Case Report - Diabetes Management (2017) Volume 7, Issue 5
Adjusting doses of U-500R insulin: the importance of using continuous blood glucose monitoring
- *Corresponding Author:
- Connie A Valdez
School of Pharmacy
University of Colorado
Aurora, Colorado, USA
E-mail: connie.valdez@ucdenver.edu
Abstract
Introduction: A U-500R Insulin Program was implemented for patients requiring >200 units of insulin. Similar to U-100 insulin, titration was based on patient BG logs. However, there was concern for nocturnal hypoglycemia, as U-500R is expected to peak while the patient is sleeping and s/he may not realize hypoglycemia is occurring. In this report, we describe how continuous blood glucose monitoring (CGM) may have prevented serious hypoglycemia in a patient receiving U-500R insulin. Case study: A 61 year-old patient was receiving U-500R. His BG log demonstrated an average: FBG of 220 mg/dL, breakfast PPBG of 209 mg/dL, lunch PPBG of 181 mg/dL, dinner PPBG of 154 mg/dL and bedtime BG of 155 mg/dL, with no values <70 mg/dL. Based on these readings, the evening dose was increased to 0.29 mL (145 units) and the morning dose remained at 0.37 mL (185 units). A two-week CGM was then implanted into the patient’s arm. Despite the lack of patient reported hypoglycemic episodes or evidence of hypoglycemia per BG logs, the patient was having nocturnal hypoglycemic episodes between midnight and 6 am where his BG was below 70 mg/dL13% of the time, with a couple of readings dropping below 50 mg/dL and one reading reaching 40 mg/dL. The dose was adjusted and repeated CGM was used to more accurately stabilize his dose. Conclusion: Had this patient’s U-500R dose been adjusted based on BG values, and not CGM, we likely would have continued to increase the nighttime dose to reduce FBG values, which may have resulted in severe hypoglycemia or death. Disclosures: The authors have declared no potential conflicts of interest.
Keywords
U-500R insulin, continuous glucose monitoring (CGM), primary care
Introduction
Patients with diabetes mellitus who are severely insulin resistant often do not achieve optimal blood glucose control as the large volumes of U-100 insulin may lead to altered absorption and leakage during administration [1]. Insulin human (regular) 500 units/mL (U-500R) is an option for patients who are severely insulin resistant requiring 200 units or more of insulin daily [2]. Since U-500R insulin is five times more concentrated than U-100 insulin, smaller-volumes are required which can improve absorption and minimize leakage. Furthermore, U-500R provides both basal and postprandial glucose control [3,4]. Hood et al. has provided two U-500R treatment approaches for patients with uncontrolled type 2 diabetes with or without oral agents. One approach is to dose U-500R twice daily with 60% of the total daily dose before breakfast and 40% before dinner. The other approach is to dose U-500R three times daily with 40% of the total daily dose before breakfast, 30% before lunch, and 30% before dinner [4].
To better manage patients who have severe insulin resistance who are uninsured or were unable to get an appointment with endocrinology, our primary care clinic developed a U-500R Insulin Program for appropriate candidates. Criteria for initiation into the U-500R Insulin Program include patients who are receiving 200 units or more of insulin daily, have minimal or no mental cognition impairment, are adherent with clinic visits and medications and are able to recognize and appropriately manage hypoglycemia. Based on the literature, a twice daily dosing protocol was implemented where 60% of the total daily dose is to be administered before breakfast and 40% before dinner. Although Hood et al. recommends a 20% dose reduction in patient who have an A1C <8% and direct dose conversion if the A1C is >8%, our protocol is more conservative, where all patients converted from U-100 to U-500R insulin have a 20% dosage reduction despite A1C values; this was incorporated into the protocol to ensure safety and reduce the likelihood of hypoglycemia [4]. Patients are instructed to record a fasting blood glucose (FBG) every morning, a 2 h post prandial (2 h PP) everyday (rotate between breakfast, lunch, dinner), and at bedtime every night. Adjustment of U-500R is based on fasting blood glucose (FBG) and post-prandial blood glucose (PPBG) values. Adjustment to the morning dose is based on dinner and bedtime averages and adjustment to the evening dose is based on FBG averages. Based on this protocol, we have transitioned and titrated several patients from U-100 to U-500R successfully and the improvement in A1C has been noted in all patients. However, understanding the physiologic implications of U-500R was unknown as the literature has demonstrated how the peak and duration varies based on the dose. There is concern of nocturnal hypoglycemia, as the U-500R is expected to peak while the patient is sleeping and the patient may not realize s/ he is experiencing hypoglycemia. Although the manufacturer does not recommend 3 am BG testing, to monitor for adverse events and hypoglycemia, Hood et al. included an algorithm where a 3 am BG check was performed within 48 h of any U-500R dosage change [4]. This additional BG checking may be beneficial for monitoring safety of U-500R, but is not realistic in actual practice. An alternative method to monitor for nocturnal hypoglycemia is to use continuous blood glucose monitoring (CGM) devices. Bergen et al. have proposed the use of Professional CGM to assist with monitoring and titrating U-500R, but literature is currently lacking regarding the use of CGM in this setting [5].
