Review Article - Imaging in Medicine (2011) Volume 3, Issue 4
Transabdominal ultrasonography of the pancreas: basic and new aspects
Friedemann G Erchinger†, Georg Dimcevski1,2, Trond Engjom1 & Odd H Gilja1,2,31Section of Gastroenterology, Department of Medicine, Haukeland University Hospital, Bergen, Norway
2Institute of Medicine, University of Bergen, Norway
3National Centre for Ultrasound in Gastroenterology, Haukeland University Hospital, Bergen, Norway
- Corresponding Author:
- Friedemann G
Erchinger†
Department of Medicine
Voss Hospital, Haukeland University Hospital
5700 Voss, Norway
Tel: +47 5653 3754
Fax: +47 5651 3072
E-mail:friedemann.erchinger@ helse-bergen.no
Abstract
Ultrasonography as a diagnostic tool has made important contributions to the technical revolution in medical imaging over the last decades. It has become the preferred method for first-line imaging in a broad spectrum of diseases. This article focuses on basic and advanced applications of transabdominal ultrasonography in pancreatic imaging. The first section describes technical principles and their consequences for image quality: harmonic imaging, Doppler, duplex, triplex ultrasonography, power Doppler, contrast-enhanced ultrasonography, panoramic imaging and elastography. The second section briefly describes examination procedures and scanning techniques with the aim to obtain complete visualization of the pancreas. In the third section we outline ultrasonographic characteristics of pancreatic diseases with emphasis on contrast-enhanced ultrasound and elastography.
Keywords
CEUS ▪ chronic pancreatitis ▪ elastography ▪ medical imaging technique ▪ pancreas ▪ transabdominal ultrasonography ▪ ultrasonography
After more than 40 years in practice, transabdominal ultrasonography is still mandatory, and the most commonly used first-line imaging modality in the diagnostic work-up of abdominal diseases. Ultrasonography is broadly available, cheap and easy to use. It has almost no side effects, and can be repeated everywhere at any time. As an ‘online’ continuous imaging modality, transabdominal ultrasonography gives an overview, localizes the ‘point of interest’ and contributes to identifying possible causes of disease. Finally, it reduces the use of other diagnostic methods, which are often costly and personnel intensive [1].
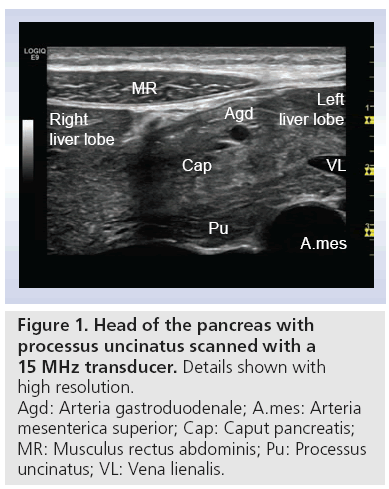
Methods, value and limitations of transabdominal ultrasonography in the diagnosis of pancreatic diseases, such as pancreatic tumors, acute and chronic pancreatitis (CP), were outlined in the 1970s [2]. Early works described evidence of pancreatic abnormality in transabdominal ultrasonography in approximately 60% of CP cases. Concerning diagnostics of pancreatic malignancy, some studies described pathological findings in all diagnosed cases, but in no case could a final diagnosis be made [2–5]. On the contrary, as early as the 1990s clinical enthusiasts equipped with modern scanners and experience in ultrasonography, refer to complete imaging of the pancreas in 90% of CP cases [6]. The overall sensitivity and specificity of transabdominal ultrasonography and its different modalities, especially contrast-enhanced, is not yet established, but they offer the possibility of easy accessible, real-time and high-resolution imaging. Figure 1 shows an image of the pancreas, liver and surrounding vessels with a 15 MHz transducer. Note the remarkably high resolution of the image. In addition, note the marked difference in echogenicity between the dorsal and ventral anlage.
Individual patient factors such as obesity and intestinal air are the most frequent limitations in scanning of the pancreas [7–12]. Experience in manipulating ultrasound transducer position, patient position and patient breathing can frequently overcome these limitations. Furthermore, using a water-filled stomach as an acoustic window will improve pancreatic scanning. Several technical modalities and methods have also ameliorated imaging quality in transabdominal ultrasonography. However, all these improvements in technology are useless if the visibility is poor. Endoscopic ultrasonography (EUS) may reduce these problems but it is a time-consuming and personnel-intensive procedure, and not as widely accessible as transabdominal ultrasonography. Despite significant improvement in ultrasound imaging, CT and MRI are still the main imaging modalities for pancreatic diseases.
The aim of this article is to describe available modalities and methods of modern ultrasound scanners when performing transabdominal ultrasonography of the pancreas. The authors suggest that transabdominal ultrasonography has a place in imaging of the pancreas, even in the age of EUS, CT, MRI and PET.
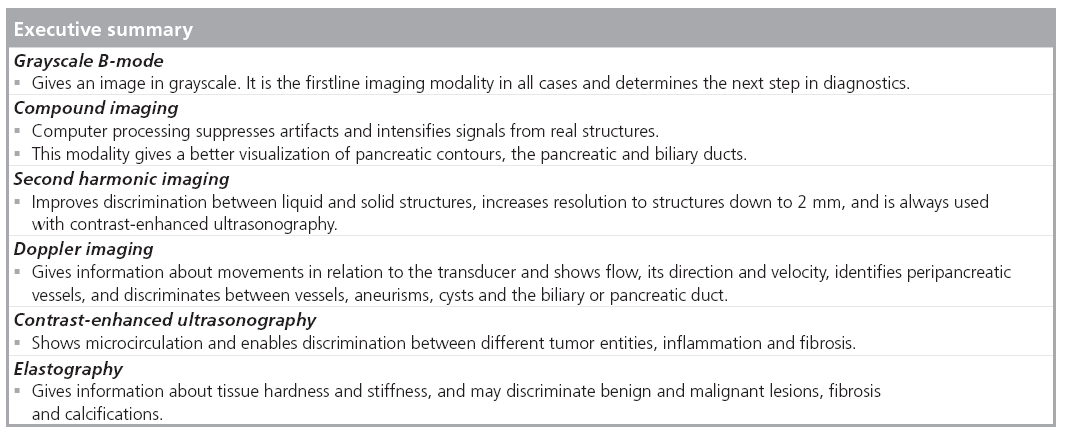
Modalities in ultrasonography
B-mode ultrasound of the pancreas enables rapid evaluation of organ size, borders echostructure, vessels and ductuli (Figure 1) . By considering these parameters, many diseases of the pancreas can be diagnosed and patients can be managed accordingly. Most frequently the echogenicity of the pancreas increases with age. However, there are several techniques that can further enhance pancreatic ultrasound scanning, and these are discussed below.
Tissue harmonic imaging improves the image quality and overcomes several of the limitations of the B-mode [13]. Sending the ultrasound beam at a lower frequency than it is received at clears up the lateral delineation and abolishes reverberation artifacts, thereby increasing the spatial and contrast resolution. This improves the discrimination between liquid and solid structures, making structures down to 2 mm in diameter visible. Some authors state that ultrasonography with tissue harmonic imaging can compete with CT and MRI, and that in some cases it may even be superior [14,15]. This ultrasound modality is a step forward, but there are three main disadvantages: reduction of frame rate, reduced penetration depth and only marginal improvement of image quality in the near field.
Doppler imaging enables flow records from vessels to be included in ultrasound imaging and to show blood flow direction and velocity. Two modalities in conventional Doppler ultrasonography exist. Continuous wave Doppler can measure high flow in, for example, stenosis, but the exact localization of stenosis cannot be shown. By contrast, pulse waved Doppler has a lower accuracy at high velocities.
