Review Article - Clinical Practice (2021) Volume 18, Issue 8
A new approach for the prevention of acute kidney injury in on-pump cardiac surgery
- Corresponding Author:
- Heyman Luckraz
Consultant Cardiothoracic Surgeon
Cardiothoracic Surgery
American Hospital, UAE
E-mail: HeymanLuckraz@aol.com
Abstract
Purpose: Cardiac Surgery Associated Acute Kidney Injury (CSA-AKI) is linked to significant patient morbidity and mortality. We assessed the use of instantaneous balanced forced diuresis provided by the RenalGuard® (RG) system in the perioperative phase and its impact on CSA-AKI.
Description: From September 2015 to June 2019, the RG system was used in 120 patients at high risk of CSA-AKI. The first 10 patients were studied as part of a device evaluation exercise, followed by a randomized study comparing the use of RG (110 patients) versus standard practice (110 patients). The balanced forced diuresis was started at induction of anesthesia, continued throughout surgery, and up to six hours post-admission on the CICU.
Evaluation: The RG system reduced CSA-AKI rates significantly without impacting the post-operative rates of atrial fibrillation, blood transfusion, and infection. Patients on the RG system produced 3 liters-5 liters of urine intra-operatively and required a higher dose of potassium supplementation (median dose 60 mmol vs. 30 mmol)
Conclusions: CSA-AKI rate is lowered by the RG system. Further studies are required to assess cost-benefit.
Keywords
On-pump cardiac surgery Prevention Acute kidney injury
Product technology
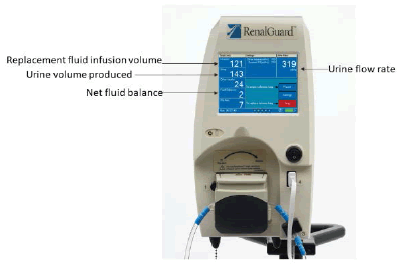
The RenalGuard® (RG) system (RenalGuard Solutions Inc., Milford, MA 01757, USA) consists of a console that measures the volume of urine (collected via a Foley’s catheter) then calculates the urine flow rate and infuses a set volume of hydration fluid to match the urine output. The console has real-time display of urine and replacement fluid volumes (FIGURE 1 and FIGURE 2), alerts to drain the urine bag or to replace the hydration fluid bag, and automatic air and occlusion detectors. It allows the medical team to set the expected fluid balance to achieve either a net fluid gain, loss, or even balance by instantaneously adjusting the volume of intravenous rehydration. Bolus infusion of fluids at the user’s request is also possible. This is a closed-loop system that rehydrates the patient as per the volume of urine output and is independent of other intravenous fluids given during the surgery and perioperative period.
■ Clinical technique
The RG system was started in the anesthetic room after the insertion of a peripheral venous line, arterial line, and urinary catheter. Forced diuresis was initiated on induction of anesthesia, with a 20 mg bolus of i.v furosemide aiming for a urine output of at least 200 ml/hour. In some patients, a furosemide infusion (titrated up to 10 mg/hour during surgery) was used to achieve the urine output rate. The RG system was set up to achieve zero balance i.e. the volume of urine output was matched in real-time to the volume of Hartmann’s fluid replacement infusion given through the system. The furosemide infusion (if required) was stopped in the OR at the end of the procedure. The device was used throughout the procedure in the Operating Room (OR) and for up to 6 hours post-transfer to Cardiac Intensive Care Unit (CICU). The patient’s intravascular volume was managed as per the usual practice of the anesthetic/intensivist teams, using a combination of colloid, crystalloid, and blood transfusion to maintain a normal Central Venous Pressure (CVP) and Mean Arterial Pressure (MAP). On the CICU, additional furosemide bolus (20 mg i.v.) was administered for patients on the RG system if urine output fell below 200 ml/hour within the first six hours of admission.
■ Anesthesia
Anaesthetic techniques included balanced anesthesia with opiates, benzodiazepines, and inhalation agents along with rocuronium or pancuronium as muscle relaxants. Propofol was the sedation agent of choice in intensive care along with opiates as required.
■ Cardiopulmonary Bypass (CPB)
CPB was carried out using the centrifugal pump in a non-pulsatile mode with a calculated flow of 2.4 L/min/m2 at mild hypothermia (34ºC for CABG and 32ºC for valve replacement). The circuit was primed using 10,000 IU Heparin, and a 2,000 ml balance of crystalloid (Baxter Compound Sodium Lactate Hartmann’s Solution) and colloid (Braun Gelofusine Ecobag) solution. Retrograde autologous priming was performed to reduce haemodilution. Myocardial protection during aortic cross-clamping involved the antegrade infusion of cold arterial blood and Harefield Hospital Formulation high strength cardioplegia solution, at 15 minute-30 minute intervals. Mean arterial pressure was maintained between 50 mmHg-80 mmHg. Hypotension (MAP<50 mmHg) was corrected using vasoactive drugs (Metaraminol or Phenylephrine: 1 mg/ml or 0.5 mg/ml). Patients were warmed to a nasopharyngeal temperature of 36.5ºC -37ºC and bladder temperature of >35ºC before discontinuation of CPB. The osmotic diuretic mannitol 10% was administered to all patients once CPB was commenced at a dose of 0.5 g/kg; with a maximum dose of 50 g. The calculated dose was given slowly over 20 minutes.
