Device Evaluations - Interventional Cardiology (2012) Volume 4, Issue 2
Accelerating endothelialization of coronary stents by capturing circulating endothelial progenitor cells
- Corresponding Author:
- Renu Virmani
CVPath Institute, Inc,19 Firstfield Road
Gaithersburg, MD 20878, USA
Tel: +1 301 208 3570
Fax: +1 301 208 3745
E-mail: rvirmani@cvpath.org
Abstract
Keywords
coronary stent,endothelial progenitor cell,endothelialization,late stent thrombosis
Introduction
Over the past few decades, percutaneous interventions have emerged as the preferred treatment of choice for coronary artery disease. In the USA alone, more than one million percutaneous interventions are performed annually and more than 80% involve the use of coronary stents. Although coronary bare metal stent (BMS) had a dramatic impact on reducing restenosis rates, restenosis still occurred in up to 30% of cases [1,2]. The restenotic process consists predominantly of smooth muscle cells (SMCs) in a proteoglycan and type III collagenous matrix. The limitations of BMS led to the development of drug-eluting stents (DES), a more promising technique for the treatment of coronary artery disease.
First-generation DES (Cypher™, Cordis Corp., FL, USA and Taxus™, Boston Scientific, MA, USA) showed a significant reduction in restenosis rates as compared to BMS and became the standard of care for the treatment of coronary artery disease [3,4]. Antiproliferative drugs, cytostatic or cytotoxic, eluted from first-generation DES successfully inhibited SMC proliferation, resulting in suppression of neointimal growth. However, these drugs are not selective in their suppression of SMCs and also inhibit endothelial cell (EC) proliferation. Therefore, it is not surprising that late stent thrombosis (LST), a rare but life-threatening complication, has emerged as a major safety concern [5,6]. In addition to antiproliferative drugs, polymers used to coat DES, along with stent malapposition, may also play a role in LST [7]. The physiopathology of LST varies with the type of DES used and the underlying target lesion being treated. Nevertheless, there is a universal finding in all DES of delayed arterial healing, which is characterized by persistent fibrin deposition, sparse SMC coverage and incomplete re-endothelialization [7,8]. Our autopsy studies demonstrate that the most powerful predictor of stent thrombosis is endothelial coverage [9].
Because antiproliferative drugs deployed on DES do not specifically target SMCs, these drugs adversely impact endothelial proliferation, migration and function [10,11]. ECs, which line the arterial lumen, play a major role in the maintenance of vascular homeostasis including, the transportation of plasma molecules, regulation of vascular tone and synthesis of a large variety of antithrombotic factors. The adverse impact of antiproliferative drugs used on DES has been demonstrated by our group in preclinical models at 14 and 28 days to inhibit the regrowth of ECs and decrease the expression of platelet endothelial cell adhesion molecule (PECAM-1) and thrombomodulin. There was also an upregulation of mRNA and a decrease in VEGF production as compared to bare metal controls [11]. Similar findings have been confirmed in autopsy samples, in other words, the lack of endothelial coverage in DES even beyond 18 months in first-generation DES which led to the development of thinner stent struts in second-generation DES (Endeavor™, Medtronic Vascular, CA, USA and Xience™ V, Abbott Vascular, CA, USA), with less or rapid drug release, and more biocompatible polymers that allow faster healing and accelerated endothelialization [12,13]. These changes have, in general, resulted in a reduction of LST, however the antiproliferative drugs are likely to remain in the diseased tissues especially in ‘off-label’ use for some time and are a concern for the continued potential of LST.
Another approach is to bypass antiproliferative drugs and instead accelerate the healing process (i.e., to restore the endothelium early after injury). During stenting, the endothelium is denuded and leads to focal fibrin and platelet aggregation, especially in the peri-strut regions where it is accompanied by inflammation, which is also dependent on the severity of injury [14]. Adherent platelets and leukocytes of nonendothelialized surfaces can release growth factors and cytokines, which initiate SMC proliferation and migration [15]. It has been demonstrated that proliferating SMCs accumulate in areas that are not fully re-endothelialized [16]. ECs produce a significant number of factors that regulate SMC differentiation and proliferation, like the cytokine TGF, angiotensin II and others similar to prostacyclin and nitric oxide that prevent platelet aggregation and SMC proliferation [17,18]. Therefore, ECs may themselves maintain the mitogenic quiescence of SMCs by growth-inhibitory factors [19].
The Genous™ stent
Besides the vessel wall cells, circulating progenitor cells have also been implicated to play a role in vascular healing following injury [20]. It has long been hypothesized that blood and ECs may share a common progenitor, known as the hemangioblast [21]. A single-cell-resolution fate map demonstrated that these cells in the early zebrafish experiments were capable of giving rise to both hematopoietic cells and ECs [22]. Asahara et al. first described endothelial progenitor cells (EPCs) and showed that circulating bone marrow- derived cells are capable of migrating to areas of vascular injury and aid in the regeneration of damaged and dysfunctional endothelium [20]. Therefore it is not surprising that biomedical engineers have thought of using circulating progenitor cells to accelerate the healing of the vessel wall.
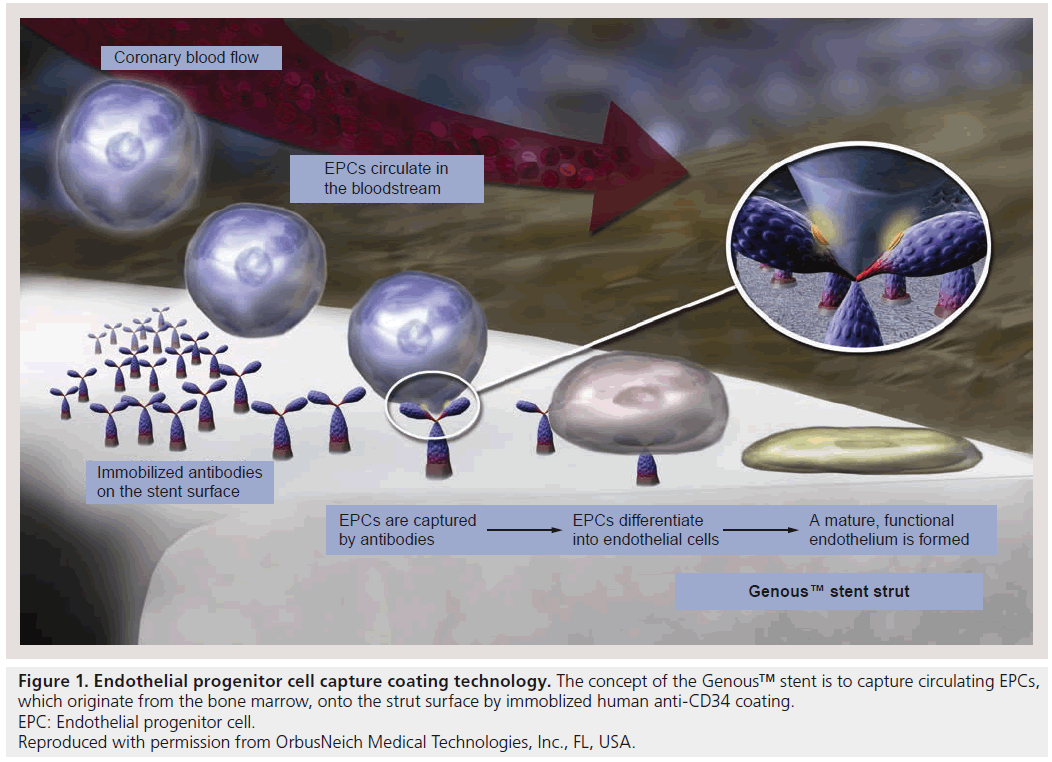
The Genous™ stent, a bioengineered EPCcapturing stent (Genous Bioengineered R stent™; OrbusNeich Medical Technologies Inc., FL, USA) is a novel ‘prohealing’ stent design that aids in recruiting circulating EPCs. The R stent has unique dual helix designed specifically for flexibility, radial strength and natural conformability. The R stent is made from medical grade 316 stainless steel with a strut thickness of 0.0040 inches (102 μm). The R stent is coated using an immobilized antihuman-CD34 monoclonal antibody designed to capture circulating EPCs (Figure 1). The design of the Genous stent is therefore to accelerate endothelialization by early and continued recruitment of EPCs. The Genous stent was introduced in 2005 in the European market for use in patients eligible for stent placement with symptomatic ischemic heart disease due to de novo and/or restenotic coronary artery lesions.
