Review Article - Pharmaceutical Bioprocessing (2016) Volume 4, Issue 4
A Review of Biological and Pharmacological Actions of Melatonin: Oxidant and Prooxidant Properties.
- Corresponding Author:
- Mohammad Ali Eghbal
Pharmacology and Toxicology Department, School of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran
E-mail: m.a.eghbal@hotmail.com
Abstract
Melatonin as an indole amine exists in most of mammals and produced by various organs. It involved in circadian regulation of physiological and neuroendocrine function. Also it modulates diverse physiological functions such as sleep and sexual behavior. During the last decade, melatonin has been shown to possess potent free radical scavenger properties against reactive oxygen species (ROS). Moreover, by induction of the expression of antioxidant enzymes and reduction of the activation of pro-oxidant enzymes, melatonin indirectly could protect cells against a variety of free radical-related diseases. Besides, melatonin has been shown to promote the generation of ROS at pharmacological concentrations in in-vitro studies. Although melatonin could potentially be useful but safety, efficacy remains uncertain. In the present report, we review the studies which document the influence of melatonin on the various oxidative stress associated diseases .We also analyze the possible mechanisms by which melatonin induce ROS formation.
Keywords
melatonin, antioxidant, anti-inflammatory, pro-oxidant, oxidative stress, free radicals
Introduction
Melatonin (N-acetyl-5-methoxytryptamine), an endogenous neurohormone, is an evolutionary conserved indolamine synthesized from tryptophan that is the mainly secreted by the pineal gland [1]. Extensive effects of melatonin and its metabolites are associated to their physiological functions such as regulating sleep(circadian rhythms) as time-giver (Zeitgeber) [2], alleviation of depression [3], synchronizing the reproductive cycle with the suitable period of the year in photoperiodic species [4], anti-aging [5], antioxidant [6], anti-inflammatory activities [7,8] and newly recognized pro-oxidative effects [9] in exogenous administration. Secondary sources of melatonin are retina, gut, platelets, skin, bone marrow and other organs, which have minor systemic roles [4]. Also melatonin can modulate a variety of neural, endocrine, and immune functions [4].
Reportedly, melatonin has shown potential therapeutic effects in several diseases such as Alzheimer [10], obesity [11], cardiovascular [12], bone disease [13] and cancer [14,15]. Besides, some prior studies have revealed that melatonin can recover colonic damage in experimental colitis [16,17]. ROS formation is a fundamental stimulant of various signaling cascades including cellular proliferation, survival, inflammation and senescent. Because of prominent antioxidant properties melatonin confronts the progression of inflammatory cascades [18].
Melatonin acts both receptor-dependently as well as independent pathways; the latter relate to its direct radical scavenging functions. Melatonin effects are via largely through G protein dependent receptors which result in the stimulation of antioxidant enzyme production against oxygen- and nitrogen-based reactive molecules [19]. Specific membrane and nuclear receptors have been recognized for melatonin in nonneural tissues from many different species [20]. There are melatonin receptors type 1 and type 2 (MT1 and MT2, respectively) or, indirectly with nuclear orphan receptors from the RORa/RZR family. Melatonin also couples to the quinone reductase II enzyme, previously defined the MT3 receptor [21]. Understanding the distribution of peripheral melatonin receptors and their function seem quite remarkable, since melatonin modulates widespread processes in the whole body including immune-modulation, regulation of endocrine, pulmonary and cardiovascular functions, as well as cancerogenesis, and aging [22]. Some evidence showed that melatonin supplementation during the phase of diabetes development protected the liver against oxidative stress and the consequences of DNA damage [23].
The present review focuses on recent insights into the various biological functions of melatonin and bolds the importance of the agent as a valuable pharmacological tool once more.
Melatonin anti-oxidant effects
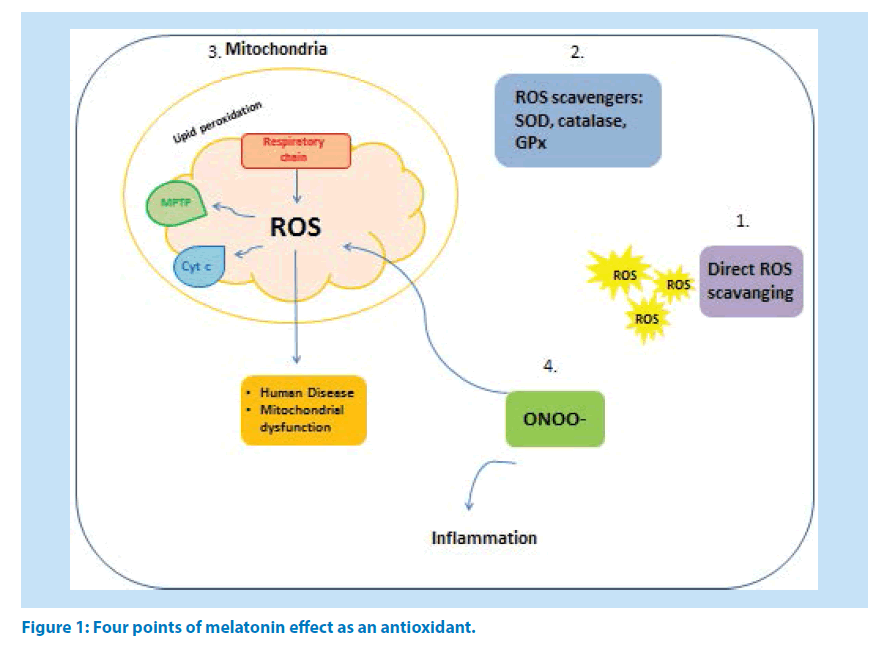
Owning an amphiphilic nature, melatonin easily enters the entire cell compartments such as nucleus [24] and mitochondria where it scavenges free radicals [25,26] and directly inhibits mitochondrial permeability transition pore (MPTP) [27]. Also, melatonin is able to prevent molecular damages resulting from toxic oxygen- and nitrogen-based reactants [28-30]. Melatonin functions in scavenging free radicals might be classified in to four main categories (Figure1): (1) as an antioxidant directly scavenges ROS [31], (2) stimulates the antioxidant enzymes production and activation [32,33] and (3) increases the efficacy of mitochondrial functions by improving MPTP, inhibition of cytochrome c release and refining of oxidative phosphorylation in mitochondrial respiratory chain which further will decrease the peroxidation of membrane lipids [34]. Melatonin inhibits peroxynitrite production and activation of poly ADP ribosesynthetase (nuclear enzyme) in macrophages of rats exposed to zymosan-induced shock [35]. Inhibition of peroxy nitrite production by melatonin cause anti-inflammatoy effects (4) [36]. Recently studies revealed the participation of nitric oxide in the mechanism of anti-inflammatory effect of melatonin [37].
It has been discovered melatonin was more effective than vitamin C in an experimental model of Alzheimer disease [38] and has exerted better protective effect than vitamin E and N-acetyl cysteine against acetaminophen induced oxidative stress toxicity in mice [39].
Melatonin as an anti-oxidant in Liver disease
Due to high amounts of intracellular concentrations of glutathione(GSH), superoxide dismutase (SOD), catalase, and lipid soluble antioxidants [40], hepatocytes are stable to injuries caused by Reactive Oxygen species (ROS) and Reactive nitrogen species (RNS). However, oxidative stress in part, is involved in different liver dysfunctions including fatty liver diseases, drug-induced liver injuries and even alcoholic liver diseases [41-44]. Hence, antioxidant therapy in context of counteracting the harmful effects of ROS and therefore preventing or treating oxidative stress-related diseases is a noteworthy therapeutic option.
Melatonin exerts anti-oxidant effects in hepatocytes and epithelium of liver by reducing lipid peroxidation and increasing the level of reduced liver glutathione [44,45]. Melatonin is a highly valuable OH and H2O2 scavenger, during its metabolism to N-acetyl-N2-formyl-5- methoxykynuramine (AFMK) [46].
It also stimulates several antioxidative enzymes such as glutathione peroxidase, glutathione reductase, SOD, and boosts the synthesis of GSH .The antioxidant properties of melatonin prevent acute liver injury induced by CCl4, acetaminophen, ischemia-reperfusion, bile duct ligation, pancreatitis nephrotoxicity and neuronal degeneration [47-56]. Also, administration of melatonin exerts hepatoprotective effects in a rat model of CPB( cardiopulmonary bypass model) and SIRS(systemic inflammatory response syndrome) by reducing the ROS generation which is well demonstrated in biochemical and histo-pathological experiments [57,58].
