Research Article - Interventional Cardiology (2022)
A novel and simply calculated cardiac load index correlates with coronary artery disease
- Corresponding Author:
- Lixian Sun
Department of Cardiology,
The Affiliated Hospital of Chengde Medical University,
Chengde,
HeBei 067000,
China,
E-mail: lixiansun01@126.com
Received date: 05-Sep-2022, Manuscript No. FMIC-22-73734; Editor assigned: 07-Sep-2022, PreQC No. FMIC-22-73734 (PQ); Reviewed date: 21-Sep-2022, QC No. FMIC-22-73734;Revised date: 28-Sep-2022, Manuscript No. FMIC-22-73734 (R); Published date: 04-Oct-2022, DOI: 10.37532/1755-5310.2022.14(S12).287
Abstract
Background: No Cardiac Load Index (CLI) has been established for patients with Coronary Artery Disease (CAD). We propose a simple method for calculating CLI and explore the association of CLI with CAD.
Methods: We enrolled 4145 consecutive inpatients with suspected stable CAD from December 2011 to June 2017 at the Chengde Medical University Affiliated Hospital. All patients were divided into the CAD (n=2914) and the NCAD groups (n=1231) according to coronary computed tomography angiography. We retrospectively collected data and calculated the CLI values of all patients. The receiver operating characteristic diagnostic test analysis was performed with CLI ≥ 77, CLI ≥ 125, CLI ≥ 171, CLI ≥ 217, and CLI ≥ 264, respectively. Multivariate logistic regression models were established to determine the risk factors of CAD.
Results: The CLI was significantly higher in the CAD group than in the NCAD group and higher in the male than in the female subgroup (both P<0.001). CLI ≥ 171 was the diagnostic cut-off value. The predictive power of CLI ≥ 171 for CAD improved after considering other risk factors. CLI ≥ 171 is a new independent risk factor for CAD, and is an independent risk factor for CAD in males and females (all P<0.001).
Conclusion: Increased CLI is an independent risk factor for CAD, it may be used as a predictor for the prevalence for CAD to improve diagnosis and prevention.
Keywords
Coronary artery disease•Cardiac load index • Risk factor
Abbreviations
CLI: Cardiac Load Index; CAD: Coronary Artery Disease; BMI: Body Mass Index; HR: Heart Rate; SBP: Systolic Blood Pressure; DBP: Diastolic Blood Pressure; LVEF: Left Ventricular Ejection Fraction; TC: Total Cholesterol; TG: Triglycerides; HDL-C: High-Density Lipoprotein Cholesterol; LDL-C, Low-Density Lipoprotein Cholesterol
Background
In previous decades, cardiovascular researchers have conducted extensive basic and clinical research to explore the pathophysiology of Coronary Artery Disease (CAD) [1,2]. Although the prevention, treatment, and care of CAD patients have greatly improved and adverse cardiovascular events and mortality have declined, the aging population experiences improved quality of life and reduced physical activity, which contributes to the prevalence of CAD remaining high and being the leading cause of death in patients with cardiovascular disease [3]. The pathogenesis of CAD is not fully understood; in addition to visceral fat, inflammatory factors, and classic clinical risk factors, researchers are committed to exploring new risk factors and biomarkers for CAD, such as the atherogenic index of plasma and the triglyceride glucose index [4,5].
Myocardial oxygen consumption depends on the wall tension, heart rate, myocardial contractility, and ejection time [6]. Systolic Blood Pressure (SBP) multiplied by Heart Rate (HR) is linearly related to myocardial oxygen consumption, and is an indicator of the cardiac workload [7]. Ejection Fraction (EF) increases with myocardial contractility, and can be considered a parameter which includes vascular pressure and blood flow, and affects the cardiac workload [8]. We have proposed a new biomarker Cardiac Load Index (CLI), which is calculated by SBP × HR/left ventricular EF (LVEF). In this study, we tested our hypothesis that CLI is associated with CAD and may be used as a new predictor for CAD.
Materials and Methods
Subjects and methods
This is a single-center, retrospectively, case-control study which included 4145 consecutive patients with suspected stable CAD treated at the Chengde Medical University Affiliated Hospital from December 2011 to June 2017. CAD was diagnosed according to coronary computed tomography angiography showing a left main, left anterior descending, circumflex, right coronary, or main branch stenosis ≥ 50% [9]. All patients were divided into the CAD (n=2914) and the NCAD groups (n=1231) and were assigned into the male and female subgroups. We excluded patients with age ≤ 18 years, severe valvular heart disease, aortic dissection, constrictive pericarditis, hypertrophic cardiomyopathy, pregnancy, or malignancy.
