Review Article - Interventional Cardiology (2021) Volume 13, Issue 1
Acute Papillary Muscle Rupture Immediately After ST-Segment Elevation Acute Myocardial Infarction: A Case Report and Review
- Corresponding Author:
- Masamichi Iwasaki
Department of Cardiovascular Surgery,
Awaji Medical Center,
Hyogo Prefectural Awaji Medical Center,
1-1-137 Shioya,
Sumoto,
Hyogo,
Japan,
E-mail: iwa_michi1114@yahoo.co.jp
Received date: November 17, 2020 Accepted date: December 01, 2020 Published date: December 08, 2020
Abstract
Papillary Muscle Rupture (PMR) is a rare complication (0.5-5%) with high mortality that usually occurs two to seven days after Acute Myocardial Infarction (AMI). However, rapid diagnosis and concomitant treatment can improve early and long-term outcomes. We describe a rare PMR occurring immediately after AMI. The patient complained of exertional chest pain and dyspnea in cardiogenic shock, and was diagnosed with acute PMR by echocardiography. Electrocardiogram showed STsegment elevation in inferior leads, and the time course of creatinine kinase elevation indicated early onset of AMI. Herein, we describe an unusual PMR immediately after AMI and further review PMR.
Keywords
Acute myocardial infarction • Papillary muscle rupture• Mitral valve replacement
Abbreviations
AMI: Acute Myocardial Infarction; IABP: Intra-Aortic Balloon Pump; MR: Mitral Regurgitation; LAD: Left Anterior Descending Artery; LCX: Left Circumflex Artery; PMR: Papillary Muscle Rupture; RCA: Right Coronary Artery
Introduction
In cases of Acute Myocardial Infarction (AMI), coagulative necrosis occurs within 24 hours of initial chest pain and progresses to softening of the infarcted myocardium; thus, granulation tissue and early collagen deposition support the area of infarction approximately one week after AMI [1]. Therefore, mechanical complications of AMI such as left ventricular free wall rupture, interventricular wall rupture, and Papillary Muscle Rupture (PMR) commonly occur two to seven days after the onset of AMI [2]. PMR is a rare and serious complication of AMI that can lead to acute mitral regurgitation, pulmonary edema, and cardiogenic shock. Prior to early reperfusion therapy, the frequency is ~3%, but early reperfusion with thrombolytic therapy and percutaneous coronary intervention can decrease the frequency to 0.7% [3,4]. We present a case report [5] whose cytological findings showed severe ischemic damage before the onset of AMI and discuss PMR as a mini-review including pathological insights.
Case Report
A 73-year-old woman was transferred to the emergency room in our hospital for a sudden onset of chest pain and dyspnea. Her consciousness was unclear, she had agonizing facial expressions, and her initial vital signs were as follows: blood pressure, 96/72 mmHg; heart rate, 130 beats/minute; respiratory rate, 32 breaths/minute; and oxygen saturation, unmeasurable using a 15-L reservoir mask. The troponin I level was 6.231 ng/mL, but the levels of creatine kinase and creatine kinase MB were not increased (119 U/L and 14 U/L, respectively). The chest radiograph showed a butterfly shadow.
