Research Article - Clinical Investigation (2018) Volume 8, Issue 4
A comparison of liprotamase, a non-porcine pancreatic enzyme replacement therapy, to porcine extracted pancrelipase in a noninferiority randomized clinical trial in patients with cystic fibrosis
- Corresponding Author:
- Michael W Konstan
Case Western Reserve University School of Medicine and Rainbow Babies and Children’s Hospital, Cleveland, Ohio, USA
E-mail: michael.konstan@case.edu
Submitted Date: 04 September 2018; Accepted Date: 25 September 2018; Published Date: 27 September 2018
Abstract
Objective: Porcine derived enzymes are used for pancreatic enzyme replacement therapy in patients with cystic fibrosis (CF). Liprotamase is a biotechnology-derived, non-porcine, enzyme replacement without enteric coating. This study compared the effects of liprotamase and porcinederived pancrelipase on Coefficient of Fat Absorption (CFA) in patients with Exocrine Pancreatic Insufficiency (EPI) due to CF.
Methods: We conducted a randomized, open-label, assessor blind, parallel group, multicenter, international trial to evaluate the noninferiority of liprotamase to porcine pancrelipase in 128 CF patients’ age ≥7 years with pancreatic insufficiency (Study NCT02279498). Subjects were randomized to liprotamase or pancrelipase, dose-matched to pre-study lipase doses. The primary endpoint was the between group difference in least square (LS) mean change from baseline in CFA, with a non-inferiority margin of -15% for the lower bound of the 95% confidence interval (CI). Key secondary endpoints compared treatment effects on CFA in the absence or presence of concomitant gastric acid suppression (GAS), and coefficient of nitrogen absorption (CNA).
Results: Liprotamase missed the noninferiority criterion for CFA (95% CI -16.0, -7.7%), but met that criterion for CNA (95% CI -1.9, -0.7%). Concomitant GAS was associated with higher CFA with liprotamase but not pancrelipase.
Conclusion: In this study, liprotamase was inferior to pancrelipase with regards to CFA, but not CNA. Higher doses and GAS may improve the efficacy of liprotamase.
Keywords
Cystic fibrosis, Pancreatic insufficiency, Pancreatic enzyme replacement, Malabsorption
Abbreviations
BMI: body mass index; BOCF: baseline observation carried forward; CF: cystic fibrosis; CFA: coefficient of fat absorption; CFTR: CF transmembrane conductance regulator; CI: confidence interval; CLEC: cross-linked enzyme crystal; CNA: coefficient of nitrogen absorption; DIOS: distal intestinal obstruction syndrome; EPI: exocrine pancreatic insufficiency; GAS: gastric acid suppression; MI: multiple imputation; mITT: modified intent to treat; PERT: pancreatic enzyme replacement therapy
Introduction
The exocrine pancreas is responsible for synthesis and secretion of bicarbonate and digestive enzymes including lipase, protease and amylase. In exocrine pancreatic insufficiency (EPI) related to cystic fibrosis (CF), the exocrine function of the pancreas is impeded or destroyed, and lipids, proteins and carbohydrates enter the distal gastrointestinal (GI) tract in less absorbable forms leading to abdominal pain and distention, flatulence, and steatorrhea. Without appropriate therapy, patients with EPI experience poor growth, weight loss, and malnutrition.
Oral, porcine-derived pancreatic enzyme replacement therapy (PERT) has been available for many years to treat EPI, and is used in over 80% of patients with CF [1,2]. Porcine derived PERTs contain a mixture of lipases, proteases, amylases, and other proteins and cofactors extracted from the pig pancreas. To protect the porcine enzymes, particularly the lipases, from destruction by gastric acid, PERTs are typically formulated into enteric-coated beads. This enteric coating is designed to protect the porcine enzymes from destruction at low gastric pH and dissolve at a pH of approximately 5.5 [3]. Some patients with CF have a lower pH recorded in the duodenum and upper jejunum, reflecting a reduced bicarbonate buffering capacity and require concomitant therapy with gastric acid suppressants (GAS) in order to increase duodenal and jejunal pH and enable release of the enzymes [4,5].
