 54, 2325-2336 (2011).
- In-Hyun Park, Lerou PH, Zhao R, Huo H, Daley GQ. Generation of human-induced pluripotentstem cells. Nat. Protoc. 3(7), 1180-1186 (2008).
- Si-Tayeb K, Noto FK, Sepac A et al. Generation of human induced pluripotent stem cells by simple transient transfection of plasmid DNA encoding reprogramming factors. BMC. Dev. Biol. 10, 81 (2010).
- Wang J, Gu Q, Hao J et al. Generation of Induced Pluripotent Stem Cells with High Efficiency from Human Umbilical Cord Blood Mononuclear Cells. Genom. Proteom. Bioinform. 11(5), 304-311 (2013).
- Stadtfeld M, Maherali N, Borkent M, Hochedlinger K. A reprogrammable mouse strain from gene-targeted embryonic stem cells. Nat. Methods. 7, 53-55 (2010).
- Heng BC, Heinimann K, Miny P et al. mRNA transfection-based, feeder-free, induced pluripotent stem cells derived from adipose tissue of a 50-year-old patient. Metab. Eng. 18, 9-24 (2013).
- Gonzalez F, Boue S, Belmonte JC. Methods for making induced pluripotent stem cells: reprogramming a la carte. Nat. Rev. Gene. 12, 231-242 (2011).
- Rohani L, Fabian C, Holland H et al. Generation of human induced pluripotent stem cells using non-synthetic mRNA. Stem. Cell. Res. 16, 662-672 (2016).
- Mohammed K, Ahmed A, Jihae H et al. Recent Advances in Disease Modeling and Drug Discovery for Diabetes Mellitus Using Induced Pluripotent Stem Cells. Int. J. Mol. Sci. 17(2), 256 (2016).
- Matveyenko A, Vella A. Regenerative Medicine in Diabetes. Mayo. Clin. Proc. 90(4), 546-554 (2015).
- Shaer A, Azarpira N, Vahdati A, Karimi MH, Shariati M. Differentiation of human-induced pluripotent stem cells into insulin-producing clusters. Exp. Clin. Transplant. 13(1), 68-75 (2015).
- Wei X, Yang X, Zhi-peng HAN et al. Mesenchymal stem cells: a new trend for cell therapy. Acta. Pharmacologica. Sinica. 34, 747-754 (2013).
- Williams AR, Hare JH. Mesenchymal Stem Cells Biology, Pathophysiology, Translational Findings, and Therapeutic Implications for Cardiac Disease. Circ. Res. 109, 907-909 (2011).
- Madec AM, Mallone R, Afonso G et al. Mesenchymal stem cells protect NOD mice from diabetes by inducing regulatory T cells. Diabetologia 52, 1391-1399 (2009).
- Moore RF, Mounayar M, Abdi R. Utility of Mesenchymal Stem Cell Therapy in Type 1 Diabetes in M.A. Hayat Stem Cells and Cancer Stem Cells, Springer, Netherlands 6, 197-203 (2012).
- Volarevic V, Arsenijevic N, Lukic ML, Stojkovic M. Mesenchymal stem cell treatment of the complications of diabetes mellitus. Stem. Cells. 29(1), 5-10 (2011).
- Jiang R, Han Z, Zhuo G et al. Transplantation of placenta-derived mesenchymal stem cells in type 2 diabetes: a pilot study. Front. Med. 5(1), 94-100 (2011).
- Wu H, Mahato R. Mesenchymal stem cell-based therapy for type 1 diabetes. Discov. Med. 17(93), 139-43 (2014).
- Pileggi A. Mesenchymal Stem Cells for the Treatment of Diabetes. Diabetes 61, 1355-1356 (2012).
- Phadnis SM, Joglekar MV, Dalvi MP et al. Human bone marrow-derived mesenchymal cells differentiate and mature into endocrine pancreatic lineage in vivo. Cytotherapy. 13, 279-293 (2011).
- Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS ONE 3, e1886 (2008).
- Ezquer M, Arango-Rodriguez M, Giraud-Billoud M, Ezquer F. Mesenchymal Stem Cell Therapy in Type 1 Diabetes Mellitus and its Main Complications: From Experimental Findings to Clinical Practice. J. Stem. Cell. Res. Ther. 4, 227 (2014).
- Bhonde RR, Sheshadri P, Sharma S, Kumar A. Making surrogate β-cells from mesenchymal stromal cells: Perspectives and future endeavours. Int. J. Biochem. Cell. Biol. 46, 90-102 (2014).
