Special Report - Imaging in Medicine (2012) Volume 4, Issue 2
Advanced retinal pigment epithelium analysis by SD-OCT to monitor dry AMD progression
Albert J Augustin*Augenklinik, Klinikum Karlsruhe, Moltkestrasse 90, 76133 Karlsruhe, Germany
- Corresponding Author:
- Albert J Augustin
Augenklinik, Klinikum Karlsruhe
Germany Moltkestrasse 90, 76133 Karlsruhe
Tel: + 49 721 9742001
Fax: + 49 721 9742009
E-mail: albertjaugustin@googlemail.com
Abstract
This article provides a short overview of developments in optical coherence tomography (OCT) technology and highlights the advantages of spectral domain OCT, which allows for high-speed, high-resolution imaging of retinal structures. Established clinical applications to diagnose and monitor macular diseases are summarized and the rapidly evolving role of OCT technology in this field is described. Recent clinical studies show that spectral domain OCT permits volumetric measurement of drusen and might be used for quantitative assessment of drusen progression. Furthermore, spectral domain OCT fundus images can be used to evaluate atrophic lesions of geographic atrophy. A new retinal pigment epithelium analysis tool is presented that should allow for automated, quantitative assessment of drusen and atrophic lesions and could potentially provide more objective and easier classification of age-related macular degeneration.
Keywords
advanced retinal pigment epithelium analysis ▪ age-related macular degeneration ▪ drusen ▪ geographic atrophy ▪ quantitative assessment ▪ spectral domain optical coherence tomography ▪ time domain optical coherence tomography
Development of OCT technology
Over the past 20 years, optical coherence tomography (OCT) has rapidly developed and today is a powerful tool for diagnosing, monitoring and managing diseases of the retina and glaucoma. OCT functions as a type of optical biopsy, providing high-resolution information on retinal pathology in situ and in real time. Thus, it provides images of the retinal structure that cannot be obtained by any other noninvasive diagnostic device [1]. In addition, recent technology developments also provide information on blood flow. Measurements with Doppler-OCT showed good repeatability and excellent correlation with visual field and clinical presentations and might contribute to a better understanding of retinal and optic nerve diseases [2].
In time domain-OCT (TD‑OCT), which was commercialized in 1996, a reflected beam of light is compared with a beam of light of a reference mirror and time delays between the two beams are measured [3–5]. TD‑OCT measurements have been standard for many years, however, the relatively slow acquisition time of TD‑OCT resulted in limited resolution of images and in artifacts due to eye movements. Furthermore, owing to the restricted acquisition of large volumetric data sets, quantitative assessment of retinal pathologies with TD‑OCT is limited.
An important milestone of retinal imaging was the development of high-speed Fourier domain or spectral detection techniques, resulting in significant improvements in OCT imaging performance [1,6]. OCT with spectral/Fourier domain detection (SD‑OCT) is a form of noninvasive, low-coherence interferometry that produces high-resolution tomograms without contacting the eye. In low-coherence interferometry, light is sent along two optical paths, one being the sample path (into the eye) and the other the reference path of the interferometer. The light source is an 840 nm superluminescent light-emitting diode. Light returning from the sample and reference paths is combined at the detector, which is a spectrometer in SD‑OCT. The spectrometer resolves the interference signals throughout the depth of each A-Scan immediately by means of a Fourier transformation. This is possible because the spectrometer resolves the relative amplitudes and phases of the spectral components scattered back from all depths of each A‑scan tissue sample, without varying the length of the reference path.
Thus, SD‑OCT measures echo time delays of light by taking the Fourier transform of the interference spectrum of the light signal. Different echo time delays of light produce different frequencies of fringes in the interference spectrum. A Fourier transform is a mathematical procedure that extracts the frequency spectrum of a signal. Different tissue structures produce different echo time delays of light so that the interference spectrum is composed of different frequencies [7]. Since SD‑OCT can measure all echoes of light from different delays simultaneously, it offers a significant sensitivity advantage, thereby providing a dramatic increase in acquisition speed in comparison with TD‑OCT [8]. With SD‑OCT 25,000–100,000 A‑scans/s are routinely acquirable, which is more than 100- times faster than TD‑OCT. This permits important sensitivity improvements and less susceptibility to eye movements. Image depth is limited by light penetration into the retinal tissue. Most commercial OCT systems use light sources with a wavelength between 800 and 900 nm allowing good imaging of the retina. For improved imaging of the choroid light, sources with wavelengths above 1000 nm have been used [9].