Recently, the clinic secured funding to purchase Professional CGM devices (Abbott FreeStyle Libre ProTM). This CGM device is intended to be used by healthcare professionals and is indicated for detecting trends and tracking patterns of BG values in adults with diabetes mellitus [6]. The device has allowed insulin doses to be adjusted based on two weeks of continuous blood glucose data rather than relying on patient logs reporting FBG and PPBG readings. One patient who was previously initiated on U-500R was offered CGM so his dose could be more accurately adjusted. During this process, it also allowed our clinicians to learn about the physiologic trends of U-500R which could help guide future dosing U-500R. In this report, we describe a patient who was receiving U-500R to control blood glucose and how CGM was used to identify blood glucose trends over a 24 h period in order to more accurately adjust the doses of U-500R.
Case Report
A 61-year-old disabled Native American man (weight, 166 kg; height, 178 cm, BMI 52) presented to clinic with a history of type 2 diabetes mellitus (diagnosed in July 2000), hypertension, dyslipidemia, GERD, chronic venous insufficiency, edema, COPD, heart failure, obesity, nicotine dependence, atherosclerotic heart disease, BPH, hematospermia, and diabetic complication including neuropathy and stage 2 chronic kidney disease. Medications included U-500R 0.37 ml (185 units) in the morning and 0.30 ml (150 units) in the evening, sitagliptin/ metformin 50/1000 orally twice daily, chlorthalidone 25 mg orally daily, valsartan 160 mg orally twice daily, metoprolol tartrate 50 mg orally twice daily, atorvastatin 80 mg orally daily, Protonix 40 mg orally daily, Combivent Respimat 1 puff into lungs four times daily, Symbicort 160/4.5 mg 2 puffs inhaled into lungs twice daily, aspirin 81 mg orally twice daily, Flomax 0.4 mg orally daily, clomiphene 50 mg orally daily, ibuprofen 600 mg orally every 6 h as needed for pain, and tizanadine 2 mg orally three times daily as needed. Patient has no known drug allergies. Social history included smoking; patient has past history of alcohol and illicit drug use, but is no longer using either. Patient’s mother and father are both deceased. Father had hypertension and unspecified heart disease and mother had an unknown medical history.
Laboratory findings included an A1C and fasting blood glucose (FBG) in March 2017 which were 8.6% and 240 mg/dL, respectively. In June, the A1C decreased to 8.3% and the FBG decreased to 142 mg/ dL. The urinary analysis was within normal limits (WNL) except protein which was 30 mg/dL, microalbumin/Cr ratio was 510 mcg/g, triglycerides were 192 mg/dL, HDL was 29 mg/dL, LDL was 67 mg/dL and total cholesterol was 134 mg/dL. Complete blood count and metabolic panel were WNL except glucose which was 200 mg/dL.
