Review Article - Imaging in Medicine (2013) Volume 5, Issue 4
Unenhanced CT in the evaluation of renal/ureteric colic
Tze M Wah*Diagnostic & Interventional Radiology, Leeds Teaching Hospitals Trust, St James’s University Hospital, Beckett Street, Leeds, LS9 7TF, UK
- Corresponding Author:
- Tze M Wah
Diagnostic & Interventional Radiology
Leeds Teaching Hospitals Trust
St James’s University Hospital
Beckett Street, Leeds, LS9 7TF, UK
Tel: +44 113 206 5525
Fax: +44 113 206 4640
E-mail: wah.tze@gmail.com
Abstract
Keywords
CTKUB ▪ renal colic ▪ stone ▪ unenhanced CT
Acute loin pain due a to renal or ureteric stone is a common diagnosis in the accident and emergency department [1–3]. A stone in the kidney alone does not warrant emergency management apart from in the context of superimposed infection, but a stone impacted within the ureter requires prompt diagnosis, urological referral and urgent intervention in specific circumstances. The classical clinical symptoms of ureteric colic, such as loin pain that radiates to the groin, vomiting and microscopic hematuria, frequently overlaps with other clinical presentations such as pyelonephritis, appendicitis, gynecological problems or diverticulitis. Therefore, imaging plays an important role in obtaining an accurate diagnosis with this clinical presentation so that the patient can be promptly triaged into the correct clinical management pathway. The majority (70%) of the stones detected are calcium oxalate and/or phosphate composition, 8% are uric acid stones and 3% cysteine stones [4]. They can present as a small stone or a staghorn calculus that fills multiple collecting systems. The treatment for a urinary tract stone may range from extracorporeal shock wave lithotripsy, retrograde ureteroscopy with laser fragmentation to percutaneous nephrolithotomy.
Intravenous urography (IVU) has been used since 1923 to investigate the symptoms of acute loin pain. Since the arrival of CT, this has revolutionized the way clinicans image urinary tract disease. Today, noncontrast enhanced CT of kidneys, ureters and the bladder (CTKUB) has replaced acute IVU and transformed the imaging approach for patients with acute loin pain presenting to the emergency department. The diagnostic utility of CTKUB was first described by Smith et al. in 1995 and has shown better detection of ureteric stones in terms of diagnostic accuracy, sensitivity and specificity when compared with IVU [5]. This was confirmed subsequently by other published series for CTKUB versus IVU with sensitivities of 94–100 versus 66–87% and specificities of 94–100 versus 92–94%, respectively [6–9]. CTKUB assessment can provide information about the size, density and distribution of stones in the kidney/site of stone impaction in the ureter [10–12] and secondary signs of obstruction such as perinephric stranding, hydronephrosis and hydroureter [13–15]. In addition, CTKUB also detects extraurinary abnormalities that may clinically mimic acute renal or ureteric colic [10–12,16].
However, there are a few potential imaging pitfalls that radiologists may encounter during CTKUB interpretation. These are other tubular structures, such as gonadal vessels or phleboliths, in the pelvis and when there is presence of a ureteral stent this can obscure the presence of a stone adjacent to it. Therefore, understanding the anatomical relationships of the ureter is crucial in assisting diagnosis. Multiplanar reformatting, such as coronal reformatting, may also clarify the location of the stone and using wide windowing during interpretation may differentiate a small stone adjacent to the ureteral stent.
Detection of the stone either in the kidney or ureter in the emergency setting is important so that patients can be referred for timely clinical management but the follow-up imaging strategy is equally important especially for patients who are treated with conservatively – that is, waiting for the stone to pass spontaneously. To date, there remain many divergent approaches as to following- up these patients. Some stones may not be visualized on plain radiography and potentially CT scout views may be used to guide whether subsequent follow-up with plain radiograph is possible depending on its visibility on initial scout view on the CTKUB [17]. Accurate imaging diagnosis is vital in order to prevent a patient having to undergo unnecessary endourological intervention.
This paper aims to describe the normal anatomy of the kidney and ureter; illustrate the salient signs on CTKUB for urinary tract stones causing renal or ureteric colic; outline the potential imaging pitfalls; and discuss the possible role of CT scout views as well as exploring Randall’s plaque theory.
Normal anatomy
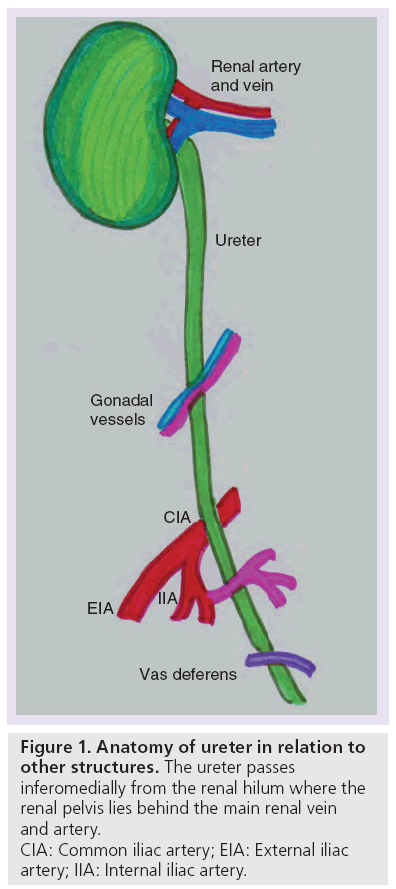
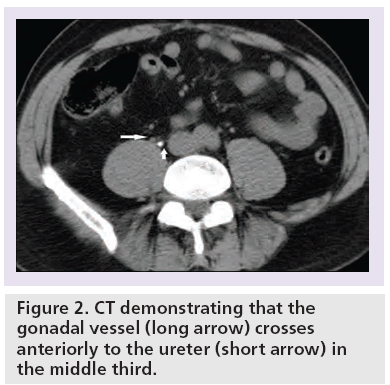
The ureter is a muscular tubular structure that measures 25 cm in length and drains via peristalsis. It is typically subdivided into: proximal (including the renal pelvis), middle, distal and the vesico–ureteric junction (VUJ) [18]. The ureter passes inferomedially from the renal hilum (Figure 1), where the renal pelvis lies behind the main renal vein and artery. The proximal two-thirds (proximal and middle) of the ureter crosses within the abdomen, and lies anterior to the psoas muscle, which separates the ureter from the underlying tips of the transverse processes of the lumbar vertebral bodies from L2 to L5. It is important to note that the gonadal vessel crosses anteriorly to the ureter in the middle third (Figure 2).
