Research Article - Clinical Investigation (2020) Volume 10, Issue 1
A retrospective study of Spondylodiscitis with clinical, imaging and therapeutic correlations
- Corresponding Author:
- Barbara Brogna
Department of Radiology, Sacred Heart of Jesus Hospital, Viale Principe di Napoli 14/A, Benevento 82100, Italy
E-mail: brogabarbara1@gmail.com
Submitted: 16 December 2019; Accepted: 18 December 2019; Published online: 29 January 2020
Abstract
Introduction: Diagnosing Spondylodiscitis (SD) can be challenging in clinical practice with highly variable outcomes. The aim of this study is to retrospectively analyze the clinical, laboratory, imaging findings of patients with SD treated at our hospital between January 2017 and December 2018. We also evaluated the SD evolution during a short follow-up at 4 and 6 weeks.
Methods: The epidemiological, clinical, microbiological, laboratory findings (White Blood Count (WBC), C-Reactive Protein (CRP) and Erythrocyte Sedimentation rate (ESR)), Imaging (CT/MRI) and treatment data of 38 patients with SD were studied retrospectively. The laboratory findings (CRP, ESR) and the CT/MRI examinations during the follow-ups at 4 and 6 weeks were evaluated. Based on imaging (CT/MRI) we divided SD into the following 5 types based on morphological features observed: spondylitis or discitis (ST/DS), SD, SD with paravertebral abscesses (SD-PA), SD with epidural abscess (SD-EP) and SD with paravertebral and epidural abscesses (SD-PEA).
Results: The most common complaint was pain (95%) and the main comorbidity was septicemia (42%). Staphylococcus aureus was found in 45% of the cases. The WBC was elevated in 32% of the patients. Both the CRP and ESR decreased during the follow-up. SD was found in 31% of the cases, SD-PA in 26% of the cases, ST/DS in 19% of the cases, SD-PEA in 13% of the cases and SD-EP in 11% of the cases. At the follow-up at week 4, SD-PA, SD-EP and SD-PEA had decreased and were found respectively in 21%, 5% and 5% of the cases. In the follow-up at week 6, SD-PA, SD-EP and SD-PEA were found respectively in 10%, 8% and 3% of the patients. Conservative treatment with antibiotic therapy was applied in 63% of the cases. Surgical treatment was given to 21% of the patients and an interventional procedure was done on 16% of the patients.
Conclusion: SD diagnosis and management continues to be based on a multidisciplinary approach. Re-imaging in the critical period of 4-6 weeks with the monitoring of systemic inflammatory markers can be a good follow-up strategy.
Keywords
spondylodiscitis • MRI • CT • spondylodiscitis morphological features • inflammatory laboratory findings
Introduction
Spondylodiscitis (SD) is a potentially devastating and time-consuming infectious disease that affects the vertebra, vertebral discs, and adjacent structures. Risk factors include being in an immunocompromised state, intravenous drug use, the use of spinal instrumentation and surgery [1]. It is a highly heterogeneous and rare disease that represents 0.15% to 5% of all osteomyelitis cases [1-3]. Men are three times more likely to be affected than women [2]. Nowadays, the incidence rate is increasing due to the improved life expectancy of patients with chronic debilitating diseases, improved case ascertainment with imaging and the increasing migration of immigrants [1-6]. Diagnosis is usually made by a multidisciplinary team and is based on the evaluation of clinical, radiological, laboratory and microbiological findings. However, it is not unusual to have a delay of 2-12 weeks between diagnosis and treatment initiation [4]. SD can be classified as granulomatous (tuberculosis, brucellosis and other mycobacterial pathogens) or pyogenic. There are three forms of dissemination: hematogenous spread from a distant septic focus, direct inoculation (either from surgery or trauma) and contiguity with an adjacent septic focus. Generally, the infection starts in the anterior portion of the vertebral body because of its rich arterial supply and then spreads through the medullary spaces, affecting the intervertebral disc by contiguity. It very frequently involves the lumbar and dorsal segments of the spine [1-7].