As Sewerynek et al has been reported, melatonin increased GSH-Px (Glutathione peroxidase) activity and at the same time reduced free radical injury to the brain and liver of rats treated with lipopolysaccharide (LPS) [59]. Administration of 4 mg/kg melatonin LPS-treated rats enhances GSH above basal levels and lowers GSSG concentrations while it has stimulatory effect on GSH-Px. One week administration of 10 mg/kg of melatonin significantly increases SOD activity in rat hepatocytes [60], while some reports show enhancement of SOD activity in rat kidney, liver and brain after a single melatonin injection (5 mg/kg) [61]. This indicates that melatonin may act on several points in the antioxidant defense system, not exclusively on GSH-Px40, which is also evident in drug induced liver injuries. Daily 3 mg/kg melatonin is expected to provide significant protection against acute and chronic liver injuries induced by thioacetamide [62,63]. However, the hepatoprotective activity of melatonin in chronic liver injuries is more favorable of acute liver injuries [64]. Besides, treatment of isolated hepatocytes with melatonin (1mM) reduces the trazodone-induced ROS formation and protects mitochondria against trazodoneinduced toxicity [65]. Taziki et al reported that 1mM of melatonin is the effective dose that provides proper protection against trazodone [65]. Simultaneous administration of taurine and melatonin prevented mitochondrial damage caused by phenytoin, and its consequences such as LPO [66]. Furthermore, 1 mM of melatonin remarkably increased glutathione contents of carbamazepine-exposed cells and decreased the GSSG levels, reactive oxygen species and TBARS production. Incubation of hepatocytes with the 1mM of melatonin decreases the death rate of hepatocytes drastically [67].
Comparison of melatonin with other known anti-oxidant agents has been investigated in some researches. Melatonin, in combination with vitamin C was also vastly efficient in reducing the lipid peroxidation in hepatic homogenates treated with 15 ml FeSO4 and100 ml H2O2 [68]. Vitamin E was found to be approximately 100-times more efficient than melatonin in reducing lipid deterioration under the experimental conditions used [68]. So this is consistent with other observations wherein, under in-vitro conditions, vitamin E was more effective than melatonin in inhibiting the oxidative breakdown of lipids [69].
Melatonin (100 nM) markedly increases the activity of the GPx liver mitochondria by eightfold, compared with the basal levels of these enzymes while, Neither NAC, ascorbate, nor Trolox are able to modify the activity of the glutathione-related enzymes. Besides, melatonin but not vitamins C and E preserves glutathione homeostasis in t-butyl hydroperoxide-induced mitochondrial oxidative stress [70].
Melatonin as an anti-oxidant in pulmonary disease
Oxidative stress is a key pathophysiological reason of airway diseases such as asthma and chronic obstructive pulmonary disease (COPD), which cause significant morbidity and mortality. ROS formation not only causes direct harm to lung structure but also exacerbates other situations such as COPD, inflammatory lung disease and increasing of the epithelial and endothelial cells of the lung apoptosis [71,72]. Oxidative stress stimulates the activation of transcription factors and signaling pathways, partly through the activation of the innate immune response which turns on several cell signaling cascades that leads to release of cytokines and chemokines [73]. It is well known that, chronic oxidative damage in COPD terminates in increased levels of highly reactive carbonyls which could be modified and become a highly immunogenic carbonyl adducts on “self ” proteins [74].
A clinical study based on measuring exhaled breath condensate (EBC) collection was organized on which malondialdehyde (MDA) was found to be increased in subjects with chronic airway disorders, particularly in COPD [75].
Life style habits also result in oxidative phenomena, as in chronically cigarette smoke exposed guinea pigs the oxidative damage to muscle proteins, which precedes the characteristic respiratory changes, may contribute to muscle loss and dysfunction in smokers and patients with COPD [76].
Numerous evidences from clinical trials have presented that exogenous melatonin has positive effects in avoidance of cell damage in acute circumstances, such as sepsis, asphyxia and also chronic metabolic disease, inflammation, and lung cancer [77-79]. Recent reports showed that the production of ROS and pro-inflammatory cytokines are extensively diminished by the treatment with melatonin, thereby melatonin may limit pulmonary injury through its antioxidant properties [80]. Pignone et al described a decreased serum melatonin and antioxidant enzymes activity in erythrocytes together with proof of increased lipid peroxidation during exacerbations in COPD and asthma [81]. There is helpful data that oxidative stress and related compounds can selectively trigger nociceptive airway afferents in bronco-pulmonary airways, initiating action potentials in unmyelinated C-fibers that conduct centrally to inducing dyspnea [82]. TNF-α production was inhibited upon pretreatment with melatonin and IL-10 releasing was increased with the result of decreased pulmonary edema [83]. Also pretreatment of rats with melatonin attenuated malondialdehyde levels in endotoxemia-induced and ventilatorinduced lung injuries [84] peritonitis-induced septic shock [85], ionizing radiation induced oxidative organ damage [86] and bleomycin induced lung injury [80]. All of these reports signifying the inhibitory role of melatonin in neutrophil infiltration and lipid peroxidation [83]. It has been published that 8-isoprostane as a Markers of oxidative stress were increased in bronco-alveolar lavage fluid from patients with sarcoidosis [87-89]. Therefore melatonin is a harmless and efficient treatment for CS (chronic pulmonary sarcoidosis) unresponsive to other treatments or when the typical remedy should be stop for dangerous side effects [90].
Melatonin as an anti-oxidant in cancer therapy
Oxidative stress appears to play an essential role in a wide range of cancers due to its association to a variety signaling pathways, apoptosis, survival, and proliferation. The stimulatory function of ROS on transcription factors such as NF-κB, AP-1, p53, HIF-1α, PPAR-γ leads to expression of different genes, including those for growth factors, inflammatory cytokines, chemokines, cell cycle regulatory molecules [91]. Besides, oxidative hazard provides the situation for chronic inflammation that is a common etiology of several diseases as well as cancer [92]. Cancer cells themselves generate active oxygen and the results by-products that cause cellular damage [93].
Also, polymorphisms in cellular oxidant/ antioxidant reservoirs have been identified in cancer susceptible patients [94]. Hence, antioxidants has been offered as a choice therapeutic option in treatment of malignancies [91,95]. As Lissoni et al works reported, Melatonin is useful in chemotherapy of cancer patient with poor clinical condition [96], advanced cancer patient with normal clinical status [96] and improve the efficacy of anthracyclines, cisplatin, 5fluorourcil, etoposide, gemcitabine, taxanes, raltitrexedand, isofosfamide [96,97].] Melatonin through numerous biological mechanisms is able to influence host–tumor interactions which may change both tumor and host characteristics [98- 100]. Melatonin as anti-proliferative [97] and also anti-inflammatory activity [101] happens as a consequence of the inhibitory effect on IL-6 secretion, which prevents anti-tumoral immunity of body [102,103].
Melatonin has been used as a supportive treatment of cancer patients by the prevention of both cancer progression-related symptoms and chemotherapy-induced toxicity [104]. So, melatonin is the first identified natural molecule that may have both therapeutic and alleviating actions in the cure of human neoplasms. Presentational agents are generally limited to endocrine-dependent tumors (breast, endometrial, prostate), whereas, melatonin is potentially effective against most solid tumors (lung, gastric, colorectal) cancer [104,105].
Bartsch et al demonstrated that the level of overnight plasma melatonin decreases due to the changing of 6-sulfatoxymelatonin metabolism and other compounds in the DMBA(Dimethyl benzanthracene) exposed rat livers [106]. So, exogenous melatonin could act as a defensive hormone against DMBA-induced carcinogenesis by effectively repairing of the antioxidant enzyme system (full protection for CAT, SOD and an adequate protection for GSH Px) [107]. Furthermore, melatonin suppresses iron related carcinogenesis by inhibiting of lipid peroxidation and sperm abnormalities in male gonads [108- 110]. Also melatonin shows higher efficacy than combination of vitamin E/ Se in antagonizing ROS effects [107].
Oxidative damage initiated by AFB1(aflatoxinB1) may be one of the underlining mechanisms for AFB1-induced cell and DNA damage, which ultimately lead to tumorigenesis [111]. Also AFB1 can trigger caspase-3 activation and lead to apoptosis in rat liver [112] and in vivo studies suggest that AFB1can cause lipid peroxidation in rat liver [113]. Melatonin treatment improve hepatic antioxidant defense system which consequently decrease the apoptosis rate in the liver [114] and according to all the previous evidences and results of some clinical studies melatonin can be used as a treatment in clinical cases of aflatoxicosis [112,115].
Melatonin as an anti-oxidant in Cardiovascular disease
There are many biological factors such as hyper-cholesterolemia, diabetes, infectious agents and an excess of free radicals could cause cardiovascular disease. According to many studies reactive oxygen species play key role in pathogenesis of coronary atherosclerosis [116- 118]. Moreover, ROS and subsequently oxidative stress been recognized in the development and maintenance of hypertension has for some time [119-121]. The accumulating data from several experiments suggest that melatonin influences the cardiovascular system [122-124].
