Device Evaluations - Interventional Cardiology (2014) Volume 6, Issue 2
Ablation of typical atrial flutter using a novel non-fluoroscopic electromagnetic catheter tracking system
- Corresponding Author:
- Philipp
Sommer
University of Leipzig, Heart Center,
Department of Electrophysiology,
Struempellstrasse 39, 04289 Leipzig, Germany
Tel: +49 341 865 1413
Fax: +49 341 865 1460
E-mail: philipp.sommer@herzzentrum-leipzig.de
Abstract
The term ‘atrial flutter’ (AFL) refers to a heterogeneous group of atrial arrhythmias defined mechanistically by a macro-reentrant circuit around fixed anatomical barriers or functional blocks to conduction.Keywords
catheter ablation, cavotricuspid isthmus, fluoroscopy time, MediGuide™ Technology System, nonfluoroscopic catheter tracking, radiation exposure, typical atrial flutter
Introduction
The term ‘atrial flutter’ (AFL) refers to a heterogeneous group of atrial arrhythmias defined mechanistically by a macro-reentrant circuit around fixed anatomical barriers or functional blocks to conduction [1,2]. In the most frequent type of AFL, the so-called typical AFL, the wavefront rotates around the tricuspid annulus in a counterclockwise direction as viewed from the cardiac apex in a left anterior oblique projection [3]. The circuit is determined by anatomical (tricuspid annulus) and functional barriers (Crista terminalis), and involves the cavotricuspid isthmus (CTI) as the critical zone of slow conduction enabling reentry to be sustained [4–7]. This CTI is situated between the inferior aspect of the tricuspid annulus and the ostium of the inferior vena cava. In other types of CTI-dependent AFL, the activation front travels in a clockwise fashion around the tricuspid annulus (‘reverse typical’ AFL) or around the inferior vena cava due to transverse conduction via the crista terminals (‘lower loop reentry’) [2]. In atypical forms of AFL, macro-reentrant circuits occur around various anatomical boundaries resulting from atrial remodeling (fibrous scarring) due to severe structural heart disease or scarring after previous cardiac surgery and atrial fibrillation ablation, respectively [2]. In this review, AFL exclusively refers to the ‘classical’ CTI-dependent forms of AFL, that is, typical and reverse typical AFL.
The overall incidence of AFL in the general population has been reported to be 0.88%, with the incidence increasing with age from 0.05% in patients <50 years to 5.87% in those ≥80 years [8]. AFL is frequently associated with other cardiovascular conditions such as hypertension, coronary artery disease, chronic pulmonary disease and sick-sinus-syndrome [3]. AFL may manifest as debilitating palpitations, chest discomfort, dyspnea, fatigue, limited exercise tolerance or thromboembolic events. The loss of atrial contribution to ventricular filling, the rapid ventricular response rate, and the irregularity of ventricular contractions may lead to myocardial ischemia, to worsening of pre-existing heart failure or to the development of a tachycardia- induced cardiomyopathy.
Current status
Role of AFL ablation
Anatomically guided radiofrequency (RF) catheter ablation targeting the CTI is a well-established therapy for the treatment of CTI-dependent AFL [1,9,10]. During an ablation procedure, continuous RF lesions are deployed to create a contiguous linear lesion across the CTI in order to interrupt the macro-reentrant circuit by connecting the non-excitable tissue of the tricuspid annulus and the inferior vena cava. Electrophysiologically proven bi-directional conduction block across the CTI is a generally accepted procedural end point and can be achieved in approximately 95% of patients [2,11,12]. This high rate of acute procedural success translates into a considerable clinical efficacy. In a meta-analysis including more than 10,000 patients who underwent catheter ablation for CTI-dependent AFL, approximately 93% of patients were free from AFL recurrences after 13.8 ± 0.3 months of follow-up if bidirectional isthmus block was sought as a procedural end point and RF ablation was performed with a large tip (8–10 mm) or an irrigated tip catheter [13]. The superiority of catheter ablation over antiarrhythmic treatment with respect to freedom from AFL recurrences during long-term follow-up has been demonstrated in randomized clinical trials [14,15]. According to the 2003 ACC/AHA/ESC Guidelines, CTI ablation is recommended as first-line therapy in patients with recurrent, well-tolerated AFL, in patients with poorly tolerated AFL, and in patients with atrial fibrillation who developed AFL during medical treatment with class IC-anti-arrhythmic agents or amiodarone (class I indication) [10].
Conventional fluoroscopy
Mapping and ablation of cardiac arrhythmias requires precise understanding of cardiac anatomy. Conventional fluoroscopy is the major imaging modality for the definition of cardiac structures, catheter visualization and catheter manipulation in a variety of interventional cardiovascular procedures. This central role of conventional fluoroscopy is based on its ability to provide instantaneous localization of intracardiac tools and to delineate the relationship of these tools to moving cardiac structures. The limitations of fluoroscopyguided mapping and catheter movement, however, are twofold: first, radiation exposure to patients and healthcare personnel remains a major issue [16–18]. Even in experienced hands, fluoroscopy time during AFL ablation usually exceeds 15 min [11,12,19,20]. Prolonged fluoroscopy times may be required if creation of bidirectional isthmus block is hampered by a complex anatomy of the CTI [21]. And second, conventional fluoroscopy only features 2D orientation in a complex 3D cardiac anatomy, which may impede catheter navigation to intended target sites and appropriate catheter tip–tissue contact, respectively. As the number and complexity of interventional electrophysiological (EP) procedures is constantly increasing, alternate mapping and navigation technologies have been introduced in an attempt to reduce radiation exposure to patients and medical staff, and to facilitate 3D orientation in cardiac chambers comprising 3D mapping systems [12,20,22,23], remote catheter navigation [24–26], real-time MRI [27,28], and non-fluoroscopic electromagnetic catheter tracking [29–34]. Except for magnetic resonance imaging-guided EP procedures, which are currently at an highly investigational stage and which have not been implemented into clinical routine, these technologies will be discussed in the context of AFL ablation in the next chapters.