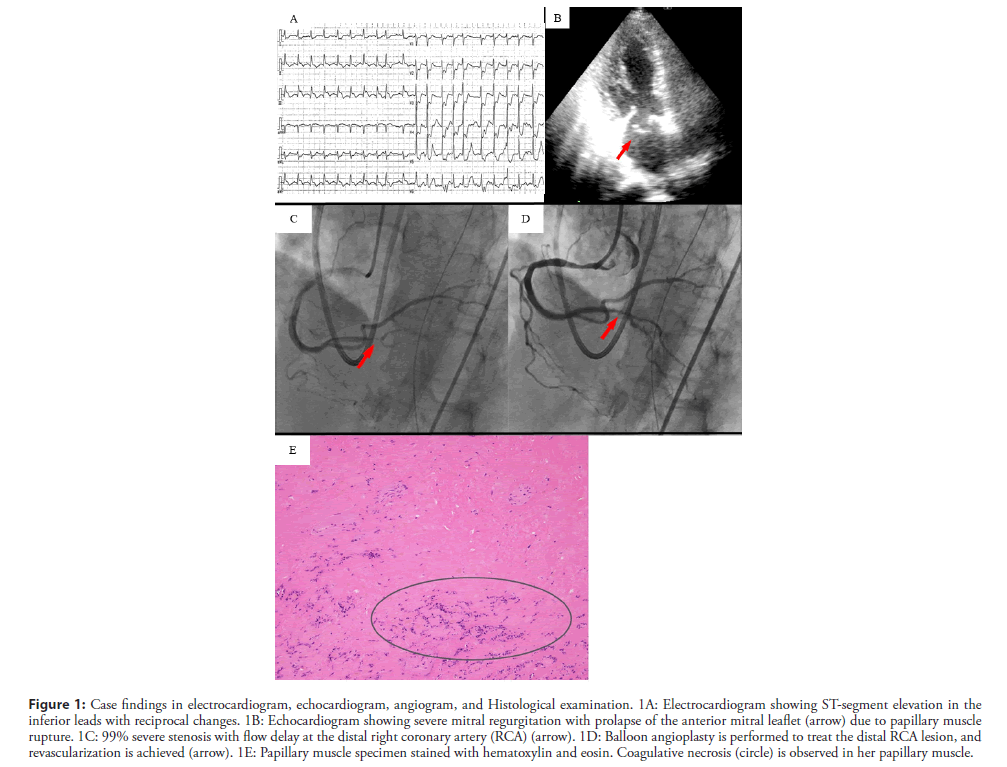
The electrocardiogram demonstrated ST-segment elevation in leads II, III, and aVF and depression in leads V2-V6 (Figure 1A). The echocardiogram demonstrated akinesis in the inferior region and severe Mitral Regurgitation (MR) due to prolapse of the anterior mitral leaflet, but the visual estimate of the left ventricular ejection fraction was 50% (Figure 1B).
Figure 1: Case findings in electrocardiogram, echocardiogram, angiogram, and Histological examination. 1A: Electrocardiogram showing ST-segment elevation in the inferior leads with reciprocal changes. 1B: Echocardiogram showing severe mitral regurgitation with prolapse of the anterior mitral leaflet (arrow) due to papillary muscle rupture. 1C: 99% severe stenosis with flow delay at the distal right coronary artery (RCA) (arrow). 1D: Balloon angioplasty is performed to treat the distal RCA lesion, and revascularization is achieved (arrow). 1E: Papillary muscle specimen stained with hematoxylin and eosin. Coagulative necrosis (circle) is observed in her papillary muscle.
We promptly performed intubation and supported her breathing with a mechanical ventilator. She was emergently transferred to the cardiac catheterization laboratory unit and had an Intra-Aortic Balloon Pump (IABP) inserted to stabilize her hemodynamics. The emergent coronary angiogram showed 99% severe stenosis with a severe flow delay in the distal Right Coronary Artery (RCA), 90% stenosis of the proximal Left Anterior Descending artery (LAD), and collateral circulation from the septal branches to the RCA. Balloon angioplasty was performed to treat the distal RCA lesion, and revascularization was achieved without a flow delay (Figures 1C and 1D).
After the percutaneous coronary intervention, the patient was referred for an emergent mitral valve replacement with concomitant coronary artery bypass grafting. The emergency surgery started 3 hours after the onset, i.e., approximately 2.5 hours after arrival at our hospital. Upon surgical inspection, the posteromedial papillary muscle was found to be completely ruptured. Mitral valve replacement was performed using a 23 mm Carpentier-Edwards PERIMOUNT biological valve (Edwards Lifesciences, Irvine, California, USA). The left intrathoracic artery was anastomosed to the LAD, and a saphenous vein graft was anastomosed to the RCA.
Postoperative serial blood test results showed that the peak values of creatine kinase and creatine kinase MB were 1380 IU/L and 137 IU/L just after surgery. The patient was successfully discharged in a good condition on postoperative day 26.