■ CICU
Post-operatively, patients were transferred to CICU and the RG system was maintained for 6 hours for those on the device. During the RCT (randomized control trial), patients in the control group were managed as per current medical practice (which did not include any forced diuresis with intravenous furosemide in OR). Otherwise, the management of the patients was similar including the need for inotropic support to maintain a MAP >65 mmHg and intravascular rehydration with colloids, crystalloids, or blood as per the patient’s need.
Clinical experience
Patients at high-risk of CSA-AKI (based on literature and our database outcomes of around 10,000 patients) were included: diabetics, eGFR of 20 mL/min/1.73 m2-60 mL/min/1.73 m2, Logistic Euro score 5 or above, procedures when CPB time likely to exceed 120 minutes and hemoglobin level of 12.5 g/dl or below. A preliminary device evaluation pilot study was agreed by the Institutional Patient Safety Improvement Group (T: PSIG/Sept2015) and 10 patients at risk of CSA-AKI were enrolled. This pilot confirmed that the RG system could be used in on-pump cardiac surgery safely and reproducibly and that there could also be a clinical benefit with lower AKI rates [1], TABLE 1. For this patient group, the median CICU stay was 1.5 (1,5) days and the median post-op stay was 6 (3,36) days. The prolonged in-hospital stay was related to one patient who developed intermittent acute bowel obstruction on a background of previous abdominal surgery and another patient who had a stroke and one death.
| Patient | Age (years) | AKI risk factor | CPB time (minutes) | Total urine (mls) | Change in creatinine | CICU stay (days) | LOS (days) |
|---|---|---|---|---|---|---|---|
| A | 51 | Diabetes | 109 | 9350 | 31% | 1 | 3 |
| B | 57 | Diabetes, renal disease | 76 | 5372 | 9% | 3 | 5 |
| C | 63 | Renal disease | 120 | 2076 | 29% | 2 | 7 |
| D | 66 | Renal disease | 102 | 3972 | 18% | 1 | 4 |
| E | 71 | Diabetes | 90 | 7226 | 14% | 1 | 7 |
| F | 74 | Renal disease | 95 | 4964 | 16% | 5 | 36 |
| G | 74 | Diabetes, renal disease | 97 | 5051 | 27% | 2 | 5 |
| H | 76 | Renal disease | 93 | 2947 | 27% | 1 | 2 |
| I | 76 | Diabetes | 152 | 5358 | 4% | 3 | 24 |
| J | 82 | Renal disease | 131 | 4539 | 1% | 1 | 9 |
| E-GFR: Estimated Glomerular Filtration Rate; CPB: Cardiopulmonary Bypass; CICU: Cardiac Intensive Care Unit; LOS: Post-Operative In-Hospital Stay. | |||||||
TABLE 1. Characteristics and outcomes of patients (first ten patients).
A subsequent RCT was undertaken after Institutional Review Board (IRB) and Ethics Committee (EC) approval (16/NI/0246, 2 December 2016) and was registered on the ClinicalTrials.gov website (NCT02974946). The results confirmed that there was a significant reduction in the CSA-AKI rate with the RG system as compared to controls (10% (11/110) vs. 20.9% (23/110), p=0.025) [2], TABLE 2. There was no significant difference in the post-operative rates of bleeding, blood transfusion, infection, and atrial fibrillation, TABLE 2. Given the significant level of diuresis with the RG system, patients required more supplemental potassium replacement over the first 24 hours (median, minimum, maximum) dose 60 (0-220) mmol vs. 30 (0-190), p<0.001) [3]. No patients on the RG system developed pulmonary edema. One patient in the RG system group and 2 patients in the control group died before hospital discharge. Causes of death were cardiogenic shock, cardiac failure, and sepsis due to pneumonia, respectively.
| RG group (n=110) | Control group (n=110) | p-Value | |
|---|---|---|---|
| Age*, years | 67.8 (9.3) | 67.0 (9.2) | 0.33 |
| Female, (n,%) | 23, 21% | 26, 24% | 0.63 |
| Diabetics, (n,%) | 80, 73% | 80, 73% | 0.66 |
| Logistic EuroScore** | 3.8 (0.9, 67.4) | 3.2 (0.9, 32.5) | 0.3 |
| CKD stage >2, (n,%) | 44, 40% | 42, 38% | 0.94 |
| CPB time*, mins | 120 (56) | 114 (46) | 0.64 |
| Total Urine output in OR*, ml | 2366 (877) | 765 (549) | <0.001 |
| Urine Volume at 24-hr CICU*, ml | 3310 (1303) | 2052 (804) | <0.001 |
| Atrial Fibrillation, (n/total,%) | 12/108, (11%) | 10/108, (9%) | 0.62 |
| Blood transfusion, (n/total,%) | 29/108, (27%) | 21/109, (19%) | 0.16 |
| *denotes mean (SD), **denotes median (minimum, maximum). e-GFR: Estimated Glomerular Filtration Rate; CKD: Chronic Kidney Disease; CPB: Cardiopulmonary Bypass; OR: Operating Room; CICU: Cardiac Intensive Care Unit. | |||
TABLE 2. Characteristics and outcomes of patients from the RCT.