3D mapping systems
Electroanatomical mapping systems are routinely used to facilitate spatial orientation, arrhythmia mapping and appropriate lesion creation, and to reduce radiation exposure during interventional EP procedures, especially when complex anatomical substrates are targeted (e.g., ablation of atrial fibrillation or ventricular tachycardias) [22,23]. These computerized systems enable the creation of a 3D geometry of cardiac structures or chambers and the real-time visualization of diagnostic and ablation catheters in relationship to these structures. Importantly, catheters can be maneuvered in the respective chamber without fluoroscopic guidance. The potential benefit of 3D mapping technologies for AFL ablation has been explored in randomized clinical studies [12,20,35]. In these studies, total fluoroscopy was consistently reduced in patients with 3D mappingguided CTI ablation. In a study by Kottkamp et al., 50 patients with typical AFL were randomly assigned to receive CTI ablation guided by conventional fluoroscopy or by additional 3D electroanatomic mapping [20]. Electroanatomic mapping significantly reduced total fluoroscopy time (22.0 ± 6.3 min vs 3.9 ± 1.5 min; p < 0.0001) and the fluoroscopy time required for CTI mapping (17.7 ± 6.5 min vs 0.2 ± 0.3 min; p < 0.0001). These data from single-center experience were confirmed in a prospective, randomized multicenter study including 210 patients with typical AFL [11]. Acute ablation success, that is, verification of bidirectional conduction block, total procedure duration and recurrence rate of typical atrial flutter after 6 months of follow- up were similar in the electroanatomically guided ablation group and the conventional ablation group, whereas fluoroscopy time was reduced by almost 50% in the electroanatomically guided group (7.7 ± 7.3 min vs 14.8 ±11.9 min; p < 0.05).
Despite this favorable effect on radiation exposure, 3D mapping systems hold some inherent limitations: first, 3D mapping systems work as independent tools unrelated to the standard working environment of live fluoroscopy. Second, they only provide static models and maps of otherwise moving cardiac chambers. In fact, intracardiac locations are affected by multiple components of organ motion (e.g., cardiac cycle, respiration). Thus, the in vivo accuracy of 3D mapping systems is limited because of the inability to reliably depict time-dependent motion of catheter position together with the target region. And third, application of a 3D mapping system is associated with a more complex workflow and increased procedural costs, especially in the setting of simple EP studies such as AFL ablation [11].
Remote catheter navigation systems
Remote navigation systems allow for catheter navigation from outside the EP laboratory either by an external low-density magnetic field (magnetic navigation) or by manipulation of steerable sheaths via a robotic arm (robotic navigation) [36]. During the procedure, the interventional electrophysiologist is sitting in a control room remote from x-ray exposure. The feasibility, efficacy and safety of remote magnetic navigation during catheter ablation for AFL were reported in an observational study including 26 patients [24]. Bi-directional isthmus block could be achieved 96% of patients. The median ablation time was 25 min (range: 12–78 min), the median procedure duration was 53 min (range: 30–130 min), and the median fluoroscopy time was 7.5 min (range: 3.2–20.8 min). The role of remote magnetic catheter navigation during AFL ablation has been further investigated in a subsequent randomized study including 90 patients undergoing de novo CTI ablation for typical AFL [25]. Patients were randomized to conventional manual ablation or remote magnetic navigation-guided ablation. Remote magnetic navigation-guided ablation was associated with a significantly reduced median fluoroscopy time (10.6 min; interquartile range: 7.6–19.9 min vs 15.0 min; range: 11.5–23.1 min; p = 0.043), whereas procedure duration was significantly prolonged (114 ± 35 min vs 77 ± 24 min; p < 0.0001). Additionally, long-term success, defined as achievement of bidirectional isthmus block and absence of AFL recurrence during 6 months of follow- up was numerically lower in the remote magnetic navigation group (73 vs 89%; p = 0.063). In a study by Steven et al., 50 patients were randomly assigned to conventional or robotic navigation-guided CTI ablation [26]. Complete bidirectional conduction block could be achieved in all patients. Fluoroscopy time and x-ray exposure to the operator were significantly longer in the conventional group compared with the robotic navigation group (8.2 ± 4.6 min vs 5.8 ± 3.6 min; p = 0.038; and 8.2 ± 4.6 min vs 1.9 ± 1.1 min; p < 0.001). Total procedure duration in the robotic navigation group was significantly longer than in the conventional group (79.2 ± 30.6 min vs 58.4 ± 17.7 min; p = 0.04). Major limitations of remote navigation technologies are the prolonged procedure duration, the requirement for additional technical equipment, and the reported lower success rate.