Melatonin could effect on circulatory function via different mechanisms. For example melatonin can maintain the availability of nitric oxide, so lead to vasodilatation and reducing blood pressure [125]. Also, melatonin has an inhibitory effect on α1-adrenergic and also increase the cholinergic tone [126]. Oxidative stress is highly involved in endothelial dysfunction and is a denominator of vascular disease. Decrease in synthesis or elevated inactivation of NO is a predictive of future cardiovascular events.
Accelerated ROS generation reduces the amount of bioactive NO by chemical inactivation to form toxic peroxynitrite [127]. So, Melatonin via acting as an antioxidant may in part improve endothelial function.
Ekmekcioglu et al suggest that recently founded MT1 and MT2 melatonin receptors in some parts of human cardiovascular system such as left ventricle, aorta, and coronary, cerebral illustrate the distinguishing role of melatonin in treatment of cardiovascular disease such as hypertension, myocardial ischemia, and stroke [128].
Some clinical studied have shown that daily administration of 1mg melatonin could reduce systolic/diastolic pressures and norepinephrine levels in young women and men [129,130]. As well, other study proved that repeated dose of oral melatonin intake 1 hour before bedtime reduced blood pressure and also enhanced sleep quality in male patients with essential hypertension [131].
In contrast to the beneficial effects of melatonin, Lusardi et al works showed that, co-administration of melatonin and nifedipine increased blood pressure heart rate in hypertensive patients [132]. These findings suggest that that melatonin may compete with nifedipine and diminish the antihypertensive effect of the calcium channel blockers, so melatonin cannot be considered a dietary supplement [132]. Besides, clinical evidences has been shown that melatonin in combination with antihypertensive drugs treatment, increased the effect of aforementioned drugs [133].
Several studies have investigated the antioxidant effect of melatonin on total cholesterol and VLDL (very low-density lipoprotein). Tengattini et al reported that plasma levels of total cholesterol and very low-density lipoprotein cholesterol as well as the low-density lipoprotein cholesterol sub-fraction was reduced by melatonin in hypercholesterolemic rats [134].
Melatonin may demonstrate the effects by enhancing endogenous cholesterol clearance. Since, melatonin has a lipophilic property, it can enter the lipid phase of the LDL particles and inhibit lipid peroxidation [135]. Also melatonin by increasing of endothelial NO production can protect human umbilical artery against the oxidation of low-density lipoprotein [136], consequently it would be protective against cardiovascular disease [134].
Pro-oxidant effect of melatonin
The pineal indolamine, melatonin, in addition to acting as a well-established antioxidant it also has been reported recently to exert prooxidant activities. Increased formation of ROS or decreased cellular antioxidant reservoir called oxidative stress results in cellular damage by oxidizing the macromolecules such as proteins, lipids and DNA [91]. Various cell functions including signal transduction pathways, host defense against invasive pathogens, autophagy, cellular proliferation and apoptosis are mediated by physiological amounts of ROS [137]. Besides ROS is produced in response to different cytokines and growth factors as a secondary messenger [138,139].
Although the imbalance between oxidant/ antioxidant particles increases ROS levels resulting in undesirable effects, ROS mediated apoptosis in a wide spectrum of cancers is the mechanism by which many chemotherapeutic agents induce cytotoxicity [140].
Melatonin by scavenging free radicals and activating of intracellular antioxidant enzymes acts as a powerful antioxidant [141]. In addition to having role in cell signaling pathways, melatonin protects normal cells against apoptosis [142,143]. Controversially, melatonin induces programmed cell death in several cancer cells as in human myeloid HL-60 cells [144], human colorectal cancer cell [145], human prostate adenocarcinoma cells [146] and B-lymphoma [147]. While the exact mechanism of melatonin induced apoptosis remains to be elucidated, its pro-oxidant activity, presumably, constitutes a mechanism that may result in tumor cell death by apoptosis. ROS induced apoptotic pathways enables melatonin to reduce cell proliferation rate in different cell types [148]. Enhancement of intracellular ROS production was observed after melatonin treatment in human pro-mylocytic leukemia HL-60 cells 9. Increase of intracellular ROS generation exacerbates intrinsic pathway of apoptosis which is usually followed by a loss of mitochondrial membrane potential [149]. Disrupting mitochondrial function, melatonin induces the liberation of cytochrome c from mitochondria into the cytosol which in turn activates caspase enzymes cascade. Inhibition of melatonin induced caspase activation by known antioxidants suggests the pro-oxidant effects of melatonin accounting for its cytotoxic role in HL-60 cells [144]. Moreover, Bejarano et al, demonstrated that melatonin provokes hydrogen peroxide induced oxidative damage accompanied by marked intracellular ROS production, mitochondrial dysfunction, reduced metabolic capacity and elevated caspase 9 activation in HL- 60 cells [150].
In addition, melatonin potentiates chemotherapeutic –induced apoptotic effects of anticancer agents that generate intracellular ROS [151]. Melatonin is involved in significant depletion of glutathione (GSH), which protects cells against oxidative hazard, causing a prooxidant mechanism related to the cytotoxic features of this indolamine. Melatonin reduces GSH level and subsequent overproduction of ROS in human liver cell line HepG2 [152]. Albertini et al, also reported a prooxidant effect of melatonin in U937 cells within the increment of intracellular oxidative species and depletion of GSH while the glutathione peroxidase activation was unchanged during the experiment [153].
Fas ligand is a trans-membrane protein subsequent to binding to its receptor plays a fundamental role in apoptosis induction. Oxidative stress induces the expression of fas receptor and fas ligand. Melatonin pro-oxidant properties triggers fas-induced cell death in human leukemic Jurkat cells [154].
Despite antagonizing deleterious oxidants generated in the cell medium, melatonin itself could be oxidized by the free radicals such as AAHP-derived peroxylnitrit radicals (ROO.) and produce melatonin radical which in turn is able to react with GSH [155].
Influencing cell signaling pathways, but not direct chemical effects of melatonin has also been proposed as the prooxidant mechanism of the substance. In this context, the activation of lypoxygenase(LOX) and phospholipase A2 (PLA2) are assumed to participate in melatonin induced ROS production [156]. Since calmodolin is a low affinity target of melatonin, their binding lead to Ca2+-independent release of PLA2. Upon moving to the cell membrane PLA2 liberates high amounts of arachidonic acid which in turn stimulate LOX activation and subsequent ROS production [157].
To note, affecting cellular redox state and prooxidant activities of melatonin are concentration dependent. Such properties have been reported in both high micro-mill molar concentrations of melatonin unlike the physiologic amounts which exert antioxidant and anti-proliferative properties [158]. Taking into the account the dual antioxidant abilities related to the antiproliferative and pro-oxidant abilities related to the cytotoxic effects of melatonin opens the door to novel indication of the indole in different cancer cells.
Anti-inflammatory Properties of Melatonin
The antioxidant and anti-inflammatory effects of melatonin extensively have been summarized in many studies6,156,159. Malondialdehyde (MDA) and inflammatory cytokines concentration have been reduced after melatonin treatment in critical situations such as asphyxia, sepsis, and surgery [160-162].
The production of inflammatory cytokines including TNF-α, IL-1β, or IL-6, subsides by melatonin in numerous experimental models of inflammation [163-166]. Different mechanisms have been reported for anti-inflammatory activity of melatonin including prevention of the activation of cyclooxygenase-2 and inducible isoform of nitric oxide synthase, as well as blocking of the transcriptional factorsthat stimulates pro-inflammatory cytokines production [167,168]. These include not only NFk-B but also, HIF, Nrf2, cAMP, CREB, STAT, PPARs, and AP-1.
Melatonin exerts anti-inflammatory effects both under in vitro and in vitro conditions. Besides, in an experimental human sepsis model, melatonin administration before endotoxemia results in significant reduction of certain markers of inflammation [169]. The protective effect of the indolamine on acute model of pancreatitis has been investigated. Caeruleininduces acute inflammation in pancreas of rats whereas, melatonin pre-treatment diminishes pro-inflammatory cytokines IL-1β and TNF-α and increases the serum levels of antiinflammatory cytokine IL-4 170. These results are consistent with the findings about acetic acid-Induced Colitis in rats that melatonin completely improves the latter inflammation [171]. In rabbit osteoarthritis (OA) model and also human chondrocytes melatonin markedly inhibits hydrogen peroxide cytotoxicity via downregulation of iNOS, and COX-2 protein and mRNA expression [172]. Furthermore, melatonin , in a dose dependent manner decreases the iNOS protein expression and TNF-α mRNA levels caused by methamphetamine in human dopaminergic neuroblastoma SH-SY5Y cells . The anti-neuroinflammatory effects of melatonin result from the inhibition of activated NF-κB in cell signaling pathway [173].
Based on mentioned observations, melatonin can be used in combination with other drugs such as omeprazole to treat ulcerative colitis [174,175]. Chen et al have reported that melatonin administration decreases abdominal and rectal pain in patients of irritable bowel syndrome176. Other clinical study demonstrate melatonin plus tryptophan treatment are effective by decreasing the plasma levels of pro-inflammatory cytokines in patients with nonalcoholic steatohepatitis [177]. Surprisingly, a clinical study on patients with rheumatoid arthritis has been revealed the combination of melatonin treatment with other medications did not improve the clinical symptoms and it has been observed concentration of some inflammatory indicators increased [178].