SD‑OCT provides more detailed images and more data with improved speed and accuracy for in-depth analysis (Figure 1). Depending on the type of information desired, different SD‑OCT scan patterns can be used; high-density, averaged cross-sectional B‑scans exhibit even small ultrastructural changes [1,10]. A dense raster scan consisting of multiple, adjacent B‑scans can be performed to cover a volume of the retina. This allows for acquiring complete 3D data sets in a short time frame, so SD‑OCT imaging can display the real retinal geometry [1,7,11,12]. Another clinically important feature is the generation of an OCT fundus image (i.e., OCT view from the fundus) similar to the one obtained by standard fundus photography. It is generated by summing the 3D‑OCT data set along the axial direction and represents an en face summary of all of the adjacent B‑scans from the data set. The OCT fundus image can be used to register individual OCT tomograms precisely and reproducibly with respect to fundus features, so exact correlations between retinal landmarks of the fundus image and the corresponding cross-sectional retinal image (B‑scan) can be achieved [1,7,13,14].
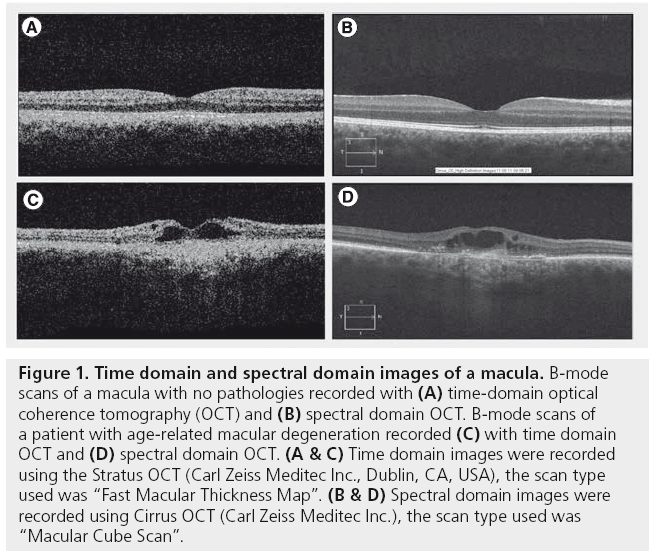
Figure 1: Time domain and spectral domain images of a macula. B‑mode scans of a macula with no pathologies recorded with (A) time-domain optical coherence tomography (OCT) and (B) spectral domain OCT. B‑mode scans of a patient with age-related macular degeneration recorded (C) with time domain OCT and (D) spectral domain OCT. (A & C) Time domain images were recorded using the Stratus OCT (Carl Zeiss Meditec Inc., Dublin, CA, USA), the scan type used was “Fast Macular Thickness Map”. (B & D) Spectral domain images were recorded using Cirrus OCT (Carl Zeiss Meditec Inc.), the scan type used was “Macular Cube Scan”.
Progressive implementation of SD‑OCT in clinical practice
As a result of these advantages SD‑OCT exhibits improved clinical value for the assessment of eyes with various retinal diseases. It offers new insights into the pathogenesis of macular abnormalities and is, therefore, progressively utilized in diagnosing and monitoring macular diseases, including vitreomacular traction syndrome, cystoid macular edema/diabetic macular edema, epiretinal membranes, full-thickness macular holes, lamellar holes, pseudoholes and microholes [15,16]. Moreover, owing to its high resolution, SD‑OCT is utilized to evaluate the antiexudative effect of intravitreal antiangiogenic drugs and has thus progressively become an important tool in the management of patients with neovascular agerelated macular degeneration (nAMD) [17–20]. Recent findings conclude that several functional parameters to detect choroidal neovascularization recurrences (e.g., subjective patient perception, Amsler grid, visual acuity and fundus examination) lead to pronounced underestimations, whereas morphologic recurrences can be detected by SD‑OCT prior to these functional deteriorations. As in nAMD any delay of treatment can result in irreversible vision loss, the authors propose to provide monthly OCT controls to detect recurrences as early as possible [21].
However, while OCT seems to be established as a helpful diagnostic tool to closely monitor the therapeutic effect of antiangiogenic drugs in nAMD, its role regarding diagnosis and progression evaluation of dry AMD (dAMD) is not yet clear, but rapidly developing.
The role of SD‑OCT in dAMD
dAMD is characterized by different clinical features like drusen, pigment abnormalities and geographic atrophy (GA). The earliest morphologic feature of dAMD is extracellular deposit accumulation between retinal pigment epithelium (RPE) and Bruch’s membrane. As these deposits slowly increase, they can be seen as soft drusen and/or localized RPE detachments, which are known to be risk factors for the progression of AMD [22,23]. As the drusen enlarge, they can contribute to either the development of new blood vessels (nAMD) and/or the slow loss of photoreceptors, which can be accompanied by thinning (atrophy) of RPE cells leading to GA, the advanced form of dAMD. Even if the biochemical processes of atrophy are not fully understood yet [24,25], pigmentary changes, as well as the increase in size and number of drusen, are used for staging AMD and for predicting the likelihood of progression [26–28].
