Rapid Communication - Interventional Cardiology (2011) Volume 3, Issue 4
Accuracy and reliability of point-of-care platelet assays in hemodialysis patients
- Corresponding Author:
- Yin Ping Liew
Department of Renal Services, RIPAS Hospital
Ministry of Health, Brunei Darussalam
Tel: +673 224 2424
Fax: +673 245 0488
E-mail: lyp2000@gmail.com
Abstract
Keywords
hemodialysis, platelet function, point-of-care assay
The point-of-care platelet assay (VerifyNow® Aspirin assay) measures platelet aggregation in response to arachidonic acid (AA). It is US FDAapproved to evaluate platelet inhibition by aspirin (ASA) in order to document ASA resistance, which is associated with worse cardiovascular disease (CVD) outcomes [1–4]. In healthy individuals, the assay is accurate, reproducible and correlates well with optical aggregometry in measuring baseline platelet aggregation [5]. However, it is often the case that a test widely used in patients without end-stage renal disease (ESRD) may give different results in patients on dialysis. Literature using this assay in the dialysis population is lacking, and so it is unclear whether it can be reliably used to document ASA resistance in patients with ESRD.
The dialysis population has a significantly higher prevalence of CVD and a higher mortality risk as compared with the general population [6]. In addition, patients with renal dysfunction appear to have diminished benefit from antiplatelet therapy [7]. Observational studies have reported wide variation in ASA use in the dialysis population and have not shown that ASA use improved CVD outcomes [8]. Furthermore, antiplatelet treatment was reported to be associated with an increased risk of bleeding in the hemodialysis (HD) cohort [9]. Literature on ASA dosing and/or ASA resistance are not available for the dialysis population. Hence, nephrologists have been using ASA for primary or secondary prevention in their dialysis patients without adequate data on proper dosing, benefits or risks. The inability of ASA to prevent CVD events in dialysis patients may be due to underdosing of ASA, ASA resistance, or other as yet unidentified factors.
Optical aggregometry is a useful method for assessing platelet function. However, it is time-consuming, not widely available and requires experienced laboratory staff [10]. For a large population or for frequent testing, the methodology should be accurate, reproducible, convenient, quick and inexpensive. In comparison to optical aggregometry, the point-of-care platelet assay is inexpensive, more portable and more convenient. For these reasons, it would be practical to use the point-of-care platelet assay to evaluate ASA resistance in a large population.
The purpose of this study is to evaluate the accuracy and reliability of the point-of-care platelet assay in assessing baseline platelet function. This study compares platelet function results from the point-of-care platelet assay to platelet function results from the gold standard assay, optical aggregometry, in a cohort of HD patients. This study evaluates the precision, reproducibility and agreement of the point-ofcare platelet assay with the gold standard assay optical aggregometry.