DM management prior to CGM placement
The patient was on a dose of U-500R with approximately 60% of the dose in the morning and 40% of the dose in the evening. He also maintained food logs to help with the interpretation of hyper and hypoglycemia episodes. When the patient was seen in March 2017 he was currently on a dose of U-500R 0.35 mL (175 units) in the morning and 0.25 mL (125 units) in the evening. At that visit, based on his BG logs and lack of hypoglycemic episodes, his dose was increased to 0.36 mL (180 units) in the morning and 0.26 mL (130 units) in the evening. The patient returned to clinic for a follow-up visit 6 weeks later (April 2017) where his dose was again increased to 0.37 mL (185 units) in the morning and 0.27 mL (135 units) in the evening. One month later, his evening dose was again increased based on detailed patient reported logs for May 2017 which demonstrated an average FBG of 220 mg/dL, average breakfast PPBG of 209 mg/dL, average lunch PPBG of 181 mg/ dL, average dinner PPBG of 154 mg/dL and average bedtime blood glucose of 155 mg/ dL, with no readings below 70 mg/dL. The evening dose was increased to 0.29 mL (145 units) and the morning dose did not change. Because the U-500R dose continued to be increased, it was felt that this patient would be a good candidate for CGM to better adjust his dose as we continued to work toward his goal of an A1C <8%; this would also allow us to see the blood glucose trends over time from U-500R in an effort to minimize hypoglycemic episodes and optimize his treatment regimen.
▪ First CGM
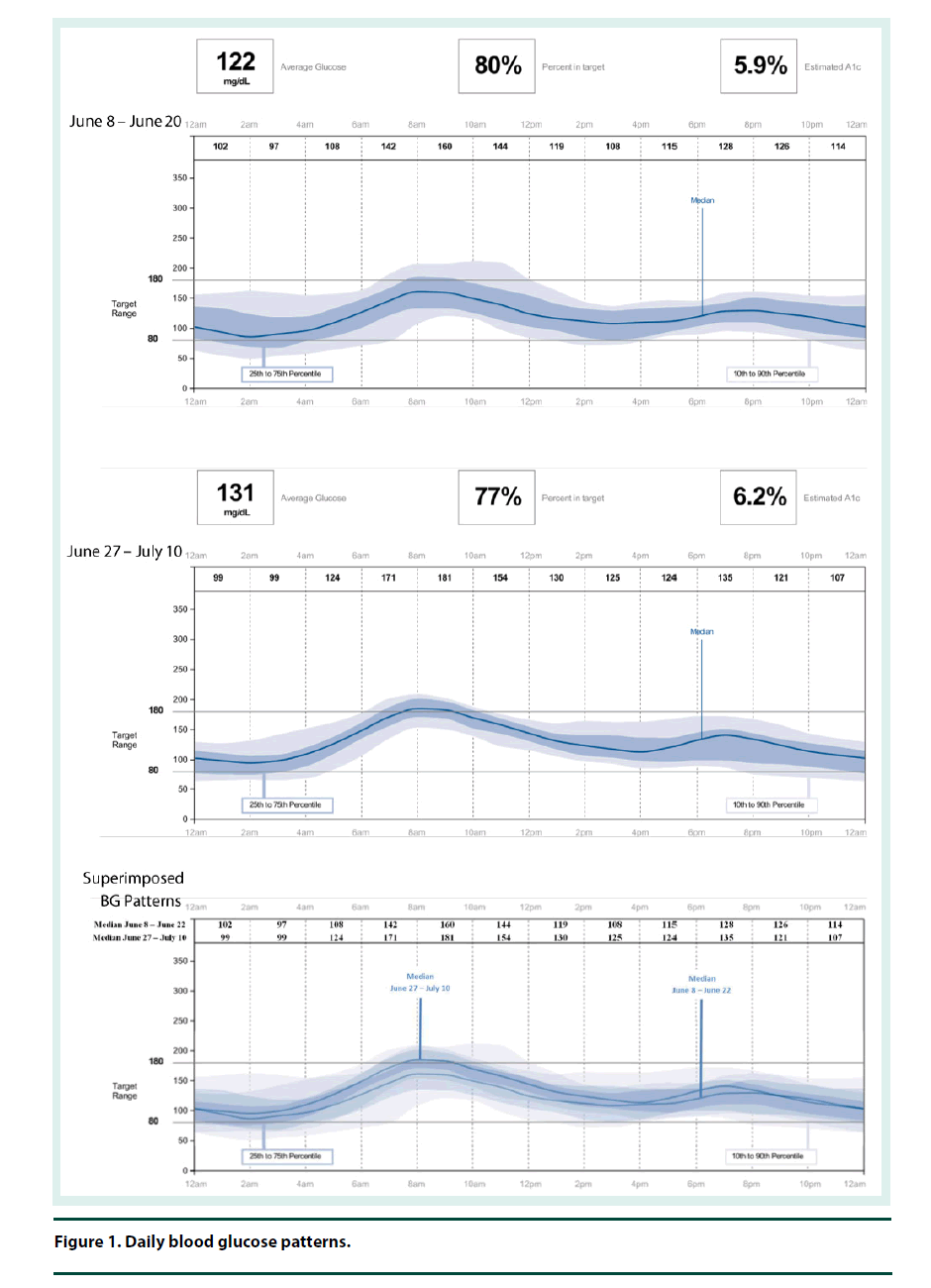
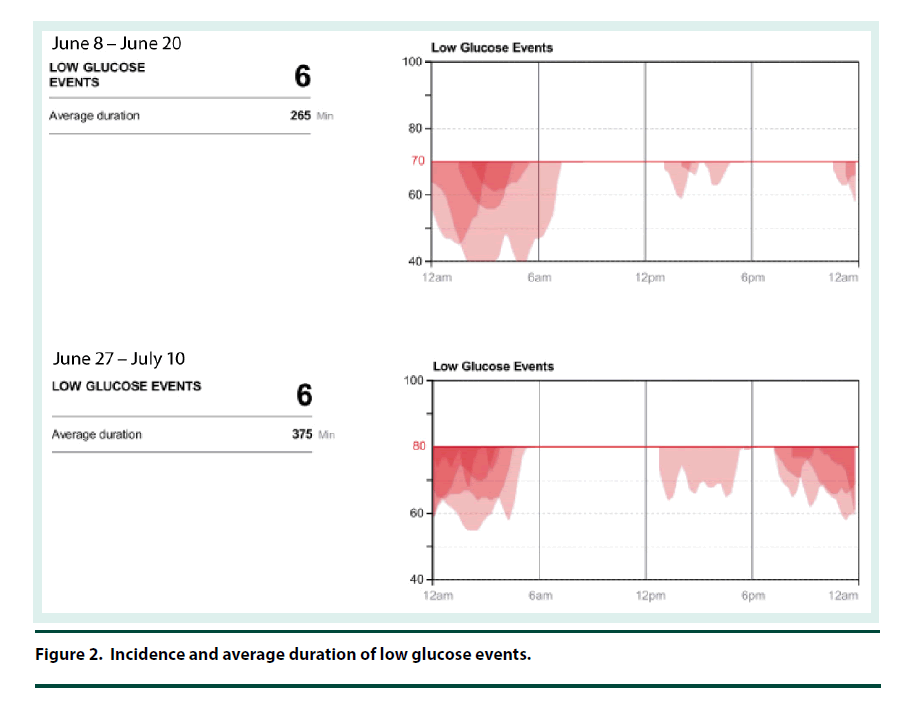
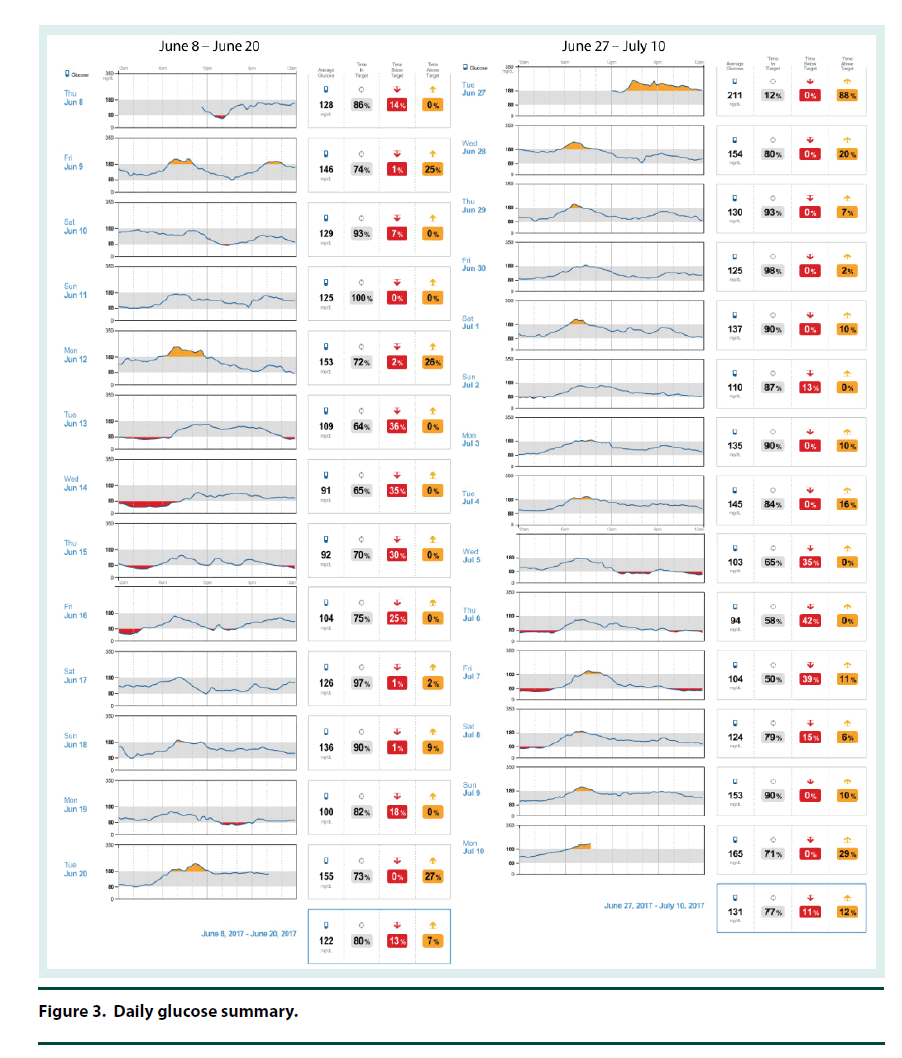
The CGM was placed on the back of the patient’s upper arm in early June 2017 and it remained in place for 12 days. At his 2 week follow-up visit, the patient brought in his CGM and the data was downloaded and reviewed. The CGM reports demonstrated that the current dose of U-500R had sufficiently been titrated to an effective dose with an average blood glucose of 122 mg/dl (Figure 1). The estimated A1C was 5.9% per CGM report and the time in goal range (70-180) was 80% (Figure 1). The CGM report also showed 6 hypoglycemic events where BG was less than 70 mg/dL and most of these events occurred between 12 am – 6 am (Figure 2). Specifically, he was below 70 mg/dL 13% of the time during the twelve day monitoring period, with a few readings dropping below 50 mg/dL and evidence that BG may have been as low as 40 mg/dL (Figures 2 and 3). The patient provided SMBG logs for the same time that the CGM was worn which showed that the average FBG was 170 mg/dL, average breakfast PPBG was 163 mg/dL, average lunch PPBG was 142 mg/dL, average dinner PPBG was 151 mg/dL and average bedtime blood glucose was 139 mg/dL, with no readings below 70 mg/dL. Despite the lack of patient reported hypoglycemic episodes or evidence of hypoglycemia per patient provided blood glucose log values, the CGM report showed the patient was having unknown nocturnal hypoglycemic episodes (Figure 2). Based on the trends from the CGM, it was identified that the evening dose needed to be reduced as the patient was actually dropping below 70 mg/dL during the early morning hrs prior to awakening. Furthermore, aggressive A1C reduction was not necessary in this patient since he had multiple comorbidities which justified a higher A1C goal of less than 8%. The U-500R dose was modified, where he was to continue to inject 0.37 ml (185 units) in the morning, but the evening dose was decreased to 0.31 mL (155 units). The patient requested to have another CGM placed to continue to better adjust his U-500R dose.
