Review Article - Diabetes Management (2018) Volume 8, Issue 1
A systematic review of psychological interventions for comorbid type 1 diabetes mellitus and eating disorders
- *Corresponding Author:
- Rosemary Banting
University of Bath
Department of Psychology
London, England
E-mail: rosiebanting123@gmail.com
Abstract
Type One Diabetes Mellitus (T1DM) is one of the most significantly impacting health conditions around the world and requires daily insulin administration to manage. Research has highlighted the impact on mental health but in particular there are elevated levels of eating disorders (ED) within T1DM. The current review aimed to systematically draw together the existing research that has explored the impact of psychological treatment within this population specifically focusing on adaptations made and the impact on insulin omission. A total of 95 papers were found and 10 were included in review. The papers ranged in methodology and rigour making comparisons difficult. The review concludes that an individualised intervention addressing both ED and T1DM difficulties appears to be the most effective. There is a lack of reporting on insulin omission within the literature and more research on the function of this behaviour is needed.
Introduction
Diabetes Mellitus (DM) is a chronic disease that affects insulin production within the pancreas. In type 1 diabetes (T1DM) the body is unable to produce insulin, whereas in type 2 (T2DM) the body uses insulin ineffectively [1]. T1DM is managed with insulin therapy, whereby the patient is required to administer long-acting insulin on a daily basis and administer short-acting insulin before meals in order to prevent hyper or hypoglycemia (Atkinson, Eisenbarth, & Michels, 2014). Insulin therapy requires the patient to adjust their mealtime insulin dose, based on the carbohydrate content of their meal [2]. DM is one of the most significantly impacting chronic health conditions globally [3] with numbers predicted to rise [4]. It is estimated that T1DM accounts for 10% of cases [5] and it is predicted that by 2020 there will be 24400 new cases of T1DM in those under the age of 14 years old [6]. A multifactor process has been suggested to account for this increase, and a number of theories involving early immunological development have been posited to contribute [7]. Poorly-managed T1DM can increase the risk of serious health conditions such as diabetic retinopathy, lower limb amputations, end-stage renal disease, hypoglycemia and ketoacidosis, as well as cardiovascular difficulties [1]. It carries an increased risk of mortality, which increases further if diabetes is poorly controlled [8-10].
▪ Impact on mental health
Mental health difficulties in people with T1DM are associated with a greater likelihood of poor illness management, although the causality of these difficulties has not been established and may well be reciprocal [11,12]. A recent meta-analysis found that parents of children with T1DM rated their child’s psychological distress as significantly worse than comparison groups, as did the children themselves [13]. Bernstein et al. [11] found that of 150 adolescents with T1DM 11.3% screen positively for depression, 21.3% for anxiety and 27% for disordered eating. Another meta-analysis found significant positive relationships between T1DM and anxiety symptoms and disorders [14]. A potential cause of mental health difficulties in T1DM is the demanding illness management regime, which can lead to people feeling overwhelmed and hopeless [15,16]. Recent research summarised T1DM as creating a psychological struggle between the successful demands of T1DM management compared to the drive to feel satisfaction of one’s own personal goals and ambitions [16]. Qualitative research exploring the impact of T1DM in women has found themes of hatred towards their bodies, feeling disadvantaged in not having the same opportunities as those without DM, struggling to feel in control, being vulnerable to the impact of DM, not feeling understood by others, worrying about the impact of DM on their futures and worrying about how others see them [17]. Young people with T1DM report feeling isolated amongst their peer group, experiencing a loss of spontaneity and health, feeling self-conscious, stigmatised by others and ultimately ‘different’ [18-20]. Those with T1DM need to not only find a way to fit in with their peer group, but also to navigate life transitions whilst taking increased responsibility for their health [21]. Interestingly research where the majority of young people were on insulin pumps, which have a positive impact on quality of life and self-esteem [22], found no significant differences in a range of different mental health problems when comparing adolescents with and without T1DM [23]. The impact of treatment regime on well-being may therefore represent a significant factor in the development of mental health difficulties.
▪ Eating disorders
The DSM-5 (Diagnostic and Statistical Manual of Mental Disorders) specifies a number of diagnoses under ‘Feeding and Eating Disorders’ including Anorexia Nervosa, (AN) characterised by excessive dieting and severe weight loss and Bulimia Nervosa (BN), which involves binging followed by purging to prevent weight gain [24] (see (Table 1) for a comprehensive list of feeding and eating disorders). Across EDs there are a number of core features that include an over or under control in eating behaviours, disturbance in body image and a range of behaviours to control weight which are deemed extreme [25].
| Name | Diagnostic Criteria |
|---|---|
| Pica | A. Persistent eating of nonnutritive, nonfood substances over a period of at least 1 month. B. The eating of nonnutritive, nonfood substances is inappropriate to the developmental level of the individual. C. The eating behavior is not part of a culturally supported or socially normative practice. D. If the eating behavior occurs in the context of another mental disorder (e.g., intellectual disability [intellectual developmental disorder], autism spectrum disorder, schizophrenia) or medical condition (including pregnancy), it is sufficiently severe to warrant additional clinical attention. |
| Rumination Disorder | A. Repeated regurgitation of food over a period of at least 1 month. Regurgitated food may be re-chewed, re-swallowed or spit out. B. The repeated regurgitation is not attributable to an associated gastrointestinal or other medical condition (e.g., gastroesophageal reflux, pyloric stenosis). C. The eating disturbance does not occur exclusively during the course of anorexia nervosa, bulimia nervosa, binge-eating disorder or avoidant/restrictive food intake disorder. D. If the symptoms occur in the context of another mental disorder (e.g., intellectual disability [intellectual developmental disorder] or another neurodevelopmental disorder), they are sufficiently severe to warrant additional clinical attention. |
| Avoidant/Restrictive Food Intake Disorder | A. An eating or feeding disturbance (e.g., apparent lack of interest in eating or food; avoidance based on the sensory characteristics of food; concern about aversive consequences of eating) as manifested by persistent failure to meet appropriate nutritional and/or energy needs associated with one (or more) of the following: 1. Significant weight loss (or failure to achieve expected weight gain or faltering growth in children). 2. Significant nutritional deficiency. 3. Dependence on enteral feeding or oral nutritional supplements. 4. Marked interference with psychosocial functioning. B. The disturbance is not better explained by lack of available food or by an associated culturally sanctioned practice. C. The eating disturbance does not occur exclusively during the course of anorexia nervosa or bulimia nervosa, and there is no evidence of a disturbance in the way in which one’s body weight or shape is experienced. D. The eating disturbance is not attributable to a concurrent medical condition or not better explained by another mental disorder. When the eating disturbance occurs in the context of another condition or disorder, the severity of the eating disturbance exceeds that routinely associated with the condition or disorder and warrants additional clinical attention. |
| Anorexia Nervosa | A. Restriction of energy intake relative to requirements, leading to a significantly low body weight in the context of age, sex, developmental trajectory and physical health. Significantly low weight is defined as a weight that is less than minimally normal or, for children and adolescents, less than that minimally expected. B. Intense fear of gaining weight or of becoming fat, or persistent behavior that interferes with weight gain, even though at a significantly low weight. C. Disturbance in the way in which one’s body weight or shape is experienced, undue influence of body weight or shape on self-evaluation, or persistent lack of recognition of the seriousness of the current low body weight. |
| Bulimia Nervosa | A. Recurrent episodes of binge eating. An episode of binge eating is characterized by both of the following: 1. Eating, in a discrete period of time (e.g., within any 2-hour period), an amount of food that is definitely larger than what most individuals would eat in a similar period of time under similar circumstances. 2. A sense of lack of control over eating during the episode (e.g., a feeling that one cannot stop eating or control what or how much one is eating). B. Recurrent inappropriate compensatory behaviors in order to prevent weight gain, such as self-induced vomiting; misuse of laxatives, diuretics, or other medications; fasting; or excessive exercise. C. The binge eating and inappropriate compensatory behaviors both occur, on average, at least once a week for 3 months. D. Self-evaluation is unduly influenced by body shape and weight. E. The disturbance does not occur exclusively during episodes of anorexia nervosa. |
| Binge-Eating Disorder | A. Recurrent episodes of binge eating. An episode of binge eating is characterized by both of the following: 1. Eating, in a discrete period of time (e.g., within any 2-hour period), an amount of food that is definitely larger than what most people would eat in a similar period of time under similar circumstances. 2. A sense of lack of control over eating during the episode (e.g., a feeling that one cannot stop eating or control what or how much one is eating). B. The binge-eating episodes are associated with three (or more) of the following: 1. Eating much more rapidly than normal. 2. Eating until feeling uncomfortably full. 3. Eating large amounts of food when not feeling physically hungry. 4. Eating alone because of feeling embarrassed by how much one is eating. 5. Feeling disgusted with oneself, depressed, or very guilty afterward. C. Marked distress regarding binge eating is present. D. The binge eating occurs, on average, at least once a week for 3 months. E. The binge eating is not associated with the recurrent use of inappropriate compensatory behavior as in bulimia nervosa and does not occur exclusively during the course of bulimia nervosa or anorexia nervosa. |
| Other Specified Feeding or Eating Disorder | 1. Atypical anorexia nervosa: All of the criteria for anorexia nervosa are met, except that despite significant weight loss, the individual’s weight is within or above the normal range. 2. Bulimia nervosa (of low frequency and/or limited duration): All of the criteria for bulimia nervosa are met, except that the binge eating and inappropriate compensatory behaviors occur, on average, less than once a week and/or for less than 3 months. 3. Binge-eating disorder (of low frequency and/or limited duration): All of the criteria for binge-eating disorder are met, except that the binge eating occurs, on average, less than once a week and/or for less than 3 months. 4. Purging disorder: Recurrent purging behavior to influence weight or shape (e.g., self-induced vomiting; misuse of laxatives, diuretics, or other medications) in the absence of binge eating. 5. Night eating syndrome: Recurrent episodes of night eating, as manifested by eating after awakening from sleep or by excessive food consumption after the evening meal. There is awareness and recall of the eating. The night eating is not better explained by external influences such as changes in the individual’s sleep-wake cycle or by local social norms. The night eating causes significant distress and/or impairment in functioning. The disordered pattern of eating is not better explained by binge-eating disorder or another mental disorder, including substance use, and is not attributable to another medical disorder or to an effect of medication. |
| Unspecified Feeding or Eating Disorder | This category applies to presentations in which symptoms characteristic of a feeding and eating disorder that cause clinically significant distress or impairment in social, occupational, or other important areas of functioning predominate but do not meet the full criteria for any of the disorders in the feeding and eating disorders diagnostic class. The unspecified feeding or eating disorder category is used in situations in which the clinician chooses not to specify the reason that the criteria are not met for a specific feeding and eating disorder, and includes presentations in which there is insufficient information to make a more specific diagnosis (e.g., in emergency room settings). |
Table 1. DSM-V feeding and eating disorders
▪ Treatment
The current treatment for EDs focuses on the restoration of nutrition as well as psychotherapeutic input to manage the causal and maintaining factors [26]. The initial aim is to help the client to eat regular meals and snacks, and alleviate anxiety around where the next meal may come from [27]. Emotional support and psychotherapeutic intervention is highly recommended and it is noted that nutritional input alone comes with low attrition rates [27].