Histological examination of the posterior papillary muscle confirmed coagulative necrosis with cytological findings consistent with an infarct age of less than seven days (Figure 1E).
Anatomic insights on PMR
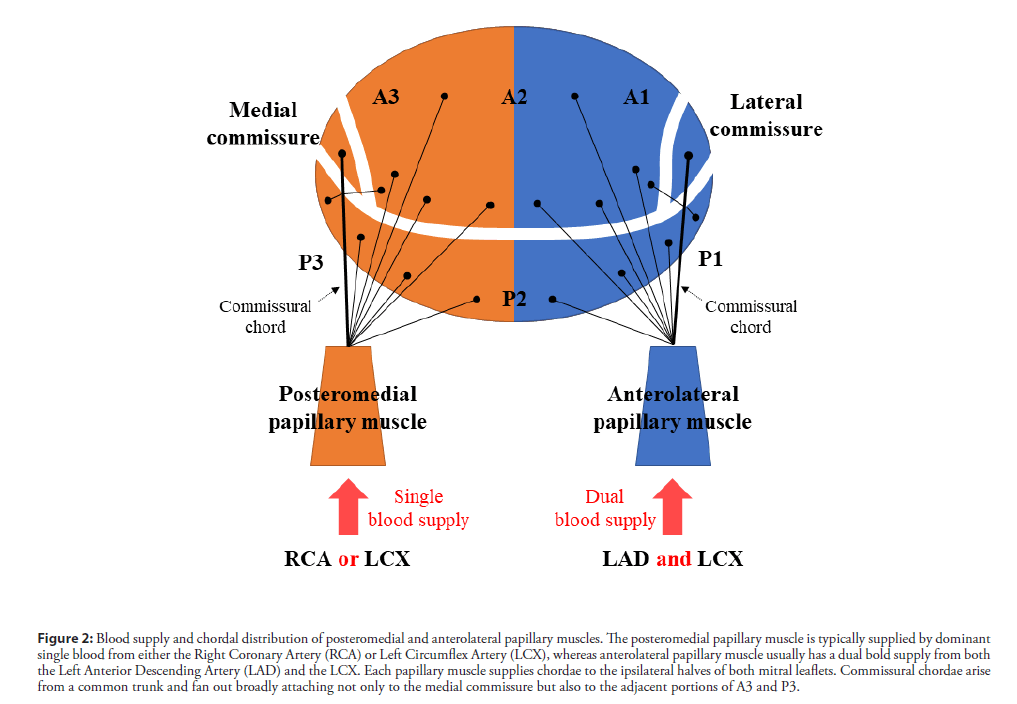
The mechanisms of PMR are tightly linked to the anatomy of the papillary muscles. The posteromedial papillary muscle tends to rupture more frequently (90%) than the anterolateral papillary muscle [3]. The posteromedial papillary muscle is typically supplied by a dominant single blood flow from the RCA or LCX, whereas the anterolateral papillary muscle usually has a dual blood supply from both the LAD and the LCX (Figure 2). Therefore, a small infarction of the supplied vessel to the posteromedial papillary muscle is often caused by PMR. Approximately 50% of PMR cases have a relatively small infarction, and their ejection fraction is preserved, which leads to increased shearing stress at the rupture site. In our case, the small inferior AMI due to the posterior descending artery was thought to be the culprit vessel, and her left ventricular contractility was increased by noninfarcted segments. This would have resulted in the hyperdynamic systolic function, and her posterior papillary muscle may not have been able to tolerate the higher shear stress.
Figure 2: Blood supply and chordal distribution of posteromedial and anterolateral papillary muscles. The posteromedial papillary muscle is typically supplied by dominant single blood from either the Right Coronary Artery (RCA) or Left Circumflex Artery (LCX), whereas anterolateral papillary muscle usually has a dual bold supply from both the Left Anterior Descending Artery (LAD) and the LCX. Each papillary muscle supplies chordae to the ipsilateral halves of both mitral leaflets. Commissural chordae arise from a common trunk and fan out broadly attaching not only to the medial commissure but also to the adjacent portions of A3 and P3.