Pyogenic SD usually spreads through the vascular arcades in the metaphysis whereas tuberculous infection commonly the result of Batson’s paravertebral venous plexus spread. Pyogenic SD mostly affects one or two adjacent vertebrae and the intervertebral disc [6-9]. The lumbar spine is more commonly involved followed by the thoracic, cervical and sacral regions [1]. Staphylococcus aureus is the most common bacterium that is responsible for pyogenic SD, followed by gram-negative pathogens such as Escherichia coli [1,4,6]. Tuberculosis is the most common cause of spinal infections in developed countries [4,6,8,9]. The frequency of it has recently increased in western countries due to the migration of immigrants from underdeveloped countries [6,8,9]. Tuberculous spondylitis involves more than two infected vertebrae and it commonly affects the thoracolumbar spine [6,8,9]. It primarily or secondarily involves the paraspinal soft tissue and posterior articular elements [1,6,8,9]. However, Tuberculous spondylitis does not
Spinal infection symptoms may be non-specific and are usually misdiagnosed. In addition to the neurological examination, early diagnosis is usually based on elevated body temperatures, increased Erythrocyte Sedimentation Rates (ESR) and increased C-reactive Proteins (CRP) [1,4,10]. Magnetic Resonance Imaging (MRI) and Computer Tomography (CT) are the standard radiological methods to diagnose SD [1,3,6,10].
Most cases of spinal infection may be successfully managed with conservative treatment such as early antibiotic therapy and spinal bracing. Surgical intervention is considered in only a few specific circumstances such as the presence of neurologic signs, vertebral collapse, progressive spinal deformity and an abscess that does not respond to antibiotics [1,4,10].
Materials and Methods
All patients with SD treated in our hospital between January 2017 and December 2018 were studied retrospectively. The diagnosis of SD was defined by clinical findings, blood count, C-Reactive Protein (CRP), Erythrocyte Sedimentation Rate (ESR), Serum Tube Agglutination (STA) test, Ziehl-Neelsen staining, culture, histopathology and radiological methods such as Magnetic Resonance Imaging (MRI) and Computed Tomography (CT) scans. The following data was collected: demographic characteristics (age at diagnosis and sex), clinical findings and comorbidities, laboratory findings (White Blood count, CRP, and ESR), microbiologic and serologic evaluations, imaging studies performed (CT/MRI) and treatment. We also evaluated the laboratory findings (CRP, ESR) and the imaging studies performed (CT/MRI) during the followup at 4 and 6 weeks. Based on imaging (CT/ MRI) we divided SD into five categories based on morphological features: Spondylitis or Discitis (ST/ DS), SD (vertebral and disc involvement), SD with Paravertebral Abscesses (SD-PA), SD with Epidural Abscess (SD-EP) and SD with Paravertebral and Epidural Abscesses (SD-PEA). MRI was the modality of choice for the diagnosis and during the follow-up. CT was often used as an alternative in cases where there were contraindications to the MRI like non-MRI-compatible pacemakers or other patient-specific factors (claustrophobic patients, patients with spine surgical interventions or patients with tuberculous SD).
Results
Demographics
The study involved 38 patients, 27 males (71%) and 11 females (29%). The ranged age at diagnosis was 28-87 years old (mean 51.4 ± 15.66).
Clinical findings and comorbidities
The most common complaints were pain (95%), high fever (47%) and neurological symptoms (16%). The main comorbidities of SD were septicemia (42%), diabetes mellitus type I (11%), unknown (11%), systemic disease (8%), diabetes mellitus type II (5%), spine surgical intervention (5%), other interventions (5%), fractures (5%), tumors (5%) and periradicular infiltration (3%) (Figure 1).
Laboratory findings
The White Blood Cell (WBC) count was elevated in 32% of the cases with a mean value of 10.800 cells/ mm3. In the initial evaluation, the mean value of ESR was 84 mm/h and the mean value of CRP was 105.76 mg/dl. In the follow-up at week 4, the ESR and CRP decreased with the mean values to 40 mm/h and 53.54 mg/dl respectively. At the 6-week follow-up, the mean values of ESR and CRP were 20 mm/h and 36.93 mg/dl.
Microbiologic evaluation
A definitive etiologic diagnosis was achieved in most of the cases (76%). In 10% of patients the etiology was presumed and in 13% it was considered undetermined. The most prevalent etiologic agent, considering certainty and presumed diagnosis, was Staphylococcus aureus (45%; n=17). It was followed by Mycobacterium tuberculosis (24%; n=9),), Brucella (5%; n=2), B-hemolytic Streptococci (5%; n=2), Candida albicans (2%; n=1), Enterococcus (2%; n=1), Pseudomonas aeruginosa (2%; n=1) and Enterobacteriaceae (2%; n=1). 13 Patients (34%) underwent a biopsy for pathogen identification and for histological diagnosis (16% guided on CT, 11% with Percutaneous Endoscopic Discectomy and Drainage (PEDD) and 8% with an open technique). Among these Patients, Staphylococcus aureus was found in 54% of the cases, Mycobacterium tuberculosis in 38% of the cases and Brucella in 8% of the cases.