Materials & methods
▪ Study subjects
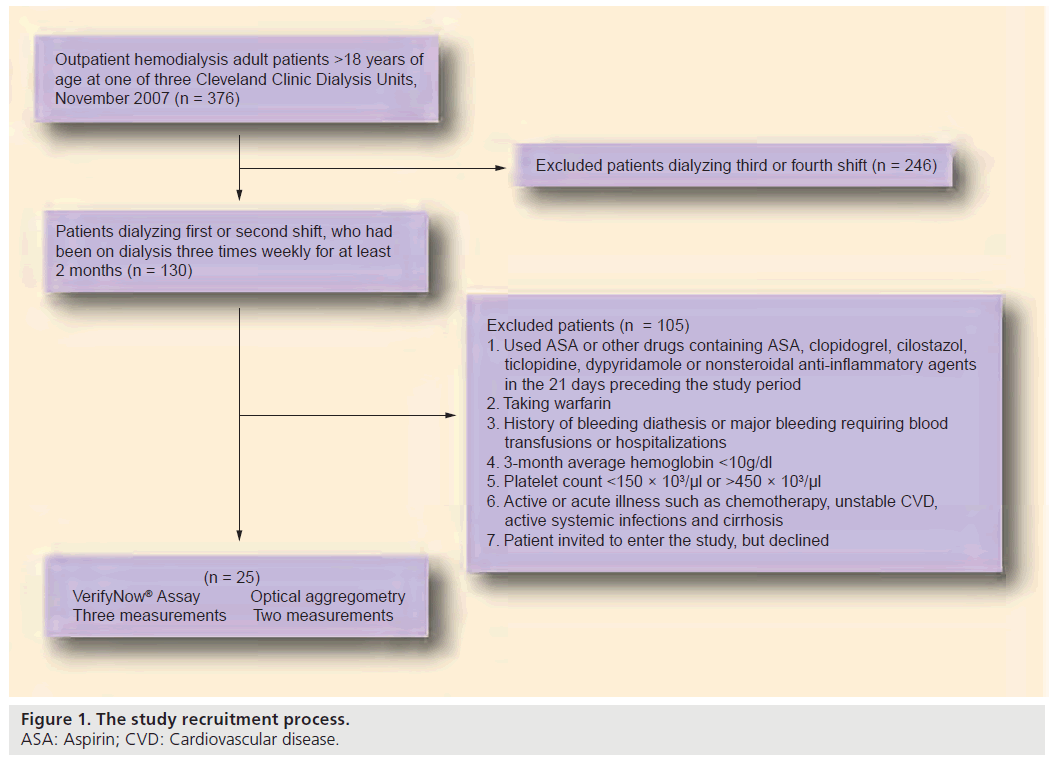
The study was approved by the Cleveland Clinic Institutional Review Board. We enrolled 25 dialysis subjects from three dialysis centers who were >18 years of age and on HD three times weekly for at least 2 months. Exclusion criteria included: the use of ASA or other drugs containing ASA, clopidogrel, cilostazol, ticlopidine, dypyridamole or nonsteroidal antiinflammatory agents in the 21 days preceding the study period; warfarin use; history of bleeding diathesis or major bleeding requiring blood transfusions or hospitalizations; 3-month average hemoglobin <10 g/dl; platelet count <150 × 103/μl or >450 × 103/μl; and active or acute illness (e.g., chemotherapy, unstable CVD, active systemic infections and cirrhosis).
▪ Research procedures
Data included demographics, frequency and duration of HD treatments, medical and surgical history, medications and allergies, collected from the Cleveland Clinic database. Baseline complete blood count was collected from each patient’s monthly labs. Medical records were reviewed and study subjects were questioned to confirm eligibility. All the clinical data were collected before platelet function assessment. After informed consent, each eligible subject underwent a baseline evaluation of platelet aggregation using two assays: the VerifyNow assay (three measurements) and optical aggregometry (two measurements). Blood samples were collected predialysis after access was obtained via arterio-venous fistula/graft or central venous catheter. Blood was collected into 3.2% buffered sodium citrate Vacutainer® tubes. The first blood sample tube was discarded. The collected blood samples (two tubes for optical aggregometry and three tubes for VerifyNow assay) were not spun or refrigerated and were delivered to the laboratory within 4 h for testing. The samples were tested as soon as they arrived at the laboratory. The VerifyNow assays were run consecutively. The optical aggregometry testing for both agonists was performed at the same time as the VerifyNow assay.
The VerifyNow ASA assay is a turbidimetricbased optical detection that uses AA as the agonist and measures platelet aggregation as an increase in light transmittance. The results are interpreted based on the extent of platelet aggregation, reported as ASA reaction units (ARUs). Platelet aggregation was considered normal for ARUs in the range of 631–676 units based on our institution’s laboratory results in healthy volunteers [5]. Optical aggregometry (10 μM adenosine diphosphate (ADP) and 0.5 mg/ml AA agonists) was performed using a BioData PAP-4® platelet aggregometer. Plateletrich plasma (adjusted to a platelet count of 300,000/μl) was evaluated as aggregating agents (ADP and AA) were added. Optical density changes are detected as platelets begin to aggregate and optical aggregometry results were reported as maximal percent platelet aggregation. The normal range for percent platelet aggregation was 66–100% for AA and 70–100% for ADP (based on our institution’s laboratory results on healthy volunteers). One laboratory technologist performed all the assays and was not blinded to the results of the other assays. Since the testing was all performed from a single blood specimen and had to be completed in a time-critical manner, it was not possible to blind the laboratory technologist to the results of the other assay. However, the results of the assays are performed by an analytical instrument with quantitative results that are not subjectively influenced by the technologist.