- Hashemian SJ, Kouhnavard M, Nasli-Esfahani E. Mesenchymal Stem Cells: Rising Concerns over Their Application in Treatment of Type One Diabetes Mellitus. J. Diabetes. Res. (2015).
- Davey GC, Patil SB, O’Loughlin A, O’Brien T. Mesenchymal stem cell-based treatment for microvascular and secondary complications of Diabetes mellitus Front. Endocrinol. 6, 5-86 (2014).
- Zhang S, Bai C, Ma Y et al. The characterisation and functional β-cell differentiation of duck pancreas derived mesenchymal cells. Br. Poult. Sci. 57(2), 201-210 (2016).
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 126(4), 76-663 (2006).
- Kaneto H, Nakatani Y, Miyatsuka T et al. PDX-1/VP16 fusion protein, together with NeuroD or NGN3, markedly induces insulin gene transcription and ameliorates glucose tolerance. Diabetes 54(4), 22-1009 (2005).
- Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature 455(7213), 32-627 (2008).
- Zhou Q, Melton DA. Extreme makeover: converting one cell into another. Cell. Stem. Cell. 3, 382-388 (2008).
- Quaranta P, Antonini S, Spiga S et al. Co-Transplantation of Endothelial Progenitor Cells and Pancreatic Islets to Induce Long-Lasting Normoglycemia in Streptozotocin-Treated Diabetic Rats. PLoS ONE 9(4), e94783 (2014).
- Akinci E, Banga A, Greder LV, Dutton JR, Slack JM. Reprogramming of pancreatic exocrine cells towards a beta cell character using Pdx1, Ngn3 and MafA. Biochem. J. 442, 539-550 (2012).
- Li W, Nakanishi M, Zumsteg A et al. In vivo reprogramming of pancreatic acinar cells to three islet endocrine subtypes. Elife 3, e01846 (2014).
- Nuria C, Bramswig L, Everett J et al. Epigenomic plasticity enables human pancreatic alpha to beta cell reprogramming. J. Clin. Invest. 123, 1275-1284 (2013).
- Fomina-Yadlin D, Kubicek S, Walpita D et al. Small-molecule inducers of insulin expression in pancreatic alpha-cells. Proc. Natl. Acad. Sci. U S A. 107(34), 104-15099 (2010).
- Yu-Ping Y, Fabrizio T, Daniel F, Pedro L, Christopher VE. Context-specific alpha-to-beta-cell reprogramming by forced Pdx1expression. Genes. Dev. 25, 1680-1685 (2011).
- Courtney M, Rabe T, Collombat P, Mansouri A. Pax4 and Arx Represent Crucial Regulators of the Development of the Endocrine Pancreas. New. J. Sci. (2014).
- Collombat P, Xu X, Ravassard P et al. The ectopic expression of Pax4 in the mouse pancreas converts progenitor cells into alpha and subsequently beta cells. Cell. 138, 449-462 (2009).
- Al-Hasani K, Pfeifer A, Courtney M et al. Adult duct-lining cells can reprogram into beta-like cells able to counter repeated cycles of toxin- induced diabetes. Dev. Cell. 26, 86-100 (2013).
- Caroline B, Ramiro J, Harry H, David T. In vitro reprogramming of pancreatic alpha cells towards a beta cell phenotype following ectopic HNF4α expression. Mol. Cell. End. 399, 50-59 (2015).
- Salpeter SJ, Klein AM, Huangfu D, Grimsby J, Dor Y. Glucose and aging control the quiescence period that follows pancreatic beta cell replication. Development. 137, 3205-3213 (2010).
- Lakshmipathi J, Alvarez-Perez JC, Rosselot C et al. PKC-ζ is essential for pancreatic beta cell replication during insulin resistance by regulating mTOR and cyclin-D2. Diabetes 65(5), 96-1283 (2016).
- Nathalie M, Taesch F, Salim F et al. Induction of Human β-Cell Proliferation and Engraftment Using a Single G1/S Regulatory Molecule, cdk6. Diabetes 59, 1926-1936 (2010).
- Shen W, Taylor B, Jin Q et al. Inhibition of DYRK1A and GSK3B induces human b-cell proliferation. Nat. Commun. 6, 8372 (2015).
- Wang P, Alvarez-Perez JC, Felsenfeld DP et al. A high-throughput chemical screen reveals that harmine mediated inhibition of DYRK1A increases human pancreatic beta cell replication. Nat. Med. 21, 383-388 (2015).
- Wei R, Hong T. Lineage Reprogramming: A Promising Road for Pancreatic β Cell Regeneration. Trends. Endocrinol. Metab. 27(3), 163-176 (2016).