We collected all patients’ data including baseline demographics (including age, sex, Body Mass Index (BMI), and smoking), CAD-related risk factor diseases (type 2 diabetes, hypertension, dyslipidemia, ischemic stroke), routine blood test results, and biochemistry. We performed echocardiography to measure the LVEF, left atrial diameter, and left ventricular end-diastolic diameter within three days of admission. The resting HR and right brachial artery blood pressure were measured by resident cardiac physicians within 1 hour of admission. We then calculated the CLI values of all patients, where CLI=SBP (mm Hg) × HR (bpm)/EF (%). The normal range of SBP is 90-140 mm Hg; of HR, 60-100 bpm; and of EF is 53-70%. The normal range of CLI should be 77-264, according to the normal ranges of SBP, HR, LVEF. The quartiles of CLI between 77 and 264 were 125, 171, and 217, respectively.
Statistical methods
Continuous variables are expressed as median and interquartile range, in accordance with the results of the Shapiro-Wilk test for normality, and the relationship between the two groups was examined using the Mann-Whitney U test. Categorical variables are presented as numbers and percentages, and the relationships between the two groups were examined using Fisher’s exact test.
The CLI were converted to binary variables according to the cut-off values of 77, 125, 171, 217, and 264. The optimal CLI cut-off value of 100.5 obtained with the Youden Index is not in line with clinical practice. We therefore performed Receiver Operating Characteristic (ROC) diagnostic test analysis with CLI ≥ 77, CLI ≥ 125, CLI ≥ 171, CLI ≥ 217, and CLI ≥ 264. Subsequently, CLI ≥ 77, CLI ≥ 125, CLI ≥ 217, and CLI ≥ 264 were compared with CLI ≥ 171, and we obtained the NRI values. Univariate regression analysis was performed to explore the relationship of CLI with the CAD risk factors. Multivariate logistic regression models were established to determine the risk factors of CAD, CAD in males, and CAD in females. P values ≤ 0.05 were considered significant. Data were analyzed using Statistical Package for Social Sciences software, version 19 (IBM SPSS Statistics for Windows, version 19 (IBM Corp., Armonk, N.Y., USA)).
Results
Clinical characteristics of the study
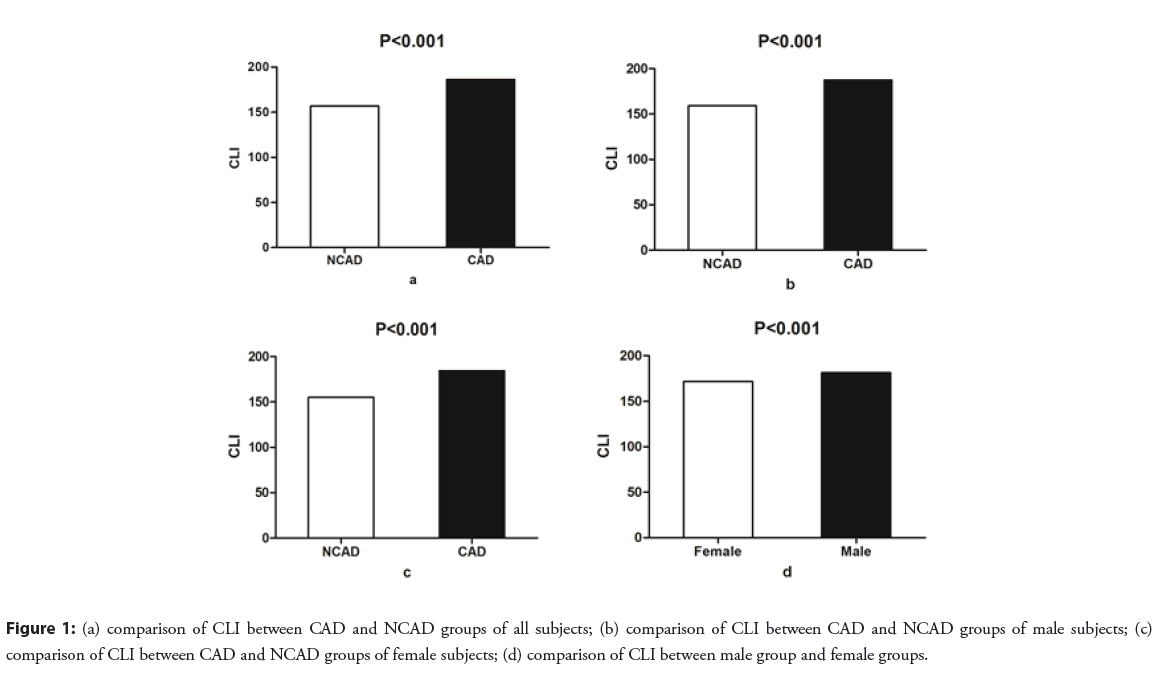
In this study, there were 2490 (60.1%) males, with the median age of 60 years. The prevalence of hypertension, type 2 diabetes, ischemic stroke, and the proportions of male gender, smoking, alcohol consumption, and chest pain were significantly higher in the CAD than in the NCAD group (all P<0.001). The median values of age, HR, SBP, left atrial diameter, and left ventricular end-diastolic diameter were higher in the CAD than in the