Although porcine-derived PERTs have been available for many years for treatment of EPI, there are several risks associated with its use, including the potential for zoonotic infection, allergic reaction to porcine proteins, and hyperuicemia due to their high purine content. Moreover, for religious reasons, some patients abstain from pork or pork products. Although no cases of infection acquired from porcine-derived PERTs have been reported to date, the risk of zoonotic viral contamination of porcine PERTs on the drug supply chain and product safety prompted the US Food and Drug Administration to suggest that future PERTs should be developed from recombinant or synthetic processes to mitigate these concerns [6].
Liprotamase is a novel, biotechnology-derived PERT that contains 3 digestive enzymes: a lipase cross-linked enzyme crystal (lipase-CLEC), a crystallized protease, and an amorphous amylase, formulated in a fixed enzyme ratio of 1:1:0.15 without enteric coating. Lipase-CLEC is engineered to enable stability of the enzyme in the low pH environment of the stomach. If liprotamase can be shown to be as effective as porcine-derived PERTs, the risks associated with porcine-derived PERT outlined above can be avoided.
In previous clinical trials in patients with CF, treatment with liprotamase resulted in a statistically significant increase in coefficients of fat absorption (CFA) and nitrogen absorption (CNA) [7,8]. Dose ranging was explored in a Phase 2 trial, and led to a fixed dose of lipase per meal (32,500 units) in the second trial without regard to fat intake or weight of subjects. Since the mean CFA achieved with liprotamase in that study was less than 80%, a trial using doses of liprotamase comparable to pancrelipase was proposed. Moreover, there have been no studies directly comparing the efficacy and safety of liprotamase to pancrelipase. In this report, we present the results of the SOLUTION trial (Study NCT02279498), which compared liprotamase to pancrelipase with initial lipase dosing of liprotamase comparable to pancrelipase.
Materials and Methods
Trial Design
The SOLUTION study was a phase 3, randomized, open-label, assessor blind, parallel-group, non-inferiority study conducted in 128 subjects with CF-related EPI age ≥7 years. The study was conducted across 46 clinical centers in Canada, Czech Republic, Hungary, Israel, Poland, Spain, and the USA. Patients had to have a fecal elastase <100 mcg/g, fair or better nutritional status, and baseline CFA of ≥80% while taking a porcine PERT other than the pancrelipase Pancreaze® for at least 30 days at a dose not exceeding 10,000 units lipase/kg/day. Subjects were ineligible if they had acute respiratory tract infection, a history of fibrosing colonopathy, recent distal intestinal obstruction syndrome (DIOS), prior liver or lung transplant, significant surgical resection of the bowel, elevated liver enzymes (greater than 5-times the upper limit of normal) or total bilirubin levels (greater than the upper limit of normal), hyperuricemia or uncontrolled diabetes, FEV1 <30% of predicted, or were receiving feeding via an enteral tube.
All subjects enrolled in this study were required to discontinue their pre-study PERT after screening and initiate study drug (liprotamase or pancrelipase [Pancreaze®]). Subjects were randomized 1:1 to liprotamase or pancrelipase and matched as close as possible to their pre-randomization PERT dose in lipase units administered with every meal or snack. Randomization, performed in blocks of 4, was stratified by age at enrollment (<17 years vs. ≥17 years) and GAS use (yes vs. no). CFA and other key efficacy endpoints were evaluated during the screening period at the end of the Primary Treatment Period, Week 7. Subjects continued to receive liprotamase or pancrelipase during the Extension Period through Week 20 for longer-term evaluation of efficacy and safety.
If warranted by clinical signs and symptoms, and if sanctioned by a blinded assessor, up to 2 dose adjustments, each up to 25% of the starting dose, were allowed during the first 2 weeks of the Primary Treatment Period. Additional dose adjustments were allowed during the Extension Period at the judgement of the Investigator. The dose of study drug was not allowed to exceed 10,000 units lipase/kg/day or 2,500 units lipase/kg/meal. Concomitant medications for treatment of cystic fibrosis, including CF transmembrane conductance regulator (CFTR) modulators, GAS, and vitamin supplements, were allowed if maintained throughout the study.
Two supervised confinement periods were used to measure CFA, CNA and stool weight, one during screening while the subject received stable, pre-study PERT, and another at Week 7 for the primary efficacy analysis. During these confinements, subjects received blue marker capsules (FD&C blue #2) at the start and end of a 72-hour controlled diet, consisting of 85-115 g fat/day and a minimum of 1.5 to 2 g of protein/kg body weight. All stool was collected after the appearance of blue stool following the 1st blue dye capsules until the appearance of blue stool due to the second blue dye capsules, and analyzed for weight, fat content, and nitrogen content [9-11].