▪ Second CGM
The second CGM was placed on the patient in late June and removed in mid-July 2017. At this visit, the CGM data was reviewed demonstrating the current dose of U-500R had reduced his episodes of lows and increased his average blood glucose to 131 mg/dL (Figures 1-3). His estimated A1C was 6.2% per CGM and the time in goal range (80-180) was 77% (Figure 1). Although the data from the first CGM included the default hypoglycemia cut-off of 70 mg/dL, the clinician downloading the data from the second CGM raised the hypoglycemia cut-off to 80 mg/dL since the patients A1C goal was <8%. Thus, the incidence of hypoglycemia was likely underreported for the first CGM data set. The total time below goal range decreased from 13% to 11% (Figure 3). Blood glucose values were above goal 12% of them time but it was for a very short duration (approximately 4 h per day); this was expected based on the fact that he has a higher A1C goal and thus higher FBG and PPBG values were acceptable (Figure 3). Based on patient reported logs for the same time period the second CGM was in place, the average FBG was 174 mg/ dL, average breakfast PPBG of 188 mg/dL, average lunch PPBG of 133 mg/dL, average dinner PPBG of 151 mg/dL and average bedtime blood glucose of 137 mg/dL, with no readings below 70 mg/dL. Although the severe hypoglycemic events (BG <50 mg/dL) identified by the first CGM report resolved with the dose increase, there was still a trend of nocturnal lows below 80 mg/dL in the early morning prior to awakening. Based on these readings, it was identified that the evening dose needed to be reduced further to 0.30 mL (150 units).
Discussion
We describe the case of a patient who had severe insulin resistant type 2 diabetes mellitus who was treated with U-500R where CGM was used to adjust the dose and better understand the physiologic trends of U-500R on his blood glucose. Prior to the application of the CGM, the patient’s dose was adjusted based on blood sugar logs, which is standard practice for adjusting insulin in most patients. We identified that although the patient’s FBG, PPBG and bedtime values appeared to be within goal or above goals prior to and during CGM he was actually experiencing hypoglycemic episodes during the morning hrs prior to awakening. This was extremely concerning as there were trends in values which may have reached a severe low and the patient was unaware that these lows were occurring. Had we not utilized CGM to identify these lows were occurring and continued to adjust his evening dose based on FBG and morning dose based on PPBG values, he could have had a life threatening event. Fortunately, we were not treating this patient as aggressively as his A1C goal was less than 8% and not less than 7%, which is the goal for most patients with diabetes. These findings were important to our clinicians at clinic as we use U-500R in a small subset of patients and there is limited literature evaluating the physiologic effects of U-500R in severely resistant patients with diabetes mellitus.
Wysham et al. evaluated the incidence of hypoglycemia associated with U-500R, comparing the twice daily regimen (60%/40%) with the three times daily regimen (40%/30%/30%). The authors found the rate of nocturnal hypoglycemia (<50 mg/dL) was significantly lower for three times daily regimens versus the twice daily regimens (P = .003). This finding is similar to our report where we have noted severe nocturnal hypoglycemia events occurring in the early morning hrs before 6 am. However, Wysham et al. also noted the twice daily regimen had more non-severe hypoglycemia which occurred in the morning and around noon whereas the three times daily dosing occurred more frequently around 6 pm [7]. In our patient who was on the twice daily dosing regimen, there were no episodes of hypoglycemia during the day, and the average BG was highest in the morning and around noon with the lowest BG levels occurring in the evening after dinner and at bedtime which is opposite to the trend Wysham found.
Ziesmer et al. also evaluated the incidence of hypoglycemia associated with U-500R. This study demonstrated severe hypoglycemia occurred eight times in their patients treated with U-500R insulin, which was similar to the rate of hypoglycemia in the same patients when they were treated with U-100 insulin [8]. A meta-analysis found similar results where severe hypoglycemia was not reported to be a problem and occurred at similar rates to U-100 regular insulin [9]. As a clinician, the concern of severe hypoglycemia is always present when using U-500R, as it is five times more concentrated and timing of peaks are less understood, especially at higher doses. However, because the literature demonstrates the rates of hypoglycemia are similar to U100, a clinician may feel more comfortable titrating U-500 more aggressively based on the patient’s log numbers, without realizing severe nocturnal hypoglycemia may be occurring as we found in our patient. Based on this case report and the literature, it appears that patients may have varying physiologic responses to U-500R and the incidence of hypoglycemia may not be identified without using CGM. For this reason, we have decided to offer CGM to all of our patients who are on U-500R, as it allows us to more accurately adjust their dose and understand the individual impact of U-500R in that patient.