So far, stereoscopic color fundus photographs are the gold standard for reading centers to assess drusen number and size as well as pigmentary changes for predicting disease progression [29,30]. Furthermore, fundus autofluorescence (FAF), based on the autofluorescence properties of RPE cells due to lipofuscin granules, is used for noninvasively imaging atrophic lesions in GA and has been used to indicate GA progression [31–33]. However, although both approaches are used for staging and predicting AMD progression, they still exhibit some limitations; measuring GA and drusen using color fundus photography is challenging and entails the risk of interobserver variability. Moreover, reproducibility and the accuracy of delineating GA by fundus photography have been reported to be moderate [31,34–36]. Attempts to implement fully automated digital techniques for evaluating color fundus photographs have not yet gained widespread acceptance for several reasons [37]. In addition, both approaches allow for 2D assessment of the macula only, but do not provide any 3D information regarding morphological changes in retinal structure. This impedes volumetric measurement of drusen and thus quantitative measurement of disease progression. Quantitative assessment of drusen, however, might be an important parameter in clinical trials to evaluate efficacy of new drugs against AMD [13].
With regard to these limitations SD‑OCT imaging offers several advantages: the same scan pattern can be used to obtain image data on both drusen and atrophic lesions of GA, and SD‑OCT allows for acquiring 3D data providing more detailed information on disease-specific changes in retinal structures and permits volumetric measurement of drusen for quantitative assessment of drusen progression.
Evaluation of drusen & atrophic lesions with SD‑OCT
It has already been demonstrated that SD‑OCT can be used for imaging the ultrastructure of drusen in vivo, because high-quality B‑scans allow the detection of new details of drusen structure and morphology. In a study with 31 eyes Khanifar et al. categorized ultrastructural characteristics of drusen with SD‑OCT and classified drusen according to shape, predominant internal reflectivity and presence of overlying hyper-reflective foci. They concluded that distinct subclasses of drusen may serve as imaging biomarkers for disease severity or risk of progression [10]. Jain et al showed good agreement in drusen detection between fundus photography and SD‑OCT [38]. Also, Freeman et al. concluded from a study with 36 eyes that drusen volume manually determined by SD‑OCT correlates with AREDS-determined drusen area and AREDS grade in nAMD and can provide additional information in grading the severity of eyes with dAMD [39]. Schlanitz et al. examined 1356 drusen in 12 eyes of AMD patients using different SD‑OCT devices. They concluded that SD‑OCT imaging provided an excellent performance in visualizing drusen-related RPE disease [40]. However, manual grading of SD‑OCT images is time consuming and carries the risk of interobserver variability. Recent studies have focused on establishing and evaluating algorithms that allow for automated volumetric assessment of drusen and thus would provide a novel strategy for following disease progression [41–43]. Yi et al. compared TD‑OCT and SD-OCT imaging in three patients with nAMD and present the first data on an automated algorithm evaluating drusen [41]. Yehoshua et al. as well as Gregori et al. evaluated efficacy and reproducibility of a new automated algorithm for drusen assessment and are described in more detail in the ‘Advanced RPE Analysis tool’ section [42,43].
Regarding GA, several studies have shown that SD‑OCT imaging provides more detailed insight in retinal alterations of GA patients than FAF and helped to expand the knowledge of disease-specific retinal alterations and disease progression [44–51]. Bearelly et al. demonstrated that SD‑OCT margins of GA can be easily identified on SD‑OCT fundus images and that B‑scans relative to these GA margins can be graded for quantifying photoreceptor losses [48]. In a study with an admittedly low number of five patients with GA Lujan et al. were the first to compare lesion size in SD‑OCT fundus images with FAF images. They reported that size and shape of atrophic areas obtained from both devices correlated well and OCT-fundus images can be used to measure and identify areas of GA [45]. Furthermore, two recent studies comparing SD‑OCT and FAF, including 21 and 81 eyes, respectively, confirmed the results described by Lujan et al. and showed good correlation between lesion size measured with both devices, while foveal involvement was more accurately determinable with SD‑OCT [50,51]. Yehoshua et al. evaluated the area and enlargement rate of atrophic lesions in a prospective study with 81 eyes and a mean follow-up time of 1.24 years. They demonstrated high reproducibility and concluded that SD‑OCT fundus images can be used to visualize and quantify GA and that precision is sufficient to reliably measure lesions as small as 0.12 mm2 (0.05 disc area) [52]. So far, both approaches (FAF and SD‑OCT) appear to reproducibly and precisely measure the central area of GA with SD‑OCT, offering more detailed insight in RPE layers. However, SD‑OCT allows detailed visualization of reactive changes in the RPE cells in the junctional zone of GA [47,48]. This contributes to a better understanding of disease-specific alterations of retinal layers and might finally help to clarify whether the primary origin of atrophic AMD is photoreceptor loss or RPE loss.