Safety was evaluated throughout the study from physical examinations, vital signs, clinical laboratory results and adverse event (AE) recordings. At the end of the 20-week study, subjects resumed their pre-study porcine PERT or, if they had been randomized to liprotamase, were invited to participate in a separate open-label extension study.
The study was conducted in accordance with the provisions of the Declaration of Helsinki, and governmental, state, and local laws. Each site obtained approval to conduct the trial from their local ethics committee or institutional review board. Informed consent was obtained from each patient prior to initiation of any study assessment or procedure. The trial started in September 2015, and the last patient completed the last study visit January 2017.
Analyses and Statistical Methods
The primary endpoint was the between group difference in least square (LS) mean change from baseline in CFA with a non-inferiority margin of 15%. Under this analysis, liprotamase would be considered non-inferior to pancrelipase if the 95% confidence intervals (CI) fall within -15 and +15%. Secondary efficacy analyses at Week 7 and/or at Week 20 included CNA, stool weight, signs and symptoms of malabsorption (abdominal pain, bloating, steatorrhea, flatulence, stool frequency, stool consistency), height, weight, body mass index (BMI), total cholesterol, vitamin (A, D, E, and K) levels, albumin, and pre-albumin.
To mitigate the potential for bias due to withdrawal from study prior to CFA measurement at the end of the primary treatment period, the CFA primary endpoint was evaluated after imputing missing CFA measures at end of the treatment period using multiple imputation (MI) methods based on regression imputation and after generation of 50 complete datasets. In this model, for each of 50 datasets, ANCOVA was to model CFA change from baseline as the dependent variable; treatment, screening CFA, age group (<17, ≥17), and acid suppression (yes, no) were used as covariates. Under this methodology, all randomized subjects who received at least one dose of study drug (the mITT population) were included in the primary and key secondary analyses of non-inferiority in change from baseline CFA and CNA.
Sensitivity analyses used ANCOVA methods with different stratification factors without multiple imputation, and baseline-observation-carried-forward (BOCF). A per protocol (PP) analysis was also conducted in the population of subjects with no major protocol deviations. Analyses of CNA were conducted using the same ANCOVA statistical methodology as for CFA. Other secondary analyses of changes from baseline in weight, height, BMI and malabsorption signs and symptoms, and subgroup analyses of treatment effects on key primary and secondary endpoints based on stratification factors of age group, and GAS use, were also analyzed using ANCOVA based on the mITT population without imputation of missing data using observed-case data only.
The planned sample size of 126 randomized subjects (approximately 63/group) was calculated to provide 92% power to test the non-inferiority of liprotamase to pancrelipase in ΔCFA assuming a non-inferiority margin of 15%, standard deviation of 18%, treatment difference of 4% in favor of active comparator, and 1-sided significance level of 0.025. A blinded interim analysis to inform sample size had been planned for when at least 50% of subjects had completed their Week 7 evaluation of CFA. However, owing to near-complete enrollment at the time of the planned analysis, this interim analysis was not conducted. No other significant changes were made to the key design elements or conduct during this trial.
Results
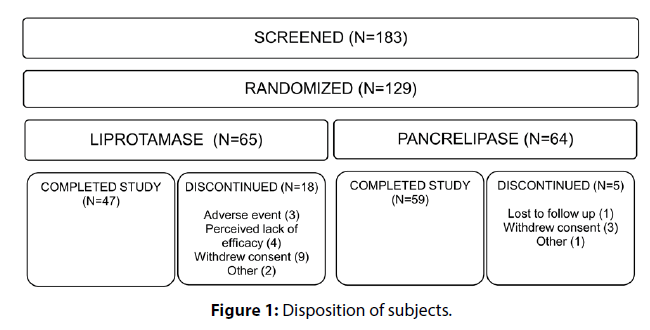
183 subjects were screened, 129 of whom were randomized (Figure 1). The majority of screen failures (61.1%) were due to failure to meet the screening criterion of CFA ≥80. One subject randomized to pancrelipase received no study drug and was excluded from all mITT analyses. Demographics and baseline disease characteristics were similar between the 2 treatment groups (Table 1). Withdrawals were more common among liprotamase subjects, and were chiefly due to withdrawal of consent, lack of efficacy or AEs (Figure 1). Week 7 CFA observations were missing and consequently imputed using multiple imputation methodology for the primary endpoint for 18.5% and 3.1% of subjects in the liprotamase and pancrelipase arms, respectively.