Comment
CSA-AKI remains a major contributor to adverse outcomes both in the short & longterm after cardiac surgery [4,5]. These include prolonged ventilatory and cardiac support, higher infection rates, prolonged CICU and in-hospital stay, higher short and long-term mortality, and poorer long-term physical activity levels. The use of the RG system set at balanced rehydration for the forced-diuresis is associated with a lower CSA-AKI rate. So far, its use has been safe and reproducible.
The RG system has previously been reported to reduce AKI in patients at risk of contrast Contrast-Induced Nephropathy (CIN) in Percutaneous Coronary Intervention (PCI) [6] and Trans-Catheter Aortic Valve Implantation (TAVI) [7]. In these studies, the RG system reduced the incidence of AKI by up to 75%. There is now evidence that the RG can be used in on-pump cardiac surgery and it is associated with a 52% reduction in AKI rates.
The exact mechanism of this protective effect is not fully understood. Furosemide (a) protects against renal hypoxia at the level of Loop of Henle and (b) has vasodilatory effects on renal cortical vessels thus improving renal blood flow whilst the forced diuresis prohibits the build-up of casts within the renal tubules. More importantly, the real-time instantaneous balanced rehydration provides additional renal protection by avoiding significant intravascular dehydration. Interestingly, during the RCT, the use of the RG system was associated with lower 6-hour NGAL (neutrophil gelatinase-associated lipocalin) levels post-operatively [8].
Despite the large volume shifts associated with the use of the CPB, open-chest cardiac surgery, and in patients on the RG system, the instantaneous intravenous fluid replacement provided a safe recovery for the patients with no increase in blood transfusion, infection, or atrial fibrillation rates as compared to patients managed as per current practice. Given the large volume of diuresis, patients on the RG system need to have their serum potassium levels monitored [3] and supplemental potassium administered as per local unit policy.
The RG system provides a new avenue to reduce CSA-AKI, especially in high-risk patients. Further studies will be required to assess costeffectiveness.
Acknowledgments
We acknowledge the assistance of the surgical, anesthetic, research, and CICU staff at the Heart and Lung Centre, Wolverhampton, UK.
Disclosures and Freedom of Investigation
RenalGuard® solutions provided an educational research grant for the study which was also funded by the National Institute of Healthcare Research (NIHR), Clinical Research Network, UK [Ref: NIHR CRN No 32769]. The authors have no financial relationship with the company. The authors confirm that they had the freedom of investigation and full control of the design of the study, methods used, outcome parameters and results, analysis of data, and production of the written report.
References
- Luckraz H, Giri R, Wrigley B, et al. The use of the RenalGuard system in cardiac surgery with cardiopulmonary bypass: a first in man prospective, observational, feasibility pilot study. Open Heart BMJ. 4, e000669 (2017).
- Luckraz H, Giri R, Wrigley B, et al. Reduction in acute kidney injury post-cardiac surgery using balanced forced diuresis: A randomized, controlled trial. Eur J Cardiothorac Surg. 59, 562-69 (2021).
- Luckraz H, Giri R, Wrigley B, et al. Balanced forced-diuresis as a renal protective approach in cardiac surgery: Secondary outcomes of electrolyte changes. J Card Surg. 36, 4125-4131 (2021).
- Vives M, Hernandes A, Parramon F, et al. Acute Kidney Injury after cardiac surgery: prevalence, impact, and management challenges. Int J Nephrol Renovasc Dis. 12, 153-166 (2019).
- Mishra PK, Luckraz H, Nandi J, et al. Long-term quality-of-life post-acute kidney injury in cardiac surgery patients. Ann Card Anaesth. 2, 41-45 (2018).
- Marenzi G, Ferrari C, Marana I, et al. Prevention of contrast nephropathy by furosemide with matched hydration the MYTHOS (Induced diuresis with matched hydration compared to standard hydration for contrast-induced nephropathy prevention) Trial. J Am Coll Cardiol Intv. 5, 90-97 (2012).
- Barbanti M, Gulino S, Capranzano P, et al. Acute kidney injury with the renalguard system in patients undergoing transcatheter aortic valve replacement. The PROTECT-TAVI Trial (PROphylactic effecT of furos Emide-induCed diuresis with matched isotonic intravenous hydra Tion in Transcatheter Aortic Valve Implantation). J Am Coll Cardiol Intv. 8, 1595-1604 (2015).
- Luckraz H, Giri R, Wrigley B, et al. Balanced forced-diuresis compared to control as a reno-protective approach in cardiac surgery: secondary outcome of a randomized controlled trial, assessment of neutrophil gelatinase-associated lipocalin levels. J Cardiothorac Surg. 16:240 (2021).