Imaging studies
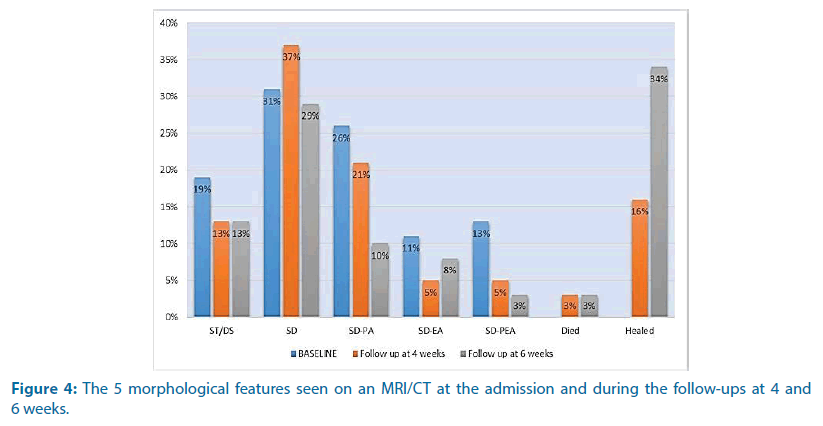
During admission, twenty patients (53%) were investigated by MRI and eighteen patients (47%) by CT. In 16% of the patients, both the CT/MRI technique was performed. The vertebral segments involved were lumbar in 21 patients (55%), thoracic in 10 patients (26%), thoracolumbar in 2 patients (5%), cervical in 2 patients (5%) and lumbosacral in 3 patients (8%). SD was found in 31% of the cases, SDPA in 26% of the cases, ST/DS in 19% of the cases, SD-PEA in 13% of the cases and SD-EP in 11% of the cases. During the follow-up, MRI imaging was performed in 74% of the cases and CT in 13% of the cases (Figures 2 and 3). In the follow-up at week 4 one patient died (3%) and six patients healed (16%). SD was found in 37% of the cases and ST/DS in 13% of the cases. SD-PA, SD-EP and SD-PEA were found respectively in 21%, 5% and 5% of the cases. In the follow-up at week 6, 1 patient died (3%) and 13 patients (34%) healed. SD-PA, SD-EP and SD-PEA were found in 10%, 8% and 3% of the cases (Figure 4).
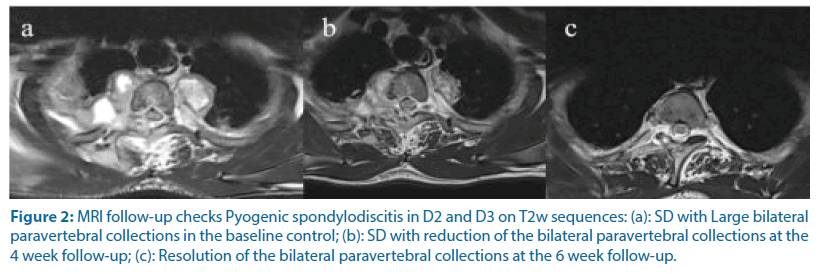
Figure 2: MRI follow-up checks Pyogenic spondylodiscitis in D2 and D3 on T2w sequences: (a): SD with Large bilateral paravertebral collections in the baseline control; (b): SD with reduction of the bilateral paravertebral collections at the 4 week follow-up; (c): Resolution of the bilateral paravertebral collections at the 6 week follow-up.