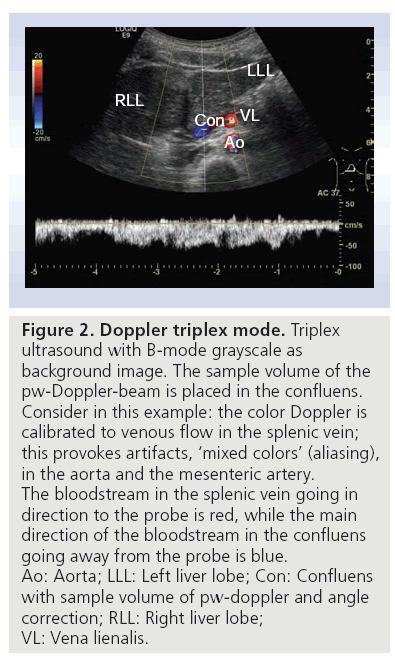
Color-coded Doppler in combination with B-mode is called duplex. Duplex in combination with continuous wave or pulse waved Doppler is called triplex ultrasound, a term which is not often used. Figure 2 shows triplex ultrasound with B-mode grayscale as the background image, and color Doppler used for imaging of the splenic vein, confluens and aorta. The sample volume of the pulse waved Doppler beam is placed in the confluens. Consider in this example: the color Doppler is calibrated to venous flow in the splenic vein; this provokes artifacts and ‘mixed colors’ (called aliasing) in the arterial vessels (aorta and arteria mesenterica). The colors in the splenic vein and confluens are both red and blue. The bloodstream in the splenic vein is red because it goes towards the probe. By contrast, the main direction of the bloodstream in the portal vein goes away from the probe, so it is blue. Using this technique and using the advantage that the pancreas is surrounded by vessels, color Doppler may show the outlines of the organ more precisely. Intrapancreatic vessels are normally small, and therefore difficult to show in conventional Doppler imaging. This method can also be used to show flows in normal versus pathological conditions, such as in tumors with high vessel infiltration, or in discriminating between cystic processes without blood flow and aneurisms. Current high-end scanners can distinguish between inflammation (high flow) and infarction (no flow) [16].
Figure 2: Doppler triplex mode. Triplex ultrasound with B-mode grayscale as background image. The sample volume of the pw-Doppler-beam is placed in the confluens. Consider in this example: the color Doppler is calibrated to venous flow in the splenic vein; this provokes artifacts, ‘mixed colors’ (aliasing), in the aorta and the mesenteric artery. The bloodstream in the splenic vein going in direction to the probe is red, while the main direction of the bloodstream in the confluens going away from the probe is blue. Ao: Aorta; LLL: Left liver lobe; Con: Confluens with sample volume of pw-doppler and angle correction; RLL: Right liver lobe; VL: Vena lienalis.
Power Doppler is another Doppler mode that enables slow velocities to be detected, but not the direction of the flow. Thus, fine flow details in small vessels can be observed. On the other hand, tissue movements such as respiration, pulse displacement or movement of the transducer may cause artifacts [17].
Methods in ultrasonography
Second-order ultrasound field imaging (SURF) uses both low and high frequency simultaneously to transmit ultrasound waves [18–20]. This reduces acoustic noise and augments the effect of contrast agents. The SURF method has been proposed to increase the sensitivity in detecting calcifications. Therefore, SURF may have a future application in imaging of CP.
■ Panoramic imaging
Conventional ultrasound has a limited field of view, with the consequence that only parts of the pancreas can be shown in one image. Assessment of the whole pancreas in one image is called the panorama technique. It is based on real-time image reconstruction, analyzing local and global movements, and computing the position of the transducer by image analysis.
■ Compound imaging
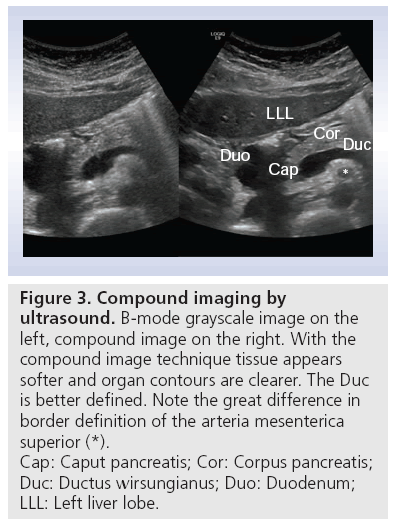
Compound imaging obtains multiple coplanar images from different angles using computed beam steering. Considerable computer processing power is necessary to suppress artifacts, intensify signals from real structures and produce reliable images with improved tissue definition (Figure 3). Using this method with several angles of view, limitations in transabdominal ultrasonography, such as cast shadows behind pancreatic calcifications or ultrasound enhancement behind pancreatic (pseudo) cysts, can be diminished or eliminated [21].
Figure 3: Compound imaging by
ultrasound. B-mode grayscale image on the
left, compound image on the right. With the
compound image technique tissue appears
softer and organ contours are clearer. The Duc
is better defined. Note the great difference in
border definition of the arteria mesenterica
superior (*).
Cap: Caput pancreatis; Cor: Corpus pancreatis;
Duc: Ductus wirsungianus; Duo: Duodenum;
LLL: Left liver lobe.
■ Contrast-enhanced ultrasonography
Contrast-enhanced ultrasonography (CEUS) is one of the most important advances that has occurred in ultrasonography over the last two decades. One example is the rapid success in detection and characterization of focal liver lesions, where CEUS in many studies has demonstrated similar accuracy in diagnostic value as CT and MRI [22,23]. Transabdominal ultrasonography with CEUS in the pancreas probably has the same potential [24], and has in some centers been established in diagnostic routine. It is most often used as a supplement in characterizing already known pancreatic lesions more precisely [25]. The existing studies show that transabdominal CEUS also has potential in differentiating malignancy and CP [26,27]. There is an important difference in the time window between a pancreatic CEUS examination and the well-established liver CEUS study: the blood supply of the pancreas is entirely arterial and the enhancement of the gland begins just after the aortic enhancement, thus the pancreatic enhancement reaches its peak between 15 and 20 s after injection of the ultrasound contrast agent. Accordingly, pancreatic tissue enhancement is earlier and shorter than that of the liver due to the absence of a venous blood supply such as the portal vein in the liver. After a marked parenchymal enhancement in the early contrast-enhanced arterial phase, there is a progressive washout of contrast medium with gradual loss of echogenicity [28]. The principle of contrast-specific ultrasound modes is suppression of ultrasound signals of tissue and augmenting ultrasound response from microbubbles. Low acoustic ultrasound pressure at a given frequency proportional to the mechanical index is used. Sulfur hexafluoride microbubbles satisfy these requirements. They have a mean diameter of 2.5 μm and this allows visualization of the microcirculation [25,29]. Ultrasound contrast agents have minimal side effects compared with x-ray or magnetic resonance contrast agents; they are not toxic, and do not influence the metabolism of the thyroid gland. The most serious adverse event is anaphylactic reaction. The frequency of anaphylaxis using registered ultrasound contrast agents is low (0.001% in ultrasound and 0.07% in x-ray) [25,30].
■ Elastography
Using ultrasound technology there are several methods that can be applied to study strain and elasticity. Ophir et al. developed ultrasonic methods for quantitative imaging of strain and elastic modulus distributions in soft tissues [31,32]. This method is based on external tissue compression with subsequent computation of the strain profile along the transducer axis. The temporal derivative of strain (i.e., the strain rate) is a measure of the rate of deformation. Strain rate imaging is a Doppler-based method that can be used to measure the strain of moving tissue [33–35], but it can also be applied to the other organs by inducing probe pressure.