▪ Comorbidity
Research has found that females with T1DM are significantly more likely to have an ED compared to women without T1DM and subclinical EDs are twice as common in women with T1DM [28,29]. Interestingly there is no significant increase in AN but there is with BN and sub-threshold ED, Eating Disorders Not Otherwise Specified (EDNOS) and Disturbed Eating Behaviour DEB.
▪ Insulin omission
Restricting insulin prevents the body from using glucose as its energy source, forcing it to use stores of fat and muscle instead [30]. This behaviour has been found to be present in 10.3% of women with T1DM [31] and in 13.3% of adolescents (data does not state gender). Insulin omission is in the DSM-V under AN and BN where it mentions those with T1DM omitting or limiting their insulin to reduce carbohydrate metabolism [24].
▪ Impact on physical health
Comorbid ED and T1DM, has been found to predict poorer glycemic control [32], resulting in higher rates of mortality and microvascular complications [33]. Those partaking in insulin omission are 8 times more likely to have poorly controlled diabetes [11] 10 and poorer physical outcomes (as noted above) [30,34-36].
▪Models of comorbidity of ED and T1DM
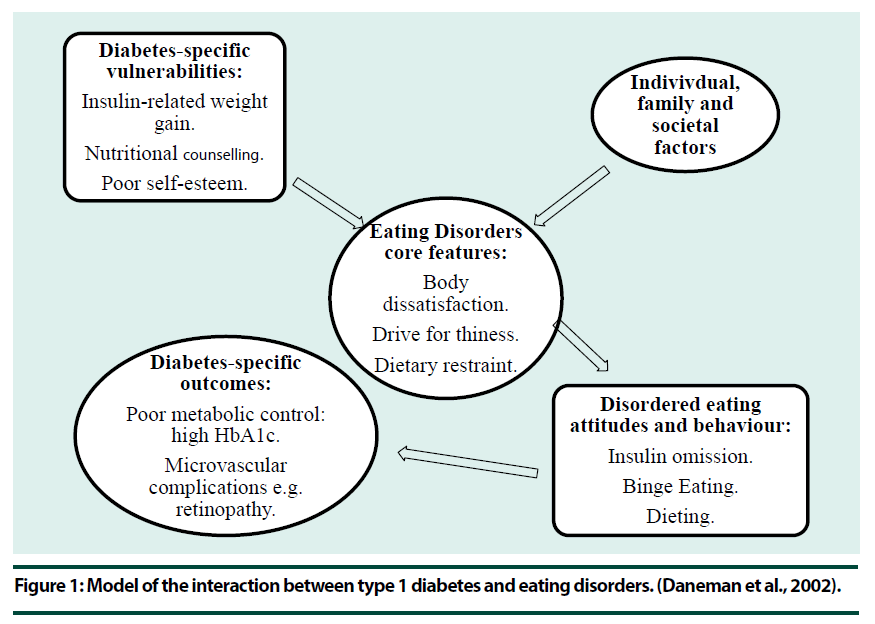
Specific risk factors for EDs include being female [25,37] living in a western culture which idolises thinness in women, body dissatisfaction, increased body weight, increased Body Mass Index (BMI), dieting, body dissatisfaction, an internalised thin ideal, negative affect including depression and low self-esteem [25,38-40] and poor family functioning [41]. A number of studies also explore the effect of biological factors such as genetics [25]. The Modified Dual Pathway model suggests three main mechanisms that heighten the chances of developing an ED in those with T1DM; food preoccupation in the form of carbohydrate counting - which is part of the treatment for T1DM, changes in weight linked to insulin use resulting in body dissatisfaction and weight gain due to the need to take on extra calories in order to prevent hypoglycemia [42]. Insulin therapy leads to weight gain which can result in subsequent emotional distress, which can initiate disordered eating behaviours such as binge eating or insulin omission [43]. Amongst girls with T1DM and comorbid ED 35% of the variance has been explained by lower self-esteem and higher BMI scores [44] Similar research with people with T1DM has found DEB is significantly predicted by weight and shape worries, higher depression rates, higher BMI scores and lower appearance-based self-worth (p <0.001) [45]. These factors accounted for 48.2% of variance, offering support for the dual pathway model Olmsted et al., Daneman et al. [46] Figure 1 and Pinhas-Hamiel et al. [47] specify that T1DM specific factors interact with individual, family and social risks to lower the threshold for an ED. The initial weight loss seen prior to a diagnosis of T1DM may be seen as positive and subsequent weight restoration and gain may have negative consequences [46]. Problematic family functioning has been found to be a risk factor for ED as well as ED and T1DM [48]. Difficulties within the family around control and autonomy may reinforce the ED behaviour as it gives the subject control and a sense of achievement [49,50] found that amongst girls with T1DM and eating disturbances self-reported more impaired mother-daughter relationships including less trust and feelings of being alienated by their mother [50].
Current Review
▪ Rationale
T1DM is a complex health condition, rising in prevalence, which requires active management on behalf of the individual. The comorbidity of T1DM and ED is a clear example of how the multifaceted interplay between mental and physical health can lead to poorer outcomes. The impact of this comorbidity on the control of diabetes and thus health outcomes is clear, however there is currently a lack of evidence or consensus on what treatment approaches to use for this specific population [51]. The management of T1DM can be seen to potentially represent a direct risk factor in the development of an ED and the use of insulin omission presents a means of losing or controlling weight in people with T1DM. Currently the NICE guidelines recommend that when working with someone with T1DM and an ED an immediate referral to psychological services should be made [51].
Aim
The aim of this review is to critically and systematically appraise the effectiveness of treatments for those with comorbid T1DM and an ED. T2DM is not included since less is known about the prevalence of T2DM and EDs [52] and T2DM is usually related to obesity [53]. Furthermore, the current review is interested specifically in insulin omission and how this is managed during treatment. Whilst insulin is always required in the management of T1DM this is not the case with T2DM [54].
The review aims to address the following questions:
What is the effectiveness of current psychological treatments for those with comorbid T1DM and an ED?
How are psychological treatments adapted to meet the specific needs of those with T1DM in terms of diabetes management and insulin omission?
What does current research into psychological treatments add to our knowledge of the clinical profiles of those with an ED and T1DM?