NCAD group; however, the median values of LVEF were higher in the NCAD than in the CAD group (P<0.001). The levels of leukocytes, low-density lipoprotein cholesterol (LDL-C), uric acid, blood urea nitrogen, and creatinine were significantly higher in the CAD than in the NCAD group, whereas the levels of high-density lipoprotein cholesterol were lower in the CAD than in the NCAD group (all P<0.001) (Table 1). The CLI is significantly higher in the CAD than in the NCAD group, and is higher in the male than the female subgroup (P<0.001) (Figure 1).
| Variables | Total (n=4145) | NCAD group (n=1231) | CAD group (n=2914) | χ2/Z | P |
|---|---|---|---|---|---|
| Male (%) | 2490 (60.1) | 514 (41.8) | 1976 (67.8) | 244.955 | <0.001 |
| Age (year) | 60 (53, 68) | 57 (51, 63) | 62 (55, 71) | -14.366 | <0.001 |
| BMI (kg/m2) | 25.2 (23.1, 27.5) | 25.4 (23.4, 27.6) | 25.1 (23.0, 27.4) | -1.589 | 0.112 |
| Smoking (%) | 1783 (40.0) | 341 (27.7) | 1442 (49.5) | 167.542 | <0.001 |
| Chest pain (%) | 2355 (57.9) | 518 (42.7) | 1837 (64.3) | 162.848 | <0.001 |
| Hypertension (%) | 2356 (56.8) | 587 (47.7) | 1796 (60.7) | 59.821 | <0.001 |
| Type 2 diabetes (%) | 787 (19.7) | 173 (14.3) | 614 (22.1) | 31.7 | <0.001 |
| Dyslipidemia (%) | 1215 (30.3) | 346 (28.6) | 869 (31.1) | 2.425 | 0.119 |
| Ischemic stroke (%) | 487 (11.7) | 85 (6.9) | 402 (13.8) | 39.628 | <0.001 |
| HR (bpm) | 72 (64, 80) | 70 (64, 78) | 72 (64, 80) | -3.524 | <0.001 |
| SBP (mmHg) | 130 (120, 150) | 130 (120, 144) | 135 (120, 150) | -4.555 | <0.001 |
| DBP (mmHg) | 80 (76, 90) | 80 (75, 90) | 80 (76, 90) | -0.806 | 0.42 |
| LVEF (%) | 60 (55, 65) | 62 (58, 66) | 58 (54, 63) | -18.156 | <0.001 |
| Left atrial diameter (mm) | 34 (30, 37) | 33 (30, 35) | 34 (31, 38) | -5.975 | <0.001 |
| Left ventricular end-diastolic diameter (mm) | 50 (46, 54) | 49 (46, 52) | 50 (47, 55) | -11.478 | <0.001 |
| Leukocytes (109/L) | 6.65 (5.446, 8.04) | 6.40 (5.35, 7.68) | 6.74 (5.50, 8.18) | -5.414 | <0.001 |
| TC (mmol/L) | 4.21 (3.58, 4.95) | 4.24 (3.61, 4.90) | 4.20 (3.57, 4.98) | -0.291 | 0.771 |
| TG (mmol/L) | 1.50 (1.05, 2.23) | 1.51 (1.06, 2.22) | 1.50 (1.04, 2.25) | -0.083 | 0.934 |
| HDL-C (mmol/L) | 1.04 (0.87, 1.26) | 1.09 (0.92, 1.33) | 1.03 (0.86, 1.24) | -6.61 | <0.001 |
| LDL-C (mmol/L) | 2.35 (1.80, 2.95) | 2.30 (1.75, 2.81) | 2.38 (1.81, 3.01) | -3.715 | <0.001 |
| Uric acid (mmol/L) | 310 (257, 370) | 294 (247, 347) | 317 (262, 376) | -7.729 | <0.001 |
| Blood urea nitrogen (mmol/L) | 5.57 (4.58 6.77) | 5.17 (4.30, 6.15) | 5.70 (4.70, 7.10) | -11.025 | <0.001 |
| Creatinine (μmol/L) | 68 (58, 80) | 63 (55, 74) | 70 (60, 82) | -11.782 | <0.001 |