Regarding to decreasing of melatonin level in many infectious patient such as HIV-1 [179], pulmonary tuberculosis [180], melatonin treatment are valuable to use against numerous bacterial and viral infections due to its antiinflammatory ,antioxidant, immune-modulating actions [181].
Conclusion
A large number of evidences have indicated a tight, cause-effect link between oxidative stress and a broad range of human diseases since it can contribute to many of cell signaling pathways and of course be hazardous in case of excess ROS generation. Hence, use of suitable antioxidant with maximum protection has always been a matter of many experiments. Melatonin due to its known powerful ROS combating properties has attracted many attentions. Definition of the role of melatonin in the patho-physiological mechanisms of inflammation has also been a growing research field. Regarding the pro-oxidant stimulating effects of melatonin clarifying the proper amount and duration of its use should be taken into the account.
Conflict of interests
The authors declare that there are no conflicts of interests.
Acknowledgments
The authors would like to thank Drug Applied Research Center of Tabriz University of Medical Sciences, Tabriz, Iran, for providing technical facilities. This is a report of a database from thesis entitled “Evaluation of the mechanisms of hepatic injuries induced by antipsychotic drugs” registered in “Drug Applied Research Center”. The authors are also thankful to the University’s “Students’ Research Committee” for providing technical supports to the study.
References
- Gürpınar T, Ekerbiçer N, Uysal N, Barut T, Tarakçı F, et al. The effects of the melatonin treatment on the oxidative stress and apoptosis in diabetic eye and brain. Sci. W. J. 2012, 498489 (2012).
- Lerchl A, Reiter RJ. Treatment of sleep disorders with melatonin. BMJ 345, e6968 (2012).
- Cardinali DP, Srinivasan V, Brzezinski A, Brown GM. Melatonin and its analogs in insomnia and depression. J. Pineal. Res. 52(4), 365-375 (2012).
- Toma CD, Svoboda M, Arrich F, Ekmekcioglu C, Assadian O, et al. Expression of the melatonin receptor (MT) 1 in benign and malignant human bone tumors. J. Pineal. Res. 43(2), 206-213 (2007).
- Pandi-Perumal SR, BaHammam AS, Brown GM, Spence DW, Bharti VK, et al. Melatonin antioxidative defense: therapeutical implications for aging and neurodegenerative processes. Neurotoxicity. Research. 23(3), 267-300 (2013).
- Galano A, Tan DX, Reiter RJ. Melatonin as a natural ally against oxidative stress: a physicochemical examination. J. Pineal. Res. 51(1), 1-16 (2011).
- Kireev RA, Tresguerres AC, Garcia C, Ariznavarreta C, Vara E, et al. Melatonin is able to prevent the liver of old castrated female rats from oxidative and pro-inflammatory damage. J. Pineal. Res. 45(4), 394-402 (2008).
- Reiter RJ, Tan DX, Jou MJ, Korkmaz A, Manchester LC, et al. Biogenic amines in the reduction of oxidative stress: melatonin and its metabolites. Neuro. Endocrinol. Lett. 29(4), 391-398 (2008).
- Bejarano I, Espino J, Barriga C, Reiter RJ, Pariente JA, et al. Pro-Oxidant Effect of Melatonin in Tumour Leucocytes: Relation with its Cytotoxic and Pro-Apoptotic Effects. Basic. Clinical. Pharmacology. Toxicology. 108(1), 14-20 (2011).
- Cardinali DP, Brusco LI, Liberczuk C, Furio AM. The use of melatonin in Alzheimer's disease. Neuro. Endocrinol. Lett. 1, 20-23 (2002).
- Prunet-Marcassus B, Desbazeille M, Bros A, Louche K, Delagrange P, et al. Melatonin reduces body weight gain in Sprague Dawley rats with diet-induced obesity. Endocrinology. 144(12), 5347-5352 (2003).
- Dominguez-Rodriguez A. Melatonin in cardiovascular disease. Expert. Opin. Investig. Drugs. 21(11), 1593-1596 (2012).
- Sánchez-Barceló EJ, Mediavilla MD, Tan DX, Reiter RJ. Scientific basis for the potential use of melatonin in bone diseases: osteoporosis and adolescent idiopathic scoliosis. J. Osteoporos. (2010).
- Sánchez-Hidalgo M, Guerrero JM, Villegas I, Packham G, de la Lastra CA. Melatonin, a natural programmed cell death inducer in cancer. Curr. Med. Chem. 19(22), 3805-3821 (2012).
- Koh W, Jeong SJ, Lee HJ, Ryu HG, Lee EO, et al. Melatonin promotes puromycin-induced apoptosis with activation of caspase-3 and 5'-adenosine monophosphate-activated kinase-alpha in human leukemia HL-60 cells. J. Pineal. Res. 50(4), 367-373 (2011).
- Pentney PT, Bubenik GA. Melatonin reduces the severity of dextran-induced colitis in mice. J. Pineal. Res. 19(1), 31-39 (1995).
- Cuzzocrea S, Mazzon E, Serraino I, Lepore V, Terranova ML, et al. Melatonin reduces dinitrobenzene sulfonic acid-induced colitis. J. Pineal. Res. 30(1), 1-12 (2001).
- Cuzzocrea S, Reiter RJ. Pharmacological actions of melatonin in acute and chronic inflammation. Curr. Top. Med. Chem. 2(2), 153-165 (2002).
- Sudnikovich EJ, Maksimchik YZ, Zabrodskaya SV, Kubyshin VL, Lapshina EA, et al. Melatonin attenuates metabolic disorders due to streptozotocin-induced diabetes in rats. Eur. J. Pharmacol. 569(3), 180-187 (2007).
- Carrillo-Vico A, Guerrero JM, Lardone PJ, Reiter RJ. A review of the multiple actions of melatonin on the immune system. Endocrine. 27(2), 189-200 (2005).
- Dubocovich ML, Markowska M. Functional MT1 and MT2 melatonin receptors in mammals. Endocrine. 27(2), 101-110 (2005).
- Slominski RM, Reiter RJ, Schlabritz-Loutsevitch N, Ostrom RS, Slominski AT. Melatonin membrane receptors in peripheral tissues: distribution and functions. Mol. Cell. Endocrinol. 351(2), 152-166 (2012).
- Grigorov I, Bogojević D, Jovanović S, Petrović A, Ivanović-Matić S, et al. Hepatoprotective effects of melatonin against pronecrotic cellular events in streptozotocin-induced diabetic rats. J. Physiol. Biochem. 70(2), 441-450 (2014).
- Erren TC, Reiter RJ. A generalized theory of carcinogenesis due to chronodisruption. Neuro. Endocrinol. Lett. 29(6), 815-821 (2008).
- Jou MJ, Peng TI, Hsu LF, Jou SB, Reiter RJ, et al. Visualization of melatonin’s multiple mitochondrial levels of protection against mitochondrial Ca2+-mediated permeability transition and beyond in rat brain astrocytes. J. pineal. res. 48(1), 20-38 (2010).
- Paradies G, Petrosillo G, Paradies V, Reiter RJ, Ruggiero FM. Melatonin, cardiolipin and mitochondrial bioenergetics in health and disease. J. Pineal. Res. 48(4), 297-310 (2010).
- Andrabi SA, Sayeed I, Siemen D, Wolf G, Horn TF. Direct inhibition of the mitochondrial permeability transition pore: a possible mechanism responsible for anti-apoptotic effects of melatonin. The. FASEB. J. 18(7), 869-871 (2004).
- Tan DX, Reiter RJ, Manchester LC, Yan MT, El-Sawi M, et al. Chemical and physical properties and potential mechanisms: melatonin as a broad spectrum antioxidant and free radical scavenger. Curr. Top. Med. Chem. 2(2), 181-197 (2002).
- Sofic E, Rimpapa Z, Kundurovic Z, Sapcanin A, Tahirovic I, et al. (2005) Antioxidant capacity of the neurohormone melatonin. J. Neural. Transm. 112(3), 349-358.
- Reiter RJ, Tan DX, Mayo JC, Sainz RM, Leon J, et al. Melatonin as an antioxidant: biochemical mechanisms and pathophysiological implications in humans. Acta. Biochim. Pol. 50(4), 1129-1146 (2003).
- Reiter RJ. Melatonin: Lowering the High Price of Free Radicals. News. Physiol. Sci. 15(5), 246-250 (2000).
- Rodriguez C, Mayo JC, Sainz RM, Antolin I, Herrera F, et al. Regulation of antioxidant enzymes: a significant role for melatonin. J. Pineal. Res. 36(1), 1-9 (2004).