Recently, in vivo quantitative mapping of viscoelasticity was also performed using the concept of supersonic shear wave imaging [36]. This technique is based on the combination of a radiation force induced in tissues by focused ultrasonic beams and very high frame rate (up to 5000 f/s) ultrasound images capable of catching in real time the transient propagation of resulting shear waves [37,38]. The local shear wave velocity is recovered using a dedicated time-of-flight estimation technique and enables the 2D quantitative mapping of shear elasticity. This imaging modality can be performed using a conventional ultrasound probe during a standard intercostal ultrasonographic examination. Three supersonic shear imaging sequences are applied successively in the left, middle and right parts of the 2D ultrasonographic image. Resulting shear elasticity images in the three regions are concentrated to provide the final image covering the entire region of interest. The ability of the supersonic shear imaging technique to provide a quantitative and local estimation of organ shear modulus with a millimetric resolution was proven in a pilot study in 15 healthy volunteers [36].
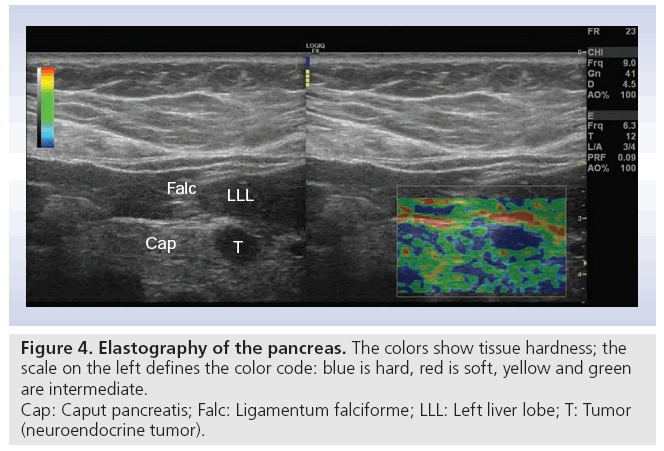
Real-time elastography images the relative difference in tissue hardness through measurements of elasticity modulus in soft tissue. The method combines B-mode ultrasonography with externally or internally applied repetitive deformations. Increased tissue hardness is a common feature of pancreatic cancers through a process called desmoplasia where tumor tissue becomes increasingly fibrotic possibly due to tumor upregulation of growth factors or their receptors. Using local differences in tissue elastic modulus as a basis of imaging may provide new diagnostic information that may improve pancreas disease diagnostics by imaging. To date, this method has mainly been applied using endoscopic ultrasound obtaining stress-induced pancreatic strain from surrounding pulsating vessels. However, in this article we present one of the very first transabdominal images of elastography of a neuroendocrine pancreatic tumor (Figure 4).
The ultrasound examination
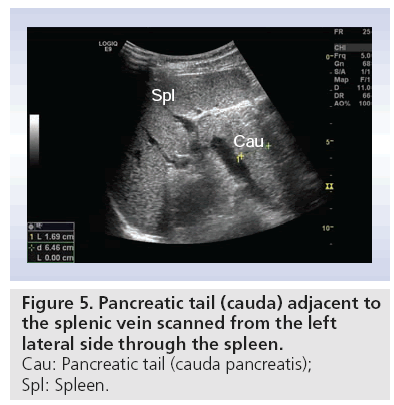
Usually patients are fasting 4–6 h before the procedure. Food provokes production of gas, with the possible consequence of total reflection of ultrasound beams. This makes scanning of the pancreas more difficult. The study of the pancreas includes transverse, longitudinal and angled oblique scans. By moving the transducer and applying graded compression, bowel gas can be removed, thus improving visualization of the pancreas. Some tricks for visualizing the whole gland include: filling the stomach with water; letting the patient stop breathing in any phase of the breathing circle; letting the patient ‘blow up the belly’; changing the patient’s position to standing or sitting; let the patient turn from the supine position to all kinds of oblique positions [39]. Often the tail of the pancreas is not easily seen in the transversal scan. Figure 5 shows the tail of the pancreas (cauda pancreatis) scanned from the left lateral side using the spleen as acoustic window.
The mandatory precondition for diagnosing the different pancreatic pathologies is to define the normal echotexture and anatomical landmarks. Pancreatic gland dimension measurements are not mandatory in routine examinations but some measures can be used as guidelines. Standard antero–posterior diameters are: the head (2.5 cm), body (1.5 cm) and tail (3.5 cm), and the pancreatic duct (<2.5 mm). The gland tends to shrink slightly with increasing age.
The surrounding vascular, ductal and abdominal organ landmarks are the portal vein, splenic vein, confluens with the mesenteric vein, vena cava, aorta, superior mesenteric artery, common hepatic artery, splenic artery, common bile duct, main pancreatic duct, duodenum, stomach and the liver. The latter is important to visualize simultaneously. Normally the echotexture is isoechogenic or hyperechogenic compared with the healthy liver [40].
Diseases of the pancreas
The following diseases of the pancreas have morphological characteristics that can be seen by transabdominal ultrasonography.
■ Acute pancreatitis with complications
The acute inflammatory process can be divided into mild with only slight enlargement of the gland due to interstitial edema, and moderate to severe with increasing presence of tissue necrosis and fluid collections. In transabdominal ultrasonography, visualization of the fine pancreatic texture and vascularity can be a problem, even when using Doppler techniques. The pancreas can appear normal in acute pancreatitis, especially in serene forms without necrosis, hemorrhage or suppuration. Necrosis is inhomogeneous; pseudocysts are without echogenicity, while abscesses are encapsulated and may have a content of low echogenicity. CEUS is always available, and seems to be ideal to differentiate between necrosis, cysts, abscesses and normal or inflamed tissue. A recent study shows that CEUS is as good as CT with contrast, with almost negligible side effects and better cost–effectiveness [41].
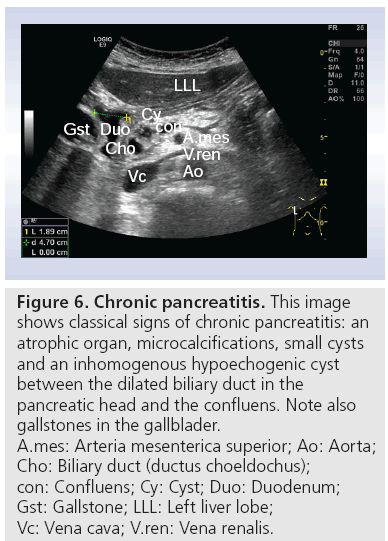
■ Chronic pancreatitis
Classical diagnostic findings of CP in transabdominal ultrasonography include pancreatic calcifications, dilated and irregular pancreatic duct, gland atrophy or enlargement, irregular gland margins, pseudo cysts and changes in gland echo texture (Figure 6). These characteristics are part of the Cambridge classification of 1983 [42]. This classification is using findings both in endoscopic retrograde pancreaticography, transabdominal ultrasonography and CT not only to diagnose CP, but also to grade the severity of morphological changes. In the 1980s, the sensitivity in grading severity of CP with ultrasound was 86%, with a correlation of 93% to the morphological gold standard endoscopic retrograde pancreaticography [43]. However, other authors cannot reproduce these results [44,45].
Figure 6: Chronic pancreatitis. This image
shows classical signs of chronic pancreatitis: an
atrophic organ, microcalcifications, small cysts
and an inhomogenous hypoechogenic cyst
between the dilated biliary duct in the
pancreatic head and the confluens. Note also
gallstones in the gallblader.