Figure 1: Endothelial progenitor cell capture coating technology. The concept of the Genous™ stent is to capture circulating EPCs,
which originate from the bone marrow, onto the strut surface by immoblized human anti-CD34 coating.
EPC: Endothelial progenitor cell.
Reproduced with permission from OrbusNeich Medical Technologies, Inc., FL, USA.
Recently, a second generation of the Genous stent (Genous Bio-engineered Cobalt Chromium stent) was introduced to the market (CE mark in April 2010). The stent platform is made from the L605 cobalt–chromium alloy with strut thickness of 0.0032 inches (81 μm), a 20% reduction in strut thickness as compared with the original Genous stainless steel design, with greater stent flexibility and reduced stent profile. As in the previous design, the surface of the Genous Bio-engineered Cobalt Chromium stent is comprised of a polysaccharide matrix with CD34+ antibodies
Bench-top & preclinical assessment of the Genous stent
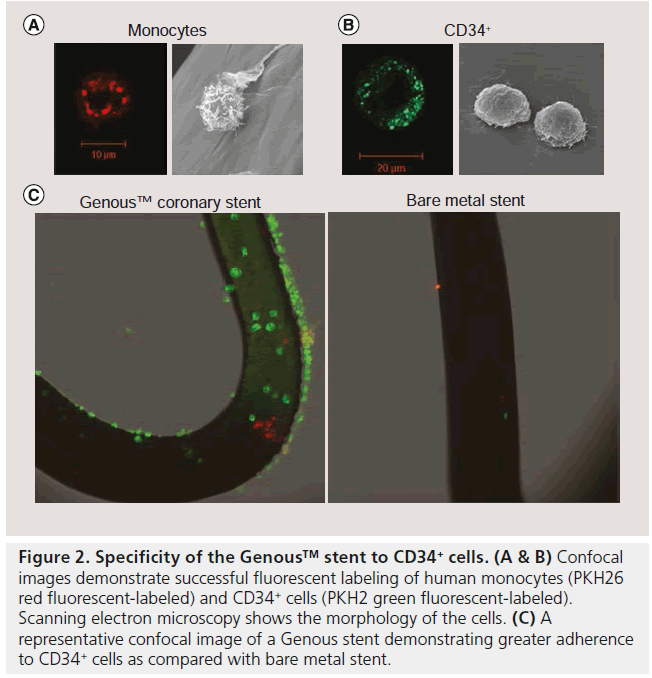
The efficacy of immobilized CD34+ antibodytreated stainless steel surfaces was first tested by Kutryk et al. demonstrating rapid enhancement of bound ECs with the antibody substrate after 5 min with confluence being reached at 60 min [23]. The biological activity was evaluated using f luorescently labeled KG1a cells (immature hematopoietic cell line that express the CD34 antigen) and demonstrated uniform distribution of adherent CD34+ cells after incubation for 1 h. Recently, a similar in vitro assessment of the specificity of the immobilized CD34 antibody was tested using human peripheral CD34+ cells [24]. In the in vitro capture system, Genous and BMS were deployed in silicone tubing and were exposed to a cell mixture of PKH26 red fluorescentlabeled human monocytes (1 × 106 cells/ml) and PKH2 green fluorescent-labeled human CD34+ cells (2 × 105 cells/ml), under a constant rotation speed of 0.3 revolutions per min for 2 h. Confocal microscopy assessment of the stent struts showed that a significantly greater number of CD34+ cells adhere to the Genous stent as compared to the BMS (500 ± 158 vs 17 ± 8 cells/cm2; p < 0.001; Figure 2), whereas monocyte adherence was not significantly different between the two stents (79 ± 44 vs 58 ± 39 cells/cm2; p = 0.07), although a trend was observed for less monocytes on the Genous stent. Overall specificity of the Genous stent to capture CD34+ cells was significantly higher when compared with the BMS, with 86% of the attached cells being CD34+ compared with only 26% on the BMS.
Figure 2: Specificity of the Genous™ stent to CD34+ cells. (A & B) Confocal images demonstrate successful fluorescent labeling of human monocytes (PKH26 red fluorescent-labeled) and CD34+ cells (PKH2 green fluorescent-labeled). Scanning electron microscopy shows the morphology of the cells. (C) A representative confocal image of a Genous stent demonstrating greater adherence to CD34+ cells as compared with bare metal stent.
Recent in vivo studies have also demonstrated enhanced endothelialization of the Genous stent, having an immobilized antihuman CD34 antibody coating. In the study by Larsen et al., acute (7 day) endothelialization rates were compared between the Genous stent and the BMS in a rabbit aorta and ilio-femoral injury model [24]. Scanning electron microscope (SEM) analysis revealed greater cell coverage between and above struts in the Genous stent versus the BMS (p < 0.01). Moreover, quantitative PCR showed increased levels of endothelial markers on the Genous stent for Tie-2 (p = 0.02) and P-selectin (p = 0.05) as compared with BMS, whereas for CD34 (p = 0.08) and CD31 (p = 0.07) levels there was a trend towards significance, thus indicating that the Genous stent promotes endothelialization.
van Beusekom et al. compared the performance of the Genous EPC capture stent to a BMS in a normal swine coronary stent model for early endothelialization (2 and 5 days) and neointimal thickness at 28 and 90 days [25]. Endothelialization by light microscopy and SEM confirmed higher rates of strut coverage in the EPC capture stent as compared with the BMS (2 days: 68 ± 29 vs 53 ± 36%; p = 0.028). At 5 days, both stent groups showed similar endotheliazation rates (>95%). Longer durations (28 and 90 days) showed no differences between the groups in terms of neointimal thickness.