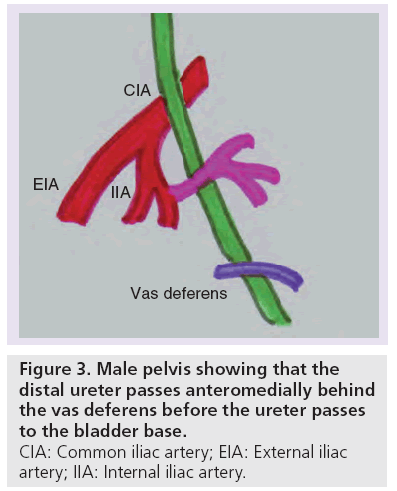
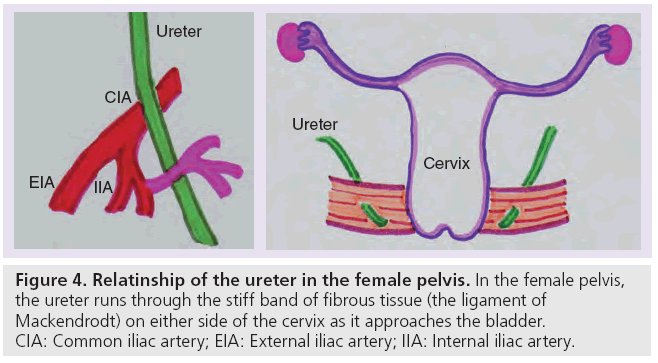
The abdominal ureter becomes the distal or pelvic ureter as it passes over the pelvic brim. The distal pelvic ureter runs over the lateral wall of the pelvis, crosses anterior to the common iliac artery and then posterior to the superior vesical branch of the internal iliac artery. In the male pelvis, the distal ureter then passes anteromedially behind the vas deferens (Figure 3) before the ureter passes to the bladder base. In the female pelvis, the ureter runs through the stiff band of fibrous tissue (the ligament of Mackendrodt) on either side of the cervix as it approaches the bladder (Figure 4). At the VUJ, the intravesical portion of the ureter passes obliquely through the muscular wall to help produce a valve-like arrangement to prevent urinary reflux [18].
Figure 4: Relatinship of the ureter in the female pelvis. In the female pelvis, the ureter runs through the stiff band of fibrous tissue (the ligament of Mackendrodt) on either side of the cervix as it approaches the bladder. CIA: Common iliac artery; EIA: External iliac artery; IIA: Internal iliac artery.
There are three recognized locations where stones may be impacted due to normal anatomical narrowing of the ureter: the pelvi–ureteric junction; at the level ureter crosses the pelvic brim; and the VUJ.
It is pertinent to be aware of the normal anatomical variants of the collecting system that can also predispose to stone formation. Duplex kidney is the most common congenital normal variant of the urinary tract that may remain concealed in the absence of associated complications [19]. However, it frequently occurs in association with other urinary tract anomalies such as ureterocele causing upper moiety obstruction, or a defective VUJ valve mechanism causing urinary reflux to the lower moiety [20]. Both situations may present on imaging with a dilated collecting system of the associated moiety [21].
CTKUB technique
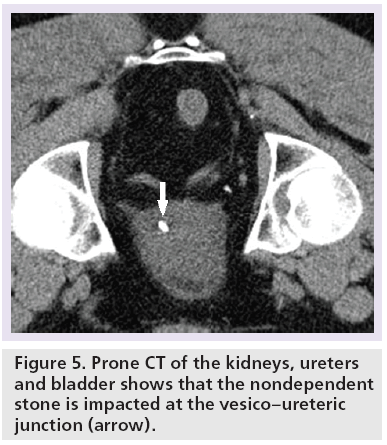
Today, multidetector CTs allow volume acquisition of information, and real-time reformatting in both the sagittal and coronal sections. CTKUB is now the ‘gold standard’ for the diagnosis of ureteric stones in the context of acute loin pain [2]. This technique is suitable for acute assessment as it requires no patient preparation and no oral or intravenous contrast is needed. Prone position scanning is preferred as this allows stones that have been passed into the bladder to be differentiated from stones that are impacted at the VUJ (Figure 5) [22]. Patients are scanned from the kidneys to the bladder base. With the CTKUB assessment, the female pelvis usually receives direct radiation exposure, and the authors have also found in their practice that female patients had a significantly lower positive rate than male patients (26.8 vs 61.6%) [3,23]. Further investigation into the young female patients in the cohort with ureteric calculus >4 mm has shown that hydronephrosis was present in 83% of cases [3]. Therefore, indiscriminate use of CTKUB in young female patients is unadvisable and a screening ultrasound scan should be used as a first-line investigation to identify the gynecological pathology and to detect the presence of hydronephrosis before performing CTKUB [3].
The CTKUB technique is a dedicated protocol to assess for urinary tract stones and is less sensitive in detecting the other intra-abdominal/pelvic pathology. However, significant extraurinary pathology may be detected on CTKUB in 9–29% of the cases [2,3,24]. If clinically indicated, this may be further assessed with administration of contrast agents.
The only drawback of CTKUB is the relatively high radiation exposure, with typical doses in the range of 4.7–6.5 mSv (and greater for multidetector CT) compared with 1.5– 3.3 mSv for an IVU series [6,25–27]. The estimated risk of lifetime induction of fatal cancer is one in 4000, one in 43,000 and one in 130,000 for a CTKUB, three-film IVU series and plain kidneys, ureters and bladder (KUB) film, respectively [27–29].
In the quest of reducing the radiation dose and maintaining the diagnostic quality of the CTKUB, a low-dose technique has been developed and is currently widely adopted [28–32]. The low-dose CTKUB technique provides comparable diagnostic accuracy with sensitivity of 97% and specificity of 95% by increasing beam width and pitch and reducing exposure factors. This can reduce the dose to as little as 0.5 mSv for men and 0.7 mSv for women [33–37]. The only pitfalls that one needs to be aware of stones <3 mm, stones impacted at VUJ or stones in obese patients may not be detected with the low-dose technique [28,31,33–35].
The attenuation of the various types of stones overlap greatly with the conventional single energy CT; hence, stone composition cannot be differentiated. However, the dual energy CT appears to have the ability to characterize stone composition, as their attenuation values differ significantly when scanned with high- and low-energy CT [38]. Dual-energy CTKUB has the potential benefit of differentiating low molecular weight stones, such as uric acid stones, from high molecular weight stones, such as calcium oxalate, or cysteine stones, which typically have higher attenuation values at lower-energy CT. The characteristic difference in attenuation values with dual energy CT scanning at 80 and 120–140 kV potentially allows the determination of stone composition [39]. This can stratify stone management where uric acid stones may benefit from medical management and consideration of percutaneous nephrolithotomy surgery for harder stones such as cysteine and certain calcium stones, which may be more resistant to extracorporeal shock wave lithotripsy. Recent literature suggests that dual-energy CT can accurately differentiate the stone composition, but there is limitation when the stone is less than 1 cm [40–43]. In addition, this technique is also associated with higher radiation doses and work is currently being carried out to reduce the radiation dose [43]. Targeted dual-energy CT for a stone detected on the conventional CTKUB has been explored [44].