A.mes: Arteria mesenterica superior; Ao: Aorta;
Cho: Biliary duct (ductus choeldochus);
con: Confluens; Cy: Cyst; Duo: Duodenum;
Gst: Gallstone; LLL: Left liver lobe;
Vc: Vena cava; V.ren: Vena renalis.
Contrast-enhanced ultrasound may give a better differential diagnosis by ultrasonography between mass-forming pancreatitis and pancreatic adenocarcinoma. Ductal adenomcarcinoma stays hypoechoic in all contrast-enhanced phases because of its intense desmoplastic reaction with low mean vascular density. The inflammatory mass shows a ‘parenchymographic’ enhancement in the early contrast-enhanced phase [24,46].
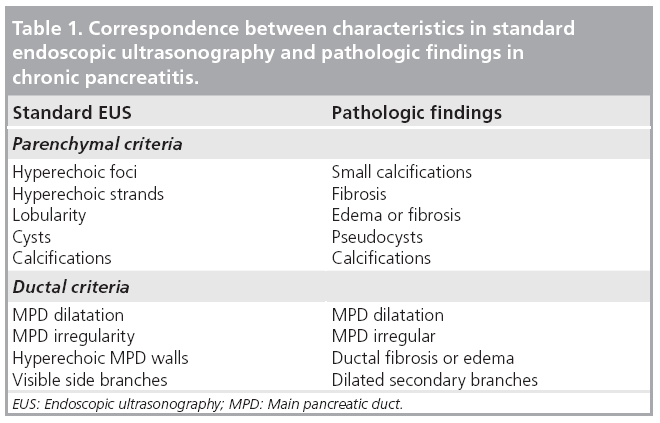
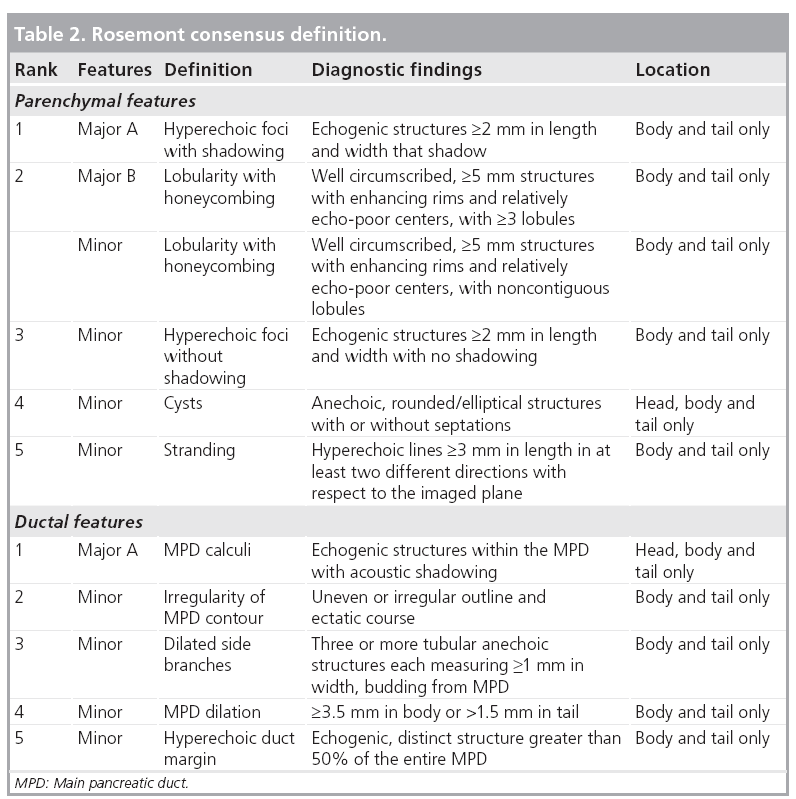
Endosongraphic ultrasound describes morphological criteria that correlate with pathological features (Table 1) [47]. These characteristics have led to the (not yet validated) Rosemont criteria (Table 2). These criteria classify sonomorphological characteristics into four groups: normal, indeterminate, suggestive of or consistent with CP [48]. As shown in Figure 6, high-end ultrasound scanners may also detect some of these criteria using transabdominal ultrasonography. Further studies comparing EUS with transabdominal ultrasonography in classifying CP after the Rosemont criteria seem to be attractive.
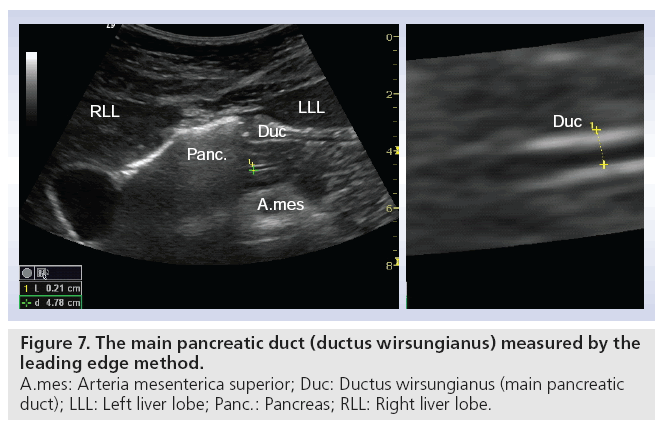
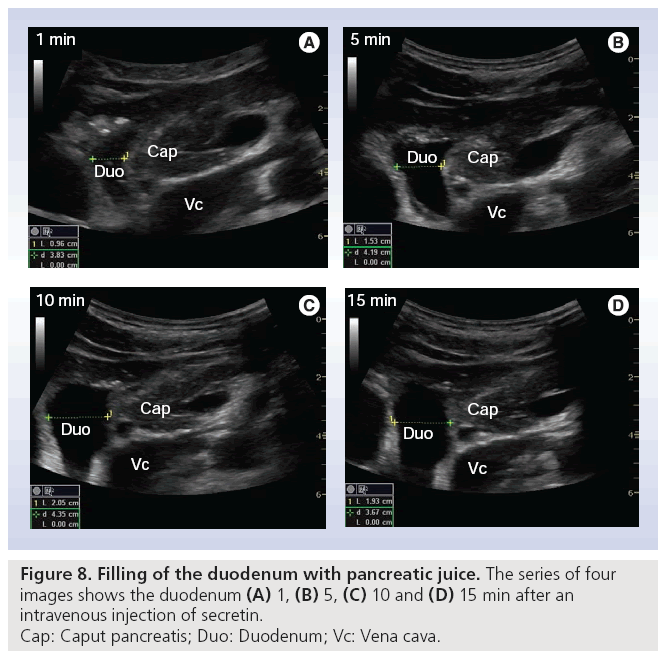
Early stage CP is often without or with minimal morphological changes, and cannot be diagnosed sufficiently by an imaging modality. On the other hand, late stage such as signs like calcifications are always diagnostic for CP, but can be absent in severe exocrine insufficiency, depending on the type of CP [49,50]. Tests of pancreatic exocrine function are therefore seen as the gold standard for diagnosing CP, especially in the early stages [51,52]. The original tests with double lumen tubes are not practical in daily clinical practice. The short endoscopic secretin-based pancreas function test has been validated and is used at present [53]. This opens the possibility to combine a functional test with an imaging modality, where ultrasonography shows the morphological changes of the pancreas and its nearby organs. Using this combined method we recorded fluid flow in the pancreatic duct and duodenum after secretin was administered. We observed that the main pancreatic duct wall has an increased echogenicity, shortly after the injection of secretin. In all subjects (>70) so far we have demonstrated a consistent, fast filling of the duodenal lumen with pancreatic juice [Erchinger FG et al., Unpublished Data]. Figure 7 shows bright echogenicity of the main pancreatic duct wall shortly after secretin stimulation. The ductal diameter is measured according to ‘the leading edge to leading edge’ principle: meaning that all measures are taken in the surface part of the interface echo. Figure 8 shows the filling of the duodenum with digestive juice during the secretin test.