Non-fluoroscopic catheter tracking
MediGuide™ Technology System: basic principles
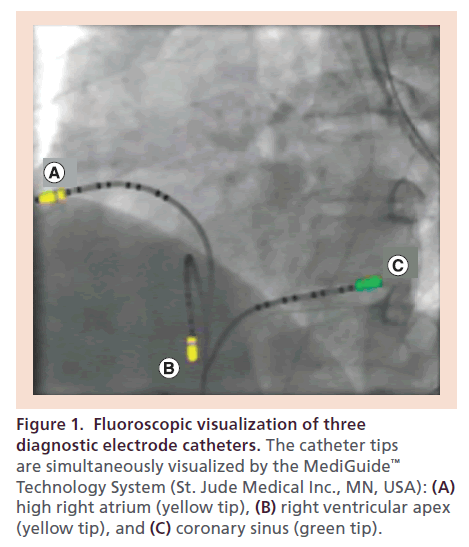
Recently, a novel 3D electromagnetic catheter tracking system (MediGuide™ Technology System, St. Jude Medical Inc., MN, USA) has been introduced for coronary interventions and invasive EP procedures that may overcome the limitations of abovementioned mapping and navigation technologies. The MediGuideT technology has been comprehensively described previously [29–34]. This system consists of three components: a transmitter unit generating a low-intensity dynamic 3D electromagnetic field; miniaturized passive single coil sensors (<1 mm3) embedded in intracardiac devices such as conventional electrode or ablation catheters; and an electromagnetic field reference sensor affixed to the patient’s chest at the level of the sternum similar to an ECG electrode in size. The electromagnetic field transmitter is installed within the fluoroscopy detector of a conventional flat-panel x-ray imaging system. Using this hardware setup, fluoroscopic imaging and electromagnetic sensor tracking are pre-aligned and auto-registered. Therefore, the tip of a sensor-integrated intracardiac device can be visualized on conventional fluoroscopy or tracked non-fluoroscopically at the identical position by the electromagnetic sensor field. Using pre-acquired fluoroscopy cine loops as background, real-time location and 3D movement of sensor-equipped devices as recorded by the electromagnetic field can be visualized simultaneously within the x-ray environment without additional fluoroscopy (Figure 1). The reference sensor provides information about the spatial relationship between the patient and the electromagnetic tracking field. The system allows both cine loops to run concurrently in a pseudo-biplane mode and therefore to visualize the catheter position in two projections. Built-in algorithms compensate catheter tracking and image display for respiration, cardiac motion and patient movement.
Figure 1. Fluoroscopic visualization of three diagnostic electrode catheters. The catheter tips are simultaneously visualized by the MediGuide™ Technology System (St. Jude Medical Inc., MN, USA): (A) high right atrium (yellow tip), (B) right ventricular apex (yellow tip), and (C) coronary sinus (green tip).
The first-in-human application of this nonfluoroscopic electromagnetic catheter tracking technology was reported by Piorkowski and Hindricks in 2011 [31]. A 26-year-old male patient underwent a complete invasive EP study for recurrent tachycardia of unknown etiology. Three diagnostic catheters were placed nonfluoroscopically in the right ventricular apex, at the His-bundle position, and in the high right atrium. Live fluoroscopy was applied in order to validate the catheter location and its in vivo alignment with the true position. This study demonstrated the feasibility of non-fluoroscopic catheter visualization within the workflow of a standard invasive diagnostic EP study. The MediGuideTM platform has been commercially available in Europe and the USA since 2011 and 2012, respectively.
MediGuide Technology System & atrial flutter ablation
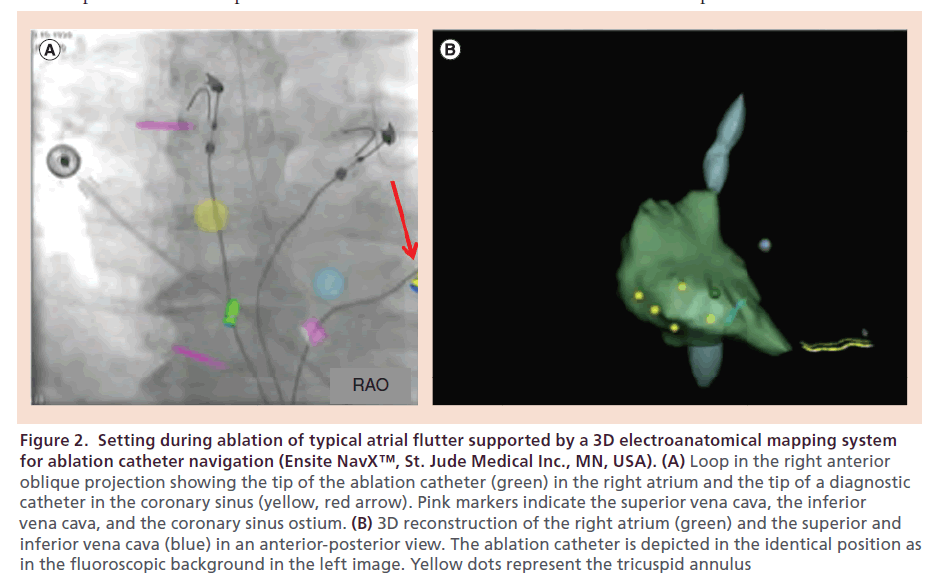
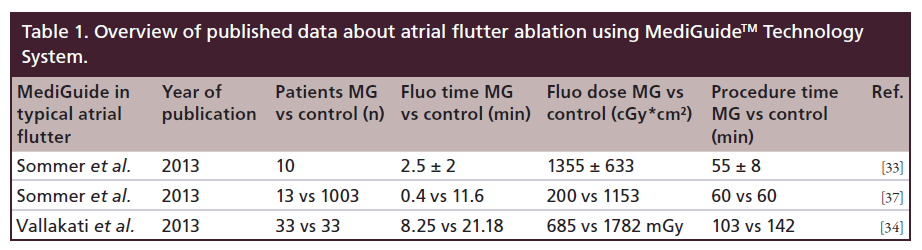
The first study that investigated safety and feasibility of the MediGuide™ Technology System during CTI-dependent AFL ablation was reported by Sommer et al. [33]. Electromagnetic catheter tracking was applied in combination with conventional fluoroscopy and a 3D electroanatomic mapping system in a cohort of ten patients (Figure 2). All patients were male, mean age was 68 ± 8 years, and structural heart disease was present in 80% of patients. At the time of study enrollment, only MediGuide-enabled diagnostic catheters but no ablation catheters were available. Before venous puncture and introduction of the decapolar diagnostic catheters, two cine loops with a length of three cardiac cycles were recorded in a right anterior oblique (30°) and left anterior oblique (60°) projection and continuously displayed on two separate screens. These cine loops served as background image for non-fluoroscopic catheter tracking. As described above, both cine loops were displayed synchronously in two projections. One decapolar catheter was inserted into the coronary sinus via the left femoral vein under the guidance of the MediGuide Technology System. In one patient, coronary sinus cannulation required