Advanced RPE Analysis tool
Manual grading of SD‑OCT images is time consuming and carries the risk of interobserver variability. Recently a new software analysis tool has been developed, which might be helpful in providing automated, quantitative assessment of changes in drusen size and volume as well as in changes of GA areas. The new software analysis tool ‘Advanced RPE Analysis’ (Carl Zeiss Meditec. Inc., CA, USA) is based on two new algorithms, one to measure elevations in the RPE for identifying drusen, and one to measure areas of sub-RPE illumination where the OCT is able to penetrate through to the choroid, indicating that the RPE is atrophic.
Maps based on each analysis, as well as a map that integrates both analyses allows a visualization of these disturbances in the RPE, and numerical results are provided to summarize them. Within the Advanced RPE Analysis tool there are two screens available for reviewing the results. The first screen shows both the RPE elevation (RPE Elevation Map) and the Sub-RPE illumination results (Sub-RPE Slab) separately as en face images. The second screen shows the integrated analysis along with calculated values.
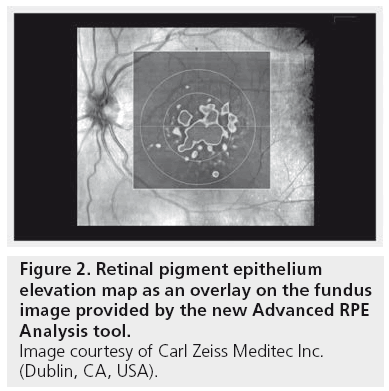
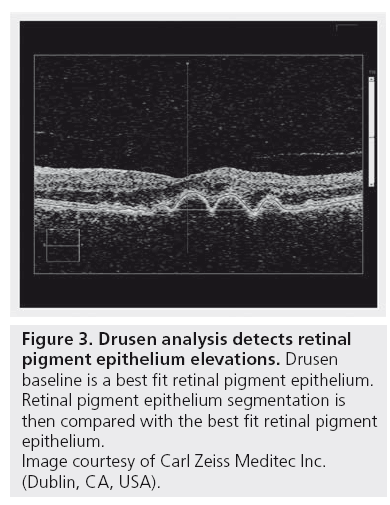
The analysis on the first screen displays an RPE Elevation Map as an overlay on the fundus image, which shows circles corresponding to 3 and 5 mm in diameter centered on the fovea. The pseudo-color aids in identifying bumps and discontinuities in the RPE. Legends at the lateral margins of the map show correlation of color to the height of the elevations (Figure 2). It is important to understand, that RPE segmentation is used as the starting point for the drusen analysis. So strictly speaking this analysis detects elevations in the RPE. To identify RPE elevations, a curve is fitted to the RPE segmentation that seeks to exclude deformations of the RPE and follow the curvature of the eye. The region between the RPE segmentation and the curve fit is designated as RPE elevation. To reduce spurious noise and to allow for some variability in fitting, only areas where the elevation exceeds ten pixels (19.5 μm) are used for calculations of RPE elevation area and volume (Figure 3).
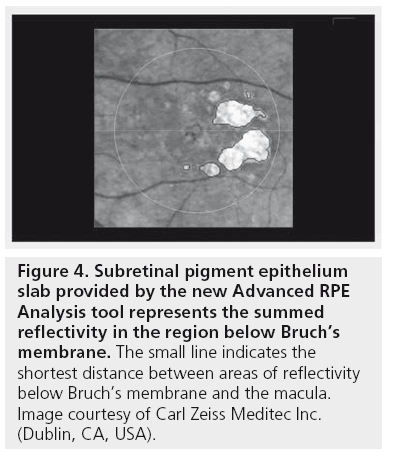
For identifying GA, the penetration of light beneath the RPE in areas of atrophy is analyzed. The OCT image volume beneath the level of the RPE is processed to create a 2D en face projection image that shows bright spots where the atrophy of RPE allows light to penetrate into the choroid. Segmentation is then performed on this en face image to identify areas with deeper light penetration (sub-RPE illumination) indicating areas of atrophic RPE. The Sub-RPE Slab represents the summed reflectivity in the region below Bruch’s membrane. This slab shows the location of the fovea with a dot marking and a circle corresponding to 5 mm in diameter centered on the fovea. It also shows a red line from the fovea to the closest area with sub-RPE illumination, which indicates the distance from the fovea to the closest area of GA (Figure 4).