■ Groove pancreatitis
Beyond the classical morphological characteristics, a rare CP can be ruled out – groove pancreatitis. The involved sites are the dorso–cranial pancreatic head, the duodenal wall and distal biliary duct. If correctly accessed and evaluated the appearance is a sickle-like groove with low or no echogenicity between the pancreatic head and the duodenal wall. This pancreatic lesion has, depending on inflammation activity and duration, low echogenicity, cloudy contours, fibrosis, necrosis, cysts and calcifications. The differential diagnosis to cancer is a challenge, but the special anatomical hints make the ultrasonographic diagnosis reliable and may avoid unnecessary personnel- and cost-intensive procedures [54].
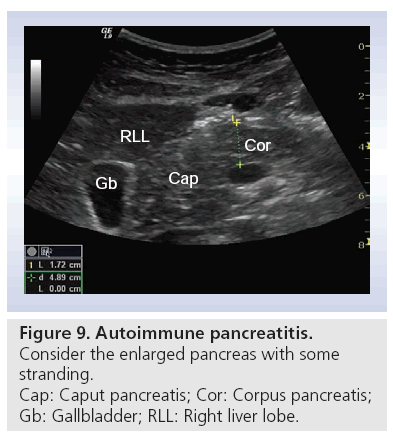
■ Autoimmune pancreatitis
Autoimmune pancreatitis is one of the most misdiagnosed benign diseases of the pancreas; in particular, focal lesions are misinterpreted as cancer. Many patients undergo unnecessarily excessive abdominal surgery such as the Whipples operation. An enlarged pancreas (Figure 9) with an absence of cysts and calcification is an important hint [55–57]. In a recently published study using CEUS, lesions caused by autoimmune pancreatitis and the surrounding pancreatic tissue typically showed a neuroendocrine tumor-like hyperenhancement pattern, whereas lesions caused by pancreatic cancer are hypoenhanced [58]. CEUS can be useful in the study of focal forms of autoimmune CP where differential diagnosis with ductal adenocarcinoma is a priority, and CT or MRI features are not absolute [24,29].
■ Adenocarcinoma
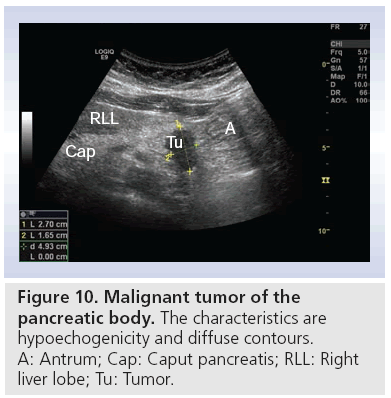
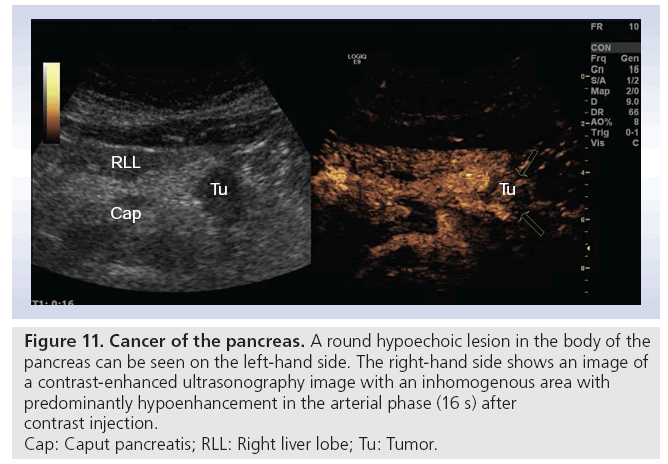
One of the biggest challenges in daily clinical activity is to differentiate between lesions caused by CP or pancreatic adenocarcinoma. Ultrasonographic findings are typically hypoechoic lesions with illdefined margins, often with spiculas, with a tendancy to alter the gland contour (Figure 10) [28]. It is here that transabdominal CEUS, with possibility to visualize the vascular pattern, has its potential. The opportunity to perform tumor characterization, adding one bolus of contrast medium, makes this method highly applicable during the examination. Typically ductal adenocarcinoma is hypoenhanced (Figure 11), while CP has more vascularity [29,59]. Some authors describe a better visualization of vascularity by CEUS than by helical CT [24,27]. Others show that different enhancement patterns also correlate with histology, and make CEUS superior to CT [60]. Malignant invasion into vessels, or thrombosis of the portal vein are cornerstones in the assessment of tumor resectability. In early thrombosis of the portal or splenic vein, normal grayscale transabdominal ultrasonography can miss the pathologic findings because the thrombus is of low or no echogenicity.
Figure 10: Malignant tumor of the
pancreatic body. The characteristics are
hypoechogenicity and diffuse contours.
A: Antrum; Cap: Caput pancreatis; RLL: Right
liver lobe; Tu: Tumor.
Figure 11: Cancer of the pancreas. A round hypoechoic lesion in the body of the
pancreas can be seen on the left-hand side. The right-hand side shows an image of
a contrast-enhanced ultrasonography image with an inhomogenous area with
predominantly hypoenhancement in the arterial phase (16 s) after
contrast injection.
Cap: Caput pancreatis; RLL: Right liver lobe; Tu: Tumor.
In these cases, duplex ultrasound and CEUS have obvious potential [6,16]. CEUS can add new information to the regional staging of pancreatic adenocarcinoma because it can confirm vascular infiltration or encasement by the neoplasm. In addition, CEUS may increase the sensitivity and specificity for the identification and characterization of liver metastases. After studying a pancreatic lesion in the arterial, pancreatic and venous phases, liver metastasis can be detected using the late hepatic contrast-enhanced phase [24].
■ Endocrine tumors: islet cell tumors
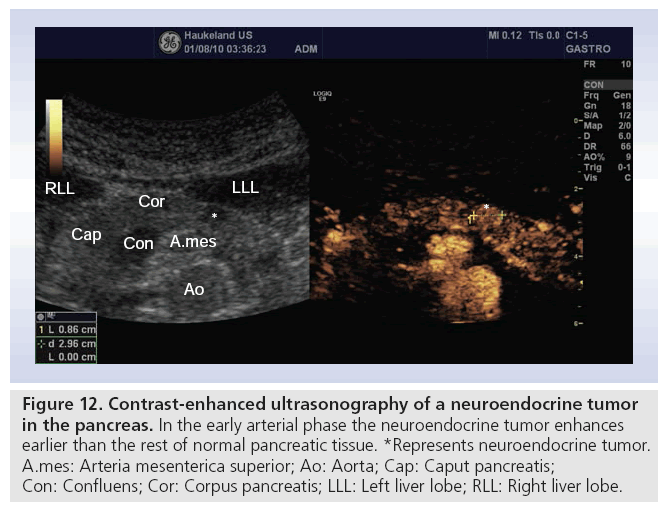
Tumors arising from neuroendocrine pancreatic cells are uncommon, representing less than 3% of all pancreatic neoplasms. However, this incidence is rising, probably due to increasing awareness of these tumors [61]. At the time of clinical presentation, tumor sizes are often small. Transabdominal ultrasonography can visualize these lesions with a sensitivity of approximately 30–60%. Older studies show that ultrasound is of the same diagnostic value as CT [62,63]. Studies with CEUS have demonstrated that neuroendocrine tumors have hypervascularity compared with hypovascularity of adenocarcinoma, or isovascularity of pancreatitis (Figure 12) [60].