the additional use of conventional fluoroscopy. The second diagnostic catheter was used to create a 3D electroanatomical map of the right atrium, the superior and inferior vena cava, the tricuspid annulus and the coronary sinus. Subsequently, the catheter was placed in the right ventricular apex and a conventional non-irrigated 8-mm tip RF catheter was introduced and displayed on the 3D electroanatomic mapping system for navigation and ablation (Figure 2). RF ablation was performed with a RF power of 70 Watt and a tip temperature of 70°C. The procedural endpoint of bidirectional isthmus block could be achieved in all patients. Procedure duration and fluoroscopy time measured 55 ± 8 min and 2.5 ± 2.0 min, respectively. Fluoroscopy dose was 1355 ± 633 cGy*cm2. Although indirect comparison of different studies should be interpreted with caution, these data demonstrated a substantial reduction of fluoroscopy times when the MediGuide Technology System was applied compared with AFL ablation procedures using conventional fluoroscopy only (fluoroscopy time usually >15 min) or in combination with a 3D mapping system (fluoroscopy time: 7.7 ± 7.3 min in the multicenter study by Hindricks et al. [11]), whereas total procedure duration was within the normal range of fluoroscopy- guided standard procedures [11,12,19,20]. In a prospective observational study by Vallakati et al., the MediGuide Technology System was utilized for catheter navigation in 45 patients undergoing an invasive EP procedure for different types of supraventricular arrhythmias (typical AFL, atrioventricular nodal reentry tachycardia, Wolff–Parkinson–White syndrome) or diagnostic purposes [34]. The study cohort was compared with 45 controls who were matched according to the type of procedure. Typical AFL was the indication for the invasive EP procedure in approximately 73% of patients. An electroanatomic mapping system was used both in the study and the control group. Again, only the diagnostic catheters were equipped with a sensor for electromagnetic catheter tracking. In the MediGuide group, both fluoroscopy time and procedure duration were significantly shorter compared with the control group (8.3 ± 4.9 min vs 21.2 ± 14.8 min, p < 0.001; and 103.8 ± 25.3 min vs 142.0 ± 55.8 min, p = 0.03). Acute procedural success could be achieved in 100% patients in both groups and no major complications or adverse events were observed during the procedure and during a 24-h period after the procedure, respectively.
Figure 2. Setting during ablation of typical atrial flutter supported by a 3D electroanatomical mapping system for ablation catheter navigation (Ensite NavX™, St. Jude Medical Inc., MN, USA). (A) Loop in the right anterior oblique projection showing the tip of the ablation catheter (green) in the right atrium and the tip of a diagnostic catheter in the coronary sinus (yellow, red arrow). Pink markers indicate the superior vena cava, the inferior vena cava, and the coronary sinus ostium. (B) 3D reconstruction of the right atrium (green) and the superior and inferior vena cava (blue) in an anterior-posterior view. The ablation catheter is depicted in the identical position as in the fluoroscopic background in the left image. Yellow dots represent the tricuspid annulus
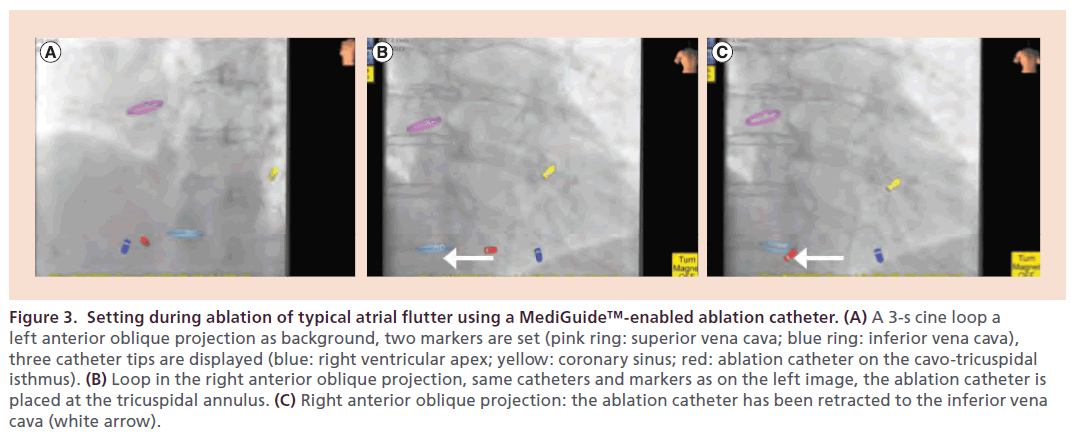
After sensor-equipped ablation catheters had become commercially available (May 2012), the MediGuide Technology System could be applied as a ‘stand-alone’ system without the support from a 3D mapping system. The performance of the MediGuide Technology System as a stand-alone system was evaluated in a subsequent study including 24 patients who underwent catheter ablation for different types of supraventricular tachycardias (atrioventricular nodal reentry tachycardia, atrioventricular reentry tachycardia, Wolff–Parkinson– White syndrome, atrial flutter and ectopic atrial tachycardia) [37]. These patients were compared with a control group of 1865 patients who were conventionally ablated in the two enrolling centers. Typical AFL was the indication for ablation in 54% of patients. RF ablation of the CTI was performed with 30–40 Watts, an irrigation rate of 30 ml/min, and a maximum temperature of 48°C. In the subgroup of AFL patients, bidirectional isthmus block could be created in all patients compared with 98% in the control group. There was no statistically significant difference with respect to the median procedure duration