▪ Statistical analysis
For baseline demographics, categorical data were presented as frequencies with percent and continuous data as mean ± standard deviation and medians. Precision was assessed with the coefficient of variation (CV) along with 95% CI for the ARUs. CV is a statistic that measures the variability of an end point relative to its average value, that is, standard deviation divided by the mean. This statistic is a measure of precision in that low values (CV <100%) indicate high precision and high values (CV >100%) indicate low precision. Reproducibility was measured with Spearman’s correlation coefficient between three replicates of ARUs per subject. The correlation coefficient ranges from -1 to +1, where 0 means no correlation (i.e., not reproducible), and values close to 1.0 in absolute value indicate high correlation (i.e., high reproducibility). The percentage of scores within 30 ARUs of each other was reported with the 95% CI [5]. Fisher’s exact testing was used to assess the agreement of platelet function testing results between the two assays. The statistical analysis was performed using SAS V9.1 (SAS Institute Inc., NC, USA).
Results
The study subjects were recruited in November 2007. A total of 25 eligible subjects were recruited in the study from the full population of HD patients at three dialysis units, as shown in Figure 1. None of the study subjects experienced adverse events related to blood draws. Table 1 shows the baseline demographic and clinical information for our study cohort. The mean age of the 25 subjects was 53 ± 17 years. The majority (72%) were African–Americans and a slightly higher proportion of the cohort was male (56%). Hypertension and diabetes mellitus were the cause of ESRD in ten of the 25 subjects, consistent with other published samples. Mean duration of HD was 221 ± 35 min. Mean hemoglobin was 12 ± 1 g/dl and mean platelet count 260 ± 76 K/μl. Mean ARUs