Figure 4: Subretinal pigment epithelium slab provided by the new Advanced RPE Analysis tool represents the summed reflectivity in the region below Bruch’s membrane. The small line indicates the shortest distance between areas of reflectivity below Bruch’s membrane and the macula. Image courtesy of Carl Zeiss Meditec Inc. (Dublin, CA, USA).
So far, only very preliminary data on the efficacy and reproducibility of this new analyzing tool are available. Two recent studies showed efficacy and reproducibility of the algorithm underlying the measurement of RPE elevations (indicating drusen). Yehoshua et al. analyzed 143 eyes with at least 6 months follow-up using the new algorithm and concluded that quantitative changes in drusen area and volume can be observed over time with SD‑OCT imaging [42]. Furthermore Gregori et al. used the novel algorithm to quantitatively assess drusen volume and area in 103 eyes from 74 patients with drusen. Measurements were taken from the entire scans as well as from 3 and 5 mm circles around the fovea. The new algorithm was shown to be highly reproducible for qualitative and quantitative assessment of drusen. The authors concluded that the ability to quantitatively assess drusen volume may be a new and useful parameter to monitor disease progression, particularly in trials for treatments of nAMD [43]. However, they pointed out that although drusen measurement with SD‑OCT seems to correlate well with fundus photography, both methods deliver different information: SD‑OCT images indicate RPE disruption, whereas fundus photographs show pigmentary changes. So, owing to threshold limitations, very shallow drusen may be missed in SD‑OCT, whereas not all RPE deformations must be associated with pigmentary changes and thus will not appear on fundus photographs. Therefore, SD‑OCT images and color fundus photographs offer complimentary information on drusen and both may have an important role in assessing drusen in patients with AMD.
Sharma et al. provided results on the new Cirrus RPE Analysis tool at the Annual Meeting of the Association for Research in Vision and Ophthalmology (ARVO) in 2011 comparing automated versus manual analysis of SD‑OCT imaging in 15 eyes with dAMD [53]. They determined drusen number, drusen area and area of GA in a 6‑mm circle centered around the fovea. Although the new Cirrus RPE Analysis tool showed higher accuracy than another automated analysis software, there was still an error rate in identifying drusen. The tool was very accurate in determining area of GA but tended to result in a larger area compared with manual measurement (4.8 vs 4.1 mm2 on average). Further studies to prove accuracy, reproducibility and feasibility of this new analysis tool are required.
Conclusion
Today SD‑OCT allows for high-speed, high-resolution imaging of retinal structures. It functions as a type of optical biopsy, 3D information providing noninvasively on retinal pathology in situ and in real time. It is progressively utilized in diagnosing and monitoring different macular diseases and in evaluating the antiexudative effect of intravitreal antiangiogenic drugs. Thus, it has progressively become an important tool in the management of patients suffering from nAMD.
Furthermore, owing to its high resolution, SD‑OCT imaging leads to new insights in microstructural processes and a more profound understanding of a patient’s underlying RPE status. This may help to more accurately estimate a patient’s prognosis in both dAMD and nAMD, because the RPE is a relevant structure in all forms of AMD. Using SD‑OCT the same scan pattern can be used to acquire 3D data permitting volumetric measurement of drusen for quantitative assessment of drusen progression and to create SD‑OCT fundus images that can be used to evaluate atrophic lesions of GA.
SD‑OCT technology in combination with the evaluation of robust algorithms that allow for automated, quantitative assessment of drusen and atrophic lesions could potentially provide more objective and easier classification of AMD. This may help to more precisely determine disease stage as well as prognosis and provides the opportunity to monitor the effectiveness of new therapies in clinical trials.