Methodology
▪ Search strategy
The current review follows the guidelines that are set out by the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRIMSA) [55,56]. Due to the prevalence the comorbidity of obesity and T2DM, with increased rates of both running parallel [54], obesity was included as a ‘not’ term within the search criteria to eliminate any articles that were included relating to obesity. The search terms (Table 2) were run in PubMed, PsychNet and Embase on the 17th January 2017.
| Database | Eating Disorder | Diabetes | Intervention | Not |
|---|---|---|---|---|
| Pub Med | Eating Attitude* OR Eating Behavior* OR Eating Disorder* OR Eating disorder not otherwise specified* OR Insulin Omission* OR “Diabulimia*” OR Subclinical disordered eating* | “Diabetes" OR "Diabetes Insipidus" OR "Diabetes Mellitus" | "Psychotherapy" OR "Psychotherapies" OR "Psycho Therapy" OR "Psycho therapies" OR Treatment Outcome* OR Cognitive Behavior Therap* OR Family Intervention* OR Group Intervention* OR "Psychoeducation" OR "Psycho education" OR School Based Intervention* | Obesity OR "Type 2 Diabetes" |
| PsychNet | "Eating Attitude*" OR "Eating Behavior*" OR "Eating Disorder*" OR "Eating Disorder Not Otherwise Specified" OR "Insulin Omission*" OR Diabulimia OR " subclinical disordered eating" | Diabetes OR "Diabetes Insipidus" OR "Diabetes Mellitus" | Psychotherapy OR Psychotherapies OR "Psycho Therapy" OR "Psycho therapies" OR Treatment Outcome OR "Cognitive Behavior Therap*" OR "Family Intervention*" OR "Group Intervention*" OR Psychoeducation OR "Psycho education" OR "School Based Intervention*" | Obesity OR "Type 2 Diabetes" |
| Embase | 'eating'/exp OR eating AND attitude* OR 'eating'/exp OR eating AND behavior* OR 'eating disorder*' OR 'eating'/exp OR eating AND ('disorder'/exp OR disorder) NOT otherwise AND specified* OR 'insulin omission*' OR 'diabulimia*' OR subclinical AND disordered AND eating* | 'diabetes' OR 'diabetes insipidus' OR 'diabetes mellitus' | 'psychotherapy' OR 'psychotherapies' OR 'psycho therapy' OR 'psycho therapies' OR treatment AND outcome* OR cognitive AND behavior AND therap* OR family AND intervention* OR group AND intervention* OR 'psychoeducation' OR 'psycho education' OR 'school based intervention*' | 'obesity' OR 'type 2 diabetes' |
Table 2. Search terms used for the different databases
▪ Inclusion/Exclusion criteria
All articles were assessed for inclusion according to the following criteria: (1) Participants must have a diagnosis of T1DM, (2) Reference to an eating disorder or disordered eating and (3) Must involve a psychological intervention for the ED. (4) Published after 1990, (5) Available in English.
▪ Search process
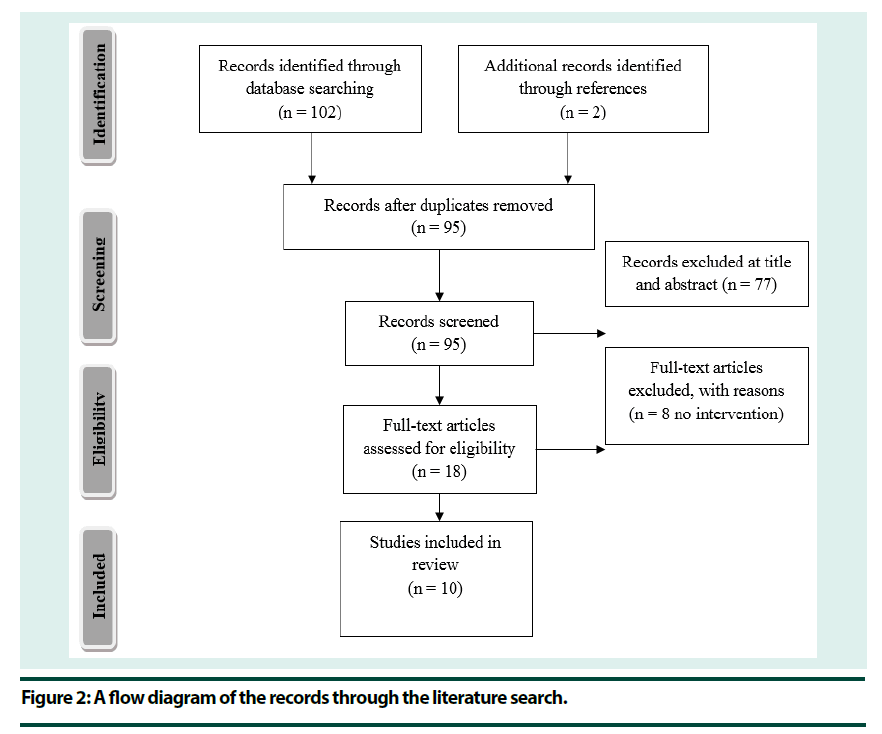
Initial searches returned 102 records with a further 2 records found through references. After duplicates were removed a total of 95 records remained. The titles and abstracts of all articles were screened for inclusion, which resulted in the exclusion of 77 records. Of the remaining 18 records a further 8 were excluded, all because they had no intervention (Figure 2). The full text articles were reviewed for inclusion by an additional researcher with 100% match.
Results
▪ Quality control
Papers were assessed using the [57] checklist designed to assess the quality of randomised and non-randomised methodology in studies exploring health interventions (see Table 3 for average scores). Random samples of papers (40%) were reviewed by a second researcher with 94% agreement. Full agreement was reached on all papers after discussion. Few papers offered a clear description of the intervention, making them difficult to replicate and reliability difficult to establish. No paper gave a list of adverse events; however a number of papers had a high average score showing that overall the majority of papers gave a clear rationale and description of their research. Overall papers demonstrated low external validity, impacting on their generalisability. Internal validity was broken down into bias and confounding. None of the papers attempted to blind the study subjects or those measuring outcomes to the interventions, which may lead to biases threatening internal validity. Only one paper made reference to adjusting analysis for the different follow up lengths and two noted compliance with the intervention, again reducing internal validity as this may have been a confounding variable. All bar three studies were rated as using appropriate statistical analysis and the majority used clearly described outcome measures. For confounding overall there was a lack of random allocation of participants which may lead to further confounding variables. The majority of papers recruited participants from the same population and over the same period of time, allowing for some control of confounding variables. Many of the studies that received no points for power did so as they were case studies. Overall there is a discrepancy in the methodological design and rigour used in each of the ten papers and there is a need for caution when interpreting the results and generalising to the wider population.
| Paper | Reporting /10 | External Validity/3 | Internal Validity Bias/7 | Internal Validity Confounding/6 | Power /5 | Total /27 |
|---|---|---|---|---|---|---|
| Alloway’01 | 8 | 0 | 3 | 3 | 3 | 17 |
| Colton’15 | 7 | 3 | 2 | 2 | 5 | 19 |
| Custal’14 | 8 | 2 | 3 | 4 | 5 | 23 |
| Dickens’14 | 7 | 3 | 4 | 3 | 0 | 17 |
| Franzese’02 | 3 | 0 | 2 | 1 | 0 | 6 |
| Olmsted’02 | 9 | 2 | 3 | 5 | 5 | 24 |
| Peveler’92 | 7 | 2 | 2 | 1 | 0 | 12 |
| Romi’08 | 3 | 0 | 1 | 1 | 0 | 5 |
| Takii’02 | 4 | 2 | 3 | 2 | 1 | 12 |
| Takii’03 | 8 | 3 | 3 | 3 | 5 | 25 |
| Average | 6.4 | 1.7 | 2.6 | 2.5 | 2.4 | 15.6 |
Table 3. Average scores given on the quality control measure
▪ Participant characteristics
BN was the most frequently included ED and was reported in five papers. Four papers included AN, three included EDNOS, two included BED, one included disturbed eating attitudes and behaviour, one included subclinical eating disorder and one made no reference to the type of ED. All participants were female and mean age ranged from 6 to 35 years old.
Interventions
A full overview of the studies included can be found in Table 4.