- Chabra A, Shokrzadeh M, Naghshvar F, Salehi F, Ahmadi A. Melatonin ameliorates oxidative stress and reproductive toxicity induced by cyclophosphamide in male mice. Hum. Exp. Toxicol. 33, 185-195 (2014).
- Acuna-Castroviejo D, Escames G, Rodriguez MI, Lopez LC. Melatonin role in the mitochondrial function. Front. Biosci. 12, 947-963 (2007).
- Maestroni GJ. The immunoneuroendocrine role of melatonin. J. Pineal. Res. 14(1), 1-10 (1993).
- Poeggeler B, Saarela S, Reiter RJ, Tan DX, Chen LD. Melatonin--a highly potent endogenous radical scavenger and electron donor: new aspects of the oxidation chemistry of this indole accessed in vitro. Ann. NY. Acad. Sci. 1, 57-60 (1993).
- Chen MF, Chen JC, Chiu DF, Ng CJ, Shyr MH. Prostacyclin analogue (OP-2507) induces delayed ex vivo neutrophil apoptosis and attenuates reperfusion-induced hepatic microcirculatory derangement in rats. Shock. 16(6), 473-478 (2001).
- Montilla-López P, Munoz-Agueda MC, Feijóo López M, Munoz-Castaneda JR, Bujalance-Arenas I. Comparison of melatonin versus vitamin C on oxidative stress and antioxidant enzyme activity in Alzheimer's disease induced by okadaic acid in neuroblastoma cells. Eur. J. Pharmacol. 451(3), 237-243 (2002).
- Sener G, Sehirli AÖ, Ayanoglu-Dülger G. Protective effects of melatonin, vitamin E and N-acetylcysteine against acetaminophen toxicity in mice: a comparative study. J. Pineal. Res. 35(1), 61-68 (2003).
- Glantzounis GK, Salacinski HJ, Yang W, Davidson BR, Seifalian AM. The contemporary role of antioxidant therapy in attenuating liver ischemia-reperfusion injury: a review. Liver. Transpl. 11(9), 1031-1047 (2005).
- Zhu H, Jia Z, Misra H, Li YR. Oxidative stress and redox signaling mechanisms of alcoholic liver disease: updated experimental and clinical evidence. J. Dig. Dis. 13(3), 133-142 (2012).
- Cichoż-Lach H, Michalak A. Oxidative stress as a crucial factor in liver diseases. World. J. Gastroenterol. 20(25), 8082-8091 (2014).
- Eftekhari A, Azarmi Y. Involvement of oxidative stress and mitochondrial/lysosomal cross-talk in olanzapine cytotoxicity in freshly isolated rat hepatocytes. Xenobiotica. 46(4), 369-378 (2015).
- Taziki S, Sattari MR, Dastmalchi S, Eghbal MA. Cytoprotective Effects of Melatonin Against Amitriptyline-Induced Toxicity in Isolated Rat Hepatocytes. Adv. Pharm. Bull. 5(3), 329-334 (2015).
- Messner M, Huether G, Lorf T, Ramadori G, Schwörer H. Presence of melatonin in the human hepatobiliary-gastrointestinal tract. Life. Sci. 69(5), 543-551 (2001).
- Gitto E, Tan DX, Reiter RJ, Karbownik M, Manchester LC, et al. Individual and synergistic antioxidative actions of melatonin: studies with vitamin E, vitamin C, glutathione and desferrrioxamine (desferoxamine) in rat liver homogenates. J. Pharm. Pharmacol. 53(10), 1393-1401 (2001).
- Montilla P, Cruz A, Padillo FJ, Tunez I, Gascon F, et al. Melatonin versus vitamin E as protective treatment against oxidative stress after extra-hepatic bile duct ligation in rats. J. Pineal. Res. 31(2), 138-144 (2001).
- Lopez PM, Finana IT, De Agueda MC, Sanchez EC, Munoz MC, et al. Protective effect of melatonin against oxidative stress induced by ligature of extra-hepatic biliary duct in rats: comparison with the effect of S-adenosyl-l-methionine. J. Pin. Res. 28(3), 143-149 (2000).
- Cruz A, Padillo FJ, Granados J, Tunez I, Munoz MC, et al. Effect of melatonin on cholestatic oxidative stress under constant light exposure. Cell. Biochem. Funct. 21(4), 377-380 (2003).
- Padillo FJ, Cruz A, Navarrete C, Bujalance I, Briceno J, et al. Melatonin prevents oxidative stress and hepatocyte cell death induced by experimental cholestasis. Free. Radic. Res. 38(7), 697-704 (2004).
- Tunez I, Montilla P, Del Carmen Munoz M, Feijóo M, Salcedo M. Protective effect of melatonin on 3-nitropropionic acid-induced oxidative stress in synaptosomes in an animal model of Huntington's disease. J. Pin. Res. 37(4), 252-256 (2004).
- Túnez I, Muñoz Mdel C, Feijóo M, Muñoz-Castañeda JR, Bujalance I, et al. Protective melatonin effect on oxidative stress induced by okadaic acid into rat brain. J. Pineal. Res. 34(4), 265-268 (2003).
- Sewerynek E, Reiter RJ, Melchiorri D, Ortiz GG, Lewinski A. Oxidative damage in the liver induced by ischemia-reperfusion: protection by melatonin. Hepatogastroenterology. 43(10), 898-905 (1996).
- Ohta Y, Kongo M, Sasaki E, Nishida K, Ishiguro I. Therapeutic effect of melatonin on carbon tetrachloride-induced acute liver injury in rats. J. Pineal. Res. 28(2), 119-126 (2000).
- Jaworek J, Brzozowski T, Konturek SJ. Melatonin as an organoprotector in the stomach and the pancreas. J. Pineal. Res. 38(2), 73-83 (2005).
- Barlas A, Çevik H, Arbak S, Bangir D, Sener G, et al. Melatonin protects against pancreaticobiliary inflammation and associated remote organ injury in rats: role of neutrophils. J. Pineal. Res. 37(4), 267-275 (2004).
- Huang H, Yin R, Zhu J, Feng X, Wang C, et al. Exogenous melatonin ameliorates hepatic injury in a rat cardiopulmonary bypass model. Eur. J. Pharmacol. 580(3), 416-424 (2008).
- Huang H, Yin R, Zhu J, Feng X, Wang C, et al. Exogenous melatonin ameliorates hepatic injury in a rat cardiopulmonary bypass model. Eur. J. Pharmacol. 580(3), 416-424 (2008).
- Sewerynek E, Abe M, Reiter RJ, Barlow-Walden LR, Chen L, et al. Melatonin administration prevents lipopolysaccharide-induced oxidative damage in phenobarbital-treated animals. J. Cell. Biochem. 58(4), 436-444 (1995).
- Ozturk G, Coşkun S, Erbaş D, Hasanoglu E. The effect of melatonin on liver superoxide dismutase activity, serum nitrate and thyroid hormone levels. Jpn. J. Physiol. 50(1), 149-153 (2000).
- Liu F, Ng TB. Effect of pineal indoles on activities of the antioxidant defense enzymes superoxide dismutase, catalase, and glutathione reductase, and levels of reduced and oxidized glutathione in rat tissues. Biochem. Cell. Biol. 78(4), 447-453 (2000).
- Saad RA, EL-Bab MF, Shalaby AA. Attenuation of acute and chronic liver injury by melatonin in rats. J. Taibah. Uni. Sci. 7(2), 88-96 (2013).
- Cruz A, Padillo FJ, Torres E, Navarrete CM, Munoz-Castaneda JR, et al. Melatonin prevents experimental liver cirrhosis induced by thioacetamide in rats. J. Pineal. Res. 39(2), 143-150 (2005).
- Pereira-Filho G, Ferreira C, Schwengber A, Marroni C, Zettler C, et al. Role of N-acetylcysteine on fibrosis and oxidative stress in cirrhotic rats. Arq. Gastroenterol. 45(2), 156-162 (2008).
- Taziki S, Sattari MR, Eghbal MA. Mechanisms of trazodone-induced cytotoxicity and the protective effects of melatonin and/or taurine toward freshly isolated rat hepatocytes. J. Biochem. Mol. Toxicol. 27(10), 457-462 (2013).
- Eghbal MA, Taziki S, Sattari MR. Mechanisms of phenytoin-induced toxicity in freshly isolated rat hepatocytes and the protective effects of taurine and/or melatonin. J. Biochem. Mol. Toxicol. 28(3), 111-118 (2014).
- Eghbal MA, Taziki S, Sattari MR, Eghbal M, Taziki S, et al. Protective role of melatonin and taurine against carbamazepine-induced toxicity in freshly isolated rat hepatocytes. Int. J. Morphol. 1, 1081-1089 (2013).
- Gitto E, Tan DX, Reiter RJ, Karbownik M, Manchester LC, et al. Individual and synergistic antioxidative actions of melatonin: studies with vitamin E, vitamin C, glutathione and desferrioxamine (desferoxamine) in rat liver homogenates. J. Pharm. Pharmacol. 53(10), 1393-1401 (2001).