Each papillary muscle supplies chordae to the ipsilateral halves of both mitral leaflets [6]; therefore, complete posteromedial PMR results in the flail of the medial halves of both leaflets. Commissural chordae arise from a common trunk and fan out broadly attaching not only to the medial commissure, but also to the adjacent portions of A3 and P3. Therefore, the medial commissure was reported to be involved in approximately two-thirds of the PMR cases, either alone or in combination with other segments [7].
Pathological findings and time course of PMR
Myocardial apoptosis has been previously observed in AMI before the occurrence of coagulative necrosis. In the following one to two days, morphological features of coagulative necrosis are observed with progressing softening of the infarcted myocardium. Following the necrotic and fragile process, granulation tissue and early collagen deposition develop approximately one week after AMI, which sustains the site of infarction [8]. Therefore, mechanical complications, including PMR, usually occur two to seven days after AMI.
However, interestingly, in our case, PMR occurred immediately after AMI. On the basis of the pathological findings in our case, we speculated that our patient suffered a silent and severe ischemic event a few days before the onset of AMI, and coagulative necrosis might have progressed in the small posterior descending artery. This would have resulted in a small inferior AMI with hyperdynamic systolic function, and her posterior papillary muscle could have been too fragile to tolerate the higher shear stress.
On the other hand, ischemic PMR without coronary artery lesion [9,10] and nonischemic PMR, such as myocarditis, Ehler- Danlos, infectious, severe annular calcification, and Takotsubo cardiomyopathy, have been reported [11]. In the ischemic PMR cases without coronary artery lesions, postoperative pathologic examination shows localized infarcted areas in the ruptured papillary muscle. Vessels in the papillary are located at the terminal of the coronary circulation and are highly vulnerable to damage induced by hypoxia or ischemia. Subendocardial supply and demand imbalance due to left ventricular hypertrophy and microvascular angina are the possible causes of ischemic PMR without coronary artery lesions.
Echocardiographic assessments
Transthoracic echocardiography is the gold standard for the diagnosis of acute PMR to assess papillary muscles, mitral valve flail, degree of MR, and left ventricular function [12]. The ruptured papillary muscle segments appear as highly mobile echodensities that display to-and-fro motion and prolapsing into the left atrium. The mitral valve flail involves one or more leaflet segments, and the most common site is the medial commissure. However, in 2D echocardiographic images, commissural flail often goes undetected and misdiagnosed as leaflet perforation [13]. Although MR due to PMR is almost always severe, the Color Doppler flow signal sometimes underestimates the MR severity. Reduced transmitral driving pressure results in atrial pressure elevation and ventricular systolic pressure decrease, and large regurgitant orifice area and the brief duration of regurgitation by rapid equalization of left ventricle and left atrial pressures or tachycardia are considered to be the cause of failed MR evaluation [14]. Left ventricular contractility is frequently hyperdynamic in the acute PMR state because of increased contractility of noninfarcted segments, left ventricle unloading from severe MR, and inotropic support. Thus, the regional wall-motion abnormality is likely to underestimate true severity [15].
Transthoracic echocardiography is the gold standard for the diagnosis of acute PMR to assess papillary muscles, mitral valve flail, degree of MR, and left ventricular function [12]. The ruptured papillary muscle segments appear as highly mobile echodensities that display to-and-fro motion and prolapsing into the left atrium. The mitral valve flail involves one or more leaflet segments, and the most common site is the medial commissure. However, in 2D echocardiographic images, commissural flail often goes undetected and misdiagnosed as leaflet perforation [13]. Although MR due to PMR is almost always severe, the Color Doppler flow signal sometimes underestimates the MR severity. Reduced transmitral driving pressure results in atrial pressure elevation and ventricular systolic pressure decrease, and large regurgitant orifice area and the brief duration of regurgitation by rapid equalization of left ventricle and left atrial pressures or tachycardia are considered to be the cause of failed MR evaluation [14]. Left ventricular contractility is frequently hyperdynamic in the acute PMR state because of increased contractility of noninfarcted segments, left ventricle unloading from severe MR, and inotropic support. Thus, the regional wall-motion abnormality is likely to underestimate true severity [15].