Image review at Picture Archiving & Communication System workstation
Image review is best performed at a Picture Archiving and Communication System (PACS) workstation. Multiplanar reformatting, especially the coronal reformat, simulates the conventional plain radiograph orientation and has been found to be most helpful in the majority of cases during interpretation [35]. Accurate stone size estimation with measurement of the maximal width can be performed using the electronic calliper, and maximal stone width is the most important predictor of spontaneous stone passage [45]. Both stone size and location are important radiological factors that influence the management strategy that varies from conservative management with simple analgesia to invasive endourological procedures [46,47].
Most CTKUB studies dating back to the 1990s were interpreted with hard-copy films on a viewing box [29,37,48,49]. The current practice uses softcopy reviewing at PACS workstations to interpret these images, where the reviewer can trace the ureter from the kidney to the bladder in a continuous sweep of observation rather than a discontinuous observation with having to flick from image to image with the hard-copy film review [29,33,34,36,50]. For image review at a PACS workstation, each ureter is reviewed in turn, and then the remainder of the abdomen and pelvis is assessed, as well as the bones, on suitable soft tissue and bone window settings.
Radiology findings
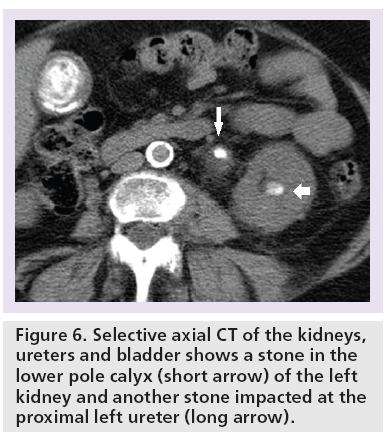
The primary sign of renal or ureteric colic on CTKUB is the identification of a high attenuation focus within the kidney or lumen of a ureter (Figures 5 & 6). Images are reviewed with soft tissue window settings on PACS. All stones are visible on CT (Hounsfield units [HU] = 200–600) apart from indinavir crystals, where regardless of the particular stone composition it will be detected against the soft tissue density of the ureteral wall [51]. Identification of a stone within the kidney or ureter on the symptomatic side gives a positive diagnosis of renal or ureteric colic.
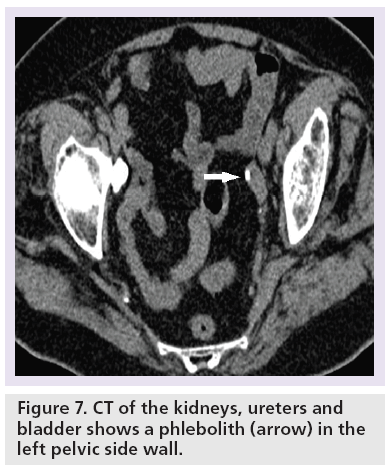
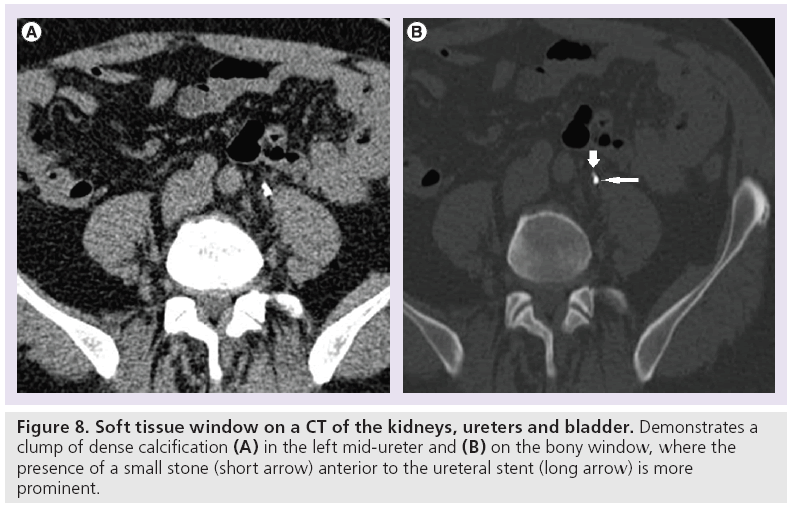
Challenges arise during interpretation when there are extraurinar calcifications, tubular structures close to the course of the ureter or the presence of a ureteral stent. Extraurinary calcifications such as phleboliths (Figure 7) – that is, foci of calcification within the walls of veins – can mimic ureteric stones. Tubular structures that course in close anatomical relation to the ureter, such as gonadal, perivesical, periprostatic and perivaginal veins, can result in a falsepositive diagnosis (Figure 2) [14]. The diagnostic confidence is worsened when there is paucity of intra-abdominal fat, and in some patients the ureter is difficult to trace from the outset. It is important to be aware that the presence of ureteral stents can obscure adjacent small ureteric stones and wide windowing such as bone window settings may be helpful in detecting this (Figure 8).