- Reiter RJ, Tan DX, Osuna C, Gitto E. Actions of melatonin in the reduction of oxidative stress. A review. J. Biomed. Sci. 7(6), 444-458 (2000).
- Martín M, Macías M, Escames G, León J, Acuña-Castroviejo D. Melatonin but not vitamins C and E maintains glutathione homeostasis in t-butyl hydroperoxide-induced mitochondrial oxidative stress. FASEB. J. 14(12), 1677-1679 (2000).
- Barnes PJ. Mediators of chronic obstructive pulmonary disease. Pharmacol. Rev. 56(4), 515-548 (2004).
- Rahman I, Adcock IM. Oxidative stress and redox regulation of lung inflammation in COPD. Eur. Respir. J. 28(1), 219-242 (2006).
- Chung KF, Marwick JA. Molecular mechanisms of oxidative stress in airways and lungs with reference to asthma and chronic obstructive pulmonary disease. Ann. N. Y. Acad. Sci. 1203(1), 85-91 (2010).
- Kirkham PA, Caramori G, Casolari P, Papi AA, Edwards M, et al. Oxidative Stress–induced Antibodies to Carbonyl-modified Protein Correlate with Severity of Chronic Obstructive Pulmonary Disease. Am. J. Res. Crit. Care. Med. 184(7), 796-802 (2011).
- Bartoli ML, Novelli F, Costa F, Malagrino L, Melosini L, et al. Malondialdehyde in exhaled breath condensate as a marker of oxidative stress in different pulmonary diseases. Mediators. Inflamm. (2011).
- Barreiro E, Peinado VI, Galdiz JB, Ferrer E, Marin-Corral J, et al. Cigarette smoke–induced oxidative stress: a role in chronic obstructive pulmonary disease skeletal muscle dysfunction. Am. J. Res. Crit. Care. Med. 182(4), 477-488 (2010).
- de Castro-Silva C, de Bruin VM, Cunha GM, Nunes DM, Medeiros CA, et al. Melatonin improves sleep and reduces nitrite in the exhaled breath condensate in cystic fibrosis--a randomized, double-blind placebo-controlled study. J .Pineal. Res. 48(1), 65-71 (2010).
- Chahbouni M, Escames G, Venegas C, Sevilla B, García JA, et al. Melatonin treatment normalizes plasma pro-inflammatory cytokines and nitrosative/oxidative stress in patients suffering from Duchenne muscular dystrophy. J .Pineal. Res. 48(3), 282-289 (2010).
- Celinski K, Konturek SJ, Konturek PC, Brzozowski T, Cichoz-Lach H, et al. Melatonin or L-tryptophan accelerates healing of gastroduodenal ulcers in patients treated with omeprazole. J. Pineal. Res. 50(4), 389-394 (2011)..
- Genovese T, Di Paola R, Mazzon E, Muia C, Caputi AP, et al. Melatonin limits lung injury in bleomycin treated mice. J. Pineal. Res. 39(2), 105-112 (2005).
- Gumral N, Naziroglu M, Ongel K, Beydilli ED, Ozguner F, et al. Antioxidant enzymes and melatonin levels in patients with bronchial asthma and chronic obstructive pulmonary disease during stable and exacerbation periods. Cell. Biochem. Funct. 27(5), 276-283 (2009).
- Taylor-Clark TE, Undem BJ. Sensing pulmonary oxidative stress by lung vagal afferents. Res. Physiol. Neurobiol. 178(3), 406-413 (2011).
- Shang Y, Xu SP, Wu Y, Jiang YX, Wu ZY, et al. Melatonin reduces acute lung injury in endotoxemic rats. Chin. Med. J. (Engl). 122(12), 1388-1393 (2009).
- Pedreira PR, Garcia-Prieto E, Parra D, Astudillo A, Diaz E, et al. Effects of melatonin in an experimental model of ventilator-induced lung injury. Am. J. Physiol. Lung. Cell. Mol. Physiol. 295(5), L820-827 (2008).
- Wu JY, Tsou MY, Chen TH, Chen SJ, Tsao CM, et al. Therapeutic effects of melatonin on peritonitis-induced septic shock with multiple organ dysfunction syndrome in rats. J. Pineal. Res. 45(1), 106-116 (2008).
- Sener G, Jahovic N, Tosun O, Atasoy BM, Yegen BC. Melatonin ameliorates ionizing radiation-induced oxidative organ damage in rats. Life. Sci. 74(5), 563-572 (2003).
- Lenz AG, Costabel U, Maier KL. Oxidized BAL fluid proteins in patients with interstitial lung diseases. Eur. Respir. J. 9(2), 307-312 (1996).
- Montuschi P, Ciabattoni G, Paredi P, Pantelidis P, du Bois RM, et al. 8-Isoprostane as a biomarker of oxidative stress in interstitial lung diseases. Am. J. Respir. Crit. Care. Med. 158(5), 1524-1527 (1998).
- Psathakis K, Papatheodorou G, Plataki M, Panagou P, Loukides S, et al. 8-Isoprostane, a marker of oxidative stress, is increased in the expired breath condensate of patients with pulmonary sarcoidosis. Chest. 125(3), 1005-1011 (2004).
- Pignone AM, Rosso AD, Fiori G, Matucci-Cerinic M, Becucci A, et al. Melatonin is a safe and effective treatment for chronic pulmonary and extrapulmonary sarcoidosis. J. Pineal. Res. 41(2), 95-100 (2006).
- Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free. Radic. Biol. Med. 49(11), 1603-1616 (2010).
- Sosa V, Moline T, Somoza R, Paciucci R, Kondoh H, et al. Oxidative stress and cancer: an overview. Ageing. Res. Rev. 12(1), 376-390 (2013).
- Mates JM, Segura JA, Alonso FJ, Marquez J. Oxidative stress in apoptosis and cancer: an update. Arch. Toxicol. 86(11), 1649-1665 (2012).
- Klaunig JE, Kamendulis LM, Hocevar BA. Oxidative stress and oxidative damage in carcinogenesis. Toxicol. Pathol. 38(1), 96-109 (2010).
- Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 160(1), 1-40 (2006).
- Lissoni P, Barni S, Mandala M, Ardizzoia A, Paolorossi F, et al. Decreased toxicity and increased efficacy of cancer chemotherapy using the pineal hormone melatonin in metastatic solid tumour patients with poor clinical status. Eur. J. Cancer. 35(12), 1688-1692 (1999).
- Lissoni P, Rovelli F, Malugani F, Bucovec R, Conti A, et al. Anti-angiogenic activity of melatonin in advanced cancer patients. Neuro. Endocrinol. Lett. 22(1), 45-47 (2001).
- Bartsch C, Bartsch H, Lippert T. The pineal gland and cancer: facts, hypotheses and perspectives. The. Cancer. J. 5(4), 194-199 (1992).
- Brzezinski A. Melatonin in humans. N. Engl. J. Med. 336(3), 186-195 (1997).
- Cos S, Fernández R, Güézmes AA, Sánchez-Barceló EJ. Influence of melatonin on invasive and metastatic properties of MCF-7 human breast cancer cells. Cancer. Res. 58(19), 4383-4390 (1998).
- Lissoni P, Rovelli F, Meregalli S, Fumagalli L, Musco F, et al. Melatonin as a new possible anti-inflammatory agent. J. Biol. Regul. Homeost. Agents. 11(4), 157-159 (1997).
- Atzpodien J, Kirchner H. Cancer, cytokines, and cytotoxic cells: interleukin-2 in the immunotherapy of human neoplasms. Klin. Wochenschr. 68(1), 1-11(1990).
- Kishimoto T. The biology of interleukin-6. Blood.74(1), 1-10 (1989).
- Lissoni P. Is there a role for melatonin in supportive care? Support. Care. Cancer. 10(2), 110-116 (2002).
- Firth BT, Christian KA, Belan I, Kennaway DJ. Melatonin rhythms in the Australian freshwater crocodile (Crocodylus johnstoni): a reptile lacking a pineal complex? J. Com. physiol. B. Biochem. Syst. Environ. Physiol. 180(1), 67-72 (2010).
- Bartsch C, Bartsch H, Lippert TH, Gupta D. Effect of the mammary carcinogen 7,12-dimethylbenz[a]anthracene on pineal melatonin biosynthesis, secretion and peripheral metabolism. Neuroendocrinology. 52(6), 538-544 (1990).
- Batcioglu K, Karagozler AA, Genc M, Celik S. Comparison of the chemopreventive potentials of melatonin and vitamin E plus selenium on 7,12-dimethylbenz[a]anthracene-induced inhibition of mouse liver antioxidant enzymes. Eur. J. Cancer. Prev. 11(1), 57-61 (2002).