Treatment for PMR
PMR carries a high mortality rate due to the development of cardiogenic shock leading to acute pulmonary edema and rapid deterioration within hours of diagnosis. Therefore, expeditious intervention is needed to achieve optimal outcomes [16]. After prompt diagnosis by echocardiography, patients should be immediately transported to a cardiac catheterization laboratory for coronary angiography and IABP insertion to improve hemodynamic stability. The benefit of concomitant revascularization has been shown in prior studies [17], so coronary artery bypass grafting and mitral valve replacement with preservation of the subvalvular apparatus are performed in most cases [18]. Mitral valve repair may be beneficial in select patients who are free from endocarditis and anticoagulation with partial PMR [19]. The papillary muscle after PMR may be necrotic and fragile, and the setting in acute PMR is usually rapidly deteriorating. Therefore, urgent mitral replacement provided promptly for hemodynamic stabilization is required for most PMR cases.
Conclusion
We describe a very rare case of PMR immediately after AMI, which may have been caused by a silent and severe ischemic event a few days before the onset of PMR. The mechanism of PMR is tightly linked with the anatomical features of papillary muscles, and optimal rapid diagnosis and surgical strategy for PMR are established.
Conflict of Interest
Authors have no conflict of interest.
References
- Veinot JP, Gattinger DA, Fliss H. Early apoptosis in human myocardial infarcts. Hum Pathol. 28(4): 485-92 (1997).
- Lavie CJ, Gersh BJ. Mechanical and electrical complications of acute myocardial infarction. Mayo Clin Proc. 65(5): 709-30 (1990).
- Russo A, Suri RM, Grigioni F, et al. Clinical outcome after surgical correction of mitral regurgitation due to papillary muscle rupture. Circulation. 118(15): 1528-34 (2008).
- Gueret P, Khalife K, Jobic Y, et al. Echocardiographic assessment of the incidence of mechanical complications during the early phase of myocardial infarction in the reperfusion era: A French multicentre prospective registry. Arch Cardiovasc Dis. 101(1): 41-7 (2008).
- Kamada T, Iwasaki M, Fujimoto W, et al. Acute papillary muscle rupture immediately after ST-segment elevation acute myocardial infarction: A case report. J Cardiol Cases. 18(6): 189-91 (2018).
- Silbiger JJ, Bazaz R. Contemporary insights into the functional anatomy of the mitral valve. Am Heart J. 158(6): 887-95 (2009).
- Jouan J, Tapia M, Lansac E, et al. Ischemic mitral valve prolapse: mechanisms and implications for valve repair. Eur J Cardiothorac Surg. 26(6): 1112-7 (2004).
- Schoen FJ, Mitchell RN. Myocardial infarction. In: Robbins and Cotran Pathologic Basis of Disease. 540-50 (2015).
- Kitada Y, Arakawa M, Miyagawa A, et al. Ischaemic papillary muscle rupture without significant coronary artery lesion. Interact Cardiovasc Thorac Surg. 29(6): 971-2 (2019).
- Sakata T, Mogi K, Sakurai M, et al. Rupture of anterolateral papillary muscle resulting from small artery occlusion. Ann Thorac Surg. 104(2): e113-e114 (2017).
- Gouda P, Weilovitch L, Kanani R, et al. Case report and review of nonischemic spontaneous papillary muscle rupture reports between 2000 and 2015. Echocardiography. 34(5): 786-90 (2017).
- Harari R, Bansal P, Yatskar L, et al. Papillary muscle rupture following acute myocardial infarction: Anatomic, echocardiographic, and surgical insights. Echocardiography. 34(11): 1702-7 (2017).
- Aubert S, Barreda T, Acar C, et al. Mitral valve repair for commissural prolapse: Surgical techniques and long term results. Eur J Cardiothorac Surg. 28(3): 443-7 (2005).
- Whiting PC, Morgan-Hughes NJ. Transesophageal echocardiographic findings in papillary muscle rupture. Anesth Analg. 101(5): 1292-3 (2005).