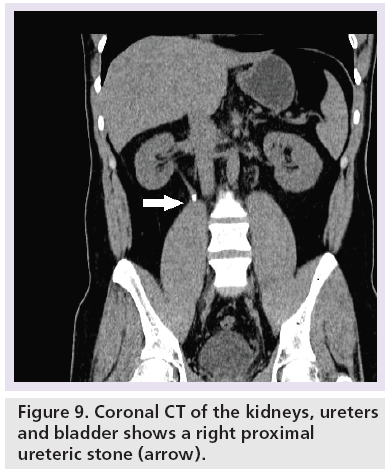
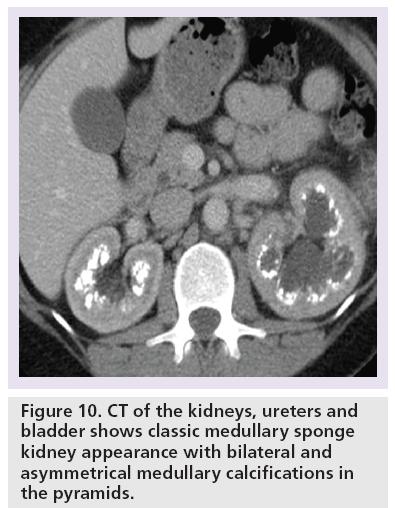
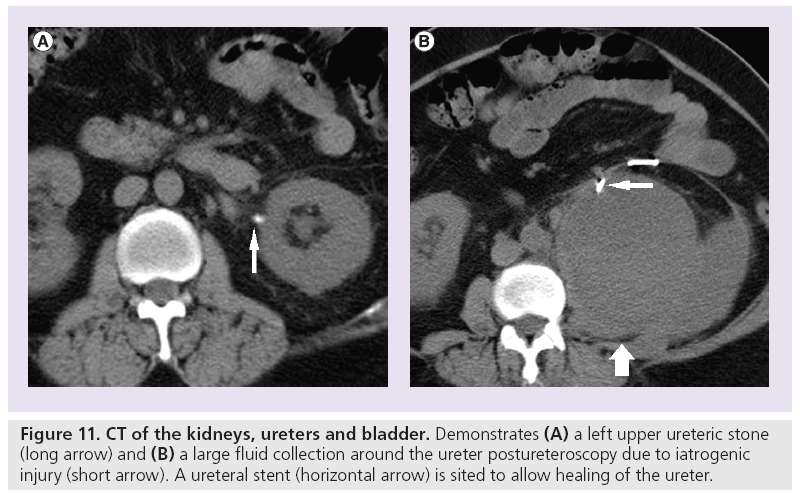
Systematic review of the CTKUB on a PACS workstation is important to detect the cause of patient’s symptoms. The observations should include primary signs of stones (Figure 9), secondary signs of stones, renal calcification that would necessitate long-term urological follow-up such as medullary sponge kidney (Figure 10), complications related to stone treatment (Figure 11), measurement of the predictor of stone free rate for extracorporeal shock wave lithotripsy, such as HU and skin to stone distance on CT [52,53] and search for alternative diagnoses to stones such as acute appendicitis, gallstones and gynecological abnormalities.
The following section will illustrate a few well-described imaging signs on CTKUB that can assist with the diagnosis of ureteric obstruction when the reader encounters an indeterminate calcific focus. These can either be direct signs that are directly associated with calcification or indirect secondary signs that are due to the effect of ureteric obstruction.
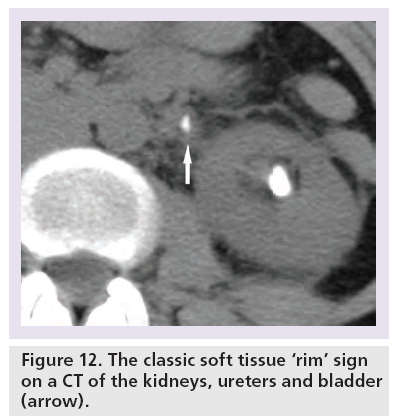
Direct sign: soft tissue rim sign
The soft tissue ‘rim’ sign describes the edematous rim of the ureteral wall surrounding the impacted stone within the lumen of the ureter. It is typically defined as a 1–2 mm rim of soft tissue measuring 20–40 HU that surrounds the impacted stone in the ureter (Figure 12) [5,54].
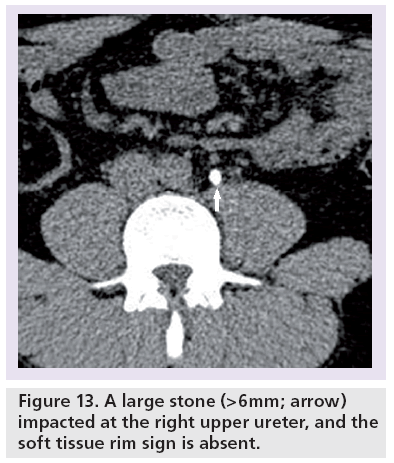
The soft tissue rim sign usually disappears when the stone is >6 mm and this is usually due to the stone stretching the wall of the ureter, thus limiting the visibility of the edema (Figure 13) [54,55].
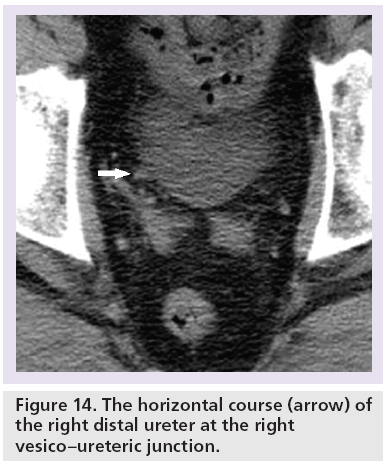
Another difficult area for interpretation is a stone at the VUJ. This is largely due to the fact the ureter has a horizontal course (Figure 14), there is very little intrapelvic fat to contrast with the rim as well as the abundant muscular wall of the intravesical portion (Figure 5). Therefore, this sign is unreliable at the VUJ [14,15,54,55]. The estimated sensitivity and specificity of the soft tissue rim sign (excluding VUJ stones) are 77 and 92%, respectively, in differentiating stones from phleboliths [55]. The positive predictive value of the soft tissue rim sign for identifying a ureteric calculus has been estimated at 84–100%, but VUJ stones (a common site of stone impaction) are excluded [54–57].
It is important to note that phleboliths can demonstrate a positive soft tissue rim sign in approximately 9% of cases [14,54,55] and this may be due to visualization of the noncalcified portion of a pelvic vein and CT volume averaging [56].
Direct sign: comet tail sign
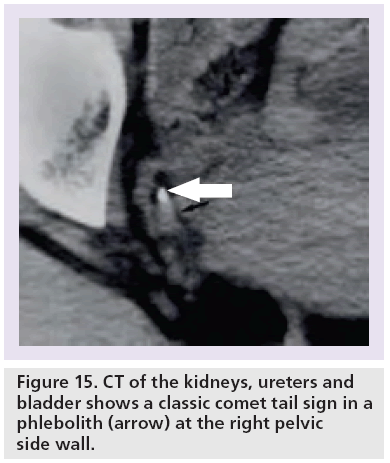
The ‘comet tail’ sign describes the adjacent eccentric tapering soft tissue mass corresponding to the noncalcified portion of the pelvic vein associated with a phlebolith (Figures 7 & 15) [48,56,57]. The comet tail sign has 100% specificity and 65% sensitivity for differentiating phleboliths from a stone in the ureter with a positive predictive value of identifying a phleobolith of 100% [48,56].