- Burcham PC. Genotoxic lipid peroxidation products: their DNA damaging properties and role in formation of endogenous DNA adducts. Mutagenesis. 13(3), 287-305 (1998).
- Kasprzak KS. Oxidative DNA and protein damage in metal-induced toxicity and carcinogenesis. Free. Radic. Biol. Med. 32(10), 958-967 (2002).
- Muller H, Knudsen LB, Lynge E. Risk of testicular cancer after vasectomy: cohort study of over 73,000 men. BMJ. 309(6950), 295-299 (1994).
- Gradelet S, Le Bon A, Berges R, Suschetet M, Astorg P. Dietary carotenoids inhibit aflatoxin B1-induced liver preneoplastic foci and DNA damage in the rat: role of the modulation of aflatoxin B1 metabolism. Carcinogenesis. 19(3), 403-411 (1998).
- Meki AR, Esmail Eel-D, Hussein AA, Hassanein HM. Caspase-3 and heat shock protein-70 in rat liver treated with aflatoxin B1: effect of melatonin. Toxicon. 43(1), 93-100 (2004).
- Shen HM, Shi CY, Lee HP, Ong CN. Aflatoxin B1-induced lipid peroxidation in rat liver. Toxicol. Appl. Pharmacol. 127(1), 145-150 (1994).
- Meki AR, Abdel-Ghaffar SK, El-Gibaly I. Aflatoxin B1 induces apoptosis in rat liver: protective effect of melatonin. Neuro. Endocrinol. Lett. 22(6), 417-426 (2001).
- Meki AR, Abdel-Ghaffar SK, El-Gibaly I. Aflatoxin B1 induces apoptosis in rat liver: protective effect of melatonin. Neuro. Endocrinol. Lett. 22(6), 417-426 (2001) .
- Papaharalambus CA, Griendling KK. Basic mechanisms of oxidative stress and reactive oxygen species in cardiovascular injury. Trends. Cardiovasc. Med. 17(2), 48-54 (2007).
- Surekha RH, Srikanth BB, Jharna P, Ramachandra RV, Dayasagar RV, et al. Oxidative stress and total antioxidant status in myocardial infarction. Singapore. Med. J. 48(2), 137-142 (2007).
- Ahmadian E, Jafari S, Yari Khosroushahi A. Role of angiotensin II in stem cell therapy of cardiac disease. J. Renin. Angiotensin. Aldosterone. Syst. 16, 702-711 (2015).
- Álvarez Y, Pérez-Girón JV, Hernanz R, Briones AM, García-Redondo A, et al. Losartan reduces the increased participation of cyclooxygenase-2-derived products in vascular responses of hypertensive rats. J. Pharmacol. Exp.Ther. 321(1), 381-388 (2007).
- Berry C, Brosnan MJ, Fennell J, Hamilton CA, Dominiczak AF. Oxidative stress and vascular damage in hypertension. Curr. Opin. Nephrol. Hypertens. 10(2), 247-255 (2001).
- Nava M, Quiroz Y, Vaziri N, Rodríguez-Iturbe B. Melatonin reduces renal interstitial inflammation and improves hypertension in spontaneously hypertensive rats. Am. J. Physiology-Renal. Physiology. 284(3), F447-F454 (2003).
- Tengattini S, Reiter RJ, Tan DX, Terron MP, Rodella LF, et al. Cardiovascular diseases: protective effects of melatonin. J. Pineal. Res. 44(1), 16-25 (2008).
- Paulis L, Simko F. Blood pressure modulation and cardiovascular protection by melatonin: potential mechanisms behind. Physiol. Res. 56(6), 671 (2007).
- Reiter RJ, Tan DX, Paredes SD, Fuentes-Broto L. Beneficial effects of melatonin in cardiovascular disease. Ann. Med. 42(4), 276-285 (2010).
- Simko F, Paulis L. Melatonin as a potential antihypertensive treatment. J. Pineal. Res. 42(4), 319-322 (2007).
- Chuang JI, Chen SS, Lin MT. Melatonin decreases brain serotonin release, arterial pressure and heart rate in rats. Pharmacology. 47(2), 91-97 (1993).
- Forstermann U. Nitric oxide and oxidative stress in vascular disease. Pflugers. Arch. 459(6), 923-939 (2010).
- Tunez I, Montilla P, Del Carmen Munoz M, Feijoo M, Salcedo M. Protective effect of melatonin on 3-nitropropionic acid-induced oxidative stress in synaptosomes in an animal model of Huntington's disease. J. Pineal. Res. 37(4), 252-256 (2004).
- Cagnacci A, Arangino S, Angiolucci M, Maschio E, Melis GB. Influences of melatonin administration on the circulation of women. Am. J. Physiol. 274(2), R335-338 (1998).
- Arangino S, Cagnacci A, Angiolucci M, Anna M.B Vacca, Giorgio Longu, et al. Effects of melatonin on vascular reactivity, catecholamine levels, and blood pressure in healthy men. Am. J. Cardiol. 83(9), 1417-1419 (1999).
- Scheer FA, Van Montfrans GA, van Someren EJ, Mairuhu G, Buijs RM. Daily nighttime melatonin reduces blood pressure in male patients with essential hypertension. Hypertension. 43(2), 192-197 (2004).
- Lusardi P, Piazza E, Fogari R. Cardiovascular effects of melatonin in hypertensive patients well controlled by nifedipine: a 24-hour study. British. J. Clin. Pharmacol. 49(5), 423-427 (2000).
- Sánchez-Barceló E, Mediavilla M, Tan D, Reiter R. Clinical uses of melatonin: evaluation of human trials. Current. Med. Chem. 17(19), 2070-2095 (2010).
- Tengattini S, Reiter RJ, Tan DX, Terron MP, Rodella LF, Rezzani R. Cardiovascular diseases: protective effects of melatonin. J. Pineal. Res. 44(1), 16-25 (2008).
- Wakatsuki A, Okatani Y, Ikenoue N, Izumiya C, Kaneda C. Melatonin inhibits oxidative modification of low-density lipoprotein particles in normolipidemic post-menopausal women. J. Pineal. Res. 28(3), 136-142 (2000).
- Wakatsuki A, Okatani Y, Ikenoue N, Shinohara K, Watanabe K, Fukaya T. Melatonin protects against oxidized low-density lipoprotein-induced inhibition of nitric oxide production in human umbilical artery. J. Pineal. Res. 31(3), 281-288 (2001).
- Jisun L, Samantha G, Jianhua Z. Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling. Biochem. J. 441(2), 523-540 (2012).
- Mao G, Kraus GA, Kim I, Spurlock ME, Bailey TB, et al. Effect of a mitochondria-targeted vitamin E derivative on mitochondrial alteration and systemic oxidative stress in mice. British. J. Nutr. 106(1), 87-95 (2011).
- Majima HJ, Indo HP, Suenaga S, Matsui H, Yen HC, et al. Mitochondria as possible pharmaceutical targets for the effects of vitamin E and its homologues in oxidative stress-related diseases. Curr. Pharm. design. 17(21), 2190-2195 (2011).
- Indran IR, Hande MP, Pervaiz S. hTERT overexpression alleviates intracellular ROS production, improves mitochondrial function, and inhibits ROS-mediated apoptosis in cancer cells. Cancer. Res. 71(1), 266-276 (2011).
- Hardeland R, Tan DX, Reiter RJ. Kynuramines, metabolites of melatonin and other indoles: the resurrection of an almost forgotten class of biogenic amines. J. Pineal. Res. 47(2), 109-126 (2009).
- Espino J, Bejarano I, Ortiz Á, Lozano GM, García JF, et al. Melatonin as a potential tool against oxidative damage and apoptosis in ejaculated human spermatozoa. Fert. Ster. 94(5), 1915-1917 (2010).
- Jou MJ, Peng TI, Yu PZ, Jou SB, Reiter RJ, et al. Melatonin protects against common deletion of mitochondrial DNA-augmented mitochondrial oxidative stress and apoptosis. J. Pineal. Res. 43(4), 389-403 (2007).
- Bejarano I, Redondo PC, Espino J, Rosado JA, Paredes SD, et al. Melatonin induces mitochondrial-mediated apoptosis in human myeloid HL-60 cells. J. Pineal. Res. 46(4), 392-400 (2009).
- García-Navarro A, González-Puga C, Escames G, López LC, López A, et al. Cellular mechanisms involved in the melatonin inhibition of HT-29 human colon cancer cell proliferation in culture. J. Pineal. Res. 43(2), 195-205 (2007).
- Kim CH, Yoo YM. Melatonin induces apoptotic cell death via p53 in LNCaP cells. Korean. J. Physiol. Pharm. 14(6), 365-369 (2010).
- Trubiani O, Recchioni R, Moroni F, Pizzicannella J, Caputi S, et al. Melatonin provokes cell death in human B-lymphoma cells by mitochondrial-dependent apoptotic pathway activation. J. Pineal. Res. 39(4), 425-431 (2005).