Abbreviations: NCAD: Non-Coronary Artery Disease; CAD: Coronary Artery Disease; BMI: Body Mass Index; HR: Heart Rate; SBP: Systolic Blood Pressure; DBP: Diastolic Blood Pressure; LVEF: Left Ventricular Ejection Fraction; TC: Total Cholesterol; TG: Triglycerides; HDL-C: High-Density Lipoprotein Cholesterol; LDL-C: Low-Density Lipoprotein Cholesterol
Table 1: Clinical characteristics of all subjects.
Receiver operating characteristic curve diagnostic test analysis
The area under the curve (AUC) of CLI ≥ 171 (0.594) is greater than that of CLI ≥ 125 (0.547), CLI ≥ 217 (0.579), and AUC of CLI ≥ 264 (0.544) (P<0.001). CLI ≥ 125, CLI ≥ 217, and CLI ≥ 264 were compared with CLI ≥ 171, indicating that the Net Reclassification Indices (NRI) were -0.090, -0.025, and -0.096, respectively. The ROC diagnostic test of CLI ≥ 77 was not statistically significant (P=0.938). Therefore, 171 is the CLI diagnostic cut-off value of this study. The predictive power of CLI ≥ 171 for CAD improves after accounting for male sex, age ≥ 45 years, smoking, type 2 diabetes, hypertension, ischemic stroke, and LDL-C ≥ 3.37 mmol/L (AUC: 0.723 vs. 0.591; P<0.001) (Table 2).
| Variables | High CLI group | Low CLI group | OR | P |
|---|---|---|---|---|
| BMI ≥25 kg/m2 | 734 (42.8) | 601 (38.5) | 1.198 | 0.011 |
| Dyslipidemia | 557 (45.8) | 1190 (42.6) | 1.14 | 0.057 |
| Hypertension | 1202 (51.0) | 614 (34.3) | 1.993 | <0.001 |
| Type 2 diabetes | 414 (52.6) | 1329 (41.5) | 1.563 | <0.001 |
| Ischemic stroke | 247 (50.7) | 1569 (42.9) | 1.37 | 0.001 |
| CAD | 1435 (79.0) | 1479 (63.5) | 2.165 | <0.001 |
Abbreviations: CLI: Cardiac Load Index; CAD: Coronary Artery Disease; BMI: Body Mass Index
Table 2: ROC diagnostic test analysis of different CLI cut-off value.
Univariate analysis of CLI and CAD risk factors
All patients were assigned into the high CLI group (CLI ≥ 171) and the low CLI group (CLI<171). The prevalence of BMI ≥ 25 kg/m2, dyslipidemia, hypertension, ischemic stroke, and CAD was significantly higher in the high CLI than in the low CLI group (P<0.05). BMI ≥ 25 kg/m2, dyslipidemia, hypertension, ischemic stroke, and CAD are associated with CLI, with Odds Ratios (OR) of 1.198, 1.140, 1.993, 1.370, and 2.165, respectively (P<0.05) (Table 3).
| Variables | High CLI group | Low CLI group | OR | P |
|---|---|---|---|---|
| BMI ≥25 kg/m2 | 734 (42.8) | 601 (38.5) | 1.198 | 0.011 |
| Dyslipidemia | 557 (45.8) | 1190 (42.6) | 1.14 | 0.057 |
| Hypertension | 1202 (51.0) | 614 (34.3) | 1.993 | <0.001 |
| Type 2 diabetes | 414 (52.6) | 1329 (41.5) | 1.563 | <0.001 |
| Ischemic stroke | 247 (50.7) | 1569 (42.9) | 1.37 | 0.001 |
| CAD | 1435 (79.0) | 1479 (63.5) | 2.165 | <0.001 |
Abbreviations: CLI: Cardiac Load Index; CAD: Coronary Artery Disease; BMI: Body Mass Index
Table 3: Univariate analysis of CLI and CAD risk factors.