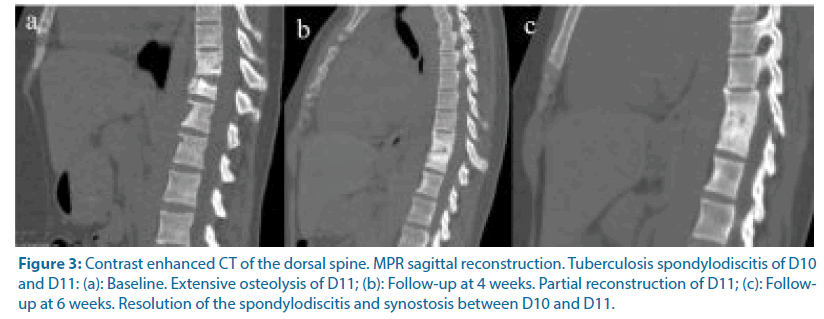
Figure 3: Contrast enhanced CT of the dorsal spine. MPR sagittal reconstruction. Tuberculosis spondylodiscitis of D10 and D11: (a): Baseline. Extensive osteolysis of D11; (b): Follow-up at 4 weeks. Partial reconstruction of D11; (c): Followup at 6 weeks. Resolution of the spondylodiscitis and synostosis between D10 and D11.
Treatment
Conservative treatment with antibiotic therapy was applied in 63% (n=24) of the cases. Antimicrobial treatment was based on suspicion of the causative organism and, if isolation was achieved, then sensitivity testing was done. The antimicrobial treatment used and its duration are summarized in Table 1. Surgical treatment was given to 21% (n=8) of the patients and an interventional procedure was done on 16% (n=6) of the patients.
| Microorganism | Treatment | Duration | |
|---|---|---|---|
| Staphylococcus aureus | Flucloxacillin | 6 wk | |
| Oxacillin susceptible | |||
| Staphylococcus aureus | Vancomycin | 6 wk | |
| Oxacillin resistent | |||
| Mycobacterium tuberculosis | Rifampicin+isoniazid+pyrazinamide+etambutol | 12 wk | |
| Brucella spp. | Doxycycline+rifampicin | 3 months | |
| B-hemolitic streptococci | Penicillin | 6 wk | |
| Enterococcus penicillin resistent | Vancomycin | 6 wk | |
| Pseudomonas | Cefepime | 6 wk | |
| Enterobacteriacee | Cefepime | 6 wk | |
| Candida spp. | Liposomal amphotericin B | 6 wk | |
| Undetermined | Vancomycin+Ciprofloxacin | 6 wk | |
Table 1. Antimicrobial treatment and duration.
Discussion
Diagnosing spinal infections can be a challenge and clinical management can be difficult. There can be severe outcomes. It is important to diagnose them in the early stage when the treatment is simple and effective [1,7,10]. There are a variety of spinal infections on a spectrum that can manifest in various places including the vertebral body (spondylitis), the intervertebral disc (discitis), the vertebral body along with the intervertebral disc (spondylodiscitis), the ligaments and the paravertebral soft tissues and the epidural space. In our study, we collected epidemiological, clinical, laboratory, microbiological imaging and therapeutic data. Our data support the view that SD is primarily a disease of old age with a male predilection [1,3,11-17]. The clinical presentation of SD in our study was generally vague and non-specific with back or neck pain in most of the cases. High fever was reported in 47% of the cases. The main cause of SD was septicemia, which was found in 42% of the cases. According to literature, the most common organism in our study was Staphylococcus aureus [1,3,10,12-16]. The second most common was Mycobacterium tuberculosis. This finding contradicts other studies [12,15,16] but is similar to a study by Karadimas et al. [17].