Figure 12: Contrast-enhanced ultrasonography of a neuroendocrine tumor in the pancreas. In the early arterial phase the neuroendocrine tumor enhances earlier than the rest of normal pancreatic tissue. *Represents neuroendocrine tumor. A.mes: Arteria mesenterica superior; Ao: Aorta; Cap: Caput pancreatis; Con: Confluens; Cor: Corpus pancreatis; LLL: Left liver lobe; RLL: Right liver lobe.
Insulinoma is a rare disease with an incidence of four per 1 million persons [64]. A recently published study describes that transabdominal ultrasonography may show a hypoechoic homogeneous nodule with smooth contour. Color Doppler flow imaging may show a short stick-like blood flow signal in the nodule. Compared to the adjacent normal pancreatic parenchyma, the enhancement pattern of insulinoma on CEUS was fast wash-in and slow wash-out. In the earlier arterial contrastenhanced phase, all the tumors showed homogeneous hypervascularity and displayed a fast wash-in enhancement pattern. The enhancement intensity in all the tumors was higher than that of the adjacent normal pancreatic parenchyma during the arterial and venous phase. In the late arterial phase, all the tumors were still hyperenhancing and displayed a slow wash-out pattern [65]. Even if transabdominal ultrasonography is not sensitive in primary localization of these often minute tumors, intra-abdominal ultrasound plays a decisive role and identifies almost all tumors [66].
■ Cystic pancreatic tumors
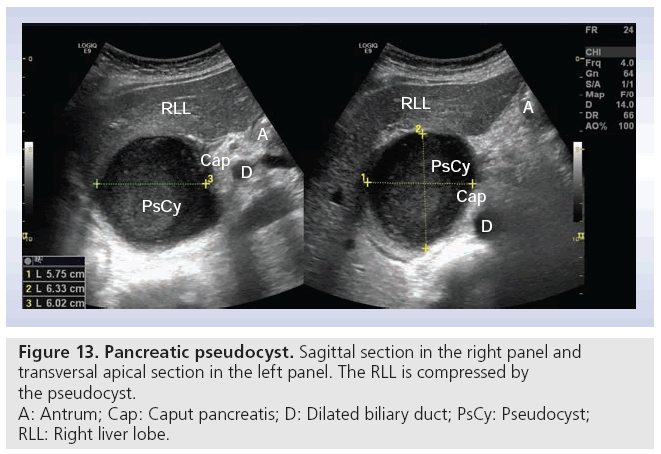
Pseudocysts are the most common pancreatic cysts; in fact, they are not real cysts because they do not have any epithelia. They are a frequent complication of acute pancreatitis (up to 30%). Anamnesis helps in differentiating from real cysts [67,68]. Figure 13 shows a pseudocyst measuring 6 × 6 × 6 cm with compression of the right liver lobe. True cysts are rare, representing only 10–15% of all pancreatic neoplasias [68]. The main cystic pancreatic neoplasias are: serous microcystic adenoma, mucinous cystadenoma and intraductal papillary mucinous neoplasia. The latter two are premalignant or malignant, and must be cured surgically if possible. CEUS can potentially differentiate between benign and malign lesions [69]. Using transabdominal ultrasonography, documentation of cystic tumors includes location (head, body and tail), size and echogenicity (echo free, hypoechoic or mixed echogenicity). Solid nodules with and without deriving septae out of the nodule also have to be described.
The presence of identifiable mass lesions within a cyst or a cystic lesion with (peripherally) located solid nodules (with or without septae) can be considered as an indicator for neoplasia. CEUS is an important tool to differentiate between benign and malign lesions when picturing an-, hyper- or hypovascularity [27]. Some authors state a high sensitivity and specificity and remark that CEUS can compete with MRI [70].
■ Lymphoma & metastases
Primary pancreatic lymphoma constitutes less than 0.5% of all pancreatic malignancies, and less than 2% of extranodal lymphomas. Most are intermediate or high-grade non-Hodgkin’s lymphoma of diffuse large B-cell type [71]. On transabdominal ultrasonography, primary pancreatic lymphoma usually appears as a bulky, homogeneous, hypoechoic mass confined to the pancreas. Transabdominal ultrasonography allows the detection of enlarged peripancreatic and periaortic lymph nodes, and dilatation of the common bile and pancreatic ducts. Duplex scanning provides helpful information about the patency of the major peripancreatic vessels, the celiac and superior mesenteric arteries, and the portal, superior mesenteric and splenic veins [72].
The most common cancers reported to metastasize to the pancreas include renal cell cancer, colon cancer, melanoma, sarcoma, breast and lung cancer [73]. In transabdominal ultrasonography they show variable echotexture and vascularity depending on origin.
■ Pancreatic & peripancreatic TB
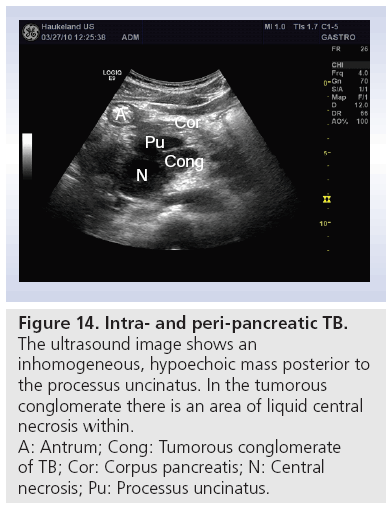
In developing countries or in immune deficient patients, an important differential diagnosis to intra- and peripancreatic tumors, metastases and lymphoma is TB. Most of the lesions are hypoechoic, and the echotexture is heterogeneous (Figure 14). Often ascites follows abdominal TB. Inhomogeneous liver, lymph nodes in the abdomen and thickened bowel walls, especially in the ileocecal region, are other findings from transabdominal ultrasonography. Technological developments that enable ultrasound equipment to be smaller and more portable will allow ultrasonography to become a valued diagnostic tool, revealing abdominal TB in resource-poor settings and being used alongside other diagnostic methods to confirm the diagnosis [74].
Figure 14: Intra- and peri-pancreatic TB.
The ultrasound image shows an
inhomogeneous, hypoechoic mass posterior to
the processus uncinatus. In the tumorous
conglomerate there is an area of liquid central
necrosis within.
A: Antrum; Cong: Tumorous conglomerate
of TB; Cor: Corpus pancreatis; N: Central
necrosis; Pu: Processus uncinatus.
Conclusion
Transabdominal ultrasonography is still the best first-line modality in pancreatic imaging. Several advancements in transabdominal ultrasonography have improved image quality and diagnostic accuracy. In showing macroand microvascularity, CEUS discriminates between pancreatic benign and malign tumors, or tumor-like changes. Harmonic imaging and computer processed imaging, such as compound imaging, enable improved contour detection of organs and pathological structures. Traditional modalities such as Doppler, duplex and triplex ultrasound, Power Doppler, and grayscale B-mode ultrasonographies still perform well. Elastography characterizes the hardness of tissue. All these modalities and methods can be combined. Together with advancements in transducer and processor technology, they have the potential to improve the diagnostic yield of ultrasound. Ultrasound in medical imaging should not be underestimated or forgotten in the modern age of CT, MRI and PET.