Indirect secondary signs
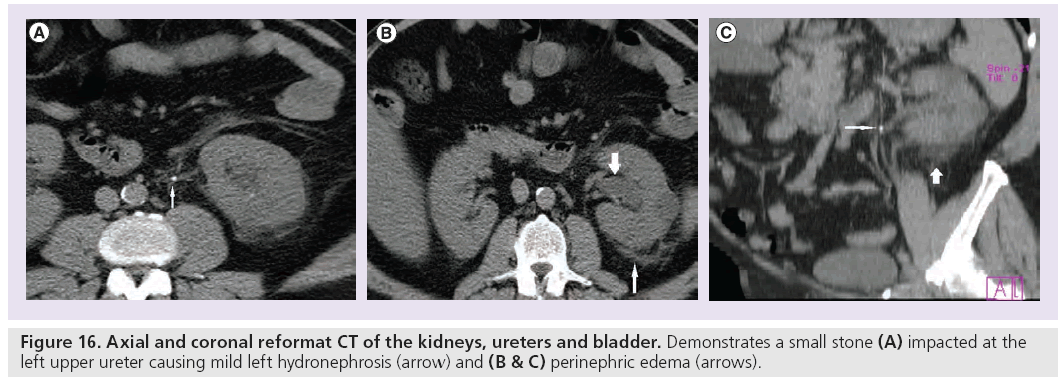
The secondary signs of urinary tract obstruction include nephromegaly, hydronephrosis, hydroureter and perinephric stranding or edema. The sensitivity and specificity are: nephromegaly 71 and 89%, hydronephrosis 69–83 and 94%, hydroureter 67–90 and 93% and perinephric edema 65–82 and 93%, respectively (Figure 16) [14,15]. In addition, the positive predictive value of hydroureter and hydronephrosis in predicting an ipsilateral obstructing stone is 99% [14,15]. The clinical suspicion of the recent passage of a stone through the ureter must be high on the differential diagnosis in patients with hydronephrosis and hydroureter on the symptomatic side in the absence of a visible stone [50].
Perinephric fluid stranding on CTKUB (in the presence of stone disease) is due to increased lymphatic flow within the perinephric space and resorbed urine due to obstructive back pressure [58]. The degree of perinephric edema is proportional to the degree of obstruction on excretory urography [49]. The extent of the stranding is also related to the time after onset of pain, which becomes apparent at 2 h and is maximal at 8 h [50]. It also persists for some time after passage of a stone. On its own, perinephric edema is nonspecific, especially in the elderly where this is of no clinical significance. Occasionally, unilateral perinephric edema may be encountered in patients with pyelonephritis or malignancy. In high pressure obstruction, rupture of the fornices may occur, resulting in urine leak from the calyces into the perinephric space.
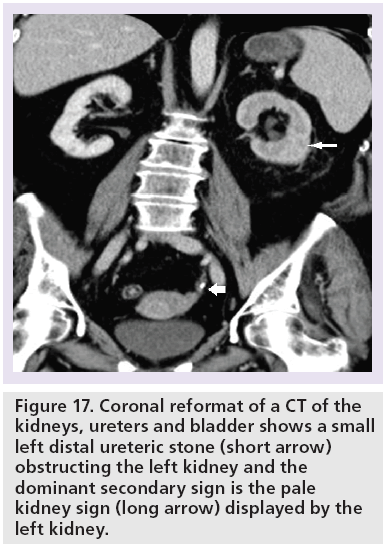
Sometimes, the ‘pale kidney sign’ may be observed when there is <5 HU in the obstructed renal parenchyma due to edema, resulting in a difference in renal parenchymal attenuation between the obstructed and unobstructed kidneys (Figure 17) [59].
Xanthogranulomatous pyelonephritis
Xanthogranulomatous pyelonephritis is a rare chronic destructive granulomatous process of renal parenchyma that occurs with chronic urinary tract obstruction and infection [60]. It affects all age groups and is more common in women. The patients are usually symptomatic with the most common symptoms being loin pain, palpable abdominal mass, lower urinary tract symptoms, weight loss, pyrexia and macroscopic hematuria. The patients usually have raised leucocytes and low hemoglobin with positive urine cultures for Escherichia coli and Proteus mirabilis. CT is the diagnostic imaging of choice for the detection of xanthogranulomatous pyelonephritis [61]. The management options are antibiotics and surgery depending on the patient’s disease status.
Extraurinary pathology
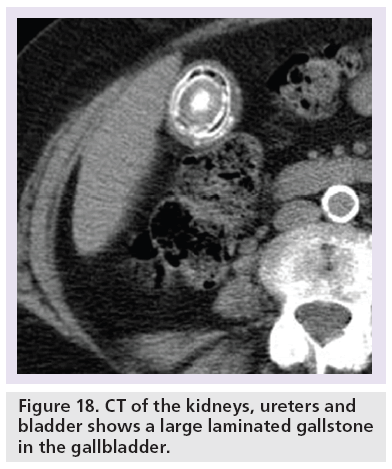
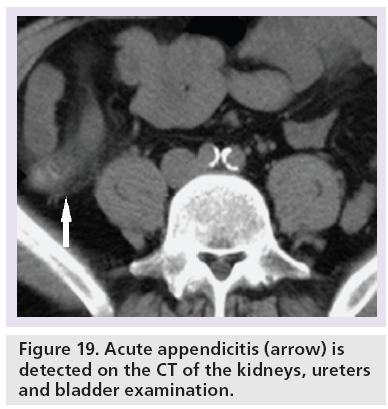
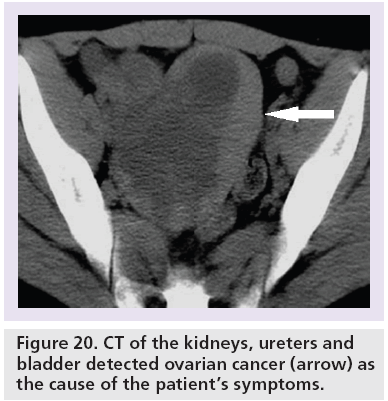
CTKUB may detect alternative symptomatic pathologies in up to 29% of patients with no accepted standard range [2,3,10,25,62–64]. The commoner causes include gallstones (Figure 18), acute appendicitis (Figure 19) and ovarian abnormalities (Figure 20). Furthermore, there is also no stated consensus on what is an acceptable positive pick-up rate for ureteric colic, with a reported range of 44–55.7% [25,62,63].
Figure 20: CT of the kidneys, ureters and bladder detected ovarian cancer (arrow) as the cause of the patient’s symptoms.
In fact, it is well recognized that local referral criteria play a crucial role in the positive stone detection rate and can be directly influenced by clinician referral. Indication creep is in fact a scenario where clinicians have broadened the indications for CTKUB after a period of time. This phenomenon has been observed with decreasing positive CTKUB for stones from 49 to 28% and increases in the alternative extraurinary pathology detection from 16 to 45% over a 12-month period [16]. Therefore, it is important to perform a cyclical audit of clinical practice to ensure no indication creep, as shown in the authors’ experience [3], and that clinicians are regularly updated with relevant education meetings and imaging protocols [3,65].