between the study group and the conventionally treated patients (60 ± 20 min vs 90 ± 45 min; p = nonsignificant). Median fluoroscopy time, however, could be significantly reduced in the study group (0.4 ± 1.6 min vs 11.6 ± 8.6 min; p < 0.001) (Figure 3). During the study, a substantial learning effect could be observed with the fluoroscopy decreasing from values between 3.0 and 4.5 min in the first four patients to values between 0.1 and 1.6 min in the following nine patients. In three out of 13 patients, bidirectional CTI conduction block could be achieved without additional fluoroscopy after acquisition of the cine loops (see Table 1). A randomized study comparing the MediGuide Technology System as a stand-alone technology for catheter navigation with conventional fluoroscopy during catheter ablation for typical AFL is currently underway (initiated by P Sommer).
Figure 3. Setting during ablation of typical atrial flutter using a MediGuide™-enabled ablation catheter. (A) A 3-s cine loop a left anterior oblique projection as background, two markers are set (pink ring: superior vena cava; blue ring: inferior vena cava), three catheter tips are displayed (blue: right ventricular apex; yellow: coronary sinus; red: ablation catheter on the cavo-tricuspidal isthmus). (B) Loop in the right anterior oblique projection, same catheters and markers as on the left image, the ablation catheter is placed at the tricuspidal annulus. (C) Right anterior oblique projection: the ablation catheter has been retracted to the inferior vena cava (white arrow).
MediGuide Technology System & complex EP procedures
The MediGuide Technology System has not only been investigated in ‘simple’ ablation procedures with a clearly defined and circumscribed substrate but also in complex interventional EP scenarios such as atrial fibrillation ablation and implantation of a left ventricular lead for cardiac resynchronization therapy [32,38,39]. In a recently published case–control study matching 49 study patients to 49 control subjects, implementation of the MediGuide Technology System in addition to a conventional 3D mapping system during an atrial fibrillation ablation procedure has been shown to be safe and feasible [32]. In this study, only a diagnostic sensor-integrated catheter was available. In the MediGuide group, median fluoroscopy time and mean irradiation dose were significantly reduced compared with the conventional control (16 min, quartiles 10 and 23 min, vs 31 min, quartiles 25 and 43 min, and 7363 ± 5827 cGy·cm2 vs 14,453 ± 7403 cGy·cm2; p < 0.001 for each comparison). After a 6-month follow-up, freedom of atrial fibrillation recurrences or atrial tachycardias was comparable in both groups (67% of patients in the MediGuide group, 69 in the control group; p = 0.83). In a subsequent study including 80 patients undergoing atrial fibrillation ablation, utilization of a MediGuide-enabled ablation catheter was associated with a median fluoroscopy time of 4.6 min (interquartile range: 2.9; 7.1 min) [38]. Fluoroscopy was predominantly used for the acquisition of background cine loops, transseptal puncture, occasional visualization of transseptal sheath position and navigation of the spiral mapping catheter. The first human application of the MediGuide platform during implantation of cardiac resynchronization devices as reported by Richter et al. [39]. Fifteen consecutive patients with a standard indication for biventricular pacing underwent conventional implantation of a right atrial and right ventricular lead. The left ventricular lead was placed using MediGuide-enabled devices, that is, two different sheaths for coronary sinus cannulation and delivery of the left ventricular sheath, a steerable decapolar catheter for coronary sinus intubation, and a guidewire for target vein access and over-the-wire deployment. The cardiac resynchronization system was successfully implanted in all patients. Overall procedure duration measured 116 ± 43 min. The median total fluoroscopy time and the fluoroscopy time for left ventricular lead placement was 5.2 min (Q1–Q3: 3.0–8.4 min) and 2.6 min (Q1–Q3: 1.6–5.6 min), respectively. No severe complications requiring acute intervention were observed during the perioperative period.
Complications
According to the available literature, application of the MediGuide Technology System does not increase the risk for intra-procedural or peri-procedural complications [32–34,37–39].