| Liprotamase (N=65) | Pancrelipase (N=63) | |
|---|---|---|
| Female (%) | 46.2 | 49.2 |
| Age | ||
| Mean, years (SD) | 22.5 (8.54) | 21.0 (8.95) |
| 7 to <12 years, n (%) | 5 ( 7.7) | 10 (15.9) |
| ≥ 12 to <17 years, n (%) | 16 (24.6) | 11 (17.5) |
| ≥ 17 years, n (%) | 44 (67.7) | 42 (66.7) |
| Race, n (%) | ||
| White | 63 (96.9) | 61 (96.8) |
| Other | 2 ( 3.0) | 2 ( 3.2) |
| Size, mean (SD) | ||
| Weight (kg) | 57.8 (14.3) | 54.4 (14.6) |
| Height (cm) | 163.3 (12.0) | 160.1 (16.4) |
| BMI (kg/m2) | 21.4 ( 3.3) | 20.7 ( 3.0) |
| Gastric acid suppression use, n (%) | ||
| Yes (any) | 27 (41.5) | 24 (38.1) |
| Proton pump inhibitor | 24 (36.9) | 22 (34.9) |
| H2 antagonist | 4 ( 6.2) | 2 ( 3.2) |
| Name of pre-randomization PERT, n (%) | ||
| Creon® | 49 (75.4) | 48 (76.2) |
| Other (e.g. Zenpep®, Pertzye®) | 16 (24.6) | 15 (23.8) |
| Use of CFTR modulators, n (%) | ||
| Ivacaftor | 1 ( 1.5) | 2 ( 3.2) |
| Lumacaftor & Ivacaftor combination | 11 (16.9) | 7 (11.1) |
| Abbreviations: SD: standard deviation; BMI: body mass index; PERT: pancreatic enzyme replacement therapy. | ||
Table 1: Demographics and Baseline Characteristics
Effects on CFA, CNA and Stool Weight
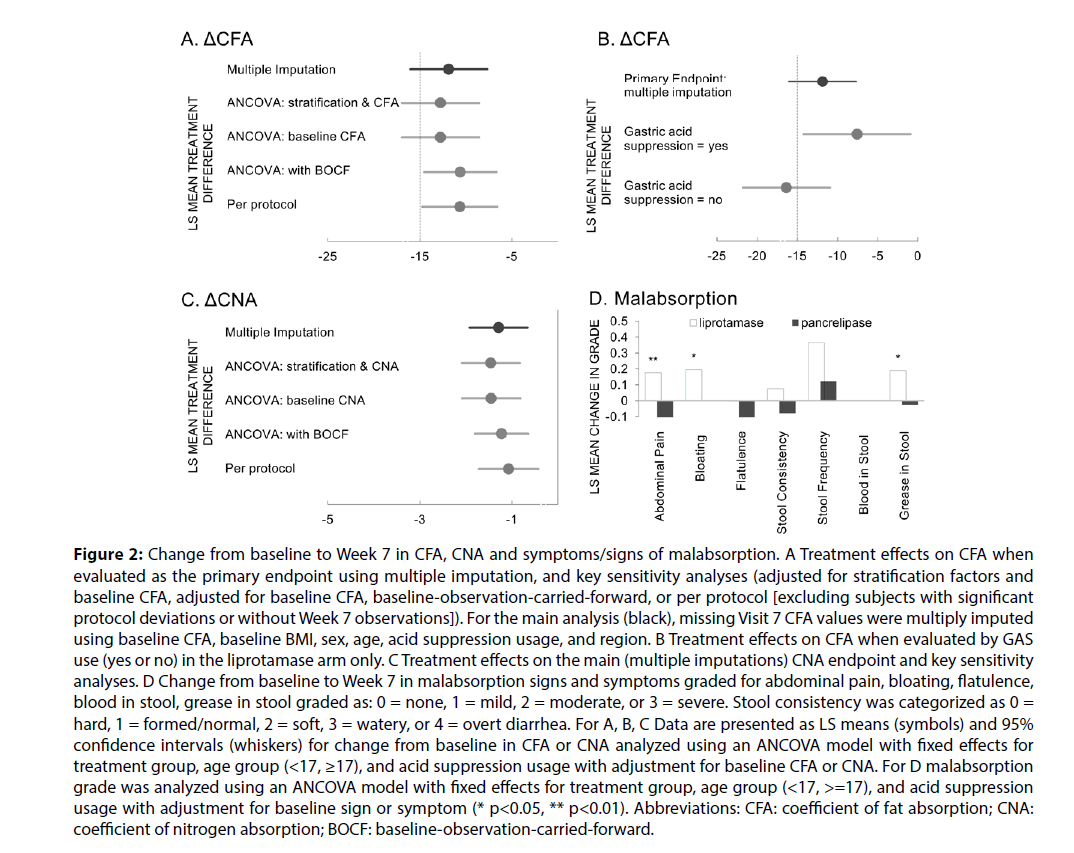
The mean CFA on pancrelipase at screening (baseline) for each group was similar, but the mean CFA at Week 7 was significantly lower for the liprotamase group compared to the pancrelipase group (76.5% vs. and 89.5%, Table 2). The primary efficacy endpoint of 15% non-inferiority margin for ΔCFA for liprotamase compared with pancrelipase was not met. LS mean for Week 7 ΔCFA for liprotamase was 11.2 and for pancrelipase was 0.62, with a treatment difference in ∆CFA of 11.85 and lower and upper 95% CI = -16.0 and -7.7 (Table 2, Figure 2A). In the pre-specified sensitivity analyses, liprotamase met the 15% non-inferiority margin for CFA when evaluated in the per protocol population, and under one of the sensitivity analyses in the mITT population, that using BOCF imputation (Figure 2A).
| Liprotamase (N=65) | Pancrelipase (N=63) | Statistical Analyses | |
|---|---|---|---|
| PERT Dose (units lipase/kg/day) | |||
| Baseline (pre-study) mean (min, max) | 6298.6 (1190.5, 9981.0) | 6264.4 (1692.0, 9962.3) | |
| Week 7 mean (min, max) | 7286.2 (4478.0, 10000.0) | 6948.8 (3099.0, 9938.0) | |
| Fat intake (g/24 hours) | |||
| Baseline mean (min, max) | 104.0 (92.5, 114.6) | 102.3 (83.8, 125.6) | |
| Week 7 mean (min, max) | 103.3 (119.3, 100.7) | 102.2 (72.7, 124.1) | |
| CFA (%) | |||
| Baseline mean (min, max) | 88.7 (80, 98) | 89.4 (79, 97) | |
| Week 7 mean (min, max) | 76.5 (31, 99) | 89.5 (64, 99) | |
| Treatment difference in ∆CFA LS mean (standard error) 95% confidence intervals |