Future perspective
Computer processed imaging is developing rapidly. We predict that transabdominal ultrasonography will soon be as accurate as EUS with regard to imaging of fine details. Furthermore, image resolution will improve, and the morphological characteristics of the Rosemont criteria may, in the near future, be visualized in transabdominal ultrasonography. Novel improvements include making ultrasound equipment smaller and more portable. This moves ultrasound technology towards bedside scanning of patients, and mobile ultrasound makes this method an easily available and widespread clinical imaging modality.
Improved characterization of pancreatic lesions by CEUS is one of the most important steps forward in pancreatic imaging today. Elastography in pancreatic imaging is sometimes used in EUS; further studies with transabdominal sonoelastography are warranted.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- Rosch T, Schusdziarra V, Born P et al. Modern imaging methods versus clinical assessment in the evaluation of hospital in-patients with suspected pancreatic disease. Am. J. Gastroenterol. 95(9), 2261–2270 (2000).
- Rettenmaier G. [Methods and results of ultrasonography in the diagnosis of pancreatic diseases]. Leber. Magen. Darm. 2(3), 88–90 (1972).
- Fontana G, Bolondi L, Conti M et al. An evaluation of echography in the diagnosis of pancreatic disease. Gut 17(3), 228–234 (1976).
- Russell JG, Vallon AG, Braganza JM, Howat HT. Ultrasonic scanning in pancreatic disease. Gut 19(11), 1027–1033 (1978).
- Swobodnik W, Meyer W, Brecht-Kraus D et al. Ultrasound, computed tomography and endoscopic retrograde cholangiopancreatography in the morphologic diagnosis of pancreatic disease. Klin. Wochenschr. 61(6), 291–296 (1983).
- Reuss J, Rettenmaier G. [Transabdominal ultrasonography in pancreatic diseases]. Schweiz. Med. Wochenschr. 123(21), 1049–1058 (1993).
- Choudhry S, Gorman B, Charboneau JW et al. Comparison of tissue harmonic imaging with conventional US in abdominal disease. Radiographics 20(4), 1127–1135 (2000).
- Fiegler W. [Artefacts in ultrasonic diagnosis]. Rofo 138(3), 340–347 (1983).
- Fiegler W. [Effect of various parameters on the resolution potential behind obstructing fatty tissue in sonography]. Rofo 139(1), 85–90 (1983).
- Fiegler W, Felix R, Langer M, Schultz E. Fat as a factor affecting resolution in diagnostic ultrasound: possibilities for improving picture quality. Eur. J. Radiol. 5(4), 304–309 (1985).
- Shmulewitz A, Teefey SA, Robinson BS. Factors affecting image quality and diagnostic efficacy in abdominal sonography: a prospective study of 140 patients. J. Clin. Ultrasound 21(9), 623–630 (1993).
- Wildgrube HJ, Dehwald H. [Value of gray scale analysis for the assessment of ultrasound detected structures in the area of the abdomen]. Bildgebung 57(1–2), 11–16 (1990).
- Hohl C, Schmidt T, Honnef D, Gunther RW, Haage P. Ultrasonography of the pancreas. 2. Harmonic imaging. Abdom. Imaging 32(2), 150–160 (2007).
- Hohl C, Schmidt T, Haage P et al. Phaseinversion tissue harmonic imaging compared with conventional B-mode ultrasound in the evaluation of pancreatic lesions. Eur. Radiol. 14(6), 1109–1117 (2004).
- Sparchez Z. Tissue harmonic imaging: is it useful in hepatobiliary and pancreatic ultrasonography? Rom. J. Gastroenterol. 12(3), 239–246 (2003).
- Bertolotto M, D’Onofrio M, Martone E, Malago R, Pozzi MR. Ultrasonography of the pancreas. 3. Doppler imaging. Abdom. Imaging 32(2), 161–170 (2007).
- Bushberg JT. Ultrasound. In: The Essential Physics of Medical Imaging. Bushberg JT, Seibert JA, Leidholdt EM, Boone JM (Eds). Lippincott Williams & Wilkins, PA, USA, 543 (2002).
- Nasholm SP, Hansen R, Masoy SE, Johansen TF, Angelsen BA. Transmit beams adapted to reverberation noise suppression using dual-frequency SURF imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 56(10), 2124–2133 (2009).
- Masoy SE, Standal O, Nasholm P, Johansen TF, Angelsen B. SURF imaging: in vivo demonstration of an ultrasound contrast agent detection technique. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 55(5), 1112–1121 (2008).
- Hansen R, Masoy SE, Johansen TF, Angelsen BA. Utilizing dual frequency band transmit pulse complexes in medical ultrasound imaging. J. Acoust. Soc. Am. 127(1), 579–587 (2010).
- Forsberg F. Ultrasonic biomedical technology; marketing versus clinical reality. Ultrasonics 42(1–9), 17–27 (2003).
- Sherman M. The radiological diagnosis of hepatocellular carcinoma. Am. J. Gastroenterol. 105(3), 610–612 (2010).
- Lencioni R, Crocetti L, Della Pina MC, Cioni D. Guidelines for imaging focal lesions in liver cirrhosis. Expert Rev. Gastroenterol. Hepatol. 2(5), 697–703 (2008).
- D’Onofrio M, Malago R, Zamboni G et al. Contrast-enhanced ultrasonography better identifies pancreatic tumor vascularization than helical CT. Pancreatology 5(4–5), 398–402 (2005).
- Claudon M, Cosgrove D, Albrecht T et al. Guidelines and good clinical practice recommendations for contrast-enhanced ultrasound (CEUS) - update 2008. Ultraschall Med. 29(1), 28–44 (2008).
- Beyer-Enke SA, Hocke M, Ignee A, Braden B, Dietrich CF. Contrast enhanced transabdominal ultrasound in the characterisation of pancreatic lesions with cystic appearance. JOP 11(5), 427–433 (2010).
- Kersting S, Konopke R, Kersting F et al. Quantitative perfusion analysis of transabdominal contrast-enhanced ultrasonography of pancreatic masses and carcinomas. Gastroenterology 137(6), 1903–1911 (2009).
- Postema M, Gilja OH. Contrast-enhanced and targeted ultrasound. World J. Gastroenterol. 17(1), 28–41 (2011).
- Faccioli N, Crippa S, Bassi C, D’Onofrio M. Contrast-enhanced ultrasonography of the pancreas. Pancreatology 9(5), 560–566 (2009).
- Goldberg M. Systemic reactions to intravascular contrast media. A guide for the anesthesiologist. Anesthesiology 60(1), 46–56 (1984).
- Ophir J, Cespedes I, Ponnekanti H, Yazdi Y, Li X. Elastography: a quantitative method for imaging the elasticity of biological tissues. Ultrason. Imaging 13(2), 111–134 (1991).
- Ophir J, Garra B, Kallel F et al. Elastographic imaging. Ultrasound Med. Biol. 26(Suppl. 1), S23–S29 (2000).
- Gilja OH, Heimdal A, Hausken T et al. Strain during gastric contractions can be measured using Doppler ultrasonography. Ultrasound Med. Biol. 28(11–12), 1457–1465 (2002).
- Heimdal A, Gilja OH. Strain rate imaging – a new tool for studying the GI-tract. In: Basic and New Aspects of Gastrointestinal Ultrasonography. Odegaard S, Gilja OH, Gregersen H (Eds). World Scientific 243–263 (2005).
- Matre K, Moen CA, Fannelop T, Dahle GO, Grong K. Multilayer radial systolic strain can identify subendocardial ischemia: an experimental tissue Doppler imaging study of the porcine left ventricular wall. Eur. J. Echocardiogr. 8(6), 420–430 (2007).
- Muller M, Gennisson JL, Deffieux T, Tanter M, Fink M. Quantitative viscoelasticity mapping of human liver using supersonic shear imaging: preliminary in vivo feasibility study. Ultrasound Med. Biol. 35(2), 219–229 (2009).