Multivariate logistic regression models of CAD
Univariate logistic regression analysis was performed to screen those variables with P<0.05, following which multivariate logistic regression models were created to identify risk factors for CAD, CAD in males, and CAD in females. In addition to the classic risk factors include male sex, age ≥ 45 years, smoking, type 2 diabetes, hypertension, ischemic stroke, and LDL-C ≥ 3.37 mmol/L were independent risk factors for CAD (all P<0.001). CLI ≥ 171 is a new independent risk factor for CAD (P<0.001) and is an independent risk factor for CAD in males and in females, with an OR of 1.966 and 1.866, respectively (both P<0.001) (Table 4).
| Variables | All subjects | Male subgroup | Female subgroup | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | |
| Male | 2.556 | 2.112-3.094 | <0.001 | - | - | - | - | - | - |
| Age ≥ 45 years | 2.205 | 1.662-2.926 | <0.001 | 2.049 | 1.459-2.808 | <0.001 | 3.426 | 1.645-7.134 | 0.001 |
| Smoking | 1.708 | 1.399-2.084 | <0.001 | 1.556 | 1.262-1.943 | <0.001 | 2.337 | 1.375-3.970 | 0.002 |
| Type 2 diabetes | 1.73 | 1.409-2.124 | <0.001 | 1.116 | 0.836-1.491 | 0.457 | 2.425 | 1.832-3.209 | <0.001 |
| Hypertension | 1.56 | 1.337-1.82 | <0.001 | 1.277 | 1.034-1.575 | 0.023 | 1.956 | 1.556-2.458 | <0.001 |
| Ischemic stroke | 1.914 | 1.462-2.506 | <0.001 | 2.115 | 1.424-3.141 | <0.001 | 1.69 | 1.161-2.461 | 0.006 |
| LDL-C ≥ 3.37 mmol/L | 1.812 | 1.437-2.285 | <0.001 | 1.832 | 1.260-2.665 | 0.002 | 1.808 | 1.339-2.441 | <0.001 |
| CLI ≥ 171 | 1.945 | 1.661-2.276 | <0.001 | 1.966 | 1.582-2.444 | <0.001 | 1.866 | 1.481-2.350 | <0.001 |
Abbreviations: CLI: Cardiac Load Index; CAD: Coronary Artery Disease; CI: Confidence Interval; LDL-C: Low-Density Lipoprotein Cholesterol
Table 4: Multivariate logistic regression models of CAD.
Discussion
This study was the first to provide the concept of SBP × HR/LVEF representing CLI. The SBP and HR product is an indirect index of cardiac work, which can predict cardiac function, morbidity, and mortality in patients with cardiovascular disease [10]. LVEF is a valuable diagnostic and prognostic tool for cardiovascular disease, and reflects cardiac function and remodeling [11]. Elevated CLI may promote inflammation and oxidative stress, active the vascular smooth muscle and leukocyte infiltration, facilitate cardiac and vascular remodeling, and, furthermore, may accelerate the occurrence of atherosclerosis and the development of CAD [12,13].
In this study, multivariate logistic regression analysis indicated that in addition to the classic risk factors of CAD, CLI ≥ 171 is also an independent risk factor for CAD and has a higher risk for CAD than do smoking and hypertension. Myocardial ischemia is associated with disorders of cardiac function. Elevated SBP × HR Indeximplies increasing myocardial oxygen consumption, and decreased LVEF indicates the myocardial in ischemic state and insufficient vascular pressure. Elevated HR is known to induce myocardial ischemia and cardiovascular events in patients with CAD [14]. Increased HR leads to endothelial oxidative stress, inflammation, and thrombosis and is conducive to atherosclerosis and plaque rupture, and eventually to adverse outcomes [15]. Hypertension is a known common pathogenic factor in CAD in developing countries. Elevated SBP promotes atherosclerosis and has a stronger predictive value for coronary heart disease, myocardial infarction, stroke, and cardiovascular mortality [16]. Ischemic heart disease is the most common cause of LVEF reduction, accounting for approximately 60% of all cases [17]. Previous studies have reported that CAD-induced cardiac failure with reduced LVEF accounts for the largest proportion of patients with cardiac failure [18]. Atherosclerosis is the most common pathophysiological mechanism for CAD; as atherosclerosis deteriorates, the stiffness of the vessel wall increases and the elasticity decreases, and coronary artery stenosis and occlusion occur, thereby reducing the blood flow and oxygen supply [19]. Elevated CLI indicates aggravated coronary inflammation and cardiovascular remodeling, which exacerbate the development of CAD and promote the occurrence of adverse cardiovascular events.