-11.85 (2.12) -16.00, -7.70 |
||
| Protein intake (g/24 hours) | |||
| Baseline mean (min, max) | 104.7 (66.3, 171.5) | 106.9 (54.3, 189.3) | |
| Week 7 mean (min, max) | 103.0 (66.3, 137.5) | 107.2 (58.1, 190.3) | |
| CNA (%) | |||
| Baseline mean (min, max) | 96.9 (92, 99) | 97.3 (94, 99) | |
| Week 7 mean (min, max) | 95.8 (87, 99) | 97.5 (93, 99) | |
| Treatment difference in ∆CNA LS mean (standard error) 95% confidence intervals |
-1.29 (0.25) -1.92, -0.66 |
||
| Stool weight (g) | |||
| Baseline mean (min, max) | 618.0 (147, 1510) | 552.2 (160, 1116) | |
| Week 7 mean (min, max) | 782.1 (107, 1706) | 551.2 (75, 1341) | |
| Treatment difference in ∆stool wt LS mean (standard error) 95% confidence intervals, p value |
204.3 (51.6) 102.1, 306.6, p<0.001 |
||
| Body Weight (kg) | |||
| Baseline mean (min, max) | 57.8 (25.0, 109.9) | 54.4 (20.0, 81.4) | |
| Week 7 mean (min, max) | 56.6 (24.3, 102.5) | 54.6 (20.3, 82.5) | p<0.001 |
| PERT=pancreatic enzyme replacement therapy; CFA=coefficient of fat absorption; CNA=coefficient of nitrogen absorption; min=minimum; max=maximum, LS=least squares, ∆=change from baseline, stool wt.=stool weight. | |||
Table 2: Baseline and Week 7 Measures of EPI
Figure 2: Change from baseline to Week 7 in CFA, CNA and symptoms/signs of malabsorption. A Treatment effects on CFA when evaluated as the primary endpoint using multiple imputation, and key sensitivity analyses (adjusted for stratification factors and baseline CFA, adjusted for baseline CFA, baseline-observation-carried-forward, or per protocol [excluding subjects with significant protocol deviations or without Week 7 observations]). For the main analysis (black), missing Visit 7 CFA values were multiply imputed using baseline CFA, baseline BMI, sex, age, acid suppression usage, and region. B Treatment effects on CFA when evaluated by GAS use (yes or no) in the liprotamase arm only. C Treatment effects on the main (multiple imputations) CNA endpoint and key sensitivity analyses. D Change from baseline to Week 7 in malabsorption signs and symptoms graded for abdominal pain, bloating, flatulence, blood in stool, grease in stool graded as: 0 = none, 1 = mild, 2 = moderate, or 3 = severe. Stool consistency was categorized as 0 = hard, 1 = formed/normal, 2 = soft, 3 = watery, or 4 = overt diarrhea. For A, B, C Data are presented as LS means (symbols) and 95% confidence intervals (whiskers) for change from baseline in CFA or CNA analyzed using an ANCOVA model with fixed effects for treatment group, age group (<17, ≥17), and acid suppression usage with adjustment for baseline CFA or CNA. For D malabsorption grade was analyzed using an ANCOVA model with fixed effects for treatment group, age group (<17, >=17), and acid suppression usage with adjustment for baseline sign or symptom (* p<0.05, ** p<0.01). Abbreviations: CFA: coefficient of fat absorption; CNA: coefficient of nitrogen absorption; BOCF: baseline-observation-carried-forward.