Imaging by MRI plays a pivotal role in the diagnosis of SD. It was the modality of choice during the initial evaluation of spinal infections and during the follow-ups. It is the most valuable tool for the diagnosis of inflammatory disorders involving soft tissue and bone marrow [1,3,7,10,11]. The MRI is highly sensitive to the early onset of inflamed edema of the vertebral and discal changes and it can fully assess abscesses within the canal [3,7]. The characteristic features of SD are the loss of disc height that appears with high signal intensity in the T2 weighted images, the low signal in T1 and high signal in T2 from the vertebral body and paravertebral or intracanal abscesses that are usually well defined after contrast medium is injected [1,3,7,14]. However, in our study, the CT with iodine-based contrast medium injection was a good alternative for patients when there were MRI contraindications. The CT is usually available and has a decreased amount of radiation exposure thanks to the improvement of CT imaging. It is now playing a much more prominent role in the evaluation of pre- and post-operative spine diseases and also in therapeutic management [3,4,7,10,11,18,19]. We also preferred to perform CTs on patients with SD caused by surgical spine treatment and those with tuberculous SD. The CT accurately delineates all bone alterations such as small vertebral foci of infections, erosions of the end plates, bone destruction, the presence of gas within an abscess and soft tissue masses with calcification that are common features of tuberculous spondylitis as seen during the follow-ups [1,3,6,7]. Intravenous injection of iodine-based medium contrast usually highlights the soft tissue extension in the paravertebral and epidural spaces but with less sensitivity than the MRI. Multiplanar Reconstructions (MPR) in sagittal and coronal planes and Volume Rendering (VR) are also useful to visualize the spatial position of the spinal devices. The use of imaging to monitor SD remains controversial [3]. In our study, imaging with MRI/ CT was essential in the initial assessment of the severity of the disease and also played an important role in monitoring the progression of the disease. Re-imaging during the critical 4-6-week period of treatment often shows an improvement, but it cannot be used on its own for monitoring the treatment of SD [10,11,20]. However, imaging by MRI or CT allows in association with clinical, laboratory/ microbiological and histopathological parameters, the diagnosis of SD [1,3,11,14,20]. In our study both the CRP and ESR decreased during the follow-up periods. Some authors suggest that CRP is the preferred marker for monitoring the patients’ response to treatment [11,14]. ESR is also a sensitive laboratory test being given to >90% of patients, with a typical range of 43 mm to 87 mm per hour [1,3,4]. In our study, the WBC was of limited diagnostic value and according to literature elevated WBC was found in 32% of cases [1,3].
Medicine nuclear imaging with a PET-CT and Gallium-67 (Ga-67) scintigraphy along with a single-photon-emission CT (SPECT) can be a good alternative particularly in cases of contraindications to contrast-enhanced MRI/CT or in cases where an MRI is inconclusive [1,3,4,10,20,21].
Identifying the pathogenic microorganism is crucial to be able to receive the appropriate antimicrobial therapy. A percutaneous CT-guided biopsy is the standard of care for the diagnosis of SD of unidentified origin. A histopathological analysis is able to distinguish between infection and contamination and between pyogenic and granulomatous disease. However, the aspirate is often inadequate and sometimes no organism can be cultured. Percutaneous Endoscopic Discectomy and Drainage (PEDD) is a better alternative to a CTguided simple technique biopsy because it provides high diagnostic accuracy. A sufficient amount of specimen from the infected disc region can be obtained directly through PEDD while avoiding the potential morbidity associated with an extensive surgical procedure. An open biopsy is usually indicated if vertebral osteomyelitis is still highly suspected after a non-diagnostic second image-guided aspiration biopsy or PEDD was performed [10,20,22,23].
The aim of SD treatment is to eliminate the focus of infection, restore spinal functionality and reduce pain.
Conservative management consists of antimicrobial therapy and non-pharmacological treatments such as physiotherapy and immobilization. In our study conservative treatment with antibiotic therapy was the most common treatment. The guidelines of the Infectious Diseases Society of America (IDSA) deem that 6-week therapy is adequate in most patients with non-specific SD [4,20]. Surgical treatment is adequate for patients with spinal cord or cauda equina compression with progressive neurological deficits [4,10,20]. A CT-guided percutaneous drainage procedure offers an effective alternative to surgery in the management of paravertebral and intradiscal abscesses [1,2,4,10,20,21].
Our study had several limitations. First, as with all retrospective studies it had very few patients. Another potential limitation was the monocenter design of the study.
Conclusion
SD is highly heterogeneous in terms of clinical and imaging presentation and the combination of several diagnostic parameters with imaging can allow prompt diagnosis of SD. MR imaging remains the modality of choice for the early diagnosis of SD and during the follow-up periods. CT is readily available and it is a good alternative to MRI for evaluating SD through the option of view in multiple plain. CT is the modality of choice in cases of Tuberculous spondylitis and SD caused by surgical treatment in the spinal region. Re-imaging in the critical period of 4-6 weeks with a careful evaluation of the clinical examination along with monitoring the systemic inflammatory markers can be a good follow-up strategy. However, the organization of a multidisciplinary team such as a radiologist, a neuroradiologist, an infectiologist, a laboratory technician, an orthopedist and a neurosurgeon is essential for the best clinical, diagnostic and therapeutic management of SD.
Funding Details
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure Statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Compliance with Ethical Standards
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Written consent was taken from the patients in accordance with the ethics committee of our institution.
References
- Mavrogenis AF, Megaloikonomo PD, Igoumenou VG, et al. Spondylodiscitis revisited. EFORT Open Rev 2: 447-461 (2017).