were 647 ± 26 (median: 655; range: 559–662). The VerifyNow assay results were precise with the low value for CV of 4% (95% CI: 3–5%) indicating high precision. The VerifyNow assay results were highly reproducible with 100% of the replicates within 30 ARUs (5% of 647) of each other, and with an overall weighted correlation coefficient of 0.65.
Figure 1: The study recruitment process.
ASA: Aspirin; CVD: Cardiovascular disease.
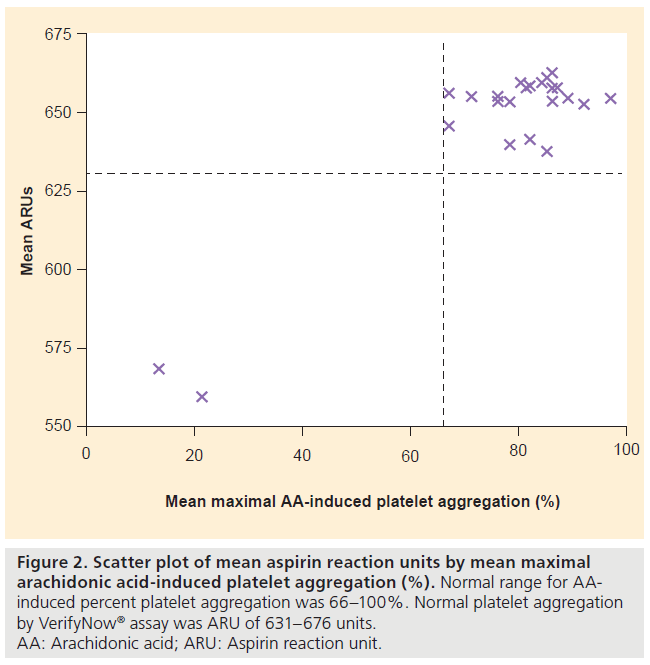
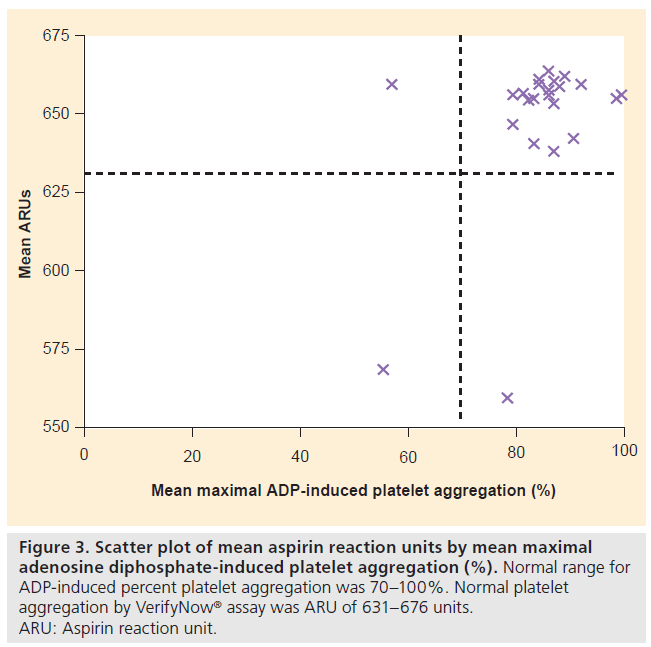
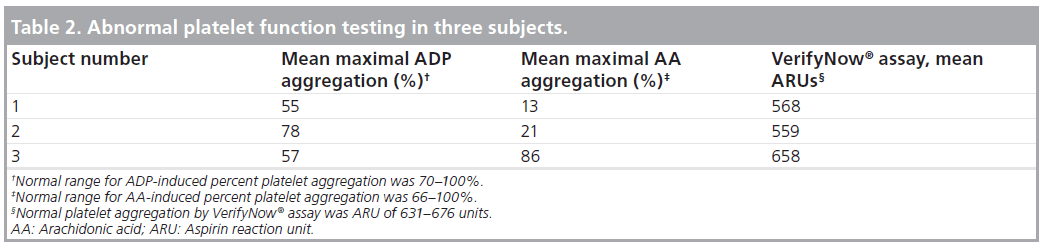
Optical aggregometry showed a mean maximal AA-induced platelet aggregation of 76 ± 19% (median: 82%) and a mean maximal ADP-induced platelet aggregation of 84 ± 10% (median: 86%). The VerifyNow assay results agreed with the AA-based optical aggregometry assay results in all subjects (Figure 2). The VerifyNow assay results agreed with the ADPbased optical aggregometry assay results in 23 of 25 patients (92%) (Figure 3). The strong agreement between the two assays was confirmed by Bland- Altman plots. One patient (subject two) had abnormal platelet aggregation by AA-based optical aggregometry and VerifyNow assay but normal aggregation by ADP-based optical aggregometry. One patient (subject three) had abnormal platelet aggregation by ADP-based optical aggregometry but normal aggregation with AA-based optical aggregometry and VerifyNow assay (Table 2). Subject one had abnormal platelet function by both VerifyNow and optical aggregometry assays (Table 2). Only three of 25 patients had abnormal platelet studies by optical aggregometry, as shown in Table 2.
Figure 2: Scatter plot of mean aspirin reaction units by mean maximal
arachidonic acid-induced platelet aggregation (%). Normal range for AAinduced
percent platelet aggregation was 66–100%. Normal platelet aggregation
by VerifyNow® assay was ARU of 631–676 units.
AA: Arachidonic acid; ARU: Aspirin reaction unit.