In an attempt to identify subjects that might be more responsive to liprotamase, both prespecified and post hoc subgroup analyses were undertaken using the mITT population. Of the preplanned analyses (age, baseline CFA, GAS), only GAS usage seemed to identify a more responsive group. Smaller mean decreases from baseline in CFA were noted in liprotamase subjects receiving GAS compared with those without GAS (LS mean ΔCFA -8.8 and -15.9, Figure 2B). In contrast, among subjects randomized to pancrelipase, observed ΔCFAs were similar in the presence or absence of GAS (-0.44 and 0.45). Post hoc analyses of fecal elastase, CFTR genotype, use of CFTR modulators, baseline lipase dose, geographic region, and BMI were unrevealing. Although total dietary fat intake was specified, and diet was to remain constant during the two stool collection periods, a specific diet was not mandated. In reviewing dietary intake, there was no obvious difference between the diet preferences of those who responded and did not respond to liprotamase.
For CNA, liprotamase met the 15% non-inferiority margin for change from baseline compared with pancrelipase in the mITT population using multiple imputation methodology (lower and upper 95% CI = -1.92 and -0.66) as well as in the per protocol population, and under sensitivity analyses in the mITT population using BOCF imputation, or no imputation with covariates of baseline CNA with or without stratification factors (Figure 2C).
A significant increase in marker-to-marker stool weight was noted at Week 7 for subjects on liprotamase compared to those on pancrelipase (LS mean change from baseline 186.1 gm vs -18.3 gm, p<0.001, Table 2).
Other measures of efficacy
Consistent with the observed effects on CFA, malabsorption symptom scores for abdominal pain, bloating and steatorrhea were generally worse in the liprotamase arm compared with pancrelipase (Figure 2D). Also in keeping with the observed effects on CFA, modest decreases from baseline were observed with liprotamase at Week 7 for total cholesterol (from 3.43 to 3.20 mmol/L [p=0.001] while pancrelipase changed from 3.21 to 3.06 mmol/L [p=0.049]), vitamin A (from 34.5 to 32.7 µg/dL [p=0.033] while pancrelipase changed from 33.6 to 34.3 µg/dL [p=0.213]) and vitamin E (from 0.82 to 0.67 mg/dL [p<0.001] while pancrelipase changed from 0.76 mg/dL to 0.74 [p=0.147]). Similar mean analyte concentrations were noted at Week 20, and no treatment differences were noted on vitamins D and K, albumin, or pre-albumin.