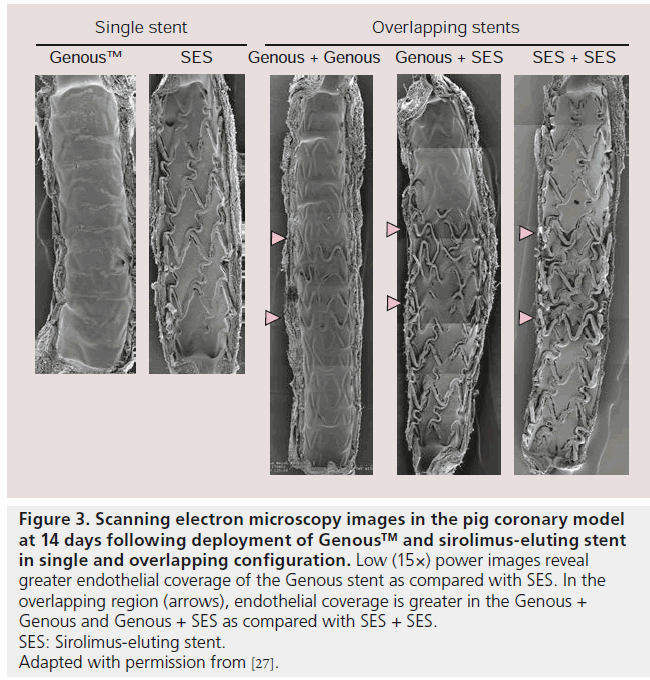
Genous stent performance has also been compared to sirolimus-eluting stents (SES) in a swine coronary model, with the results showing significantly greater endothelial coverage at 14 days by SEM analysis on the Genous stent [26]. Confocal microscopy also showed greater endothelial maturation by quantitative analysis of PECAM-1/CD31 expression on the Genous stent as compared with SES. Neointimal evaluation by optical coherence tomography revealed similar neointimal thickness between the Genous, SES and everolimus-eluting stents (Xience V) at 28 days (0.30 ± 0.16 vs 0.36 ± 0.23 vs 0.31 ± 0.25), however on histologic examination, there was a trend towards greater neointimal thickness for the Genous stent, rather than the SES or everolimus-eluting stents (0.29 ± 0.12 vs 0.21 ± 0.02 vs 0.15 ± 0.05, respectively). Nakazawa et al. compared the performance of the Genous stent versus SES for endothelialization in the porcine model and showed by SEM and confocal microscopy greater endothelialization as well as higher PECAM-1 expression in the Genous stent as compared with the SES at 3 days (endothelialization 76 ± 8 vs 7 ± 3%; PECAM-1 expression 59 ± 25 vs 0 ± 0%) and 14 days, (endothelialization 98 ± 2 vs 53 ± 20%; PECAM-1 expression 96 ± 7 vs 41 ± 20%) [27]. Endothelialization was also compared when the Genous stent was overlapped with another Genous stent as compared with Genous + SES, and SES + SES overlapped. SEM of the overlapping zone showed enhanced rate of endothelial coverage above the strut in the Genous + Genous group (95 ± 6%) and the Genous + SES group (79 ± 5%) compared with SES + SES group (36 ± 14%; p = 0.007) (Figure 3). The nonoverlapping proximal and distal segments from all three combinations showed higher endothelialization rates above the Genous segments (98 ± 3 and 100 ± 0% in Genous + Genous) as compared with the Genous + SES or SES + SES segments (62 ± 33% in Genous; 46 ± 20% in SES; p = 0.0003). Thus confirming that the Genous stent irrespective of whether it was overlapped with SES or not, always showed greater endothelialization versus SES.
Figure 3: Scanning electron microscopy images in the pig coronary model at 14 days following deployment of Genous™ and sirolimus-eluting stent in single and overlapping configuration. Low (15×) power images reveal greater endothelial coverage of the Genous stent as compared with SES. In the overlapping region (arrows), endothelial coverage is greater in the Genous +
Genous and Genous + SES as compared with SES + SES.
SES: Sirolimus-eluting stent.
Adapted with permission from [27].
Genous clinical results
▪ First-in-man
The HEALING First-In-Man (HEALINGFIM) registry investigated the safety and feasibility of the Genous stent in a single-center, prospective, nonrandomized study [28]. A total of 16 stable or unstable angina or silent ischemia patients were enrolled. Patients received dual antiplatelet therapy (DAPT) for 1 month after stent deployment. At 6 month angiographic follow-up, Genous stent showed a 0.63 ± 0.52 mm late lumen loss and in-stent restenosis of 27.2 ± 20.9% as determined by intravascular ultrasound (IVUS). The 9 month composite major adverse cardiac event (MACE) rate (included cardiac death, stroke, myocardial infarction [MI] and target vessel revascularization) was 6.3% with no evidence of stent thrombosis.
▪ Genous clinical trials in stable patients
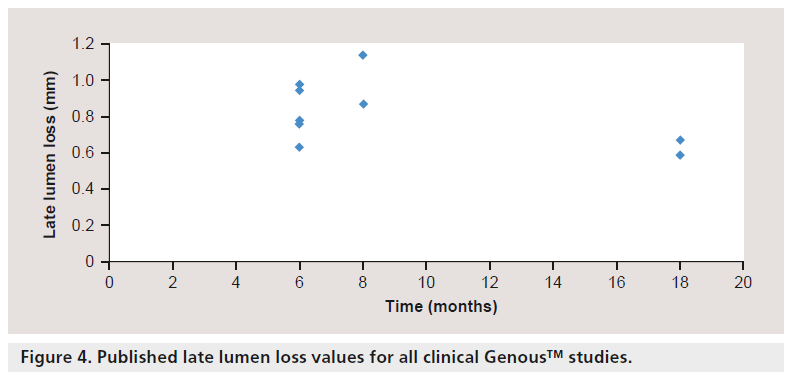
Duckers et al. further evaluated the safety and efficacy of the Genous EPC capture stent in a multicenter, prospective nonrandomized registry study (HEALING II) [29]. Sixty three patients with single de novo native coronary artery lesions were enrolled. The composite MACE (death, MI, emergency coronary artery bypass graft and clinically driven target lesion revascularizaion [TLR]) rate was 6.3% at 9 months and 7.9% at 18 months, and the clinically driven TLR was 6.3% at both 9 and 18 months. Patients received DAPT for 1 month, similar to BMS, and showed no incidence of acute or subacute thrombosis. A significant late regression of neointimal hyperplasia was also observed between 6 and 18 months on quantitative coronary angiography (late lumen loss: 6 months = 0.78 ± 0.39 mm vs 18 months = 0.59 ± 0.31 mm; p = 0.001; 24.4% reduction) (Figure 4) and by IVUS (in-stent volume obstruction: 6 months = 22.94 ± 13.65% vs 18 months = 20.29 ± 14.34%; 11.4% reduction). The authors also correlated neointimal late loss to circulating levels of EPCs and showed that patients with normal EPC titers (>6.5/100 μl) at 6 months follow-up had low lumen loss (0.53 ± 0.06 mm, n = 25) as opposed to patients with low EPC titers (late lumen loss = 1.01 ± 0.07 mm, n = 24) [30]. In addition, statin therapy was associated with a 1.9-fold increase in EPC numbers (10.5 ± 1.12 vs 5.4 ± 0.94; p = 0.001) and improved angiographic outcome (late lumen loss: 0.50 ± 0.05 vs 0.65 ± 0.05 mm; p < 0.001).
The beneficial outcome of statin therapy in the HEALING II study lead to the design of the HEALING IIB study that aimed to assess the safety and efficacy of the Genous stent in conjunction with optimal statin therapy to stimulate EPC recruitment in 100 elective patients with de novo native coronary artery lesion [31]. Within 2 weeks after initiation of high-dose atorvastatin (80 mg once daily) pharmacotherapy, relative EPC levels increased by 5.6-fold and maintained elevated levels during a 30 day follow-up. Despite effective EPC recruitment, angiographic follow-up of late lumen loss data demonstrated no significant differences as compared to HEALING II at 6 months (0.76 ± 0.50 vs 0.78 ± 0.39 mm). Remarkably, comparable with the HEALING II study, angiographic late loss was shown to be significantly reduced from 6 to 18 months (late lumen loss: 0.76 ± 0.50 vs 0.67 ± 0.54 mm; 11.8% reduction; p = 0.001).