| Study | Design & Aim | Outcome Measures | Participants | Interventions | Key Results | Implications | Limitations |
|---|---|---|---|---|---|---|---|
| Alloway, Toth, and McGargar (2001) | Group comparison. PE compared to waitlist. Improvements to DM management, ED symptoms and general psychopathology in women with T1DM and Subclinical Disordered Eating. | BMI. EAT and EDI subscales. HbA1C. Self-care Inventory. Two questions about omitted insulin. Rosenberg Self Esteem Scale (SES). BDI. BSI. | N = 8 PE. N = 6 wait list. All female. All Sub Clinical Eating Disorder. Age treatment M 32.5 (SD 9.3) and control M 31.0 (SD 10.3). | 6 Week PE Group or Waitlist. No adaptations noted. | Outcome: Significant time effect for metabolic control (p=0.008). Significant time effect for depression (p=0.003) and general emotional distress (p=0.001) but no interaction. Group Differences: At baseline control group had significantly greater emotional distress (p=0.009). |
Psychoeducation programme no more effective than wait list control. T1DM may develop a particularly intractable form of ED requiring intensive individual work. | No control over other interventions or help seeking which may be why both changed equally. Very small sample size. |
| Colton, Olmsted, Wong, and Rodin (2015) | Cohort study. Effectiveness of intensive CBT based day hospital treatment. Characterise clinical presentation of those with T1DM seeking treatment. | BMI. Eating Disorder Examination Diagnostic Interview. HbA1C. Diabetes medical complications. Day hospital treatment; offered, provided and length recorded. | N = 95 ED and T1DM. Offered day hospital N=70. Attended day hospital N=37. Control non T1DM N=801. All Female. Age attenders M 25.6 (SD 6.4) and non-attenders M 27.1 (SD 9.1). BN N=45, AN N=14, EDNOS N=36. | Transdiagnostic, non-dieting approach, based on CBT principles. Extra DM education if needed. Adherence to normal DM clinic. | Outcome: Significantly poorer outcomes in those with comorbid ED and T1DM (p=0.002). Significant reduction pre and post day hospital for T1DM and ED treatment completers (≥4 weeks) for objective binge eating episodes (p=<0.001), self-induced vomiting (p=0.004), insulin omission (p=<0.001), and laxative abuse (p=0.03). Group Differences: Attenders had significantly more insulin omission (p=0.01). |
ED and T1DM may feel poorly understood. Behavioural change burden heavier due to DM management. Can be slow weight gain as need the adequate caloric intake and consistency with insulin dosage. Conflicting advice from care givers and health professionals. | Control not matched across demographics Retrospective chart review design. Missing DM data. Small sample who attended day hospital. High numbers chose not to engage. |
| Custal, et al (2014) | Cohort comparison study. Clinical, psychopathological and personality features between two samples of ED with T1DM and ED. Compare differences in outcomes for CBT and day hospitalisation. |
EDI-2. Weight. Binges, vomits, laxatives and diuretics per week. Perceived intensity of ED, need for treatment, impairment and worry. HbA1c. Insulin Adherence. TCI-R. Motivational stage of change. | T1DM and ED N=20, ED N=20. Age T1DM M 25.3 years (SD 8) and non-T1DM M 28.0 years (SD 8.4). All Female. BN and subthreshold N= 25%, AN N=10%, EDNOS N=55%, BED N=10%. | 16 sessions CBT for BN, BED and EDNOS, and 3 months day hospitalisation for AN. No adaptations noted. | Outcome: No significant difference between partial or full remission. T1DM and ED stopped treatment significantly earlier (p=0.034). Group differences: Vomiting and laxative abuse more prevalent in non-DM group. T1DM significantly less likely to self-harm (p<.012), have suicidal ideation (p<.030) and behaviour (p<.039). T1DM significantly lower on motivation to change, level of consciousness about their ED (d=0.63) and persistence personality trait (p=.038). |
Lower self-harm may indicate insulin omission to regulate mood rather than weight loss. Worse outcomes linked with motivation levels and personality traits. | No control group. Small sample size. Reliability of patient reporting. Possible influence of T1DM on ED measures. |
| Dickens, Haynos, Nunnemaker, Platka-Bird, and Dolores. (2014) | Cohort study. Exploring residential treatment impact on blood sugar and ED/psychological symptoms. Examine impact of duration of treatment on physical and psychological outcomes. | EDI 3 (drive for thinness, Bulimia, body dissatisfaction, eating disorder risk composite and general psychological maladjustment composite). HbA1c. | T1DM and ED N=29. All Female. Age M 22.55 (SD 9.03). 23 (79.3%) BN, 3(10.3%) AN binge eating /purging subtype, and 3 (10.3%) EDNOS. | Residential CBT. Adaptation that an Intuitive eating nutrition approach used. | Outcome: Significant improvements in HBa1c levels (p<.001). Significant positive interactions for BD (p=.020) BN (p=.009), Eating disorder risk composite (p=.012) and General Psychological Maladjustment Composite (p=.031) and duration of treatment. Significant reductions on all EDI-3 subscales with moderate effect sizes BD (p=.001), BN (p=.001), DT (p=.001), EDRC (p=.001) and GPMC (p=.001). Group Differences: Duration of treatment higher for T1DM and ED. |
Advantages for residential treatment as quicker medical stabilization, greater ease of coordinating MD treatment, and high initial treatment dose. Adequate duration needed to have impact on ED symptoms and psychological concerns. | No comparison group. Unknown still if respond differently to those without T1DM. No follow up data. |
| Franzese, Valerio, Buono, Mozzillo, Gritti, and Lucariello. (2002). | Case study. Describe the successful treatment of AN and T1DM in a child. | HbA1c. | T1DM and ED N=1. AN N=1. 6 years old. | Enteral nutrition. Intensive individual psychoanalysis. No adaptations noted. | Outcome: Accepted and maintained a normal and complete oral nutrition. HbA1c 6.9-7.5% Group Differences: None. |
Treatment complicated by management of diabetes. Symptoms of ED started after "honey moon" period. | No comparison. Aims not clear. Cannot be replicated as intervention is not clear. No outcome measures. |
| Olmsted, Daneman, Rydall, Lawson and Rodin. (2002) | RCT. Evaluate the effect of a brief PE group compared to TAU on disturbed eating attitudes and behaviour and metabolic control in adolescent girls with T1DM. | DSED – modified to include diabetes specific item (intentional insulin omission). EDI (DTS, BS, BDS). EDE including diabetes related questions. Objective binge episodes. Insulin omission. HbA1c. | Invited to screening N = 291. Attended screening N = 212. Eligible to partake N = 130. Agreed to partake N = 85. All Female. Age M 16 years (SD 2). All disturbed eating attitudes and behaviour | PE consisting of 6 weekly 90 minute sessions. TAU quarterly visits to DM clinic and adherence to meal plan. Adapted to included specific education on the relationship between ED and T1DM. DM clinic refrained from comments about eating. | Outcome: No improvements on insulin omission or HbA1c levels. Significant time effect on EDI (p<.00005) and EDE (p<.00005) for Psychoeducation Group but not TAU. Interaction effects significant for restraint and eating concern (p<.00005 and p<.0004) improvements over time in PE Group. Group Differences: Responders significantly lower HbA1c levels pre-treatment than non-responders (p<.0005). |
More relaxed attitude to eating but no reduction in binge eating or insulin omission. Brief intervention not enough to diminish risk of complications in DM. | Refusal of some subjects. Low attrition of TAU over follow up. |
| Peveler and Firburn (1992) | Case series. To explore what modifications are needed when using CBT to treat BN with DM. | EAT, EDI, and BSQ. HbA1c. SCL-90. | N=6. BN N = 6. Age range 18 - 35 years old. | CBT for bulimia nervosa (Fairburn 1981, 1985). Adapted when need on clinical grounds. Cognitive restructuring included DM. Therapist helped make insulin dose changes. | Outcome: CBT was effective for some cases however adjunctive psychological treatment may be required. Usually treatment showed an improvement in ED habits and glycemic control. Group differences: None. |
CBT may be resisted due to monitoring behaviours as already required to for DM. IPT more effective when CBT was not, but unclear if progress was due to a resolve in depression and interpersonal problems or lack of monitoring. There is a conflict between CBT modifications to eating and DM management advice. One therapist with expertise in both is preferred. | Small numbers and follow up period short. Unable to determine why IPT was successful. No control. |
| Romi and Kowen (2008) | Case study. To improve metabolic control. | None. | N=1. Age 15 years old. | Multidisciplinary co-therapy Structural-systemic family intervention. No adaptations noted. | Outcome: HbA1c levels dropped in first 3 months. Diabetes management stabilised after 6 months. Proper eating habits regained at 1 year. Good metabolic control in 5 year follow up. Group Differences: None |
Treating with parent allowed parent to be part of the team. Sick child effects the whole system, and parents are often the most significant support and have an impact on the handling of the illness. MD teamwork essential. ED specialist brought in to develop approach. | Case study. No measures. No control. No statistics. Does not mention what ED. |
| Takii, Uchigata, Komaki, Nozaki, Kawai, Nishikata, Kawai, Morioka, Iwamoto, and Kubo. (2002) | Group Comparison. BN inpatient, BN outpatient, BED inpatient and BED outpatient. Describe outpatient and integrated inpatient therapy and assess effectiveness for BN and BED. | EDI. Clinical interview for binge eating and ICB during the preceding 3 months. HbA1c. Zung SDS. Trait-anxiety scale of the STAI. | BN inpatient N=9, BN outpatient N=9, BED inpatient N=2 and BED outpatient N=8. | Outpatient counselling at first visit then return to referring physician. If HbA1c levels did not fall by 1% in 6-12 months then encouraged to undergo Integrated Inpatient Therapy (IIT). | Outcome: BN inpatients significantly lower HbA1c levels at discharge and all follow ups to 36mths. BED outpatients HbA1c significantly lower at 24, 30 and 36. BN inpatients at follow up at 36 months after discharge were significantly lower than at first visit on EDI, SDS, STAI, and frequency/amount of binge eating. BED outpatients SDS score and frequency/amount of binge eating at follow up and 36 months rom first visit with significantly lower, and the STAI tended to be lower than first (p<0.1). BN inpatients, significantly lower rate of ICB and insulin omission at follow up. Group Differences: Baseline BN in and outpatients had significantly higher HbA1c than BED outpatients. BN inpatient had most severe psychological/behavioural disturbances re ED. BN in and out were more depressed and anxious than BED outpatients |
Therapy effective for T1DM with binge eating. Diagnosis of ED at first visit helped predict responses to therapeutic intervention. Improvements shown after initial counselling session by but not BN. BN treated with IIT improved. Fits with previous study that showed that BN have more severe psychological and behavioural distortions compared to BED and may be why BED can change due to first visit counselling. | No mention of BED inpatient in comparison of group differences. No comparison between groups in examination of data. Limited reporting of statistics. |
| Takii, Uchigata, Komaki, Nozaki, Kawai, Iwamoto, and Kubo (2003) | Cohort study. Describe inpatient therapy and assess effectiveness for patients with BN. | BMI. EDI. Clinical interview for binge eating and ICB during the preceding 3 months. HbA1c. Trait-anxiety scale of the STAI. | BN N=19. Inpatient group N=9. Non-inpatient group N=10. Age in years inpatient M 23.8 (SD 5) and non-inpatient M 21.3 (SD 4). All Female. | Outpatient counselling at first visit then return to referring physician. If HbA1c levels did not fall by 1% in 6-12 months then encouraged to undergo Integrated Inpatient Therapy (IIT). | Outcome: HbA1c for inpatient levels at discharge significantly lower than at first visit (p=.0001). No difference for non-inpatient. BMI significantly higher for inpatient group at 30 and 36 months (p=.0259). Not for non-inpatient. Significant difference at first visit and follow up for inpatient on EDI (p=.0004), SDS (p=.0036), STAI trait-anxiety (p=.0100), BE frequency (p=.0003), BE calories (p=.0011), rate of patients with ICB (p=<.02) and insulin omission (p=<.05). Non inpatient showed no significant differences and STAI tended to be higher. Group Differences: Inpatient significantly greater frequency of binge eating (p=.0357). |
Counselling alone at first visit ineffective. Motivation most successful factor and complex psychological reasons may reason for initial treatment refusal. Self-monitoring initially taken on by nurse. Inflexible thinking may be stronger than in ED alone as twofold: desire to be thin and incorrect information about DM management. Hospital admission allows time to be devoted to recovery. Half relapsed to binge eating, relapse needs to be taught. | No group comparisons made and no control. Not randomised to control for variables. |
Table 4. A table giving an overview of all papers included in the review
▪ Location
Interventions were delivered in an outpatient setting in five of the papers, during a hospital admission in three of the papers and two compared the effectiveness of inpatient and outpatient treatment.