Future perspective
Regarding AMD research, SD‑OCT imaging in combination with reliable algorithms that allow for automated quantitative assessment of atrophic lesions and drusen offer new opportunities and may become a powerful tool in diagnosing and monitoring AMD. As growth of GA has become a primary end point in clinical trials of drugs for the treatment of dAMD, it might offer the feasibility to automatically and objectively quantitate GA and the progression of GA. Moreover, it allows for automated qualitative and quantitative assessment of drusen, which may become a useful clinical tool for evaluation of disease progression. As drusen as well as atrophic lesions can be evaluated from the same scan pattern, all forms of AMD can be assessed quantitatively. Finally, it allows for the detection of these morphologic alterations over time, which should – in conjunction with functional testing – aid in the stratification of stages of disease progression and allow for monitoring progression of disease over time. Of course, in this context it is of utmost importance to define and evaluate reliable and reproducible parameters that allow for grading AMD using SD‑OCT. Moreover, quantifying changes in drusen volume and atrophic lesions over time with SD‑OCT provides a novel strategy for identifying novel clinical trial end points for clinical studies on new therapeutic drugs in AMD. Furthermore, SD‑OCT imaging permits simultaneous measurement of GA along with loss of photoreceptors and RPE and, thus, may contribute to a better understanding of the relationship between GA enlargement, loss of photoreceptors/RPE and visual loss. Another future possibility might be SD‑OCT examination of photoreceptor layer. Schuman et al. detected a significant thinning of the photoreceptor layer over drusen [54]. Thus, photoreceptor changes might be used to investigate even earlier events in drusen formation. Furthermore, polarization-sensitive OCT, a novel technology detecting the depolarizing properties of the RPE in addition to information obtained by conventional SD‑OCT scans, might provide even more detailed information on the RPE status and may help to better estimate the functional prognosis of AMD [55].
Financial & competing interests disclosure
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.

References
- Drexler W, Fujimoto JG. State-of-the-art retinal optical coherence tomography. Prog. Retinal Eye Res. 27(1), 45–88 (2008).
- Wang Y, Fawzi AA, Varma R et al. Pilot study of optical coherence tomography measurement of retinal blood flow in retinal and optic nerve diseases. Invest. Ophthalmol. Vis. Sci. 52, 840–845 (2011).
- Huang D, Swanson EA, Lin CP et al. Optical coherence tomography. Science 254, 1178–1181 (1991).
- Hee MR, Izatt JA, Swanson EA et al. Optical coherence tomography of the human retina. Arch. Ophthalmol. 113, 325–332 (1995).
- Puliafito CA, Hee MR, Lin CP et al. Imaging of macular diseases with optical coherence tomography. Ophthalmology 102, 217–229 (1995).
- Fercher AF, Hitzenberger CK, Kamp G, El-Zaiat SY. Measurement of intraocular distances by backscattering spectral interferometry. Optics Commun. 117, 43–48 (1995).
- Wojtkowski M, Srinivasan V, Fulimoto JG et al. Three-dimensional retinal imaging with high-speed ultrahigh-resolution optical coherence tomography. Ophthalmology 112, 1734–1746 (2005).
- Leitgeb R, Hitzenberger C, Fercher A. Performance of Fourier domain vs. time domain optical coherence tomography. Opt. Express 11, 889–894 (2003).
- Huber R, Adler DC, Srinivasan VJ, Fujimoto JG. Fourier domain mode locking at 1050 nm for ultra high-speed optical coherence tomography images of the retina in the presence of epiretinal membranes. Am. J. Ophthalmol. 145, 1023–1030 (2008).
- Khanifar AA, Koreishi AF, Izatt JA, Toth CA. Drusen ultrastructure imaging with spectral domain optical coherence tomography in age-related macular degeneration. Ophthalmology 115, 1883–1890 (2008).
- Wojtkowski M, Srinivasan V, Ko T et al. Ultra high resolution, high speed, Fourier domain optical coherence tomography and methods for dispersion compensation. Opt. Express 12, 2404–2422 (2004).
- Leitgeb R, Drexler W, Unterhuber A et al. Ultrahigh resolution Fourier domain optical coherence tomography. Opt. Express 12, 2156–2165 (2004).
- Yehoshua Z, Rosenfeld PJ, Gregori G, Penha F. Spectral domain optical coherence tomography imaging of dry age-related macular degeneration. Ophthalmic. Surg. Lasers Imaging 41(Suppl.), S6–S14 (2010).
- Lujan BJ, Wang F, Gregori G et al. Calibration of fundus images using spectral domain optical coherence tomography. Ophthalmic. Surg. Lasers Imaging 39(Suppl.), S15–S20 (2008).
- Wolf S, Wolf-Schnurrbusch U. Spectraldomain optical coherence tomography use in macular diseases: a review. Ophthalmologica 224(6), 333–340 (2010).
- Schmidt-Erfurth U, Leitgeb RA, Michels S et al. Three dimensional ultra highresolution optical coherence tomography of macular diseases. Invest. Ophthalmol. Vis. Sci. 46, 3393–3402 (2005).
- Kiss CG, Geitzenauer W, Simader C et al. Evaluation of ranibizumab-induced changes in high-resolution optical coherence tomographic retinal morphology and their impact on visual function. Invest. Ophthalmol. Vis. Sci. 50, 2376–2383 (2009).