More subjects in the liprotamase arm had dose adjustments through the Week 7 CFA assessment compared with pancrelipase (43.1% vs. 14.3%, p=0.0045), and the mean dose of study drug at that time was slightly higher with liprotamase compared with pancrelipase (7286.2 [range 4478 - 10000] vs. 6948.8 [range 3099 - 9938] units lipase/kg/day, p=0.30). Of note, 37 subjects (28.9% of the population randomized) were receiving a pre-study dose of PERT >8,000 units lipase/kg/day and, owing to the protocol-defined maximum dose of 10,000 units lipase/kg/day, were limited to less than a 25% increase in dose even if warranted by clinical signs or symptoms. More than half of these subjects (n=20) were receiving PERT ≥9,000 units lipase/kg/day prior to study and consequently unable to receive more than a 11% increase in dose.
A decline in body weight by Week 7 was observed among subjects in the mITT population randomized to liprotamase. This decline was statistically significant compared with pancrelipase at Week 7 (0.84 kg, p<0.001), however weight was maintained thereafter in the Extension Period. Weight, height and BMI were generally stable throughout the 20-week treatment period.
Safety
Liprotamase was generally well-tolerated with similar numbers of subjects reporting treatment-emergent AEs (63.1% vs 60.3% with pancrelipase) and serious AEs (10.8% vs 9.5%) compared with pancrelipase. Three subjects discontinued from the liprotamase arm due to an AE: constipation, gastritis, and CF-related hepatic disease, none of which was considered treatment related. The most frequently-reported AEs were related to CF lung manifestations including infective pulmonary exacerbation of CF and general respiratory disorders. The most commonly-reported serious adverse event was pulmonary exacerbation of CF reported in 4.6% and 7.9% of subjects randomized to liprotamase and pancrelipase, respectively. No serious AE was deemed to be related to study drug. No deaths were reported in this study.
Discussion
Liprotamase failed to achieve the primary endpoint in the SOLUTION trial. The change from baseline CFA to end of study was minimal for pancrelipase-treated patients, while the liprotamase-treated group had a drop from 89% to 77%. The pre-specified non-inferiority analysis required that the lower limit of the 95% confidence interval of the difference in CFA between liprotamase and Pancreaze arms, baseline vs end of Week 7, be greater than -15% (i.e. -14.9% or greater); the observed lower 95% confidence limit was -16%. Among the baseline characteristics evaluated, including CFTR genotype and usage of CFTR modulators, only the usage of gastric acid suppression (GAS) seemed to identify a more responsive population, perhaps signifying that a higher pH is required for greater enzyme activity. In contrast to the effect of liprotamase on CFA, the effect on CNA was 87% or greater in all subjects.
The biochemistry and physical chemistry of the lipase moiety in liprotamase differs considerably from pancrelipase. The mammalian pancreas secrets a number of forms of lipases, co-lipases and phospholipases, and the ideal lipase replacement therapy must have activity against a broad array of substrates. Unlike pancrelipase, the lipase component of liprotamase is expressed from a single, non-mammalian gene. It is able to digest diverse triglycerides (preferring hydrolysis of short- and middle- length fatty acid chains over long-chain), without requiring co-factors [12]. During manufacturing, it is chemically cross-linked and crystalized, in order to render it resistant to low pH in the stomach. Despite its engineered pH stability and substrate diversity, it is possible that the use of a single physicochemically-altered lipase in liprotamase may not adequately replace the multiple natural porcine lipases that are present in pancrelipase. One possibility for why some subjects responded well to liprotamase while others did not, may be related to variable pancreatic reserve and relative availability of endogenous lipases. Alternatively, perhaps the variability in response may be diet related, with liprotamase being most effective with certain lipid substrates. In either of these cases, solving the puzzle of variable liprotamase success may be very challenging.