▪ Duration
Duration of interventions range from 6 weekly 90 minute sessions up to an average of 112.3 days stay in hospital.
▪Model
Two interventions were based on psychoeducation, four were on the principles of CBT, two used Integrated Inpatient Therapy (IIT), one used psychoanalysis and one looked at systemic therapy.
▪ Design
Two studies were group comparisons, four were cohort studies that involved data dredging, two were single case studies, one was a case series and one involved the random allocation of participants to groups.
Impact on ED symptoms
▪ Psychoeducation
Of the two papers that measured the effectiveness of psychoeducation on ED symptoms one found no impact [58]. The other [59] found a positive significant effect of time on the EDI (p<.00005) and EDE (p<.00005) when compared to treatment as usual (TAU), specifically for restraint and eating concern (p<.00005 and p<.0004). The opposite was found for the impact of T1DM management. In the study by Olmsted et al. those who responded to treatment had significantly lower HbA1c levels (p<.0005) than non-responders. This could be an indicator of better T1DM management, therefore these participants may be more similar to ED-only participants where psychoeducation has been found to be effective in ED symptom reduction but not in preventing ED pathology [60]. Given the complex nature of ED and T1DM it is not surprising that psychoeducation alone is not effective. Research is needed to draw an informed conclusion as it may be that psychoeducation, as part of a treatment package or in cases where diabetes is reasonably well-controlled, is helpful.
▪ Cognitive behavioural therapy (CBT)
Three of the CBT papers used data dredging [61,62] and one was a case series [63]. Of the three that used past data one compared those with and without T1DM in a day hospital group intervention [61], one compared outpatient CBT for BN, BED and ENDOS and day hospital for AN for those with and without T1DM [62] and one looked at individual/family residential treatment [64].
Colton et al. [61] found that participants with T1DM who had attended day hospital and completed treatment (≥4 weeks) showed significant reductions in binge episodes (p<0.001), vomiting (p=0.004), insulin omission (p<0.001) and laxative use (p=0.03). The data showed none of those with AN had a ‘good outcome’ and 80% had a ‘poor outcome’, - much higher than those with BN. However this comparison is not explored further statistically [61]. Those with T1DM and an ED did significantly worse when compared to those without T1DM [61]. This was not a controlled comparison of data and participants were not matched, so other confounding variables may have had a role to play. Custal et al. [62] did compare two groups and still found that those without T1DM had greater responses in terms of symptom reduction (75% vs 50%). They found no difference in outcome for ED type [62]. Dickens [64] explored the impact of residential treatment on ED symptoms for women with T1DM by combining CBT with person-centered, feminist and family approaches. Patients received two to three individual sessions a week as well as attending groups [64]. The intervention was designed to restore an awareness of hunger and satiety cues; noting that dieting may put those with T1DM at risk of an ED. Treatment focused on development of cognitive and emotional skills to manage thoughts and urges [64]. There was a significant reduction in scores across all EDI-3 subscales (p<.001) and all were positively associated with the duration of treatment [64]. The paper does not compare outcomes to women without T1DM so it is unclear if the results of those with T1DM are favourable to those without [64]. This was the only CBT study to note a significant improvement on Hba1c levels [64]. Peveler and Fairburn [63] report on six case studies where they used CBT for the treatment of T1DM and BN however three patients did not respond as expected and these are discussed in detail. In two cases initial attempts at CBT weren’t successful and alternative interventions were required, in one case Interpersonal Psychotherapy (IPT) and the other required inpatient settings, both to address low mood. In the first case subsequent CBT was helpful and advances were maintained at six month follow-up [63]. The other began to take insulin again during inpatient admission but disengage upon discharge and was referred back six months later [63]. The third case appears to have responded to CBT and there is a reduction in BN behaviours however this is not discussed further so it is unclear if any adaptations were made or what aspect of therapy was important [63]. The authors note the complexities and idiosyncratic factors associated with working with those with T1DM and ED and overall note that further thorough evaluation is needed and recommend that treatment should be offered in a “stepped-care” approach.
▪ Integrated inpatient therapy (IIT)
Two papers by the same authors based in Japan described a new intervention, IIT, which had been designed for the treatment of those with T1DM and an ED [65,66]. IIT has three main stages; recovery for the mind and body, modification of behaviours and cognitions and restoration of family relationships and is designed to address both the ED and the T1DM through removing control of both and then phasing it back in [65,66]. The earlier paper [66] looked at the effectiveness of IIT on females with T1DM and BN or BED, whilst the latter only explored the impact on BN [65]. In both papers participants were allocated into groups dependent on their ED type and then whether they attended inpatient following a one off outpatient session [65,66]. The one-off appointment is designed to relieve stress and promote self-care without a specially prescribed diet. If a reduction in HbA1c levels over the next 6-12 months was not noted then they were encouraged to receive IIT [65,66]. BN inpatients showed a significant reduction on scores on EDI, State-Trait Anxiety Inventory (STAI) and the Zung Self-Rating Depression Scale (SDS), as well as the frequency and amount of binge episodes at discharge and 36 months post discharge [65,66]. The latter paper found only two participants still met criteria for an ED at follow up, but nine outpatient participants did [65]. Those with BED who attended inpatient (n=2) are not mentioned in the results or discussion of the study. The SDS scores, and amount and frequency of binge episodes of the outpatient group was significantly lower at 26 months after their first visit [66]. In the 2003 paper the non-inpatient BN group had higher scores in the STAI at 24 month follow up but there were no differences on any other measures [65]. Takii et al. [65] found that in the inpatient group ED behaviours and HbA1c levels rose at six months but fell again and note the importance of teaching patients to recovery from relapses. They mention that the calming of the patients mind and modifying behaviour, lifting the controls little by little and coaching them to recovery from conflicts are all important parts of this process [65]. However there is no data to support this and further research would benefit from measuring this to support these claims. Of the two studies that used IIT they found a significant positive impact on HbA1c levels for BN inpatients (p<.0001) and BED outpatients (p<.05) [65,66] also found a significant positive effect of IIT for those with BN who attended inpatient that was maintained at 36 month follow up (p=.0002) whereas those who had not attended showed no difference. The two papers are based on a cohort study in which participants elected to partake in inpatient treatment or not. Whilst the later paper gives an ethical justification of the decision to not delay treatment for those who were choosing to engage due to the impact on physical health, there is no control for any extraneous variables such as motivation levels or additional therapeutic intervention sought. The study also concludes that T1DM and BN is best treated in inpatient settings and whilst both Takii et al. [65,66] papers do support inpatient effectiveness they do not compare their intervention to an active outpatient treatment.
Takii et al. [65] hypothesise that the intervention is effective as it addresses both the patient’s own vulnerabilities and incorrect information about T1DM via education and CBT. They note the importance of therapist control and the difficulties in doing so in outpatient settings. Further research is needed looking at the effectiveness of the treatment in outpatient to explore the importance of having the necessity of delivering the intervention in inpatient settings.
▪ Psychoanalytic
One case study notes an intervention with a 6 year old with T1DM and AN [67]. Enteral nutrition was delivered to the child for six months; however there is no description of this. The child received intensive psychoanalysis leading to the expression of obsessive-phobic symptoms [68]. The development of AN occurred after a time in T1DM called the “honeymoon” period1 when greater control and more insulin is required. The authors hypothesise that the AN developed due to these changes, combined with starting school. The overall quality of the paper is low, particularly due to a lack of reported data collection or analysis. The authors’ claim that the AN ‘completely-disappeared’ is not supported by any evidence. Exploring the onset of an ED in context of T1DM in young children is vastly under researched. It is important for future research to expand on this to enhance understanding of the development subsequent prevention. To explore the benefits of a psychoanalytic approach future research will need to be done with stronger methodological rigor.