- Witkin AJ, Vuong LN, Srinivasan VJ et al. High-speed ultrahigh resolution optical coherence tomography before and after ranibizumab for age-related macular degeneration. Ophthalmology 116, 956–963 (2009).
- Sayanagi K, Sharma S, Yamamoto T, Kaiser PK. Comparison of spectral-domain versus time-domain optical coherence tomography in management of age-related macular degeneration with ranibizumab. Ophthalmology 116, 947–955 (2009).
- Cukras C, Wang YD, Meyerle CB, Forooghian F, Chew EY, Wong WT. Optical coherence tomography-based decision making in exudative age-related macular degeneration: comparison of time- vs spectral-domain devices. Eye 24(5), 775–783 (2010).
- Hoerster R, Muether PS, Hermann MM et al. Subjective and functional deterioration in recurrences of neovascular AMD are often preceded by morphologic changes in optic coherence tomography. Br. J. Ophthalmol. 95(10), 1424–1426 (2011).
- Pauleikhoff D, Barondes MJ, Minassian D, Chisholm IH, Bird AC. Drusen as risk factors in age-related macular disease. Am. J. Ophthalmol. 109, 38–43 (1990).
- Bressler SB, Maguire MG, Bressler NM, Fine SL. Relationship of drusen and abnormalities of the retinal pigment epithelium to the prognosis of neovascular macular degeneration. The Macular Photocoagulation Study Group. Arch. Ophthalmol. 108, 1442–1447 (1990).
- Sunness JS, Gonzalez-Baron J, Applegate CA et al. Enlargement of atrophy and visual acuity loss in the geographic atrophy form of age-related macular degeneration. Ophthalmology 106, 1768–1779 (1999).
- Dreyhaupt J, Mansmann U, Pritsch M et al. Modelling the natural history of geographic atrophy in patients with age related macular degeneration. Ophthalmic. Epidemiol. 12, 353–362 (2005).
- Seddon JM, Sharma S, Adelman RA. Evaluation of the clinical age-related maculopathy staging system. Ophthalmology 113, 260–266 (2006).
- Mitchell P, Foran S. Age-related eye disease study severity scale and simplified severity scale for age-related macular degeneration. Arch. Ophthalmol. 123, 1598–1599 (2005).
- Ferris FL, Davis MD, Clemons TE et al.; Age-related eye disease study research group. A simplified severity scale for age-related macular degeneration: AREDS report no. 18. Arch. Ophthalmol. 123, 1570–1574 (2005).
- Klein R, Davis MD, Magli YL et al. The Wisconsin age-related maculopathy grading system. Ophthalmology 98, 1128–1134 (1991).
- Davis MD, Gangnon RE, Lee LY et al. Age-Related Eye Disease Study Research Group. The age-related eye disease study severity scale for age-related macular degeneration: AREDS report no. 17. Arch. Ophthalmol. 123, 1484 –1498 (2005).
- Holz FG, Bindewald-Wittich A, Fleckenstein M et al. Progression of geographic atrophy and impact of fundus autofluorescence patterns in age-related macular degeneration. Am. J. Ophthalmol. 143, 463–472 (2007).
- Holz FG, Bellman C, Staudt S et al. Fundus autofluorescence and development of geographic atrophy in age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 42, 1051–1056 (2001).
- Schmitz-Valckenberg S, Bindewald-Wittich A, Dolar-Szczasny J et al. Correlation between the area of increased autofluorescence surrounding geographic atrophy and disease progression in patients with AMD. Invest. Ophthalmol. Vis. Sci. 47, 2648–2654 (2006).
- Sunness JS, Ziegler MD, Applegate CA. Issues in quantifying atrophic macular disease using retinal autofluorescence. Retina 26, 666–672 (2006).
- Scholl HP, Peto T, Dandekar S et al. Inter- and intra-observer variability in grading lesions of age-related maculopathy and macular degeneration. Graefes Arch. Clin. Exp. Ophthalmol. 241, 39–47 (2003).
- Pirbhai A, Sheidow T, Hooper P. Prospective evaluation of digital non-stereo color fundus photography as a screening tool in age-related macular degeneration. Am. J. Ophthalmol. 139, 455–461 (2005).
- Smith RT, Chan JK, Nagasaki T et al. Automated detection of macular drusen using geometric background leveling and threshold selection. Arch. Ophthalmol. 123, 200–206 (2005).
- Jain N, Farsiu S, Khanifar AA et al. Quantitative comparison of drusen segmented on SD-OCT versus drusen delineated on color fundus photographs. Invest. Ophthalmol. Vis. Sci. 51, 4875–4883 (2010).