Risk factors for CAD differ between males and females due to differences in the genes carried by the X and Y chromosomes and in the sex hormones [20,21]. Women display greater cardiac strength values than men, but male myocardia fibers are more sensitive to calcium than are female fibers [22]. There are differences in the degree of vascular wall stiffness, HR dynamics, and HR variability between males and females [23,24]. We found that CLI was higher in the male than the female subgroup, that CLI ≥ 171 was an independent risk factor for CAD in both the male and female subgroups, and the risk for CAD in males is slightly higher than in females.
In addition, CLI is associated with BMI ≥ 25 kg/m2, dyslipidemia, hypertension, and ischemic stroke. These variables are known risk factors for CAD and influence the occurrence and development of atherosclerosis. Patients with one or more of these risk factors may develop higher CLI; moreover, higher CLI may aggravate the development of overweight, dyslipidemia, hypertension, and ischemic stroke and promote the development of CAD.
Conclusion
This study explores the potential of CLI as a new biomarker, which can be used to predict CAD along with other clinical risk factors. Exploring new risk factors and predictors improves the diagnosis and prevention of CAD. In conclusion, CLI is a novel risk indicator in patients with CAD and may be used as a predictor for the prevalence of CAD. Furthermore, this novel index differs between male and female patients, the risk for CAD in males is slightly higher than in females.
Limitations of the Study
This study has several limitations. This study is a single-center case-control study evaluating retrospective data. Further studies are required to elucidate whether the findings are applicable to other populations.
Ethics Approval and Consent to Participate
We conducted this study according to the standards of the Declaration of Helsinki on medical research, and the study protocol was approved by the Institutional Review Boards of the Affiliated Hospital of Chengde Medical University. All subjects provided written informed consent.
Availability of Data and Materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors Contributions
YS contributed to the design of the study, performed the research, analyzed the data, and wrote the paper. JL, CH and XJ performed the research and collected the data. WF,TL performed the research. LS analyzed and interpreted the data and critically revised the manuscript for important intellectual content. All the authors approved the final version of the manuscript for publication.
Acknowledgments
The authors are grateful for the assistance of the cardiology doctors and nurses at the Affiliated Hospital of Chengde Medical University.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Funding
This study was supported by grants from Hebei Province Government Science and Technology Agency (Grant no. 17277769D) and Hebei Provincial Department of Education Graduate Innovation Funding Project (Grant no. CXZZSS2018138).
References
- Sechtem U, Brown D, Godo S, et al. Coronary microvascular dysfunction in stable ischaemic heart disease (non-obstructive coronary artery disease and obstructive coronary artery disease). Cardiovasc Res. 116(4): 771-786 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Shao C, Wang J, Tian J, et al. Coronary artery disease: From mechanism to clinical practice. Adv Exp Med Biol. 1177: 1-36 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Piepoli MF, Hoes AW, Agewall S, et al. ESC Scientific Document Group. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention and Rehabilitation (EACPR). Eur Heart J. 37(29): 2315-2381 (2016).
[CrossRef] [Google Scholar] (All versions) [PubMed]
- Guo Q, Zhou S, Feng X, et al. The sensibility of the new blood lipid indicator--Atherogenic Index of Plasma (AIP) in menopausal women with coronary artery disease. Lipids Health Dis. 19(1): 27 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Alizargar J, Bai CH, Hsieh NC, et al. Use of the Triglyceride-Glucose index (TyG) in cardiovascular disease patients. Cardiovasc Diabetol. 19(1): 8 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Fox KAA, Metra M, Morais J, et al. The myth of 'stable' coronary artery disease. Nat Rev Cardiol. 17(1): 9-21 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Nelson RR, Gobel FL, Jorgensen CR, et al. Hemodynamic predictors of myocardial oxygen consumption during static and dynamic exercise. Circulation. 50(6): 1179-89 (1974).