▪ Systemic
Romi and Kowen [69] report the treatment of a 15 year old with T1DM who met the criteria for an ED according to the DSM-IV, though the details of which ED are not given. Concerns about family difficulties led to a structural-systemic family intervention to aid the patient to leave the “triangular relationship with her feuding parents” (page 6) [69]. The intervention included co-therapists and in four three month cycles the intervention focused on nutrition, the mother and daughter’s views of coping with eating habits and chronic disease, difficulties including family, self, eating and the environment and finally the internalising of eating habits and person difficulties and interactions [69]. After a year eating habits were ‘normal’ and physical appearance had improved however no outcome data is presented to support this claim. As no ED type is given the results cannot be replicated or applied to other clinical work. The study concluded that being able to involve parents helped them to become part of her diabetes team [69]. In the psychoeducation paper Olmsted et al. [59] note that parents and daughters were given the same content but in different groups to allow for privacy, Takii et al. [65,66] simply note that they include a section on family relationships and Dickens [64] notes family therapy sessions were offered once or twice a month. Unfortunately there is no further mention of these aspects in either paper. Given the vast attention to family therapy interventions given in ED literature [70] there is a lack of research exploring such approaches within the T1DM population and it will be important for research to further explore this.
The “honeymoon period” is a time where following the introduction of insulin therapy for newly-diagnosed individuals there is a restoration of cell functioning (Alzaman, Siegel, & Schaefer, 2014). There is then a fall in the need for external insulin in order to maintain good metabolic control (Alzaman et al.,) and during this time there is less of an emphasis on diet and T1DM management (Franzese et al.,).
Insulin Omission
▪ Outcome
Insulin omission is not consistently measured as a variable. CBT was effective in the reduction of insulin omission in one paper [61]. In one case presented by Peveler and Fairburn [63] CBT had no impact on insulin omission and in another whilst insulin omission is mentioned in the case presentation is not reported in the results. There was no impact in either psychoeducation study with both concluding that a brief psychoeducation programme may not be enough [58,59]. Both IIT papers found a significant reduction in insulin omission at follow up for their BN inpatient group [65,66] which led to the improvements of glycemic control [65]. Franzese et al. [67] note that when parents administer insulin omission does not happen in young people. It may be plausible that the positive impact found in the two IIT papers is partly because members of staff are initially responsible for the patient’s insulin adherence and the intervention addresses T1DM management [65]. The paper does not offer much information on the intervention related to T1DM management; however it could be hypothesised that working with both the ED and T1DM together allows for the subsequent reduction in insulin omission. Further research detailing this aspect of IIT, as well as more research exploring the impact of interventions on insulin omission is needed in order to better understand this behaviour.
▪ Function
One paper operationalised insulin omission, defining it as an inappropriate compensatory behaviour (ICB) which requires omission of at least one in four injections or the whole insulin dose [65]. Both Takii et al. [65] and Custal et al. [62] attribute the function of insulin omission to weight control, however neither describe how this conclusion was drawn. The only paper to consider the function of insulin omission is Custal et al. [62] who note that those with T1DM reported significantly less self-injurious behaviours (SIB) and suicidal ideation and conclude that insulin omission may be linked to emotion regulation rather than weight loss. The use of vomiting and laxatives were the only behaviours more established in the non-T1DM group [62] which could be used to argue that insulin omission is in fact a behaviour linked to weight loss; this is if purging is seen as a weight loss behaviour and not as a way to regulate emotion. Within the ED literature it has been proposed that the function of ED behaviour can be seen as the result of poor emotion regulation [71,72] reports that a number of studies have advocated for the idea that purging and binging behaviours increase SIB. Similar research has also been found that whilst purging behaviours are not significantly associated with a presence or absence of SIB in the ED population (p=.25) they may be used to manage emotion dysregulation [73]. Colton et al. [49] propose that insulin omission may be a maladaptive way of avoiding T1DM and that to engage in T1DM self-care anger and grief related to diagnosis may first need addressing [49]. However they and no other study in the review state whether they worked with participants to come to terms with their diagnosis. Research into risk factors of insulin omission found that, amongst other things, inference of T1DM on daily living was a significant predictor, whilst fear of weight gain was not [74,75]. The authors suggest that addressing pain and embarrassment, which were also associated with insulin omission, may reduce the emotional burden and enhance adherence. As noted by Peyrot et al. [75] Custal et al. [62], it will be important for those working with ED and T1DM to assess the function of insulin omission prior to treatment; assumptions that it is automatically linked to an ED should not be made.
▪ Clinical profile
There appears to be a difference in the clinical profile of those who attended treatment and respond to treatment and those who do not. One paper notes that those who did attend day hospital had significantly greater insulin omission and had a lower BMI than those who did not [61]. Takii et al. [65] found that those who attended inpatient treatment had significantly more binge eating episodes (p=.0357) and higher rates of psychological difficulties and mood disorders [65]. They hypothesise that higher attendance to treatment amongst this group may be due to a greater awareness of their distress and the impact of this, which may also be true for the participants in the Colton et al. [61] paper. This may highlight the need for early interventions. Colton et al. [61] experienced low take-up rates to treatment and Custal et al. [62] found those with T1DM and ED terminated treatment significantly sooner than those without. It is hypothesised that this may be due to the increased pressures from health professionals coupled with a low readiness to change or concerns that their needs may not be met by ED services [61]. Custal et al. [62] found those with T1DM and ED had lower motivation and persistence which may support this hypothesis and account for high drop outs, as low persistence is linked to high frustration. Other papers in the review did not include dropout rate comparisons so conclusions on this cannot be taken further. The clinical profile for the different subtypes of ED appears to differ and Takii et al. [66] found patients with BN were more depressed and anxious when compared to the BED patients and had greater HbA1c levels. This is consistent with their previous findings [76] and they conclude this account for the lack of improvement without IIT in the BN patients. One study that compared ED subtypes found no differences in responses to treatment or dropout rates [62], whereas another found high droprates specifically in those with AN [61]. From the limited studies in this review it is unclear if ED subtype has an impact on treatment outcomes and further research is needed.
Adaptations to Eating Disorder Treatment
Some studies do not report on any adaptations made [62,67,69] or do not report the interventions delivered to non-T1DM patients so adaptations made are not clear [64-66]. One mentions that extra T1DM education was given if needed but does not clarify the content of this or if anyone requested it [61]. The two psychoeducation-based interventions both note adaptations with both adding extra information on T1DM [58,59]. Some studies note the need for interventions to be run by those with expertise in both T1DM and ED [58,59,63,69]. This is in order to help monitor and adjust insulin doses through treatment, as well as using cognitive restructuring with T1DM related fears [63]. It is also deemed helpful to have a link between ED and T1DM teams that may be giving conflicting advice. The ED team may be advising that all foods are incorporated into the diet whereas the T1DM clinicians may be encouraging the avoidance of certain food, such as carbohydrates [61,63]. Dietary restriction may actually put people with T1DM at risk of developing an ED [63,64]. A number of studies note that their intervention is aimed at helping participants respond to their own feelings of hunger and satiety [59,64-66]. Dickens et al. [64] note that to achieve this participant were required to deliver half of their insulin before and half after they had eaten and meals were eaten under the supervision of staff members. The burden of behavioural change is noted in that the change required by those with T1DM is greater than those without [61] and Peveler and Fairburn [63] note their difficulties in getting one of their cases to monitor their eating and T1DM habits. This is addressed in IIT and by Dickens [64] through residential interventions. Takii [65] notes the similarities of IIT with CBT but that it has been modified so that the therapist can have a more direct impact on the behaviour via intensive observation and therapist control, which takes away the difficulties of participants monitoring their behaviours. Once the patient is able to stick to an agreed amount of food and insulin they move to phased removal of control by the hospital staff which is extended to the family home. Similar to this Dickens [64] notes that patients underwent daily medical monitoring of their T1DM management and that food was eaten under observation. Custal et al. [62] and Colton et al. [61] both used CBT in day hospital treatment but neither note if there was a similar level of observation therefore they can’t add any support. There is a need for further scientifically robust research exploring the impact of day or residential treatment compared to outpatient.
Overall, little adaptations have been made, with the most popular being to add extra education about T1DM to the pre-existing ED treatment. The main conclusion drawn from the papers is that those delivering the intervention should ideally be knowledgeable and trained in the treatment of both ED and T1DM management [63,69]. Dickens [64] notes that the duration of treatment offered should be addressed, with a longer treatment offering greater impact. Papers note the need for future research to explore adaptations to treatment to address T1DM specific aspects [59,61,62].