Randall’s plaque theory
The Randall’s plaque theory suggests that a renal stone forms from deposition of calcium phosphate immediately under the renal papillary epithelium, known as Randall’s plaque, which initiates more calcium adherence and results in renal stone formation [66]. Randall’s plaque is frequently observed in calcium oxalate stone formers [67]. Therefore, current exploratory works have suggested that the recurrent stone former has higher density measurement in the papillary region and measurement of the density on the CTKUB in the papillary region could potentially be used to identify the high-risk recurrent stone former, so to warrant longer-term follow-up [68,69].
Imaging follow-up in patients with urinary stone disease
CTKUB is now the first-line investigation for acute loin pain with clinical suspicion of urinary stone disease and pre-CTKUB has now been omitted from the authors’ departmental imaging pathway since 2008, given that 50% of the patients with suspected ureteric colic do not have stone disease [65]. The challenge is for the group of patients with stones in the ureter who have been managed conservatively. Repeated CTKUB examinations would not be an ideal way of following-up these patients owing to cost and radiation burden. As a result of this, interpretation of CT scout radiographs that are routinely performed during CTKUB has been increasingly incorporated into the management algorithm. The sensitivities of the scout view of CTKUB and plain KUB in detecting stones range from 40 to 52 versus 52 to 60%, respectively [17,70–73].
It is well documented that the majority of ureteric stones <5 mm will pass spontaneously, especially for distal ureteric stones [74]. Though the sensitivity is not as good for the detection of stones <4 mm with CT scout radiographs, these patients are unlikely to need intervention and hence follow-up imaging. The authors also noted that the sensitivity of stone detection based on location is superior when the stones are located in the upper tract compared with the distal ureter/VUJ. In clinical practice, upper tract stones are more likely to need follow-up, as they are likely to be treated if they are large and have the potential to cause obstruction if they migrate distally. The positive predictive value of a stone visible on the CT scout view is reported to be 100%, in the authors’ experience [17]. Therefore, if stones are visible on CT scout radiographs, then baseline plain KUB is unnecessary. However, if stones are not visible on CT scout radiographs, plain KUB should be performed at the time of the CTKUB. The subsequent follow-up investigation will then depend on the visibility of the stone on plain KUB. If the stone is not visible on plain KUB, then follow-up imaging will need to be either ultrasound, to detect the resolution of hydronephrosis, or potential repeat CTKUB depending on the clinical indication and assessment by the urologists.
Conclusion
CTKUB is the standard investigation for acute renal/ureteric colic in current clinical practice and promptly triaged patients referred from the emergency department. Understanding the normal anatomy for the ureter will assist in the interpretation and awareness of direct and indirect radiological signs and will enhance the differentiation of stones from other conditions. Furthermore, it is important to examine the rest of the CT scan carefully in order to pick up alternative diagnoses that may mimic the nonspecific acute loin pain in this cohort of patients. The follow-up strategy, with both cost and radiation taken into account, is equally important in monitoring the recurrent stone former and also to assess whether the ureteric stone has passed with conservative management in patients with stones detected on initial CTKUB.
Future perspective
Dual-energy CT is a promising technique to be incorporated into clinical practice in the future if further work could be used to facilitate the reduction of the radiation dose associated with the technique. This can assist the differentiation of the different soft and hard stones which can be triaged appropriately for their stone management.
Financial & competing interests disclosure
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- Sandhu C, Anson KM, Patel U. Urinary tract stones – part I: role of radiological imaging in diagnosis and treatment planning. Clin. Radiol. 58(6), 415–421 (2003).
- Chowdhury FU, Kotwal S, Raghunathan G, Wah TM, Joyce A, Irving HC. Unenhanced multidetector CT (CT KUB) in the initial imaging of suspected acute renal colic: evaluating a new service. Clin. Radiol. 62(10), 970–977 (2007).
- Patatas K, Panditaratne N, Wah TM, Weston MJ, Irving HC. Emergency department imaging protocol for suspected acute renal colic: re-evaluating our service. Br. J. Radiol. 85(1016), 1118–1122 (2012).
- Prien EL, Frondel C. Studies in urolithiasis; the composition of urinary calculi. J. Urol. 57(6), 949–994 (1947).
- Smith RC, Rosenfield AT, Choe KA et al. Acute flank pain: comparison of non-contrastenhanced CT and intravenous urography. Radiology 194(3), 789–794 (1995).
- Pfister SA, Deckart A, Laschke S et al. Unenhanced helical computed tomography vs intravenous urography in patients with acute flank pain: accuracy and economic impact in a randomized prospective trial. Eur. Radiol. 13(11), 2513–2520 (2003).
- Miller OF, Rineer SK, Reichard SR et al. Prospective comparison of unenhanced spiral computed tomography and intravenous urogram in the evaluation of acute flank pain. Urology 52(6), 982–987 (1998).
- Sourtzis S, Thibeau JF, Damry N, Raslan A, Vandendris M, Bellemans M. Radiologic investigation of renal colic: unenhanced helical CT compared with excretory urography. AJR Am. J. Roentgenol. 172(6), 1491–1494 (1999).
- Chen MY, Zagoria RJ. Can noncontrast helical computed tomography replace intravenous urography for evaluation of patients with acute urinary tract colic? J. Emerg. Med. 17(2), 299–303 (1999).
- Smith RC, Verga M, McCarthy S, Rosenfield AT. Diagnosis of acute flank pain: value of unenhanced helical CT. AJR Am. J. Roentgenol. 166(1), 97–101 (1996).
- Newhouse JH, Prien EL, Amis ES Jr, Dretler SP, Pfister RC. Computed tomographic analysis of urinary calculi. AJR Am. J. Roentgenol. 142(3), 545–548 (1984).
- Hillman BJ, Drach GW, Tracey P, Gaines JA. Computed tomographic analysis of renal calculi. AJR Am. J. Roentgenol. 142(3), 549–552 (1984).
- Sommer FG, Jeffrey RB Jr, Rubin GD et al. Detection of ureteral calculi in patients with suspected renal colic: value of reformatted noncontrast helical CT. AJR Am. J. Roentgenol. 165(3), 509–513 (1995).
- Smith RC, Verga M, Dalrymple N, McCarthy S, Rosenfield AT. Acute ureteral obstruction: value of secondary signs of helical unenhanced CT. AJR Am. J. Roentgenol. 167(5), 1109–1113 (1996).
- Katz DS, Lane MJ, Sommer FG. Unenhanced helical CT of ureteral stones: incidence of associated urinary tract findings. AJR Am. J. Roentgenol. 166(6), 1319–1322 (1996).
- Chen MY, Zagoria RJ, Saunders HS, Dyer RB. Trends in the use of unenhanced helical CT for acute urinary colic. AJR Am. J. Roentgenol. 173(6), 1447–1450 (1999).