[CrossRef] [Google Scholar] [PubMed]
- Monge García MI, Jian Z, Settels JJ, et al. Determinants of left ventricular ejection fraction and a novel method to improve its assessment of myocardial contractility. Ann Intensive Care. 9(1): 48 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Task Force Members, Montalescot G, Sechtem U, et al. 2013 ESC guidelines on the management of stable coronary artery disease: The Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 34(38): 2949-3003 (2013).
[CrossRef] [Google Scholar] [PubMed]
- Verma AK, Sun JL, Hernandez A, et al. Rate pressure product and the components of heart rate and systolic blood pressure in hospitalized heart failure patients with preserved ejection fraction: Insights from ASCEND-HF. Clin Cardiol. 41(7): 945-952 (2018).
[CrossRef] [Google Scholar] [PubMed]
- Marwick TH. Ejection fraction pros and cons: JACC State-of-the-Art Review. J Am Coll Cardiol. 72(19): 2360-2379 (2018).
[CrossRef] [Google Scholar] [PubMed]
- Chatterjee M, Rath D, Schlotterbeck J, et al. Regulation of oxidized platelet lipidome: Implications for coronary artery disease. Eur Heart J. 38(25): 1993-2005 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Nakahara T, Dweck MR, Narula N, et al. Coronary artery calcification: From mechanism to molecular imaging. JACC Cardiovasc Imaging. 10(5): 582-593 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Ferrari R, Fox K. Heart rate reduction in coronary artery disease and heart failure. Nat Rev Cardiol. 13(8): 493-501 (2016).
[CrossRef] [Google Scholar] [PubMed]
- Fox KM, Ferrari R. Heart rate: A forgotten link in coronary artery disease? Nat Rev Cardiol. 8(7): 369-79 (2011).
[CrossRef] [Google Scholar] [PubMed]
- Nichols WW, Edwards DG. Arterial elastance and wave reflection augmentation of systolic blood pressure: Deleterious effects and implications for therapy. J Cardiovasc Pharmacol Ther. 6(1): 5-21 (2001).
[CrossRef] [Google Scholar] [PubMed]
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 128(16): e240-327 (2013).
[CrossRef] [Google Scholar] (All versions) [PubMed]
- Cao J, Dong R, Zhang K, et al. Effects of myocardial viability and left ventricular remodeling on survival of patients with heart failure and reduced ejection fraction after coronary artery bypass grafting. Cardiovasc Diagn Ther. 10(2): 183-192 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Mori H, Torii S, Kutyna M, et al. Coronary artery calcification and its progression: What does it really mean? JACC Cardiovasc Imaging. 11(1): 127-142 (2018).
[CrossRef] [Google Scholar] [PubMed]
- Florijn BW, Bijkerk R, van der Veer EP, et al. Gender and cardiovascular disease: Are sex-biased microRNA networks a driving force behind heart failure with preserved ejection fraction in women? Cardiovasc Res. 114(2): 210-225 (2018).
[CrossRef] [Google Scholar] [PubMed]
- Arnold AP, Cassis LA, Eghbali M, et al. Sex hormones and sex chromosomes cause sex differences in the development of cardiovascular diseases. Arterioscler Thromb Vasc Biol. 37(5): 746-756 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Bening C, Weiler H, Vahl CF. Effects of gender, ejection fraction and weight on cardiac force development in patients undergoing cardiac surgery--an experimental examination. J Cardiothorac Surg. 8: 214 (2013).
[CrossRef] [Google Scholar] [PubMed]
- Nethononda RM, Lewandowski AJ, Stewart R, et al. Gender specific patterns of age-related decline in aortic stiffness: A cardiovascular magnetic resonance study including normal ranges. J Cardiovasc Magn Reson. 17(1): 20 (2015).
[CrossRef] [Google Scholar] [PubMed]
- Ryan SM, Goldberger AL, Pincus SM, et al. Gender- and age-related differences in heart rate dynamics: Are women more complex than men? J Am Coll Cardiol. 24(7): 1700-7 (1994).
[CrossRef] [Google Scholar] [PubMed]