Discussion
The point-of-care platelet assay VerifyNow correlates well with optical aggregometry in the general population [5] and, in this study, accurately assessed the baseline platelet function in HD patients. This study has demonstrated that the VerifyNow assay for point-of-care platelet testing in this HD cohort was precise, reproducible and agreed well with optical aggregometry. Using AA as the agonist, the two assays were in agreement on platelet function results in all the patients. This study finding supports the use of the point-of-care platelet assay in future studies to evaluate the effect of ASA on platelet inhibition in a cohort of HD patients. The results of this study also support use of the point-of-care platelet assay to document prevalence of ASA resistance within a cohort of HD patients who receive current standard of care ASA dosing, and also to test associations between ASA dosing and resistance, and CVD outcomes. Ultimately, the goal of future studies is to determine optimal dosing of ASA that inhibits platelets effectively to reduce or prevent CVD events and does not pose an increased risk of bleeding in the HD cohort.
In addition, this study also provides a reference value for baseline platelet aggregation results by point-of-care assay that can be used in future studies to evaluate the platelet inhibition effect of ASA in a cohort of HD patients. The mean ARUs were similar to the healthy subjects reported by Karha et al. with ARUs of 647 ± 26 compared with 640.3 ± 52.6 [5]. A recent study by Tanrikulu et al. reported an ASA resistance rate of 46.1% in a HD cohort of 115 patients. However, the cohort followed in this study was historically reported to have a high prevalence of platelet dysfunction related to multiple factors, some of which are listed later, and the study investigators did not examine baseline platelet function in their HD cohort [11].
In this study, only three of 25 subjects had abnormal platelet function testing. Further clinical assessment of the three patients did not reveal any medical conditions or medications that could interfere with platelet function. Previous reports have found uremic platelet dysfunction to be common and associated with an increased risk of bleeding in ESRD [12]. Causes of uremic platelet dysfunction were thought to be multifactorial related to uremic toxins, vessel wall alterations, effects of HD procedures and anemia [12]. In this study, the blood samples were drawn predialysis to avoid the previously reported platelet dysfunction due to the transient effect of HD [13]. Therefore, this may explain the relatively low prevalence of platelet dysfunction in this study cohort. Although our study subject number is small, if verified in larger numbers, platelet function may improve with better dialysis regimens, biocompatible dialyzers, erythropoietin use or other factors.
This study has several limitations. First, this study has a small sample size. The cohort is composed of a relatively young HD cohort, with a majority of African–Americans, and so may not be representative of the general HD population. Even though the study sample size is small, the study was able to demonstrate the precision and reproducibility of the assay results with repeated measurement of three blood samples, and has shown a strong agreement with the average of two measurements of optical aggregometry. Second, this study only measured baseline platelet function, but did not measure the antiplatelet effect of ASA. This study should ideally be undertaken as a crossover study where baseline platelet function was measured and antiplatelet effect of ASA measured after starting patients on ASA. It is, however, not ethical to start patients on ASA just for study purposes without good clinical indication, especially since currently there is no strong clinical data to support the use of ASA as primary prevention of CVD outcomes in the HD population who tend to have an increased risk of bleeding. More importantly, this study serves as a basis for future studies to test the reliability of this assay in measuring ASA responsiveness in patients who are already on ASA in the HD cohort. Third, the VerifyNow assay used in this study only measured platelet aggregation in response to AA for platelet function assessment. Of course, aggregation assays can test platelet aggregation response to many more agonists, but the AA comparison performed in this study is a reasonable, if limited, comparison. In addition to AA, we also used ADP-based optical aggregometry to confirm that the VerifyNow assay can be reliably used in assessing baseline platelet function in the HD cohort and it is shown that overall the VerifyNow assay results agreed well with ADP-based optical aggregometry.
Conclusion
This study has demonstrated that the pointof- care platelet assay VerifyNow is reliable in measuring baseline platelet function with a high degree of accuracy in a HD cohort.