- Yap WW, Belfield JC, Bhatnagar P, Kennish S, Wah TM. Evaluation of the sensitivity of scout radiographs on unenhanced helical CT in identifying ureteric calculi: a large UK tertiary referral centre experience.
- Br. J. Radiol. 85(1014), 800–806 (2012). 18 Ellis H. The urinary tract. In: Clinical Anatomy a Revision and Applied Anatomy for Clinical Students 10th Edition. Ellis H (Ed.). Blackwell Publishing, NY, USA, 112–122 (2002).
- Privett JT, Jeans WD, Roylance J. The incidence and importance of renal duplication. Clin. Radiol. 27(4), 521–530 (1976).
- Hartman GW, Hodson CJ. The duplex kidney and related abnormalities. Clin. Radiol. 20(4), 387–400 (1969).
- Nusbacher N, Bryk D. Hydronephrosis of the lower pole of the duplex kidney: another renal pseudotumor. AJR Am. J. Roentgenol. 130(5), 967–969 (1978).
- Levine J, Neitlich J, Smith RC. The value of prone scanning to distinguish ureterovesical junction stones from ureteral stones that have passed into the bladder: leave no stone unturned. AJR Am. J. Roentgenol. 172(4), 977–981 (1999).
- Eikefjord EN, Thorsen F, Rorvik J. Comparison of effective radiation doses in patients undergoing unenhanced MDCT and excretory urography for acute flank pain. AJR Am. J. Roentgenol. 188(4), 934–939 (2007).
- Rucker CM, Menias CO, Bhalla S. Mimics of renal colic: alternative diagnoses at unenhanced helical CT. Radiographics 24(Suppl. 1), S11–S28; discussion S28–S33 (2004).
- Greenwell TJ, Woodhams S, Denton ER, MacKenzie A, Rankin SC, Popert R. One year’s clinical experience with unenhanced spiral computed tomography for the assessment of acute loin pain suggestive of renal colic. BJU Int. 85(6), 632–636 (2000).
- Mendelson RM, Arnold-Reed DE, Kuan M et al. Renal colic: a prospective evaluation of non-enhanced spiral CT versus intravenous pyelography. Australas. Radiol. 47(1), 22–28 (2003).
- Denton ER, MacKenzie A, Greenwell T, Popert R, Rankin SC. Unenhanced helical CT for renal colic – is the radiation dose justifiable? Clin. Radiol. 54(7), 444–447 (1999).
- Poletti PA, Platon A, Rutschmann OT et al. Abdominal plain film in patients admitted with clinical suspicion of renal colic: should it be replaced by low-dose computed tomography? Urology 67(1), 64–68 (2006).
- Liu W, Esler SJ, Kenny BJ, Goh RH, Rainbow AJ, Stevenson GW. Low-dose nonenhanced helical CT of renal colic: assessment of ureteric stone detection and measurement of effective dose equivalent. Radiology 215(1), 51–54 (2000).
- Rogers LF. Dose reduction in CT: how low can we go? AJR Am. J. Roentgenol. 179(2), 299 (2002).
- Hamm M, Knopfle E, Wartenberg S, Wawroschek F, Weckermann D, Harzmann R. Low dose unenhanced helical computerized tomography for the evaluation of acute flank pain. J. Urol. 167(4), 1687–1691 (2002).
- Meagher T, Sukumar VP, Collingwood J et al. Low dose computed tomography in suspected acute renal colic. Clin. Radiol. 56(11), 873–876 (2001).
- Poletti PA, Platon A, Rutschmann OT, Schmidlin FR, Iselin CE, Becker CD. Low-dose versus standard-dose CT protocol in patients with clinically suspected renal colic. AJR Am. J. Roentgenol. 188(4), 927–933 (2007).
- Kluner C, Hein PA, Gralla O et al. Does ultra-low-dose CT with a radiation dose equivalent to that of KUB suffice to detect renal and ureteral calculi? J. Comput. Assist. Tomogr. 30(1), 44–50 (2006).
- Kim BS, Hwang IK, Choi YW et al. Low-dose and standard-dose unenhanced helical computed tomography for the assessment of acute renal colic: prospective comparative study. Acta Radiol. 46(7), 756–763 (2005).
- Tack D, Sourtzis S, Delpierre I, de Maertelaer V, Gevenois PA. Low-dose unenhanced multidetector CT of patients with suspected renal colic. AJR Am. J. Roentgenol. 180(2), 305–311 (2003).
- Heneghan JP, McGuire KA, Leder RA, Delong DM, Yoshizumi T, Nelson RC. Helical CT for nephrolithiasis and ureterolithiasis: comparison of conventional and reduced radiation-dose techniques. Radiology 229(2), 575–580 (2003).
- Bellin MF, Renard-Penna R, Conort P et al. Helical CT evaluation of the chemical composition of urinary tract calculi with a discriminant analysis of CT-attenuation values and density. Eur. Radiol. 14(11), 2134–2140 (2004).
- Eiber M, Holzapfel K, Frimberger M et al. Targeted dual-energy single-source CT for characterisation of urinary calculi: experimental and clinical experience. Eur. Radiol. 22(1), 251–258 (2012).
- Manglaviti G, Tresoldi S, Guerrer CS et al. In vivo evaluation of the chemical composition of urinary stones using dual-energy CT. AJR Am. J. Roentgenol. 197(1), W76–W83 (2011).
- Thomas C, Patschan O, Ketelsen D et al. Dual-energy CT for the characterization of urinary calculi: in vitro and in vivo evaluation of a low-dose scanning protocol. Eur. Radiol. 19(6), 1553–1559 (2009).
- Thomas C, Heuschmid M, Schilling D et al. Urinary calculi composed of uric acid, cystine, and mineral salts: differentiation with dual-energy CT at a radiation dose comparable to that of intravenous pyelography. Radiology 257(2), 402–409 (2010).
- Graser A, Johnson TR, Bader M et al. Dual energy CT characterization of urinary calculi: initial in vitro and clinical experience. Invest. Radiol. 43(2), 112–119 (2008).
- Silva AC, Morse BG, Hara AK, Paden RG, Hongo N, Pavlicek W. Dual-energy (spectral) CT: applications in abdominal imaging. Radiographics 31(4), 1031–1046; discussion 1047–1050 (2011).
- Nadler RB, Stern JA, Kimm S, Hoff F, Rademaker AW. Coronal imaging to assess urinary tract stone size. J. Urol. 172(3), 962–964 (2004).
- Coll DM, Varanelli MJ, Smith RC. Relationship of spontaneous passage of ureteral calculi to stone size and location as revealed by unenhanced helical CT. AJR Am. J. Roentgenol. 178(1), 101–103 (2002).