The largest study completed to date to test the safety and efficacy of the Genous stent is the eHEALING registry, a worldwide, multicenter prospective study [32]. Approximately 5000 patients were included between October 2005 and 2007 from 144 centers in Europe, Asia/Pacific, Middle East, Africa and Latin America. The objective of the eHEALING registry was to assess the clinical outcome up to 12 months after placement of the Genous stent in a ‘real world’ population with a nonurgent percutaneous coronary intervention (PCI). Patients undergoing PCI with at least one lesion suitable for stenting with the Genous stent (diameter: 2.5–4.00 mm, length 9–33 mm) were enrolled. DAPT was administered to 83% of patients for 30 days, 59% at 6 months and 34% at 12 months. The cumulative event rate of cardiac death, MI and TLR, was 7.9 % at 12 months. Target vessel failure (TVF) was 1.7, 5.7 and 8.4% at 30 days, 6 months and 12 months followup, respectively. Definite stent thrombosis was 0.6%, with the majority of cases having subacute (n = 17, 0.3%), followed by late (n = 8, 0.2%) and acute (6, 0.1%) stent thrombosis. In a posthoc analysis of the eHEALING study, patients who continued DAPT from 30 days through 6 months (n = 2654) were compared with patients on DAPT for only 30 days (n = 4249) and showed a similar incidence of cumulative event rate (6.5 vs 6.3%, p = 0.89). Definite or probable stent thrombosis increased, although not significantly between patients with continued DAPT as compared to those who stopped (0.2 vs 0.6%, p = 0.16) [33]. Several substudies focusing on elderly patients undergoing nonurgent PCI [34] and diabetic patients [35] have been published from the eHEALING registry. The data demonstrated that TVF occurred significantly more often in elderly patients compared with younger patients (age <65 years; 7.0% vs age 65–74 years; 11.7% vs age ≥75 years; 11.7%; p < 0.001).
Recently, a prospective randomized trial evaluating the Genous stent in combination with or without a paclitaxel-coated balloon was performed in 120 patients with de novo coronary artery lesion [36]. Angiographic follow-up at 6 months (follow-up rate 96%) demonstrated treatment with paclitaxel-coated balloon plus the Genous stent was superior to Genous stent alone, with an in-stent late loss of 0.34 ± 0.45 versus 0.88 ± 0.48 mm (p < 0.001). The re-stenosis rate was reduced from 23.2 to 5.1% (p = 0.006) with no definite or probable stent thrombosis in either group.
Overall, in stable patients, the Genous stent has performed well with MACE rates no greater than 17.3% at 1 year with the exception of a single- center prospective study where MACE rates were 28.0% at 1 year for the treatment of older patients (≥75 years) with de novo lesions [37]. In this study, a total of eight deaths occurred, five of which were cardiac deaths. Additionally, two patients suffered from nonfatal acute MI and 22 patients had clinically justified TLR. Definite ST was also observed in an additional two patients.
▪ Genous clinical trials in high-risk patients
A summary of the clinical results of the Genous stent in high-risk patients is provided in Table 1. High risk, in general, was defined as meeting two or more of the following criteria: diabetes, acute coronary syndrome, heart failure, proximal vessel disease, multivessel disease, B2/C type lesion, bifurcation lesion and long lesion. Miglionico et al. studied the outcome of 80 high-risk patients treated with Genous stent with 14 ± 4 months follow-up who received aspirin indefinitely, whereas clopidogrel was discontinued 2 months after angioplasty (patients with acute coronary syndromes were continued for 9 months) [38]. The incidence of MACE was 16%; ten patients underwent percutaneous TLR and three patients had reintervention of a nontarget vessel. There was no incidence of stent thrombosis. Similarly, the study by Low et al. showed no evidence of Academic Research Consortium defined stent thrombosis at a mean follow-up of 34 months with MACE rates at 16% in patients with ST elevation MI (STEMI) [39].
| Study type | Study name | Year | Patients | Indication | Mean FU | Event | Genous | Angiographic or IVUS FU (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| or first | (n) | (months) | type | event rate | |||||
| author | (%) | ||||||||
| First-in-man | |||||||||
| Single-center | HEALING-FIM | 2005 | 16 | Stable or unstable angina or silent | 9 | MACE | 6.3 | 6 month FU – IVUS (100%) | [28] |
| prospective | ischemia | ST | 0.0 | ||||||
| Stable patients | |||||||||
| Multicenter | HEALING-II | 2007 | 63 | De novo stable or unstable angina | 9 | MACE | 7.9 | 6 month FU – angiographic (92.1%), | [29] |
| prospective | or silent ischemia | ST | 0.0 | IVUS (92.1%) | |||||
| 18 | MACE | 7.9 | Angiographic (49.2%), IVUS | ||||||
| ST | 0.0 | (49.2%) | |||||||
| Single-center | Azzarelli | 2010 | 100 | Older patients with de novo | 12 | MACE | 28.0 | FU (73%) | [37] |
| prospective | lesions | ST | 2.0 | ||||||
| Multicenter | HEALING-IIB | 2011 | 96 | De novo coronary lesion | 6 | MACE | 9.4 | 6 month FU – angiographic (89.9%) | [31] |
| prospective | 12 | ST | 2.1 | No routine angiographic or IVUS FU | |||||
| MACE | 15.6 | ||||||||
| 18 | ST | 3.1 | 18 month FU – angiographic | ||||||
| MACE | 15.6 | (78.1%) | |||||||
| 24 | ST | 3.1 | No routine angiographic or IVUS FU | ||||||
| MACE | 16.6 | ||||||||
| Worldwide, | eHEALING | 2011 | 4939 | ‘Real world’ population with a | 12 | TVF | 8.4 | No routine angiographic or IVUS FU | [32] |
| multicenter | nonurgent PCI | ST | 0.6 | ||||||
| postmarketing | |||||||||
| registry | |||||||||
| Single-center | Martin-Yuste | 2011 | 78 | Chronic anti-vitamin K regimen | 14 ± 8 | MACE | 22.0 | No routine angiographic or IVUS FU | [58] |
| prospective | ST | 0.0 | |||||||
| Multicenter | PERfECT | 2011 | 120 | De novo coronary lesion | 6 | TVF | 17.2 | 6 month FU – angiographic (96%) | [36] |
| prospective | ST | 0 | |||||||
| randomized | |||||||||
| High-risk patients | |||||||||
| [38] | |||||||||
| Single-center | Miglionico | 2008 | 80 | High risk | 14 ± 4 | MACE | 16.0 | No routine angiographic or IVUS FU | |
| prospective | ST | 0.0 | |||||||
| Two-center | Co | 2008 | 120 | STEMI | 12 | MACE | 9.2 | No routine angiographic or IVUS FU | [43] |
| prospective | ST | 1.7 | |||||||
| Single-center | Chong | 2010 | 95 | AMI | 24 | MACE | 13.7 | No routine angiographic or IVUS FU | [44] |
| prospective | ST | 3.2 | |||||||
| [47] | |||||||||
| Single-center | Bystron | 2010 | 50 | STEMI | 6 | MACE | 24.0 | Angiographic (88 and 94%), IVUS | |