- Sobottke R, Seifert H, Fätkenheuer G, et al. Current diagnosis and treatment of spondylodiscitis. Dtsch Arztebl Int 105: 181-187 (2008).
- Skaf GS, Domloj NT, Fehlings MG, et al. Pyogenic Spondylodiscitis: an overview. J Infect Public Health 3: 5-16 (2010).
- Herren C, Jung N, Pishnamaz M, et al. Spondylodiscitis: diagnosis and treatment options: A systematic review. Dtsch Arztebl Int 114: 51-52 (2017).
- Aagaard T, Roed C, Dahl B, et al. Long-term prognosis and causes of death after spondylodiscitis: a Danish nationwide cohort study. Infect Dis 48: 201-208 (2016).
- Trecarichi EM, Di Meco E, Mazzotta V, et al. Tuberculous spondylodiscitis: epidemiology, clinical features, treatment, and outcome. Eur Rev Med Pharmacol Sci 16: 58-72 (2012).
- Leone A, Dell’Atti C, Magarelli N, et al. Imaging of spondylodiscitis. Eur Rev Med Pharmacol Sci 16: 8-19 (2012).
- Kumar Y, Gupta N, Chhabra A, et al. Magnetic resonance imaging of bacterial and tuberculous spondylodiscitis with associated complications and non-infectious spinal pathology mimicking infections: a pictorial review. BMC Musculoskelet Disord 18: 1-10 (2017).
- Lee KY. Comparison of pyogenic spondylitis and tuberculous spondylitis. Asian Spine J 8: 216-223 (2014).
- Gouliouris T, Sani HA, Nicholas MB. Spondylodiscitis: update on diagnosis and management. J Antimicro Chemoth 65: 11-24 (2010).
- Homagk L, Marmelstein D, Homagk N, et al. SponDT (Spondylodiscitis Diagnosis and Treatment): spondylodiscitis scoring system. J Orthop Surg Res 14: 1-8 (2019).
- Lebre A, Velez J, Rabadão E, et al. Infectious spondylodiscitis: a retrospective study of 140 patients. Infect Dis Clin Pract 22: 223-228 (2014).
- Kourbeti IS, Tsiodras S, Boumpas DT. Spinal infections: evolving concepts. Curr Opin Rheumatol 20: 471-479 (2008).
- Pola E, Taccari F, Autore G, et al. Multidisciplinary management of pyogenic spondylodiscitis: epidemiological and clinical features, prognostic factors and long-term outcomes in 207 patients. Eur Spine J 27: 229-236 (2018).
- Kaya S, Ercan S, Aktas U, et al. Spondylodiscitis: evaluation of patients in a tertiary hospital. J Infect Dev Ctries 8: 1272-1276 (2014).
- Aagaard T, Roed C, Dragsted C, et al. Microbiological and therapeutic challenges in infectious spondylodiscitis: a cohort study of 100 cases, 2006-2011. Scandinavian J Infect Dis 45: 417-424 (2013).
- Karadimas EJ, Bunger C, Lindblad BE, et al. Spondylodiscitis. A retrospective study of 163 patients. Acta Orthopaedica 79: 650-659 (2008).
- Splendiani A, D’Orazio F, Patriarca L, et al. Imaging of post-operative spine in intervertebral disc pathology. Musculoskeletal Surg 101: 75-84 (2017).
- Sans N, Faruch M, Lapègue F, et al. Infections of the spinal column-spondylodiscitis. Diagn Interventional Imaging 93: 520-529 (2012).
- Berbari EF, Kanj SS, Kowalski TJ, et al. Infectious Diseases Society of America (IDSA) clinical practice guidelines for the diagnosis and treatment of native vertebral osteomyelitis in adults. Clin Infect Dis 61: e26-e46 (2015).
- Guerado E, Cerván AM. Surgical treatment of Spondylodiscitis. An update. Int Orthop 36: 413-420 (2012).
- Di Martino A, Papapietro N, Lanotte A, et al. Spondylodiscitis: standards of current treatment. Curr Med Res Opin 28: 689-699 (2012).
- Yang SC, Fu TS, Chen LH, et al. Identifying pathogens of spondylodiscitis: percutaneous endoscopy or CT-guided biopsy. Clin Orthop Relat Res 466: 3086-3092 (2008).