- Masarani M, Dinneen M. Ureteric colic: new trends in diagnosis and treatment. Postgrad. Med. J. 83(981), 469–472 (2007).
- Boridy IC, Nikolaidis P, Kawashima A, Goldman SM, Sandler CM. Ureterolithiasis: value of the tail sign in differentiating phleboliths from ureteral calculi at nonenhanced helical CT. Radiology 211(3), 619–621 (1999).
- Boridy IC, Kawashima A, Goldman SM, Sandler CM. Acute ureterolithiasis: nonenhanced helical CT findings of perinephric edema for prediction of degree of ureteral obstruction. Radiology 213(3), 663–667 (1999).
- Jindal G, Ramchandani P. Acute flank pain secondary to urolithiasis: radiologic evaluation and alternate diagnoses. Radiol. Clin. North Am. 45(3), 395–410, vii (2007).
- Spencer BA, Wood BJ, Dretler SP. Helical CT and ureteral colic. Urol. Clin. North Am. 27(2), 231–241 (2000).
- Pareek G, Armenakas NA, Fracchia JA. Hounsfield units on computerized tomography predict stone-free rates after extracorporeal shock wave lithotripsy. J. Urol. 169(5), 1679–1681 (2003).
- Pareek G, Hedican SP, Lee FT Jr, Nakada SY. Shock wave lithotripsy success determined by skin-to-stone distance on computed tomography. Urology 66(5), 941–944 (2005).
- Kawashima A, Sandler CM, Boridy IC, Takahashi N, Benson GS, Goldman SM. Unenhanced helical CT of ureterolithiasis: value of the tissue rim sign. AJR Am. J. Roentgenol. 168(4), 997–1000 (1997).
- Heneghan JP, Dalrymple NC, Verga M, Rosenfield AT, Smith RC. Soft-tissue ‘rim’ sign in the diagnosis of ureteral calculi with use of unenhanced helical CT. Radiology 202(3), 709–711 (1997).
- Bell TV, Fenlon HM, Davison BD, Ahari HK, Hussain S. Unenhanced helical CT criteria to differentiate distal ureteral calculi from pelvic phleboliths. Radiology 207(2), 363–367 (1998).
- Guest AR, Cohan RH, Korobkin M et al. Assessment of the clinical utility of the rim and comet-tail signs in differentiating ureteral stones from phleboliths. AJR Am. J. Roentgenol. 177(6), 1285–1291 (2001).
- Hartman RP, Kawashima A, Leroy AJ. Helical CT in the diagnosis of urolithiasis. In: New Techniques in Uroradiology (1st Edition). Morcos S, Cohan RH (Eds). Taylor and Francis, PA, USA, 13–33 (2006).
- Georgiades CS, Moore CJ, Smith DP. Differences of renal parenchymal attenuation for acutely obstructed and unobstructed kidneys on unenhanced helical CT: a useful secondary sign? AJR Am. J. Roentgenol. 176(4), 965–968 (2001).
- Li L, Parwani AV. Xanthogranulomatous pyelonephritis. Arch. Pathol. Lab. Med. 135(5), 671–674 (2011).
- Rajesh A, Jakanani G, Mayer N, Mulcahy K. Computed tomography findings in xanthogranulomatous pyelonephritis. J. Clin. Imaging Sci. 1, 45 (2011).
- Boulay I, Holtz P, Foley WD, White B, Begun FP. Ureteral calculi: diagnostic efficacy of helical CT and implications for treatment of patients. AJR Am. J. Roentgenol. 172(6), 1485–1490 (1999).
- Katz DS, Scheer M, Lumerman JH, Mellinger BC, Stillman CA, Lane MJ. Alternative or additional diagnoses on unenhanced helical computed tomography for suspected renal colic: experience with 1000 consecutive examinations. Urology 56(1), 53–57 (2000).
- Levine JA, Neitlich J, Verga M, Dalrymple N, Smith RC. Ureteral calculi in patients with flank pain: correlation of plain radiography with unenhanced helical CT. Radiology 204(1), 27–31 (1997).
- Kennish SJ, Bhatnagar P, Wah TM, Bush S, Irving HC. Is the KUB radiograph redundant for investigating acute ureteric colic in the non-contrast enhanced computed tomography era? Clin. Radiol. 63(10), 1131–1135 (2008).
- Evan AP, Lingeman JE, Coe FL et al. Randall’s plaque of patients with nephrolithiasis begins in basement membranes of thin loops of Henle. J. Clin. Invest. 111(5), 607–616 (2003).
- Low RK, Stoller ML. Endoscopic mapping of renal papillae for Randall’s plaques in patients with urinary stone disease. J. Urol. 158(6), 2062–2064 (1997).
- Eisner BH, Iqbal A, Namasivayam S et al. Differences in computed tomography density of the renal papillae of stone formers and non-stone-formers: a pilot study. J. Endourol. 22(10), 2207–2210 (2008).
- Bhuskute NM, Yap WW, Wah TM. A retrospective evaluation of Randall’s plaque theory of nephrolithiasis with CT attenuation values. Eur. J. Radiol. 72(3), 470–472 (2009).
- Ege G, Akman H, Kuzucu K, Yildiz S. Can computed tomography scout radiography replace plain film in the evaluation of patients with acute urinary tract colic? Acta Radiol. 45(4), 469–473 (2004).
- Assi Z, Platt JF, Francis IR, Cohan RH, Korobkin M. Sensitivity of CT scout radiography and abdominal radiography for revealing ureteral calculi on helical CT: implications for radiologic follow-up. AJR Am. J. Roentgenol. 175(2), 333–337 (2000).
- Chu G, Rosenfield AT, Anderson K, Scout L, Smith RC. Sensitivity and value of digital CT scout radiography for detecting ureteral stones in patients with ureterolithiasis diagnosed on unenhanced CT. AJR Am. J. Roentgenol. 173(2), 417–423 (1999).
- Johnston R, Lin A, Du J, Mark S. Comparison of kidney-ureter-bladder abdominal radiography and computed tomography scout films for identifying renal calculi. BJU Int. 104(5), 670–673 (2009).
- Segura JW, Preminger GM, Assimos DG et al. Ureteral Stones Clinical Guidelines Panel summary report on the management of ureteral calculi. The American Urological Association. J. Urol. 158(5), 1915–1921 (1997).
• Ultrasound screening in young females is recommended rather than an indiscriminate use of CT scan of the kidneys, ureters and bladder (CTKUB), following review of a large CTKUB practice at a tertiary referral institution.
• • Suggests that the composition of stone’s ability can be differentiated with the dual-energy CT.
• • Current literature demonstrating the limitation of dual-energy CT in determining the stone composition, when stone size is <1 cm.
• • Paper on the application of dual-energy CT in the urinary tract.