- Bercoff J, Pernot M, Tanter M, Fink M. Monitoring thermally-induced lesions with supersonic shear imaging. Ultrason. Imaging 26(2), 71–84 (2004).
- Bercoff J, Tanter M, Fink M. Supersonic shear imaging: a new technique for soft tissue elasticity mapping. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 51(4), 396–409 (2004).
- Dietrich CF. Ultraschall-kurs. In: Deutscher Ärzteverlag. Dietrich (Ed). Deutscher Ärzte-Verlag GmbH, Dieselstr, Germany, 116 (2006).
- Martinez-Noguera A, D’Onofrio M. Ultrasonography of the pancreas. 1. Conventional imaging. Abdom. Imaging 32(2), 136–149 (2007).
- Rickes S, Monkemuller K, Malfertheiner P. Acute severe pancreatitis: contrast-enhanced sonography. Abdom. Imaging 32(3), 362–364 (2007).
- Sarner M, Cotton PB. Classification of pancreatitis. Gut 25(7), 756–759 (1984).
- Jones SN, Lees WR, Frost RA. Diagnosis and grading of chronic pancreatitis by morphological criteria derived by ultrasound and pancreatography. Clin. Radiol. 39(1), 43–48 (1988).
- Siddiqi AJ, Miller F. Chronic pancreatitis: ultrasound, computed tomography, and magnetic resonance imaging features. Semin. Ultrasound CT MR 28(5), 384–394 (2007).
- Kinney TP, Freeman ML. Recent advances and novel methods in pancreatic imaging. Minerva Gastroenterol. Dietol. 54(1), 85–95 (2008).
- Numata K, Ozawa Y, Kobayashi N et al. Contrast-enhanced sonography of autoimmune pancreatitis: comparison with pathologic findings. J. Ultrasound Med. 23(2), 199–206 (2004).
- Sahai AV. EUS and chronic pancreatitis. Gastrointest. Endosc. 56(Suppl. 4), S76–S81 (2002).
- Seicean A. Endoscopic ultrasound in chronic pancreatitis: where are we now? World J. Gastroenterol. 16(34), 4253–4263 (2010).
- Ammann RW, Muench R, Otto R, Buehler H, Freiburghaus AU, Siegenthaler W. Evolution and regression of pancreatic calcification in chronic pancreatitis. A prospective long-term study of 107 patients. Gastroenterology 95(4), 1018–1028 (1988).
- Lankisch PG, Otto J, Erkelenz I, Lembcke B. Pancreatic calcifications: no indicator of severe exocrine pancreatic insufficiency. Gastroenterology 90(3), 617–621 (1986).
- Conwell DL, Zuccaro G, Purich E et al. Comparison of endoscopic ultrasound chronic pancreatitis criteria to the endoscopic secretin-stimulated pancreatic function test. Dig. Dis. Sci. 52(5), 1206–1210 (2007).
- Lankisch PG. Exocrine pancreatic function tests. Gut 23(9), 777–798 (1982).
- Stevens T, Dumot JA, Zuccaro G Jr et al. Evaluation of duct-cell and acinar-cell function and endosonographic abnormalities in patients with suspected chronic pancreatitis. Clin. Gastroenterol. Hepatol. 7(1), 114–119 (2009).
- Seitz K, Rettenmaier G, Stolte M. Groove pancreatitis – its pathological anatomy and sonographic findings. Ultraschall Med. 6(3), 131–133 (1985).
- Hirche TO, Ignee A, Barreiros AP et al. Indications and limitations of endoscopic ultrasound elastography for evaluation of focal pancreatic lesions. Endoscopy 40(11), 910–917 (2008).
- Dietrich CF, Hirche TO, Ott M, Ignee A. Real-time tissue elastography in the diagnosis of autoimmune pancreatitis. Endoscopy 41(8), 718–720 (2009).
- Law R, Bronner M, Vogt D, Stevens T. Autoimmune pancreatitis: a mimic of pancreatic cancer. Cleve. Clin. J. Med. 76(10), 607–615 (2009).
- Hocke M, Ignee A, Dietrich CF. Contrastenhanced endoscopic ultrasound in the diagnosis of autoimmune pancreatitis. Endoscopy 43(2), 163–165 (2010).
- Dietrich CF, Braden B, Hocke M, Ott M, Ignee A. Improved characterisation of solitary solid pancreatic tumours using contrast enhanced transabdominal ultrasound. J. Cancer Res. Clin. Oncol. 134(6), 635–643 (2008).
- Sofuni A, Iijima H, Moriyasu F et al. Differential diagnosis of pancreatic tumors using ultrasound contrast imaging. J. Gastroenterol. 40(5), 518–525 (2005).
- Verbeke CS. Endocrine tumours of the pancreas. Histopathology 56(6), 669–682 (2010).
- Galiber AK, Reading CC, Charboneau JW et al. Localization of pancreatic insulinoma: comparison of pre- and intraoperative US with CT and angiography. Radiology 166(2), 405–408 (1988).
- Zimmer T, Ziegler K, Bader M et al. Localisation of neuroendocrine tumours of the upper gastrointestinal tract. Gut 35(4), 471–475 (1994).
- Mathur A, Gorden P, Libutti SK. Insulinoma. Surg. Clin. North Am. 89(5), 1105–1121 (2009).
- An L, Li W, Yao KC et al. Assessment of contrast-enhanced ultrasonography in diagnosis and preoperative localization of insulinoma. Eur. J. Radiol. (2010).
- Grover AC, Skarulis M, Alexander HR et al. A prospective evaluation of laparoscopic exploration with intraoperative ultrasound as a technique for localizing sporadic insulinomas. Surgery 138(6), 1003–1008 (2005).
- Diagnostic Ultrasound. Rumack CM, Wilson SR, Charboneau JW (Eds). Mosby Year Book, St. Louis, MO, USA, 145–177 (1991).
- Weissleder R, Wittenberg J, Harisinghani MG, Chen JW. Primer of Diagnostic Imaging. Mosby, PA, USA, 220–228 (1997).
- Itoh T, Hirooka Y, Itoh A et al. Usefulness of contrast-enhanced transabdominal ultrasonography in the diagnosis of intraductal papillary mucinous tumors of the pancreas. Am. J. Gastroenterol. 100(1), 144–152 (2005).
- D’Onofrio M, Megibow AJ, Faccioli N et al. Comparison of contrast-enhanced sonography and MRI in displaying anatomic features of cystic pancreatic masses. AJR Am. J. Roentgenol. 189(6), 1435–1442 (2007).
- Sugishita H, Watanabe Y, Yamamoto Y et al. Primary pancreatic lymphoma: the role of surgical treatment. Case Rep. Gastroenterol. 4(1), 104–110 (2010).
- Merkle EM, Bender GN, Brambs HJ. Imaging findings in pancreatic lymphoma: differential aspects. AJR Am. J. Roentgenol. 174(3), 671–675 (2000).
- Konstantinidis IT, Dursun A, Zheng H et al. Metastatic tumors in the pancreas in the modern era. J. Am. Coll. Surg. 211(6), 749–753 (2010).
- Nagar AM, Raut AA, Morani AC, Sanghvi DA, Desai CS, Thapar VB. Pancreatic tuberculosis: a clinical and imaging review of 32 cases. J. Comput. Assist. Tomogr. 33(1), 136–141 (2009).
• • Mandatory for all ultrasound performers who deal with contrast agents.
• A nice overview.
• All articles in the series ‘Ultrasonography of the pancreas’ can be recommended.