Discussion
The current review aimed to explore the effectiveness of psychological treatments for those with T1DM and an ED, recognising the high prevalence rates of this dual-diagnosis and the impact on physical and psychological well-being. The review aimed to highlight adaptions made to treatment, particularly in relation to insulin omission and to synthesise results that add to the current knowledge of the clinical profiles of those with T1DM and an ED. Overall there is mixed support for the effectiveness of current interventions for those with T1DM and EDs. Psychoeducation was effective in only one of two studies and within that study those with T1DM did worse than those without. CBT seemed to be helpful in all four studies, but there were high dropout rates. IIT and one CBT paper used a more integrative approach, addressing both the DM and the ED, which had positive effects on DM management and ED behaviours. Systemic aspects of interventions were unfortunately limited and not well addressed in results or discussions. Both the systemic and psychoanalytic paper lack outcome measures and statistical analysis. Considering the vast amount of research into systemic approaches within the ED literature and the mention of systemic factors in the development and maintenance models of ED within T1DM research may wish to focus here. Group interventions did not appear to be beneficial which maybe because they are entering a treatment programme where they have an extra diagnosis [61,77] notes in group psychotherapy interactions between group members is the vehicle for change. It could be hypothesised that those with comorbid T1DM and ED, feel they do not fit within the group and have a sense of difference, preventing learning from other’s experiences. This coupled with extra demands due to illness management may result in those with T1DM requiring an idiosyncratic intervention. Future research should consider group processes and adhesion, as well as comparing groups for T1DM and ED alone with mixed participant groups. The adaptations made to interventions are not always clear as some papers do not state how they deviate from those delivered to people with ED alone. The main adaptation made to current ED treatments appears to be the inclusion of T1DM-specific information. Studies where control was initially taken away as part of the intervention and control was gradually brought back appear to have been the most effective. This shows us that allowing time to therapeutically address T1DM difficulties may be an important step in behaviour change. Those working with people who engage in insulin omission may want to consider this as a treatment option. However further outcomebased research is needed. Insulin omission is not clearly defined across papers. The majority present it as part of ED pathology, however the function of the behaviour is only explored in one paper [62]. Whilst there is evidence that insulin omission may be linked to weight loss, this paper also presents the idea that insulin omission may be a way to regulate emotion akin to other SIB. Further research is again needed to understand the function of insulin omission as this has treatment implications and clinicians would benefit from completing thorough functional assessments. It is unclear whether one particular subtype of ED responds better to treatment than another. However it appears that when someone is in treatment, higher than expected drop-out rates may be caused by the added pressure of managing T1DM and ED treatment, especially when the treatment has not been adapted to take into account the extra stresses faced by those with T1DM. Those who attended treatment were experiencing more severe symptoms than those who did not. Within the ED literature the importance of early intervention is noted [78] and its effectiveness is beginning to be explored [79]. Research may wish to focus on early intervention approaches with those with T1DM and ED. As noted in a number of the papers, those working with people with T1DM and ED should be knowledgeable in the management of both conditions. This should prevent conflicting information being given to patients. Similar to the Modified Dual Pathway Model [80] studies noted that the dieting advice given by DM clinic teams may be a risk factor in the development of BN. In addition, the therapist can aid in the changing of insulin regimes. In practice this may mean having a specialist of both diagnoses sitting within an ED team and T1DM team. Particularly within the T1DM this will be important in order to identify those who may be at risk of developing an ED. The role of the ED and T1DM specialist within the T1DM may be one of early intervention support to those at risk to stop them progressing and needing further input. Limitations of the current review include the small amount of eligible papers and inability to conduct meta-analysis due to methodological diversity. Additionally the variation in methodology makes comparison across papers difficult, particularly as there are omissions in data reporting. Future research would benefit from using more methodological sound designs, such as randomised controlled trials, to account for confounding variables. However the papers do show that a variety of approaches are being trailed with those with an ED and T1DM and that there is international recognition and interest in this area. In conclusion, results from this review suggest that the treatment of those with an ED and T1DM should aim to address both difficulties in using a person-centered approach. There are many additional stresses and difficulties faced by those with T1DM which may impact on their mental health and thus it can be argued that this makes them a heterogeneous and complex population. Initial research is promising, but further, more methodologically robust studies are needed to address the growing need for a clear and specialised treatment pathway. However in terms of a specialist pathway the review suggests that the involvement of psychoeducation, individual and group interventions may all play a role within the context of ED and T1DM.
References
- WHO, World Health Organization. Global Report on Diabetes. Retrieved from France. (2016).
- Atkinson M, Eisenbarth G, Michels A. Type 1 diabetes. Lancet. 383(9911), 69–82 (2014).
- Shaw J, Sicree R, Zimmet P. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes. Res. Clin. Pract. 87(1), 4–14 (2010).
- Guariguata L, Whiting D, Hambleton I et al. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes. Res. Clin. Pract.103(2), 137–149 (2014).
- Team D. D. P. Making Every Young Person with Diabetes Matter: Report of the Children and Young People with Diabetes Working Group: Department of Health. (2007).
- Patterson C, Dahlquist G, Gyürüs E et al. Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–20: a multicentre prospective registration study. Lancet. 373(9680), 2027–2033 (2009).
- Egro F. Why is type 1 diabetes increasing? J. Mol. Endocrinol. 51(1), R1–13 (2013).
- Lind M, Svensson A, Kosiborod M et al. Glycemic Control and Excess Mortality in Type 1 Diabetes. N. Engl. J. Med. 371(21), 1972–1982 (2014).
- Livingstone S, Levin D, Looker H et al. Estimated life expectancy in a Scottish cohort with type 1 diabetes, 2008-2010. JAMA. 313(1), 37–44 (2015).
- Patterson C, Guariguata L, Dahlquist G et al. Diabetes in the young – a global view and worldwide estimates of numbers of children with type 1 diabetes. Diabetes. Res. Clin. Pract. 103(2), 161–175 (2014).
- Bernstein C, Stockwellb M, Gallagher M et al. Mental Health Issues in Adolescents and Young Adults With Type 1 Diabetes. Clin. Pediatr. 52(1), 10–15 (2013).
- Plener P, Molz E, Berger G et al. Depression, metabolic control, and antidepressant medication in young patients with type 1 diabetes. Pediatr. Diabetes. 16(1), 58–66 (2015).
- Reynolds S, ClarkeS, Smith H et al. Randomised controlled trial of parent- enhanced CBT compared with individual CBT for Obsessive Compulsive Disorder in young people. J. Consult. Clin. Psychol. 81(6), 1021–1026 (2013).
- Smith K, Beland M, Clyde M et al. Association of diabetes with anxiety: A systematic review and meta- analysis. J. Psychosom. Res. 74(2), 89–99 (2013).
- Polonsky W. Emotional and quality-of- life aspects of diabetes management. Curr. Diab. Rep. 2(2), 153–159 (2002).
- Watts S, Hara L, Trigg R. Living with Type 1 diabetes: A by- person qualitative exploration. Psychol. Health. 25(4), 491-507 (2010).
- Kay C, Davies J, Gamsu D et al. An Exploration of the Experiences of Young Women Living with Type 1 Diabetes. J. Health. Psychol. 14(2), 242–250 (2009).
- Balfe M, Doyle F, Smith D et al. What’s distressing about having type 1 diabetes? A qualitative study of young adults’ perspectives. BMC. Endocr. Disord. 13(1), 25 (2013).
- Kime N. Join us on our journey; Exploring the experiences of children and young people with type 1 diabetes and their parents. 31(1), (2014).
- Marshall M, Carter B, Rose K et al. Living with type 1 diabetes: perceptions of children and their parents. J. Clin. Nurs. 18(12), 1703–1710 (2009).
- Monaghan M, HelgesonV, Wiebe D. Type 1 Diabetes in Young Adulthood. Curr. Diabetes. Rev. 11(4), 239–250 (2015).
- Ghazanfar H, Rizvi S, Khurram et al. A Impact of insulin pump on quality of life of diabetic patients. Indian. J. Endocrinol. Metab. 20(4), 506-511 (2016).
- Sivertsen B, Petrie K, Wilhelmsen-Langeland A et al. Mental Health in Adolescents with Type 1 Diabetes: Results from a Large Population- based Study. BMC. Endocr. Disord. 63, A627–A627 (2014).
- APA, American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders : DSM-5 (Fifth edition. ed.). Arlington, Va.: Arlington, Va. : American Psychiatric Publishing.
- Striegel-Moore R, Bulik C. Risk Factors for Eating Disorders. Am. Psychol. 62(3), 181–198 (2007).
- Treasure J. Eating disorders. Medicine. 44(11), 672–678 (2016).
- Mairs R, Nicholls D. Assessment and treatment of eating disorders in children and adolescents. Arch. Dis. Child. 101(12), 1168–1175 (2016).
- Jones J, Lawson M, Daneman D et al. Eating disorders in adolescent females with and without type 1 diabetes: cross sectional study. BMJ. 320(7249), 1563 (2000).
- Mannucci E, Rotella F, Ricca V et al. Eating disorders in patients with Type 1 diabetes: A meta- analysis. J. Endocrinol. Invest. 28(7), 417–419 (2005).
- Goebel-Fabbri A. Disturbed eating behaviors and eating disorders in type 1 diabetes: Clinical significance and treatment recommendations. Curr. Diab. Rep. 9(2), 133–139 (2009).
- Neumark-Sztainer D, Patterson J, Mellin A et al. Weight control practices and disordered eating behaviors among adolescent females and males with type 1 diabetes - Associations with sociodemographics, weight concerns, familial factors, and metabolic outcomes. Diabetes. Care. 25(8), 1289–1296 (2002).
- Young V, Eiser C, Johnson B et al. Eating problems in adolescents with Type 1 diabetes: a systematic review with meta-analysis. Diabet. Med. 30(2), 189–198 (2012).
- Peveler R, Bryden K, Neil H et al. The relationship of disordered eating habits and attitudes to clinical outcomes in young adult females with type 1 diabetes. Diabetes. Care. 28(1), (2005).