Alternative technologies
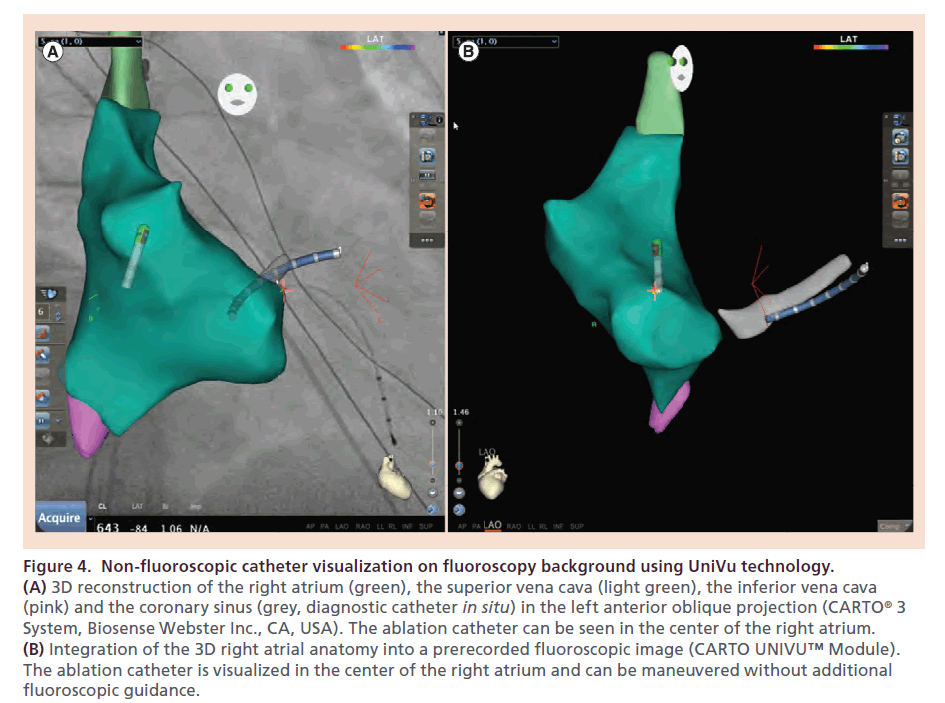
Another novel non-fluoroscopic catheter visualization system that combines fluoroscopic images with 3D electroanatomical maps into a single 3D view on the CARTO® 3 system is available since 2013 (CARTOUNIVU ™ Module, Biosense Webster Inc., CA, USA) (Figure 4). In our experience, application of this technology during an EP procedure is associated with a substantial reduction in fluoroscopy times and radiation exposure, respectively. Systematic clinical data concerning this technology, however, are currently lacking.
Figure 4. Non-fluoroscopic catheter visualization on fluoroscopy background using UniVu technology. (A) 3D reconstruction of the right atrium (green), the superior vena cava (light green), the inferior vena cava (pink) and the coronary sinus (grey, diagnostic catheter in situ) in the left anterior oblique projection (CARTO® 3 System, Biosense Webster Inc., CA, USA). The ablation catheter can be seen in the center of the right atrium. (B) Integration of the 3D right atrial anatomy into a prerecorded fluoroscopic image (CARTO UNIVU™ Module). The ablation catheter is visualized in the center of the right atrium and can be maneuvered without additional fluoroscopic guidance.
Conclusion
It can be assumed that a variety of additional cardiovascular devices will be equipped with the MediGuide Technology System in the nearer future. Thus, the non-fluoroscopic electromagnetic catheter tracking will serve as a platform in a broad spectrum of invasive procedures including interventional cardiology, electrophysiology and angiology. This will lead to a substantial reduction in overall radiation exposure to patients and healthcare personnel. In the field of interventional electrophysiology, the MediGuide Technology System potentially enables almost non-fluoroscopic procedures except for the radiation that is used for the acquisition of the cine loops as soon as the full range of diagnostic EP catheters, ablation catheters and intravascular sheaths will be equipped with the sensor technology. In addition to the reduction of x-ray exposure, which currently is the only measurable clinical benefit of non-fluoroscopic electromagnetic catheter tracking, this platform may be applied in order to improve the clinical efficacy of EP procedures, for example, reducing the rate of non-response after implantation of a cardiac resynchronization device by providing a detailed analyses of left ventricular wall motion and thereby helping to identify the optimal site for left ventricular lead placement.
Catheter ablation is the treatment of choice in the vast majority of patients with typical or reverse typical AFL. Radiation exposure to patients and medical staff during an ablation procedure, however, remains a major concern. Remote catheter navigation and 3D mapping systems have the potential to significantly reduce x-ray exposure. In the setting of an AFL ablation procedure these technologies are limited by a more complex workflow, increased procedural costs and prolonged procedure duration. Recently, the MediGuide Technology System has been introduced as a novel non-fluoroscopic electromagnetic catheter tracking system that allows integration of 3D catheter navigation into the environment of prerecorded conventional 2D fluoroscopy. The feasibility, safety and efficacy of this system have consistently been demonstrated in a variety of invasive EP procedures. In three nonrandomized clinical trials, utilization of the MediGuide Technology System for AFL ablation was associated with a substantial reduction in fluoroscopy time and radiation exposure without affecting periprocedural safety, acute procedural success or workflow in the EP laboratory.
Financial & competing interests disclosure
S Rolf has received moderate lecture fees from St. Jude Medical. G Hindricks has received moderate lecture fees from St. Jude Medical and is member of advisory board of St. Jude Medical and Biosense Webster. P Sommer has received moderate lecture fees from St. Jude Medical, Biotronik and Siemens, and is a member of the advisory board of St. Jude Medical. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Executive Summary
Background
• Catheter ablation is the treatment of choice in the vast majority of patients with cavotricuspid isthmusdependent atrial flutter.
• Radiation exposure to patients and healthcare personnel during conventional fluoroscopy-guided catheter mapping and navigation remains a major issue.
• 3D mapping systems and remote navigation systems substantially reduce x-ray exposure during ablation for atrial flutter at the expense of a prolonged procedure duration, a more complex workflow and increased procedural costs.