| prospective | ST | 6.0 | (76 and 72%) | ||||||
| randomized | |||||||||
| Single-center | TRIAS | 2010 | 98 | Stable or unstable angina or a | 12 | TVF | 17.3 | 8 month FU – angiographic (54 and | [40] |
| prospective | non-STEMI | ST | 0.0 | 39%) | |||||
| randomized | |||||||||
| Single-center | Lee | 2010 | 321 | STEMI | 12 | MACE | 12.2 | No routine angiographic or IVUS FU | [46] |
| prospective | ST | 0.9 | |||||||
| Single-center | Beijk | 2010 | 178 | De novo bifurcation lesion | 12 | TVF | 12.4 | No routine angiographic or IVUS FU | [45] |
| prospective | ST | 0.6 | |||||||
| Single-center | Scacciatella | 2011 | 61 | High risk and no option for DES | 24 | MACE | 18.0 | No routine angiographic or IVUS FU | [59] |
| prospective | ST | 1.6 | |||||||
| Single-center | TRIAS | 2011 | 98 | Nonurgent de novo with a high | 24 | TVF | 20.4 | No routine angiographic or IVUS FU | [41] |
| prospective | risk of restenosis | ST | 0.0 | ||||||
| randomized pilot | |||||||||
| study | |||||||||
| Single-center | Low | 2011 | 95 | STEMI | 34 | MACE | 16.0 | 8 month FU – angiographic (82%) | [39] |
| prospective | ST | 0.0 | |||||||
| Multicenter | TRIAS HR | 2011 | 304 | Nonurgent de novo with a high | 12 | TVF | 17.4 | No routine angiographic or IVUS FU | [42] |
| prospective | risk of restenosis | ST | 2.0 | ||||||
| randomized | |||||||||
| Worldwide, | eHEALING | 2011 | 2651 | Patients <65 years with a | 12 | TVF | 7.0 | No routine angiographic or IVUS FU | [34] |
| multicenter | (substudy) | nonurgent PCI | ST | 0.6 | |||||
| postmarketing | 1403 | Patients between 65 and 74 years | TVF | 8.8 | |||||
| registry | |||||||||
| with a nonurgent PCI | ST | 0.7 | |||||||
| 869 | Patients ≥75 years with a | TVF | 11.7 | ||||||
| nonurgent PCI | ST | 0.7 | |||||||
| Worldwide, | eHEALING | 2011 | 1292 | Nonurgent PCI (low-risk patients) | 12 | TVF | 7.0 | No routine angiographic or IVUS FU | [60] |
| multicenter | (substudy) | ST | 0.5 | ||||||
| postmarketing | 2949 | Nonurgent PCI (high-risk patients) | TVF | 8.8 | |||||
| registry | |||||||||
| ST | 0.6 | ||||||||
| Single-center | Klomp | 2011 | 405 | High risk | 12 | TVF | 13.3 | No routine angiographic or IVUS FU | [48,61] |
| retrospective | ST | 0.5 | |||||||
| 36 | TVF | 18.3 | |||||||
| ST | 0.5 |
Table 1. Overview of Genous™ clinical studies.
In the TRIAS trial, 193 patients with lesions carrying a high risk of restenosis were randomly treated with the Genous stent or the paclitaxeleluting stent (PES) [40,41]. At 1 year, the rate of TVF was 17.3% in the Genous stent and 10.5% for the PES. No incidence of stent thrombosis was observed in the Genous stent, however in the PES, four patients (4.2%) on DAPT had a definite stent thrombosis. At 2 years, a nonsignificant difference in TLR between the Genous stent and PES was observed (20.4 vs 15.8%). Stent thrombosis was again absent in the Genous group at 2 years as compared to five lesions (in four patients) in the PES group. However, results of the TRIAS HR trial, an investigator-initiated, prospective, multicenter, single-blind, randomized clinical trial did not establish noninferiority when comparing the Genous endothelial capturing stent with DES in patients carrying lesions with a high risk of restenosis at 12 months (target lesion failure [TLF]: 17.4 vs 7.0%) [42].
Co et al. assessed the use of the Genous stent in primary percutaneous intervention in 120 patients with acute STEMI without cardiogenic shock [43]. DAPT was given for 1 month and statin therapy started immediately after the procedure. The cumulative MACE event rate was 4.2% at 30 days, 5.8% at 6 months and 9.2% at 1 year. Definite stent thrombosis rate at 1 year was 1.7%, one patient presented with an acute and another patient with subacute stent thrombosis, and no incidence of LST.
Chong et al. studied the intermediateterm efficacy and safety of the Genous with a sirolimus-eluting bioabsorbable polymer stent (CURA) and BMS in patients undergoing primary PCI for acute MI. The number of patients enrolled in the study was 366, treated with 95 were treated with the Genous stent, 53 with CURA and 218 with BMS [44]. At 2 years, MACE rates for the Genous stent was 13.7% which were comparable with BMS (19.7%) and DES (15.1%) (p = 0.38). One patient in the Genous group had an acute stent thrombosis with no incidence of LST.
Beijk et al. evaluated 1-year clinical outcome in patients treated with the Genous stent for bifurcation lesions using provisional T-stenting techniques and compared these to historical control groups with an identical BMS [45]. A total of 178 consecutive patients with de novo bifurcation lesions treated with the Genous stent were compared with 465 consecutive patients treated with BMS. At 1 year, the cumulative rate of the primary end point was 12.4% in the Genous group as compared with 17% in the BMS group. The cumulative rate of definite ST was 0.6% in the Genous group as compared with 0.4% in the BMS group. These results showed favorable outcomes in the treatment of bifurcation lesions, however, the results were statistically nonsignificant.
In the largest high risk patient study, Lee et al. investigated the effectiveness of the Genous stent in treatment of patients with acute STEMI [46]. A total of 321 patients receiving 357 Genous stents with a cumulate MACE event rate of 8.1% at 30 days, 10.0% at 6 months and 12.2% at 1 year. One patient developed acute stent thrombosis and another two had subacute stent thrombosis with no incidence of LST. Similar MACE rates were also observed in other high-risk studies except in a single study where the authors report MACE rates of 24% at 6 months with highest incidence of stent thrombosis (6%) in the Genous stent as compared with none in the bare cobalt–chromium group [47].
In a more recent study, Klomp et al. assessed the 1 and 3 year clinical outcome in a large cohort of unselected patients treated with the Genous stent [45,48]. Four hundred and five unselected patients were treated with the EPCcapturing Genous stent, with the majority of patients having complex lesions and high risk of restenosis. At 1 year, TLF (the composite cardiac death, MI and TLR) was 13.3% with the occurrence of definite LST at 0.5%. At 3 years, TLF rates were at 18.3% with no further incidence of very LST. Patients with a high risk of restenosis also showed a higher incidence of TLF as compared with those with low risk (1 year: 16.4 vs 8.7%; p = 0.03). Overall, Genous stent outcomes of high-risk patients have been promising, in particular in patients unable to receive DAPT for long periods of time.