An additional possibility for lack of efficacy of liprotamase in this study may be that dose was not optimized. The cross-linked lipase in liprotamase is less soluble than the lipases in porcine PERTs at pH below 6.0 and subjects on GAS achieved higher CFAs, suggesting that variable solubility in subjects might explain the variable response, and that higher doses of liprotamase might be more effective. The protocol-defined maximum dose (10,000 lipase units/kg/day) and maximum dose adjustments (no more than 50% increase over pre-study dose) may have limited the ability to optimize the liprotamase dose during this trial. At the end of the Primary Treatment Period, the mean liprotamase dose was approximately 24% higher than the mean dose at randomization among the 43% of liprotamase subjects who received 1 or more dose adjustments. Of note, upon enrollment into the study, approximately one-fourth of the subjects were at or near the maximum allowed liprotamase dose and were not able to undertake meaningful dose adjustments. To determine if higher doses of liprotamase than was used in the SOLUTION trial study would provide better efficacy, a similar phase 3 study, the RESULT trial, was recently completed [Study NCT03051490]. Emerging data from this study (communicated in a press release by Anthera Pharmaceuticals, March 12 2018) suggest that dose alone may not provide sufficient lipid digestion by the current formulation of liprotamase. Perhaps refinement in the formulation of liprotamase or a change to the extent of lipase cross-linking might improve its efficacy.
For the present, the goal of bringing a non-porcine derived PERT to market with an alternative production process not dependent upon animal herds to patients with CF related pancreatic enzyme insufficiency remains elusive.
Acknowledgement
We thank all the patients, study investigators and coordinators, the United States Cystic Fibrosis Therapeutics Development Network, the European Clinical Trials Network, and the Cystic Fibrosis Foundation Data and Safety Monitoring Board for their support of this trial.
Funding
This study was funded by Anthera Pharmaceuticals Inc. with the support of a research award from the Cystic Fibrosis Foundation (USA).
- Bruno MJ, Haverkort EB, Tytgat GN, van Leeuwen DJ. Maldigestion associated with exocrine pancreatic insufficiency: implications of gastrointestinal physiology and properties of enzyme preparations for a cause-related and patient-tailored treatment. Am J Gastroenterol 90: 1383-1393 (1995).
- https://www.cff.org/Research/Researcher-Resources/Patient-Registry/2016-Patient-Registry-Annual-Data-Report.pdf
- Kraisinger M, Hochhaus G, Stecenko A, Bowser E, Hendeles L. Clinical Pharmacology of Pancreatic Enzymes in Patients with Cystic Fibrosis and In Vitro Performance of Microencapsulated Formulations. J Clin Pharmacol 34: 158-166 (1994).
- Gelfond D, Ma C, Semler J, Borowitz D. Intestinal pH and gastrointestinal transit profiles in cystic fibrosis patients measured by wireless motility capsule. Dig Dis Sci 58: 2275-2281 (2013).
- Regan P, Malagelada JR, DiMagno EP, Glanzman SL, Go VL. Comparative effects of antacids, cimetidine and enteric coating on the therapeutic response to oral enzymes in severe pancreatic insufficiency. N Engl J Med 297:854-858 (1977).
- https://www.fda.gov/ohrms/dockets/ac/08/briefing/2008-4402b1-01-FDA.pdf.
- Borowitz D, Goss CH, Limauro S, et al. Study of a novel pancreatic enzyme replacement therapy in pancreatic insufficient subjects with cystic fibrosis. J Pediatr 149: 658-662 (2006).
- Borowitz D, Stevens C, Brettman LR, et al. International phase III trial of liprotamase efficacy and safety in pancreatic-insufficient cystic fibrosis patients. J Cyst Fibros 10: 443-452 (2011).
- Konstan MW, Borowitz D, Mayer-Hamblett N, et al. Study design considerations for evaluating the efficacy and safety of pancreatic enzyme replacement therapy in patients with cystic fibrosis. Clin Investig (Lond) 3: 731-741 (2013).
- Korpi-Steiner NL, Ward JN, Kumar V, McConnell JP. Comparative analysis of fecal fat quantitation via nuclear magnetic resonance spectroscopy (1H NMR) and gravimetry. Clin Chim Acta 400: 33-36 (2009).
- Ward JN, Kloke KM, Chezick PA, et al. Rapid accurate quantitation of nitrogen in urine and feces using the Elementar Rapid NIII analyzer. Clin Chem 48: A71 (2002).
- Sugihara A, Ueshima M, Shimada Y, Tsunasawa S, Tominaga Y. Purification and characterization of a novel thermostable lipase from Pseudomonas cepacia. J Biochem 112: 598-603 (1992).