Limitations
Per the FDA, the clinical data used to approve this CGM evaluated 75 subjects with 3 subjects withdrawing from the study. Of the 72 subjects evaluated, 82% had Type 1 diabetes mellitus (T1DM) and 18% had Type 2 diabetes mellitus (T2DM) with an average BMI of 28.3% [10]. The CGM used in this case report was primarily studied in patients with T1DM and not in severely insulin resistant patients with T2DM. The subjects wore 2 sensors for 14 days while continuing to perform finger stick blood glucose checking. During the study venous BG was analyzed and compared to BG readings from the CGM every 15 min during 3 different sessions that lasted for 8 hrs each. The device established safety and efficacy in detecting trends and patterns of BG values, not absolute values, which are to be interpreted by a healthcare professional in this setting. Based on this study, the FDA required Abbott to include a warning that when the CGM reports values in the hypoglycemic range (<60 mg/dL), the true glucose values were actually in the range of 80-160 mg/dL 40% of the time [10]. Thus, it is recommended to use trending to adjust doses for sustained hypoglycemia and not just a single value. The FDA also noted that this device may be beneficial in identifying individuals who become hypoglycemic overnight. Another limitation is patient perception as he noted that completing the corresponding food logs while wearing the CGM caused him to make better dietary choices. The results should be interpreted with this in mind as the patient’s diet may be different when not being monitored.
Conclusion
This case report demonstrated the benefit of using CGM in diabetic patients who are on U-500R. Had this patient’s U-500R dose been adjusted based on BG values alone we likely would have continued to increase the nighttime dose to reduce FBG values, which may have resulted in severe hypoglycemia or death. This report illustrated how a patient had no idea he was experiencing lows based on symptoms, FBG, PPBG or bedtime blood glucose values when in fact, he had several episodes of nocturnal hypoglycemia. The CGM allowed the U-500R dose be more accurately adjusted as well as provide more insight about the physiologic impact of U-500R in this patient.
References
- Binder C. Absorption of injected insulin. A clinical-pharmacological study. Acta. Pharmacol. Toxicol. 27(2), 1–84.
- http://uspl.lilly.com/humulinru500/humulinru500.html#pi
- Ballani P, Tran M, Navar M et al. Clinical experience with U-500 regular insulin in obese, markedly insulin-resistant type 2 diabetic patients. Diabetes. Care. 29(11), 2504–2505 (2006).
- Hood RC, Arakaki RF, Wysham C et al. Two treatment approaches for human regular U-500 insulin in patients with type 2 diabetes not achieving adequate glycemic control on high-dose U-100 insulin therapy with or without oral agents: a randomized, titration-to-target clinical trial. Endocr. Pract. 21(7), 782–793 (2015).
- Bergen PM, Kruger DF, Taylor AD et al. Translating U-500R Randomized Clinical Trial Evidence to the Practice Setting. Diabetes Educator. 43(3), 311–323 (2017).
- http://www.freestylelibrepro.us/safety-information.html
- Wysham C, Hood RC, Warren ML. Effect of total total daily dose on efficacy, dosing, and safety of 2 dose titration regimens of human regular U500 insulin in severely insulin-resistant patients with type 2 diabetes. Endocr. Pract. 22(6), 665 (2016).
- Ziesmer AE, Kelly KC, Guerra PA et al. U500 regular insulin use in insulin-resistant type 2 diabetic veteran patients. Endocrine Practice. 18(1), 34–38 (2012).
- Reutrakul S, Wroblewski M, Brown R. Clinical Use of U-500 Regular Insulin: Review and Meta-Analysis. J. Diabetes Sci. Technol. 6(2), 412–420 (2012).
- http://www.accessdata.fda.gov/cdrh_docs/pdf15/p150021b.pdf