Technological advancement of the Genous coronary stent
The ability to capture circulating EPCs on the stent surface to accelerate healing could have an advantage over BMS or DES, especially in high-risk patient populations. EPCs have the capability to migrate, proliferate and differentiate into endothelial lineage cells [20]. In culture, EPCs have been shown to differentiate into mature ECs as assessed by molecular markers and function [49]. EPC-derived ECs have also been used to develop endothelialized blood vessels, a field that routinely seeks novel autologous cell sources to develop patient-specific tissue, as nonendothelialized blood vessels are prone to thrombosis [50]. Moreover, enhancement of EPCs has been shown indirectly to inhibit in-stent restenosis in preclinical models [51,52].
The immobilized antihuman CD34 coating of the Genous stent is unique, as it represents the only coronary stent on the market that promotes healing by sequestering circulating EPCs. The Genous bioengineered surface features antibodies immobilized on the stent surface, a significant bioengineering feat that provides a stable shelflife technology that allows the capture of circulating EPCs. Although there are many surface antigens present in circulating EPCs (e.g., CD133, CD34, CD31, CD45, von Willebrand factor, CD146 and VEGFR2) that promote EPC mobilization, CD34 has been shown to be more specific for capturing EPCs [53]. However it is worth noting that not all captured CD34+ EPCs will either differentiate into mature ECs or accelerate endothelial adhesion, as the CD34+ marker used to phenotype EPCs is nonspecific, and it is also expressed by both hematopoietic stem cells and mature ECs [54]. Overall, the circulating peripheral blood contains <1% of circulating endothelial precursor cells that express CD34, VEGFR2 and AC133 [54]. A subpopulation of CD34+ hematopoietic stem cells, which has been shown to play a role in vascular maintenance and repair, are the CD34+/KDR+ cells [55]. These cells (CD34+/KDR+) are not directly mobilized from bone marrow but are generated from circulating multipotent CD34+ cells following interaction at platelet-rich sites of vascular injury and exposure to shear stress [55]. This mechanism is consistent with the EPC capture in a recently stented vessel implanted with the Genous stent. Ultimately though, the heterogeneity in cell capture will need to be better characterized, nevertheless, the therapeutic concept of rapid endothelialization using autologous progenitor cells is exciting. Other attempts to modify the stent surface to promote greater cellular healing include increasing surface roughness [56] and binding of the Arg-Gly-Asp (RGD) peptide [57], however, these technologies need further validation.
Conclusion
DAPT certainly helps prevent thrombotic events following stenting, however, DES still remain at risk of LST. Autopsy studies of patients dying from LST have shown that stents with >30% uncovered struts are at nine-times greater risk of thrombosis. Given the increased concern of late thrombosis following DES implantation, EPC-based technologies and strategies that enhance endothelialization are of great interest in order to accelerate healing. The Genous stent was designed to capture circulating EPCs with the idea that DAPT following stenting can be shortened or eliminated as compared with DES in patients at high risk of thrombosis. Moreover, the recovery of endothelial function would prevent platelet and inflammatory cell adhesion to stented surfaces and decrease late loss. Preclinical studies have successfully demonstrated that Genous stents endothelialize at a faster rate as compared with BMS and DES. In clinical studies, the overall safety and efficacy of the Genous stent has been demonstrated in several nonrandomized studies and large registries. Angiographic follow-up studies have shown peak late lumen loss at 6–12 months in the range of 0.6 to 1.1 mm, with significant reduction at 18 months. Clinical results have also shown, in stable patients at 6–24 months, that MACE rates ranged from 6 to 20%. Furthermore, stent thrombosis rates were lower in comparison to DES. In STEMI patients, stent thrombosis rates have shown similar incidences of stent thrombosis in the Genous stent as compared with BMS, except in one randomized study. Further studies are warranted to demonstrate the efficacy of the Genous stent in randomized trials comparing the Genous and BMS as well as comparison to current DES in both stable and unstable patients with increased risk of stent thrombosis including acute MI, left main and vein grafts.
Future perspective
Although the Genous stent has displayed excellent results in terms of stent thrombosis, the late lumen loss has been inferior to current DES. Therefore, a next-generation Genous stent, the ‘Combo’ stent, has been developed in which the EPC-capturing technology (on the luminal surface) is combined with an antiproliferative drug (on the abluminal surface) to minimize the hyperproliferative reaction to the damaged vessel wall to suppress late loss. The efficacy and safety of the Combo stent has recently been investigated in a preclinical model demonstrating superior results in terms of endothelialization and equivalency of neointimal thickness as compared to first- and second-generation DES [26,27].
Fully biodegradable coronary stents are currently being introduced to the market because they could potentially ease adverse events such as LST. As these stents degrade over time, the potential for LST due to nonendothelialized strut or remaining polymer is alleviated. Moreover, biodegradable stents can negate some of the other issues related to permanent metallic stents such as overhang at ostial lesions and the potential for long-term positive remodeling of the stented vessel (i.e., no long-term ‘jailing’ of the vessel).
Major changes for optimal treatment of coronary disease, however, may not come from new devices but rather from accurate assessment of the extent and type of disease by catheterbased imaging techniques. Second-generation IVUS and, more impressively, optical coherence tomography, can provide plaque morphology of tissue components, including identification of high-risk thin cap fibroatheroma. This will require clinical proof and the economic cost will have to be weighed before they can be utilized in routine clinical practice.
Financial & competing interests disclosure
R Virmani is a consultant for Medtronic AVE, Abbott Vascular, WL Gore, Biosensors International, Terumo, Atrium Medical, Arsenal Medical and Lutonix. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Background
▪▪ Delayed healing, characterized by incomplete endothelialization, is the primary substrate underlying drug-eluting stent (DES) thrombosis.
▪▪ Endothelial progenitor cells (EPCs) possess the ability to migrate to areas of vascular injury and differentiate into mature endothelial cells.
▪▪ Recruiting EPCs to injured arterial segments after stenting is an attractive approach to accelerate healing.
Genous™ bioengineered stent
▪▪ A novel ‘prohealing’ stent coated with immobilized antihuman CD34 monoclonal antibody was designed to capture circulating EPCs.
▪▪ The Genous is based on the R stent platform, a unique dual helix design made from 316 stainless steel. Recently, a second generation
has been developed from cobalt–chromium with thinner struts (81 μm).
▪▪ Preclinical studies have shown accelerated endothelialization of the Genous stent as compared with bare metal stents and DES.
▪▪ A first-in-man study provided the safety and efficacy of the Genous stent with no evidence of stent thrombosis at 9 months.
Genous clinical performance
▪▪ In de novo stable lesions the Genous stent major adverse cardiac event rates range from 6 to 20% at 6–24 months with a low rate of
stent thrombosis (<2%).
▪▪ In high-risk ST elevation myocardial infarctions, the major adverse cardiac event rates range from 12.2 to 28% at 12 months with stent
thrombosis between 0 and 6%.
▪▪ Late lumen loss at 6–12 months are in the range of 0.6 to 1.1 mm, greater than DES, however, late loss has been shown to decrease
significantly at 18 months.
Future perspective
▪▪ Prohealing approach in combination with antiproliferate drugs will help reduce late loss while maintaining low stent thrombosis.
▪▪ Fully biodegradable stents could potentially alleviate adverse events associated with permanent polymer metallic DES.
▪▪ Better characterization of the plaque prior to stenting may help lower late stent thrombosis.
References
Papers of special note have been highlighted as:
▪▪ of considerable interest
- Serruys PW, de Jaegere P, Kiemeneij F et al. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. Benestent study group. N. Engl. J. Med. 331(8), 489–495 (1994).