Description of the technology
• The MediGuide™ Technology System (St. Jude Medical Inc., MN, USA) platform has been introduced as a novel non-fluoroscopic electromagnetic catheter tracking system that enables integration of 3D catheter navigation into the environment of prerecorded conventional 2D fluoroscopy.
• Thus, this technology allows for non-fluoroscopic real-time catheter navigation in complex cardiac structures.
• Built-in algorithms compensate catheter tracking and image display for respiration, cardiac motion and patient movement.
Alternative technologies
• CARTOUNIVU™ technology (Biosense Webster Inc., CA, USA) is another currently available technology that enables non-fluoroscopic catheter navigation by combining fluoroscopic images with 3D electroanatomical maps into a single 3D view.
Clinical benefit & potential use
• Application of the MediGuide platform is associated with a clinically relevant reduction in fluoroscopy time and radiation in different electrophysiological scenarios including ablation of atrial flutter.
• Currently, this technology does not seems to negatively affect acute procedural success, periprocedural safety and workflow in the electrophysiological laboratory.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- Kottkamp H, Hindricks G. Catheter ablation of atrial flutter. Thorac. Cardiovasc. Surg. 47(3), 357–361 (1999).
- Lee G, Sanders P, Kalman JM. Catheter ablation of atrial arrhythmias: state of the art. Lancet 380(9852), 1509–1519 (2012).
- Wellens HJ. Contemporary management of atrial flutter. Circulation 106(6), 649–652 (2002).
- Olgin JE, Kalman JM, Saxon LA, Lee RJ, Lesh MD. Mechanism of initiation of atrial flutter in humans: site of unidirectional block and direction of rotation. J. Am. Coll. Cardiol. 29(2), 376–384 (1997).
- Cosio FG, Arribas F, Barbero JM, Kallmeyer C, Goicolea A. Validation of double-spike electrograms as markers of conduction delay or block in atrial flutter. Am. J. Cardiol. 61(10), 775–780 (1988).
- Kalman JM, Olgin JE, Saxon LA, Fisher WG, Lee RJ, Lesh MD. Activation and entrainment mapping defines the tricuspid annulus as the anterior barrier in typical atrial flutter. Circulation 94(3), 398–406 (1996).
- Olshansky B, Okumura K, Hess PG, Waldo AL. Demonstration of an area of slow conduction in human atrial flutter. J. Am. Coll. Cardiol. 16(7), 1639–1648 (1990).
- Granada J, Uribe W, Chyou PH et al. Incidence and predictors of atrial flutter in the general population. J. Am. Coll. Cardiol. 36(7), 2242–2246 (2000).
- Cosio FG, López-Gil M, Goicolea A, Arribas F, Barroso JL. Radiofrequency ablation of the inferior vena cava-tricuspid valve isthmus in common atrial flutter. Am. J. Cardiol. 71(8), 705–709 (1993).
- Blomström-Lundqvist C, Scheinman MM, Aliot EM et al. ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias. Circulation 108(15), 1871–1909 (2003).
- Hindricks G, Willems S, Kautzner J et al. Effect of electroanatomically guided versus conventional catheter ablation of typical atrial flutter on the fluoroscopy time and resource use: a prospective randomized multicenter study. J. Cardiovasc. Electrophysiol. 20(7), 734–740 (2009).
- Willems S, Weiss C, Ventura R et al. Catheter ablation of atrial flutter guided by electroanatomic mapping (CARTO): a randomized comparison to the conventional approach. J. Cardiovasc. Electrophysiol. 11(11), 1223–1230 (2000).
- Pérez FJ, Schubert CM, Parvez B, Pathak V, Ellenbogen KA, Wood MA. Long-term outcomes after catheter ablation of cavo-tricuspid isthmus dependent atrial flutter: a metaanalysis. Circ. Arrhythm. Electrophysiol. 2(4), 393–401 (2009).
- Natale A, Newby KH, Pisanó E et al. Prospective randomized comparison of antiarrhythmic therapy versus first-line radiofrequency ablation in patients with atrial flutter. J. Am. Coll. Cardiol. 35(7), 1898–1904 (2000).
- Da Costa A, Thévenin J, Roche F et al. Results from the Loire-Ardèche-Drôme-Isère-Puy-de-Dôme (LADIP) trial on atrial flutter, a multicentric prospective randomized study comparing amiodarone and radiofrequency ablation after the first episode of symptomatic atrial flutter. Circulation 114(16), 1676–1681 (2006).
- Kovoor P, Ricciardello M, Collins L, Uther JB, Ross DL. Risk to patients from radiation associated with radiofrequency ablation for supraventricular tachycardia. Circulation 98(15), 1534–1540 (1998).
- Wagner LK, Eifel PJ, Geise RA. Potential biological effects following high X-ray dose interventional procedures. J. Vasc. Interv. Radiol. 5(1), 71–84 (1994).
- Lindsay BD, Eichling JO, Ambos HD, Cain ME. Radiation exposure to patients and medical personnel during radiofrequency catheter ablation for supraventricular tachycardia. Am. J. Cardiol. 70(2), 218–223 (1992).
- Anselme F, Savouré A, Cribier A, Saoudi N. Catheter ablation of typical atrial flutter: a randomized comparison of 2 methods for determining complete bidirectional isthmus block. Circulation 103(10), 1434–1439 (2001).
- Kottkamp H, Hügl B, Krauss B, Wetzel et al. Electromagnetic versus fluoroscopic mapping of the inferior isthmus for ablation of typical atrial flutter: a prospective randomized study. Circulation 102(17), 2082–2086 (2000).