- Hall L (2012) Eating Disorders and Diabetes. Retrieved from http://www.eatingdisordersonline.com/articles/eating-disorders/eating-disorders-and-diabetes
- Hasken J, Kresl L, Nydegger T et al. Diabulimia and the Role of School Health Personnel. J. Sch. Health. 80(10), 465–69 (2010).
- Takii M, Uchigata Y, Tokunaga S et al. The duration of severe insulin omission is the factor most closely associated with the microvascular complications of Type 1 diabetic females with clinical eating disorders. Int. J. Eat. Disord. 41(3), 259–264 (2008).
- Davey G. Psychopathology : research, assessment and treatment in clinical psychology (2nd ed.). Chichester: Chichester. Wiley (2014).
- Investigators M. Risk factors for the onset of eating disorders in adolescent girls: Results of the McKnight longitudinal risk factor study. Am. J. Psychiatry. 160(2), 248–254 (2003).
- Jackson T, Chen H. Risk Factors for Disordered Eating During Early and Middle Adolescence: A Two Year Longitudinal Study of Mainland Chinese Boys and Girls. J. Abnorm. Child. Psychol. 42(5), 791–803 (2014).
- Rodgers R. A Biopsychosocial Model of Body Image Concerns and Disordered Eating in Early Adolescent Girls. Journal of Youth & Adolescence, 43(5), 814–824. (2014).
- Holtom-Viesel A, Allan S. A systematic review of the literature on family functioning across all eating disorder diagnoses in comparison to control families. Clin. Psychol. Rev. 34(1), 29–43 (2014).
- Peterson C. Topical Review: A Comprehensive Risk Model for Disordered Eating in Youth With Type 1 Diabetes. J. Pediatr. Psychol. 40(4), 385–391 (2015).
- Yasuhara D, Inui A. Pathogenesis and treatment of eating disorders associated with type 1 diabetes mellitus. Nat. Clin. Pract. Endocrinol. Metab. 3(9), 612–613 (2007).
- Colton P, Olmsted M, Daneman D et al. Natural history and predictors of disturbed eating behaviour in girls with Type 1 diabetes. Diabet. Med. 24(4), 424–429 (2007).
- Olmsted M, Colton P, Daneman D et al. Prediction of the onset of disturbed eating behavior in adolescent girls with type 1 diabetes. Diabetes. Care. 31(10), 1978–1982 (2008).
- Daneman D, Rodin G, Jones J et al. Eating Disorders in Adolescent Girls and Young Adult Women With Type 1 Diabetes. Diabetes. Spectr. 15(2), 83–105 (2002).
- Pinhas-Hamiel O, Hamiel U, Levy-Shraga Y. Eating disorders in adolescents with type 1 diabetes: Challenges in diagnosis and treatment. World. J. Diabetes. 6(3), 517–526 (2015).
- Rodin G, Olmsted M, Rydall A et al. Eating disorders in young women with type 1 diabetes mellitus. J. Psychosom. Res. 53(4), 943–949 (2002).
- Colton P, Olmsted M, Daneman D et al. Eating Disorders in Girls and Women With Type 1 Diabetes: A Longitudinal Study of Prevalence, Onset, Remission, and Recurrence. Diabetes. Care. 38(7), 1212–1217 (2015).
- Maharaj S, Rodin G, Connolly J et al. Eating Problems and the Observed Quality of Mother–Daughter Interactions Among Girls With Type 1 Diabetes. J. Consult. Clin. Psychol. 69(6), 950–958 (2001).
- Bernstein Allan A. Understanding poor outcomes in women with type 1 diabetes and eating disorders. J. Diabetes. Nurs. 19(3), 99–103 (2015).
- García-Mayor R, García-Soidán F. Eating disorders in type 2 diabetic people: Brief Review. Diabetes. Metab. Syndr. 11(3),221–224 (2017).
- Diabetes U. Diabetes: facts and stats. Diabetes UK, London (2014).
- Nyenwe E, Jerkins T, Umpierrez G et al. Management of type 2 diabetes: evolving strategies for the treatment of patients with type 2 diabetes. Metabolism. 60(1), 1–23 (2011).
- Moher D, Liberati A, Tetzlaff J et al. Preferred reporting items for systematic reviews and meta- analyses: the PRISMA statement. Ann. Intern. Med. 151(4),264-269 (2009).
- Shamseer L, Moher D, Clarke M et al. Preferred reporting items for systematic review and meta- analysis protocols (PRISMA- P): Elaboration and explanation. BMJ. 349 (2015).
- Downs S, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non- randomised studies of health care interventions. J. Epidemiol. Community. Health. 52(6), 377–384 (1998).
- Alloway S, Toth E, McCargar L. Effectiveness of a group psychoeducation program for the treatment of subclinical disordered eating in women with type 1 diabetes. Can. J. Diet. Pract. Res. 62(4), 188–192 (2001).
- Olmsted M, Daneman D, Rydall A et al. The effects of psychoeducation on disturbed eating attitudes and behavior in young women with type 1 diabetes mellitus. Int. J. Eat. Disord. 32(2), 230–239 (2002).
- Stice E, Shaw H. Eating Disorder Prevention Programs: A Meta- Analytic Review. Psychol. Bull. 130(2), 206–227 (2004).
- Colton P, Olmsted M, Wong H et al. Eating Disorders in Individuals with Type 1 Diabetes: Case Series and day Hospital Treatment Outcome. Eur. Eat. Disord. Rev. 23(4), 312-317 (2015).
- Custal N, Arcelus J, Agüera Z et al. Treatment outcome of patients with comorbid type 1 diabetes and eating disorders. BMC. Psychiatry. 14, 140–140 (2014).
- Peveler R, Fairburn C. The treatment of bulimia nervosa in patients with diabetes mellitus. Int. J. Eat. Disord. 11(1), 45–53 (1992).
- Dickens Y. Multidisciplinary Residential Treatment of Type 1 Diabetes Mellitus and Co- Occurring Eating Disorders. Eat. Disord. 23(2), 134–144 (2015).
- Takii M, Uchigat Y, Komaki G et al. An integrated inpatient therapy for type 1 diabetic females with bulimia nervosa: A 3- year follow- up study. Journal of Psychosomatic Research, 55(4), 349–356 (2003).
- Takii M, Uchigata Y, Komaki G et al. A cognitive/ behavioral approach to type 1 diabetic females with recurrent binge eating: a 3- year follow- up study. International Congress Series 1241, 291–296 (2002).
- Franzese A, Valerio G, Buono P et al. Comorbidity of type 1 diabetes and anorexia nervosa in a 6- year- old girl. Diabetes. Care. 25(4), 800 (2002).
- Alzaman N, Siegel R, Schaefer E. Prolonged Honeymoon Period in Type 1 Diabetic Patient with Low Carbohydrate Intake. In Diabetes Pathophysiology and Treatment; Glucose Metabolism (pp. SUN-0974-SUN-0974): Endocrine Society (2014).
- Romi T, Kowen G. Multidisciplinary Family- Based Intervention in the Treatment of a Diabetic Adolescent with an Eating Disorder. J. Family. Psychotherapy. 17(1), 21–36 (2006).
- Murray S, Grange D. Family Therapy for Adolescent Eating Disorders: An Update. Curr. Psychiatry. Rep. 16(5), 1–7 (2014).
- Danner U, Sternheim L, Evers C. The importance of distinguishing between the different eating disorders (sub)types when assessing emotion regulation strategies. Psychiatry. Res. 215(3), 727–732 (2014).
- Gonçalves S, Machado B, Silva C et al. The Moderating Role of Purging Behaviour in the Relationship Between Sexual/ Physical Abuse and Nonsuicidal Self Injury in Eating Disorder Patients. Eur. Eat. Disord. Rev. 24(2), 164–168 (2016).
- Vieira A, Ramalho S, Brandão I et al. Adversity, emotion regulation, and non- suicidal self- injury in eating disorders. Eat. Disord. 24(5),440–452 (2016).
- Farsaei S, Radfar M, Heydari Z et al. Insulin adherence in patients with diabetes: Risk factors for injection omission. Prim. Care. Diabetes. 8(4), 338–345 (2014).
- Peyrot M, Rubin R, Kruger D et al. Correlates of insulin injection omission. Diabetes. Care. 33(2), 240–245 (2010).
- Takii M, Komaki G, Uchigata Y et al. Differences between bulimia nervosa and binge- eating disorder in females with type 1 diabetes: The important role of insulin omission. J. Psychosom. Res. 47(3), 221–231(1999).
- Whitfield G. Group cognitive- behavioural therapy for anxiety and depression. Adv. Psychiatr. Treat. 16(3), 219–227(2010).
- Treasure J, Russell G. The case for early intervention in anorexia nervosa: theoretical exploration of maintaining factors. Br. J. Psychiatry.199(1), 5–7 (2011).
- Pinhas L, Wong J, Woodside D. Early Intervention in Eating Disorders. Early Intervention in Psychiatry: EI of Nearly Everything for Better Mental Health, 288 (2014).
- Stice E. A Prospective Test of the Dual- Pathway Model of Bulimic Pathology: Mediating Effects of Dieting and Negative Affect. J. Abnorm. Psychol. 110(1), 124–135 (2001).