- Fischman DL, Leon MB, Baim DS et al.A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. Stent restenosis study investigators. N. Engl. J. Med. 331(8), 496–501 (1994).
- Moses JW, Leon MB, Popma JJ et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N. Engl. J. Med. 349(14), 1315–1323 (2003).
- Stone GW, Ellis SG, Cox DA et al.A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N. Engl. J. Med. 350(3), 221–231 (2004).
- Farb A, Boam AB. Stent thrombosis redux – the FDA perspective. N. Engl. J. Med. 356(10), 984–987 (2007).
- Daemen J, Wenaweser P, Tsuchida K et al. Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large two-institutional cohort study. Lancet 369(9562), 667–678 (2007).
- Joner M, Finn AV, Farb A et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J. Am. Coll. Cardiol. 48(1), 193–202 (2006).
- Finn AV, Nakazawa G, Joner M et al. Vascular responses to drug eluting stents: importance of delayed healing. Arterioscler. Thromb. Vasc. Biol. 27(7), 1500–1510 (2007).
- Finn AV, Joner M, Nakazawa G et al. Pathological correlates of late drug-eluting stent thrombosis: strut coverage as a marker of endothelialization. Circulation 115(18), 2435–2441 (2007).
- van Beusekom HM, Sorop O, van den Heuvel M et al. Endothelial function rather than endothelial restoration is altered in paclitaxel-as compared to bare metal-, sirolimus and tacrolimus-eluting stents. EuroIntervention 6(1), 117–125 (2010).
- Joner M, Nakazawa G, Finn AV et al. Endothelial cell recovery between comparator polymer-based drug-eluting stents. J. Am. Coll. Cardiol. 52(5), 333–342 (2008).
- Mcfadden EP, Stabile E, Regar E et al. Late thrombosis in drug-eluting coronary stents after discontinuation of antiplatelet therapy. Lancet 364(9444), 1519–1521 (2004).
- Moreno R, Fernandez C, Hernandez R et al. Drug-eluting stent thrombosis: results from a pooled analysis including 10 randomized studies. J. Am. Coll. Cardiol. 45(6), 954–959 (2005).
- Farb A, Sangiorgi G, Carter AJ et al. Pathology of acute and chronic coronary stenting in humans. Circulation 99(1), 44–52 (1999).
- Carter AJ, Laird JR, Farb A, Kufs W, Wortham DC, Virmani R. Morphologic characteristics of lesion formation and time course of smooth muscle cell proliferation in a porcine proliferative restenosis model. J. Am. Coll. Cardiol. 24(5), 1398–1405 (1994).
- Haudenschild CC, Schwartz SM. Endothelial regeneration. II. Restitution of endothelial continuity. Lab. Invest. 41(5), 407–418 (1979).
- Tanaka H, Suzuki A, Schwartz D, Sukhova G, Libby P. Activation of smooth muscle and endothelial cells following balloon injury. Ann. NY Acad. Sci. 748, 526–529 (1995).
- Newby AC, Southgate KM, Assender JW. Inhibition of vascular smooth muscle cell proliferation by endothelium-dependent vasodilators. Herz 17(5), 291–299 (1992).
- Dubey RK, Jackson EK, Luscher TF. Nitric oxide inhibits angiotensin II-induced migration of rat aortic smooth muscle cell. Role of cyclic-nucleotides and angiotensin 1 receptors. J. Clin. Invest. 96(1), 141–149 (1995).
- Asahara T, Masuda H, Takahashi T et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ. Res. 85(3), 221–228 (1999).
- de Val S. Key transcriptional regulators of early vascular development. Arterioscler. Thromb. Vasc. Biol. 31(7), 1469–1475(2011).
- Vogeli KM, Jin SW, Martin GR, Stainier DY. A common progenitor for haematopoietic and endothelial lineages in the zebrafish gastrula. Nature 443(7109), 337–339 (2006).
- Kutryk M, Kuliszewski M. In vivo endothelial progenitor cell seeding for the accelerated endothelialization of endovascular devices. Am. J. Cardiol. 92(6), 94L (2003).
- Larsen K, Cheng C, Tempel D et al. Capture of circulatory endothelial progenitor cells and accelerated re-endothelialization of a bio-engineered stent in human ex vivo shunt and rabbit denudation model. Eur. Heart J. 33(1), 120–128 (2012).
- van Beusekom HM, Ertas G, Sorop O, Serruys PW, van der Giessen WJ. The genous endothelial progenitor cell capture stent accelerates stent re-endothelialization but does not affect intimal hyperplasia in porcine coronary arteries. Catheter. Cardiovasc. Interv.79(2), 231–242 (2012).
- Granada JF, Inami S, Aboodi MS et al. Development of a novel prohealing stent designed to deliver sirolimus from a biodegradable abluminal matrix. Circ. Cardiovasc. Interv. 3(3), 257–266 (2010).
- Nakazawa G, Granada JF, Alviar CL et al. Anti-CD34 antibodies immobilized on the surface of sirolimus-eluting stents enhance stent endothelialization. JACC Cardiovasc. Interv. 3(1), 68–75 (2010).
- Aoki J, Serruys PW, van Beusekom H et al. Endothelial progenitor cell capture by stents coated with antibody against CD34: the HEALING-FIM (healthy endothelial accelerated lining inhibits neointimal growth-first in man) registry. J. Am. Coll. Cardiol. 45(10), 1574–1579 (2005).
- Duckers HJ, Soullie T, den Heijer P et al. Accelerated vascular repair following percutaneous coronary intervention by capture of endothelial progenitor cells promotes regression of neointimal growth at long term follow-up: final results of the Healing II trial using an endothelial progenitor cell capturing stent (Genous
- Duckers HJ, Silber S, de Winter R et al. Circulating endothelial progenitor cells predict angiographic and intravascular ultrasound outcome following percutaneous coronary interventions in the Healing II trial: evaluation of an endothelial progenitor cell capturing stent. EuroIntervention 3(1), 67–75 (2007).
- den Dekker WK, Houtgraaf JH, Onuma Y et al. Final results of the HEALING IIB trialto evaluate a bio-engineered CD34 antibody coated stent (Genous stent) designed to promote vascular healing by capture of circulating endothelial progenitor cells in CAD patients. Atherosclerosis 219(1), 245–252 (2011).
- Silber S, Damman P, Klomp M et al. Clinical results after coronary stenting with the Genous bio-engineered R stent: 12month outcomes of the eHEALING (Healthy Endothelial Accelerated Lining Inhibits Neointimal Growth) worldwide registry. EuroIntervention 6(7), 819–825 (2011).
- Damman P, Klomp M, Silber S et al. Duration of dual antiplatelet therapy and outcomes after coronary stenting with the Genous bio-engineered R stent in patients from the e-HEALING registry. Catheter. Cardiovasc. Interv. 79(2), 243–252 (2012).
- Damman P, Iniguez A, Klomp M et al. Coronary stenting with the Genous™ bio-engineered R stent™ in elderly patients. Circ. J. 75(11), 2590–2597 (2011).
- Damman P, Klomp M, Beijk MA et al. Twelve-month outcomes after coronary stenting with the Genous bio-engineered R stent in diabetic patients from the eHEALING registry. J. Interv. Cardiol. 24(4), 285–294 (2011).