- Da Costa A, Faure E, Thévenin J et al. Effect of isthmus anatomy and ablation catheter on radiofrequency catheter ablation of the cavotricuspid isthmus. Circulation 110(9), 1030–1035 (2004).
- Ben-Haim SA, Osadchy D, Schuster I, Gepstein L, Hayam G, Josephson ME. Nonfluoroscopic, in vivo navigation and mapping technology. Nat. Med. 2(12), 1393–1395 (1996).
- Wittkampf FH, Wever EF, Derksen R et al. LocaLisa: new technique for real-time 3-dimensional localization of regular intracardiac electrodes. Circulation 99(10), 1312–1317 (1999).
- Arya A, Kottkamp H, Piorkowski C et al. Initial clinical experience with a remote magnetic catheter navigation system for ablation of cavotricuspid isthmus-dependent right atrial flutter. Pacing Clin. Electrophysiol. 31(5), 597–603 (2008).
- Vollmann D, Lüthje L, Seegers J, Hasenfuss G, Zabel M. Remote magnetic catheter navigation for cavotricuspid isthmus ablation in patients with common-type atrial flutter. Circ. Arrhythm. Electrophysiol. 2(6), 603–610 (2009).
- Steven D, Rostock T, Servatius H et al. Robotic versus conventional ablation for common-type atrial flutter: a prospective randomized trial to evaluate the effectiveness of remote catheter navigation. Heart Rhythm 5(11), 1556–1560 (2008).
- Piorkowski C, Grothoff M, Gaspar T et al. Cavotricuspid isthmus ablation guided by real-time magnetic resonance imaging. Circ. Arrhythm. Electrophysiol. 6(1), e7–e10 (2013).
- Sommer P, Grothoff M, Eitel C et al. Feasibility of real-time magnetic resonance imaging-guided electrophysiology studies in humans. Europace 15(1), 101–108 (2013).
- Jeron A, Fredersdorf S, Debl K et al. First-in-man (FIM) experience with the Magnetic Medical Positioning System (MPS) for intracoronary navigation. EuroIntervention 5(5), 552–557 (2009).
- Flugelman MY, Shiran A, Nusimovici-Avadis D et al. Medical positioning system: a technical report. EuroIntervention 4(1), 158–160 (2008).
- Piorkowski C, Hindricks G. Nonfluoroscopic sensor-guided navigation of intracardiac electrophysiology catheters within prerecorded cine loops. Circ. Arrhythm. Electrophysiol. 4(4), e36–e38 (2011).
- Rolf S, Sommer P, Gaspar T et al. Ablation of atrial fibrillation using novel 4-dimensional catheter tracking within autoregistered left atrial angiograms. Circ. Arrhythm. Electrophysiol. 5(4), 684–690 (2012).
- Sommer P, Wojdyla-Hordynska A, Rolf S et al. Initial experience in ablation of typical atrial flutter using a novel three dimensional catheter tracking system. Europace 15(4), 578–581 (2013).
- Vallakati A, Reddy YM, Emert M et al. Impact of nonfluoroscopic MediGuide™ tracking system on radiation exposure in radiofrequency ablation procedures (LESS-RADS registry) – an initial experience. J. Interv. Card. Electrophysiol. 38(2), 95–100 (2013).
- Schneider MA, Ndrepepa G, Dobran I et al. LocaLisa catheter navigation reduces fluoroscopy time and dosage in ablation of atrial flutter: a prospective randomized study. J. Cardiovasc. Electrophysiol. 14(6), 587–590 (2003).
- Schmidt B, Chun KR, Tilz RR, Koektuerk B, Ouyang F, Kuck KH. Remote navigation systems in electrophysiology. Europace 10(Suppl. 3), iii57–iii61 (2008).
- Sommer P, Piorkowski C, Gaspar T et al. MediGuide in supraventricular tachycardia: initial experience from a multicentre registry. Europace 15(9), 1292–1297 (2013).
- Rolf S, John S, Gaspar T et al. Catheter ablation of atrial fibrillation supported by novel nonfluoroscopic 4D navigation technology. Heart Rhythm 10(9), 1293–1300 (2013).
- Richter S, Döring M, Gaspar T et al. Cardiac resynchronization therapy device implantation using a new sensor-based navigation system: results from the first human use study. Circ. Arrhythm. Electrophysiol. 6(5), 917–923 (2013).
• In this study Willems et al. could demonstrate in 2000 that additional application of 3D mapping systems for ablation of atrial flutter can contribute to shorter fluoroscopy duration in these procedures.
• In this article the potential risks for operators and patients during SVT ablations are explained and stratified.
• A randomized study where ablation of atrial flutter was performed either with conventional fluoroscopy or supported by 3D mapping systems.
• The largest patient cohort ablated for atrial flutter using remote magnetic catheter navigation (Stereotaxis).
• Rostock et al. describe their experience comparing conventional catheter ablation of atrial flutter with robotic navigation.
• This article focusses on ablation of atrial fibrillation, but the MediGuide™ Technology System is nicely explained.
•• The very first article on the application of MediGuide Technology Systems in ablation procedures – still without having a MediGuide-enabled ablation catheter available.
•• Recently published article on the experience of Kansas University using MediGuide in atrial flutter – also in inexperienced operators.
•• Application of MediGuide technology in several SVT – also in atrial flutter with dramatic reduction of fluoroscopy time to 30 s in the MediGuide group.
• Another article focussing on atrial fibrillation. The technological aspects of MediGuide are highlighted.
• Initial experience in application of the MediGuide Technology System in CRT implantation – with a significant reduction of fluoroscopy time and dose.