- Freeman SR, Kozak I, Cheng L et al. Optical coherence tomography-raster scanning and manual segmentation in determining drusen volume in age-related macular degeneration. Retina 30, 431–435 (2010).
- Schlanitz FG, Ahlers C, Sacu S et al. Performance of drusen detection by spectral-domain optical coherence tomography. Invest. Ophthalmol. Vis. Sci. 51, 6715–6721 (2010).
- Yi K, Mujat M, Park BH et al. Spectral domain optical coherence tomography for quantitative evaluation of drusen and associated structural changes in non neovascular age-related macular degeneration. Br. J. Ophthalmol. 93, 176–181 (2009).
- Yehoshua Z, Wang F, Rosenfeld PJ et al. Natural history of drusen morphology in age-related macular degeneration using spectral domain optical coherence tomography. Ophthalmology 118(12), 2434–2441 (2011).
- Gregori G, Wang F, Rosenfeld PJ et al. Spectral domain optical coherence tomography imaging of drusen in non-exudative age-related macular degeneration. Ophthalmology 118, 1373–1379 (2011).
- Fleckenstein M, Charbel Issa P, Helb HM et al. High-resolution spectral domain-OCT imaging in geographic atrophy associated with age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 49, 4137–4144 (2008).
- Lujan BJ, Rosenfeld PJ, Gregori G et al. Spectral domain optical coherence tomographic imaging of geographic atrophy. Ophthalmic Surg. Lasers Imaging 40, 96–101 (2009).
- Wolf-Schnurrbusch UE, Enzmann V, Brinkmann CK, Wolf S. Morphologic changes in patients with geographic atrophy assessed with a novel spectral OCT-SLO combination. Invest. Ophthalmol. Vis. Sci. 49, 3095–3099 (2008).
- Brar M, Kozak I, Cheng L et al. Correlation between spectral domain optical coherence tomography and fundus autofluorescence at the margins of geographic atrophy. Am. J. Ophthalmol. 148, 439–444 (2009).
- Bearelly S, Chau F, Koreishi A et al. Spectral domain optical coherence tomography imaging of geographic atrophy. Ophthalmology 116, 1762–1769 (2009).
- Kaluzny JJ, Wojtkowski M, Sikorski BL et al. Analysis of the outer retina reconstructed by high-resolution, three-dimensional spectral domain optical coherence tomography. Ophthalmic. Surg. Lasers Imaging 40, 102–108 (2009).
- Schmitz-Valckenberg S, Fleckenstein M, Göbel AP et al. Optical coherence tomography and autofluorescence findings in areas with geographic atrophy due to age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 52, 1–6 (2011).
- Sayegh RG, Simader C, Scheschy U et al. A systematic comparison of spectral domain optical coherence tomography and fundus autofluorescence in patients with geographic atrophy. Ophthalmology 118(9), 1844–1851 (2011).
- Yehoshua Z, Rosenfeld PJ, Gregori G et al. Progression of geographic atrophy in age-related macular degeneration imaged with spectral domain optical coherence tomography. Ophthalmology 118, 679–686 (2011).
- Sharma S, Huo S, Kaiser P. Comparison of manual versus automated analysis of spectral domain optical coherence tomography (SDOCT) scans in non-neovascular age related macular degeneration. Presented at: The Annual Meeting of the Association for Research and Vision in Ophthalmology. Fort Lauderdale, FL, USA, 1–5 May 2011.
- Schuman SG, Koreishi AF, Farsiu S et al. Photoreceptor layer thinning over drusen in eyes with age related macular degeneration imaged in vivo with spectral domain optical coherence tomography. Ophthalmology 116(3), 488–496 (2009).
- Ahlers C, Götzinger E, Pircher M et al. Imaging of the retinal pigment epithelium in age-related macular degeneration using polarization-sensitive optical coherence tomography. Invest. Ophthalmol. Vis. Sci. 51, 2149–2157 (2010).
• This article reviews developments in optical coherence tomography (OCT) technology in detail and highlights advantages of spectral domain (SD-OCT).
• • Interesting review article that summarizes relevant studies on SD-OCT imaging in dry age-related macular degeneration.
• Review article that summarizes relevant studies on SD-OCT imaging in various macular diseases.
• • Clinical study evaluating the reproducibility of the novel algorithm for qualitative and quantitative assessment of drusen using SD-OCT.
• • Clinical study evaluating the reproducibility of the novel algorithm for qualitative and quantitative assessment of drusen using SD-OCT.
• This study, comparing images of geographic atrophy obtained by SD-OCT and fundus autofluorescence, shows good correlation between both methods with SD-OCT providing more anatomic information.
• • Clinical study evaluating SD-OCT for quantitative assessment of geographic atrophy.