- Wohrle J, Birkemeyer R, Markovic S et al. Prospective randomised trial evaluating a paclitaxel-coated balloon in patients treated with endothelial progenitor cell capturing stents for de novo coronary artery disease. Heart 97(16), 1338–1342 (2011).
- Azzarelli S, Galassi AR, Grosso G et al. Clinical and angiographic outcomes in elderly patients treated with endothelial progenitor cell capture coronary stents: results from a prospective single-center registry. J. Invasive Cardiol. 22(12), 594–598 (2010).
- Miglionico M, Patti G, D’Ambrosio A, Di Sciascio G. Percutaneous coronary intervention utilizing a new endothelial progenitor cells antibody-coated stent: a prospective single-center registry in high-risk patients. Catheter. Cardiovasc. Interv. 71(5), 600–604 (2008).
- Low AF, Lee CH, Teo SG et al. Effectiveness and safety of the Genous endothelial progenitor cell-capture stent in acute ST elevation myocardial infarction. Am.J. Cardiol. 108(2), 202–205 (2011).
- Beijk MA, Klomp M, Verouden NJ et al. Genous endothelial progenitor cell capturing stent vs. the Taxus Liberte stent in patients with de novo coronary lesions with a high-risk of coronary restenosis: a randomized, single-centre, pilot study. Eur. Heart J. 31(9), 1055–1064 (2010).
- Beijk MA, Klomp M, van Geloven N et al. Two-year follow-up of the Genous endothelial progenitor cell capturing stent versus the taxus liberte stent in patients with de novo coronary artery lesions with a high-risk of restenosis: a randomized, single-center, pilot study. Catheter. Cardiovasc. Interv. 78(2), 189–195 (2011).
- Klomp M, Beijk MA, Varma C et al. 1 year outcome of TRIAS HR (TRI-stent adjudication study-high risk of restenosis) a multicenter, randomized trial comparing Genous endothelial progenitor cell capturing stents with drug-eluting stents. JACC Cardiovasc. Interv. 4(8), 896–904 (2011).
- Co M, Tay E, Lee CH et al. Use of endothelial progenitor cell capture stent (Genous bio-engineered R stent) during primary percutaneous coronary intervention in acute myocardial infarction: intermediate-to long-term clinical follow-up. Am. Heart J. 155(1), 128–132 (2008).
- Chong E, Poh KK, Liang S et al. Two-year clinical registry follow-up of endothelial progenitor cell capture stent versus sirolimus-eluting bioabsorbable polymer-coated stent versus bare metal stents in patients undergoing primary percutaneous coronary intervention for ST elevation myocardial infarction. J. Interv. Cardiol. 23(2), 101–108 (2010).
- Beijk MA, Klomp M, Koch KT et al. One-year clinical outcome after provisional T-stenting for bifurcation lesions with the endothelial progenitor cell capturing stent compared with the bare-metal stent. Atherosclerosis 213(2), 525–531 (2010).
- Lee YP, Tay E, Lee CH et al. Endothelial progenitor cell capture stent implantation in patients with ST segment elevation acute myocardial infarction: one year follow-up. EuroIntervention 5(6), 698–702 (2010).
- Bystron M, Cervinka P, Spacek R et al. Randomized comparison of endothelial progenitor cells capture stent versus cobalt-chromium stent for treatment of ST elevation myocardial infarction. Six-month clinical, angiographic, and IVUS follow-up.
- Klomp M, Beijk MA, Damman P et al. Three-year clinical follow-up of an unselected patient population treated with the Genous endothelial progenitor cell capturing stent.
- Tillman BW, Yazdani SK, Geary RL, Corriere MA, Atala A, Yoo JJ. Efficient recovery of endothelial progenitors for clinical translation. Tissue Eng. Part C Methods 15(2), 213–221 (2009).
- Yazdani SK, Tillman BW, Berry JL, Soker S, Geary RL. The fate of an endothelium layer after preconditioning. J. Vasc. Surg. 51(1), 174–183 (2010).
- Walter DH, Rittig K, Bahlmann FH et al. Statin therapy accelerates reendothelialization: a novel effect involving mobilization and incorporation of bone marrow-derived endothelial progenitor cells. Circulation 105(25), 3017–3024 (2002).
- Walter DH, Cejna M, Diaz-Sandoval L et al. Local gene transfer of phVEGF2 plasmid by gene-eluting stents: an alternative strategy for inhibition of restenosis. Circulation 110(1), 36–45 (2004).
- Plouffe BD, Kniazeva T, Mayer JE Jr, Murthy SK, Sales VL. Development of microfluidics as endothelial progenitor cell capture technology for cardiovascular tissue engineering and diagnostic medicine. FASEB J. 23(10), 3309–3314 (2009).
- Peichev M, Naiyer AJ, Pereira D et al. Expression of VEGFR2 and AC133 by circulating human CD34+ cells identifies a population of functional endothelial precursors. Blood 95(3), 952–958 (2000).
- de Boer HC, Hovens MM, van Oeveren-Rietdijk AM et al. Human CD34+/KDR+ cells are generated from circulating CD34+ cells after immobilization on activated platelets. Arterioscler. Thromb. Vasc. Biol. 31(2), 408–415 (2011).
- Samaroo HD, Lu J, Webster TJ. Enhanced endothelial cell density on NiTi surfaces with sub-micron to nanometer roughness.Int. J. Nanomed. 3(1), 75–82 (2008).
- Blindt R, Vogt F, Astafieva I et al. A novel drug-eluting stent coated with an integrin-binding cyclic Arg-Gly-Asp peptide inhibits neointimal hyperplasia by recruiting endothelial progenitor cells. J. Am. Coll. Cardiol. 47(9), 1786–1795 (2006).
- Martin-Yuste V, Brugaletta S, Ferreira-Gonzalez I et al. Endothelial progenitor cell capturing stent and short dual antiplatelet therapy in patients on chronic anti-vitamin K regimen undergoing percutaneous coronary interventions: long-term outcomes of a single centre registry. EuroIntervention 6(7), 831–837 (2011).
- Scacciatella P, Meliga E, D’Amico M et al. Percutaneous coronary interventions with an endothelial progenitor cell capture stent (EPC) for high risk patients with no option for drug eluting stents: long term clinical outcomes of a single centre registry. EuroIntervention 6(7), 826–830 (2011).
- Klomp M, Damman P, Beijk MA et al. Applying the National Institute for Clinical Excellence criteria to patients treated with the Genous™ Bio-engineered R stent™: a sub-study of the eHEALING (Healthy Endothelial Accelerated Lining Inhibits Neointimal Growth) worldwide registry. Heart Vessels doi:10.1007/s00380-011-0167-8(2011) (Epub ahead of print).
- Klomp M, Beijk MA, Tijssen JG, de Winter RJ. One-year clinical outcome in an unselected patient population treated with the Genous endothelial progenitor cell capturing stent. Catheter. Cardiovasc. Interv. 77(6), 809–817 (2011).
▪▪ First report of delayed arterial healing in drug-eluting stents at autopsy.
▪▪ First to demonstrate the role of endothelial progenitor cells in vascular repair.
▪▪ Details the specificity of the Genous™ stent in capturing CD34+ cells in vitro and ex vivo.
▪▪ First-in-man clinical results of the Genous stent.
nn Largest clinical study to date showing good clinical results of the Genous stent with low incidence of repeat revascularization and stent thrombosis.