Future perspective
Point-of-care platelet assay is reliable and accurate in measuring baseline platelet function in the HD cohort. This study serves as a basis for future platelet function studies in a large HD cohort on ASA effect, dosing, ASA resistance, and the association between ASA resistance and CVD outcomes.
Acknowledgements
The authors thank Riana Naude, Leslie Smith, Susan Busch and Karen Mallow for their assistance in patient enrollment, Teresa Kaldus for laboratory testing and Accumetrics for providing the cartridges for VerifyNow® assay free of charge.
Financial & competing interests disclosure
Deepak L Bhatt has received research grants from Astra Zeneca, Bristol-Myers Squibb, Eisai, Ethicon, Heartscape, Sanofi Aventis and The Medicines Company. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- Chen WH, Cheng X, Lee PY et al. Aspirin resistance and adverse clinical events in patients with coronary artery disease. Am. J. Med. 120, 631–635 (2007).
- Chen WH, Lee PY, Ng W, Tse HF, Lau CP. Aspirin resistance is associated with a high incidence of myonecrosis after non-urgent percutaneous coronary intervention despite clopidogrel pretreatment. J. Am. Coll. Cardiol. 43, 1122–1126 (2004).
- Bhatt DL. Aspirin resistance: more than just a laboratory curiosity. J. Am. Coll. Cardiol. 43, 1127–1129 (2004).
- Gum PA, Kottke-Marchant K, Welsh PA, White J, Topol EJ. A prospective, blinded determination of the natural history of aspirin resistance among stable patients with cardiovascular disease. J. Am. Coll. Cardiol. 41, 961–965 (2003).
- Karha J, Rajagopal V, Kottke-Marchant K, Bhatt DL. Lack of effect of enteric coating on aspirin-induced inhibition of platelet aggregation in healthy volunteers. Am. Heart J. 151(5), 976. e7–11 (2006).
- Cheung AK, Sarnak MJ, Yan G et al. Cardiac diseases in maintenance hemodialysis patients: results of the HEMO Study. Kidney Int. 65, 2380–2389 (2004).
- Dasgupta A, Steinhubl SR, Bhatt DL et al. Clinical outcomes of patients with diabetic nephropathy randomized to clopidogrel plus aspirin versus aspirin alone (a post hoc analysis of the clopidogrel for high atherothrombotic risk and ischemic stabilization, management, and avoidance [CHARISMA] trial). Am. J. Cardiol. 103, 1359–1363 (2009).
- Ethier J, Bragg-Gresham JL, Piera L et al. Aspirin prescription and outcomes in hemodialysis patients: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am. J. Kidney Dis. 50, 602–611 (2007).
- Chan KE, Lazarus JM, Thadhani R et al. Anticoagulant and antiplatelet usage associates with mortality among hemodialysis patients. J. Am. Soc. Nephrol. 20, 872–881 (2009).
- Chambless LE, McMahon R, Finch A et al. ARIC hemostasis study – III. Quality control. Atherosclerosis risk in communities. Thromb. Haemost. 70, 588–594 (1993).
- Tanrikulu AM, Ozben B, Koc M et al. Aspirin resistance in patients with chronic renal failure. J. Nephrol. DOI: 10.5301/ JN.2011.6259 (2011) (Epub ahead of print).
- Escolar G, Diaz-Ricart M, Cases A. Uremic platelet dysfunction: past and present. Curr. Hematol. Rep. 4, 359–367 (2005).
- Sreedhara R, Itagaki I, Lynn B et al. Defective platelet aggregation in uremia is transiently worsened by hemodialysis. Am. J. Kidney Dis. 25, 555–563 (1995).
▪ Aspirin resistance is associated with an increased risk of adverse clinical outcomes in stable patients with coronary artery disease.
▪ Aspirin resistance was associated with an increased risk of myonecrosis following nonurgent percutaneous coronary intervention.
▪ Aspirin resistance is associated with an increased risk of cardiovascular events.
▪▪ Point-of-care platelet assessment correlated well with the gold standard of laboratory-based optical aggregometry in healthy individuals.