- Danielczyk K, Dziegiel P. Receptory melatoninowe MT1 oraz ich rola w onkostatycznym dzialaniu melatoniny, MT1 melatonin receptors and their role in the oncostatic action of melatonin. Postepy. Hig. Med. Dosw. 63, 425-434 (2014).
- Cao R, Jia J, Ma X, Zhou M, Fei H. Membrane localized iridium (III) complex induces endoplasmic reticulum stress and mitochondria-mediated apoptosis in human cancer cells. J . Med. Chem. 56(9), 3636-3644 (2013).
- Bejarano I, Espino J, Marchena AM, Barriga C, Paredes SD, et al. Melatonin enhances hydrogen peroxide-induced apoptosis in human promyelocytic leukaemia HL-60 cells. Mol. Cell. Biochem. 353(1-2), 167-176 (2011).
- Uguz AC, Cig B, Espino J, Bejarano I, Naziroglu M, et al. Melatonin potentiates chemotherapy-induced cytotoxicity and apoptosis in rat pancreatic tumor cells. J. Pineal. Res. 53(1), 91-98 (2012).
- Osseni RA, Rat P, Bogdan A, Warnet J-M, Touitou Y. Evidence of prooxidant and antioxidant action of melatonin on human liver cell line HepG2. Life. sci. 68(4), 387-399 (2000).
- Albertini MC, Radogna F, Accorsi A, Uguccioni F, Paternoster L, et al. Intracellular Pro-oxidant Activity of Melatonin Deprives U937 Cells of Reduced Glutathione without Affecting Glutathione Peroxidase Activity. Ann. NY. Acad. Sci. 1091(1), 10-16 (2006).
- Wölfler A, Caluba HC, Abuja PM, Dohr G, Schauenstein K, et al. Prooxidant activity of melatonin promotes fas-induced cell death in human leukemic Jurkat cells. FEBS. lett. 502(3), 127-131 (2001).
- Ximenes VF, Pessoa AS, Padovan CZ, Abrantes DC, Gomes FH, et al. Oxidation of melatonin by AAPH-derived peroxyl radicals: Evidence of a pro-oxidant effect of melatonin. Biochimica. Et. Biophysica. Acta. (BBA)-Gen. Sub. 1790(8), 787-792 (2009).
- Radogna F, Diederich M, Ghibelli L. Melatonin: a pleiotropic molecule regulating inflammation. Biochem. Pharmacol. 80(12), 1844-1852 (2010).
- Radogna F, Sestili P, Martinelli C, Paolillo M, Paternoster L, et al. Lipoxygenase-mediated pro-radical effect of melatonin via stimulation of arachidonic acid metabolism. Toxicol. Appl. Pharmacol. 238(2), 170-177 (2009).
- Sánchez-Sánchez AM, Martín V, García-Santos G, Rodríguez-Blanco J, Casado-Zapico S, et al. Intracellular redox state as determinant for melatonin antiproliferative vs cytotoxic effects in cancer cells. Free. rad. res. 45(11), 1333-1341.
- Bonnefont-Rousselot D, Collin F. Melatonin: action as antioxidant and potential applications in human disease and aging. Toxicol. 278(1), 55-67 (2010).
- Gitto E, Romeo C, Reiter R, Impellizzeri P, Pesce S, et al. Melatonin reduces oxidative stress in surgical neonates. J. Ped. Surg. 39(2), 184-189.
- Kücükakin B, Gögenur I, Reiter RJ, Rosenberg J. Oxidative stress in relation to surgery: is there a role for the antioxidant melatonin? J. Surg. Res. 152(2), 338-347 (2009).
- Carrillo-Vico A, Lardone PJ, Naji L, Fernández-Santos JM, Martín-Lacave I, et al. Beneficial pleiotropic actions of melatonin in an experimental model of septic shock in mice: regulation of pro-/anti-inflammatory cytokine network, protection against oxidative damage and anti-apoptotic effects. J. Pineal. Res. 39(4), 400-408 (2005).
- Veneroso C, Tuñón MJ, González-Gallego J, Collado PS. Melatonin reduces cardiac inflammatory injury induced by acute exercise. J. Pineal. Res. 47(2), 184-191 (2009).
- Agil A, Reiter RJ, Jiménez-Aranda A, Ibán-Arias R, Navarro-Alarcón M, et al. Melatonin ameliorates low-grade inflammation and oxidative stress in young Zucker diabetic fatty rats. J. Pineal. Res. 54(4), 381-388 (2013).
- Tsai MC, Chen WJ, Tsai MS, Ching CH, Chuang JI. Melatonin attenuates brain contusion-induced oxidative insult, inactivation of signal transducers and activators of transcription 1, and upregulation of suppressor of cytokine signaling-3 in rats. J. Pineal. Res. 51(2), 233-245 (2011).
- Lin XJ, Mei GP, Liu J, Li YL, Zuo D, et al. Therapeutic effects of melatonin on heatstroke-induced multiple organ dysfunction syndrome in rats. J. Pineal. Res. 50(4), 436-444 (2011).
- Korkmaz A, Reiter RJ, Topal T, Manchester LC, Oter S, et al. Melatonin: an established antioxidant worthy of use in clinical trials. Mol. Med. 15(1), 43 (2009).
- Deng WG, Tang ST, Tseng HP, Wu KK. Melatonin suppresses macrophage cyclooxygenase-2 and inducible nitric oxide synthase expression by inhibiting p52 acetylation and binding. Blood. 108(2), 518-524 (2006).
- Alamili M, Bendtzen K, Lykkesfeldt J, Rosenberg J, Gögenur I. Melatonin suppresses markers of inflammation and oxidative damage in a human daytime endotoxemia model. J. Crit.Care. 29(1), e9-e13 (2014).
- Carrasco C, Marchena AM, Holguín-Arévalo MS, Gervasio Martín-Partido, Ana B. Rodríguez et al. Anti-inflammatory effects of melatonin in a rat model of caerulein-induced acute pancreatitis. Cell Biochem. Func. 31(7), 585-590 (2013).
- Tahan G, Gramignoli R, Marongiu F, Aktolga S, Cetinkaya A, et al. Melatonin Expresses Powerful Anti-inflammatory and Antioxidant Activities Resulting in Complete Improvement of Acetic-Acid-Induced Colitis in Rats. Dig. Dis. Sci. 56(3), 715-720 (2011).
- Lim H-D, Kim Y-S, Ko S-H, Yoon IJ, Cho SG, et al. Cytoprotective and anti-inflammatory effects of melatonin in hydrogen peroxide-stimulated CHON-001 human chondrocyte cell line and rabbit model of osteoarthritis via the SIRT1 pathway. J. Pineal. Res. 53(3), 225-237 (2012).
- Permpoonputtana K, Govitrapong P. The anti-inflammatory effect of melatonin on methamphetamine-induced proinflammatory mediators in human neuroblastoma dopamine sh-sy5y cell lines. Neurotox. Res. 23(2), 189-199 (2013).
- Terry PD, Villinger F, Bubenik GA, Sitaraman SV. Melatonin and ulcerative colitis: evidence, biological mechanisms, and future research. Inflam. Bow. Dis. 15(1), 134-140 (2009).
- Celinski K, Konturek SJ, Konturek PC, Brzozowski T, Cichoz-Lach H, et al. Melatonin or l-tryptophan accelerates healing of gastroduodenal ulcers in patients treated with omeprazole. J. Pineal. Res. 50(4), 389-394.
- Chen CQ, Fichna J, Bashashati M, Li YY, Storr M. Distribution, function and physiological role of melatonin in the lower gut. W. J. Gastroenterol. 17(34), 3888 (2011).
- Cichoz-Lach H, Celinski K, Konturek P, Konturek S, Slomka M. The effects of L-tryptophan and melatonin on selected biochemical parameters in patients with steatohepatitis. J. Physiol. Pharmacol. 61(5), 577 (2010).
- Forrest CM, Mackay GM, Stoy N, Stone TW, Darlington LG. Inflammatory status and kynurenine metabolism in rheumatoid arthritis treated with melatonin. British. J. Clin. Pharm. 64(4), 517-526 (2007).
- Nunnari G, Nigro L, Palermo F, Leto D, Pomerantz R, Cacopardo B. Reduction of serum melatonin levels in HIV-1-infected individuals’ parallel disease progression: correlation with serum interleukin-12 levels. Infection. 31(6), 379-382 (2003).
- Ozkan E, Yaman H, Cakir E, Deniz O, Oztosun M, et al. Plasma melatonin and urinary 6-hydroxymelatonin levels in patients with pulmonary tuberculosis. Inflammation. 35(4), 1429-1434 (2012).
- Srinivasan V, Mohamed M, Kato H. Melatonin in bacterial and viral infections with focus on sepsis: a review. Recent. Pat. Endocr. Metab. Immune. Drug. Discov. 6(1), 30-39 (2012).