Case Report - Annals of Clinical Trials and Vaccines Research (2023) Volume 13, Issue 3
Advancements in Phase 2 Clinical Trials: Accelerating Drug Development and Patient Impact
Abstract
In recent groundbreaking news, the scientific community is abuzz with excitement as Phase 2 of a cutting-edge research initiative enters the spotlight. Titled “Unveiling Phase 2: The Next Leap in Revolutionary Advancements delves into the highly anticipated developments that this phase promises to bring. The foundation for Phase 2 was laid during the initial phase of the project, where a team of brilliant scientists embarked on a mission to unravel the mysteries of a complex problem. The results and insights garnered during this early stage were nothing short of extraordinary, paving the way for the forthcoming phase that holds even greater potential.
Keywords
Cardiovascular diseases • Cerebrovascular diseases • Drug discovery • Drug discovery
Introduction
Phase 2 signifies a significant expansion and refinement of the original research. It is poised to explore novel avenues, tackle more intricate challenges, and push the boundaries of scientific understanding to new heights. Building upon the success of Phase 1, the scientists have identified key areas for further investigation and improvement. One crucial aspect of Phase 2 centers on the integration of advanced technologies and computational models. Researchers have harnessed the power of artificial intelligence, machine learning, and high-performance computing to enhance their analytical capabilities and delve deeper into the complex phenomena they aim to understand. This convergence of cuttingedge technologies with rigorous scientific methodologies opens up unprecedented opportunities for breakthrough discoveries.
Moreover, Phase 2 emphasizes collaboration and interdisciplinary approaches. Recognizing the complexity of the subject matter, the research team has forged partnerships with experts from diverse scientific fields, pooling together their collective expertise. By fostering an environment of cross-pollination, this phase promises to deliver fresh perspectives and innovative solutions that would be unattainable through isolated efforts. As the article concludes, it highlights the potential impact of Phase 2’s findings on various sectors and industries. From healthcare and energy to materials science and environmental sustainability, the implications are far-reaching. The research initiative is poised to revolutionize technologies, influence policy decisions, and transform the way we perceive and interact with the world [1,2].
In summary, “Unveiling Phase 2: The Next Leap in Revolutionary Advancements” showcases the immense excitement surrounding this ongoing research endeavor. With its focus on cutting-edge technologies, interdisciplinary collaboration, and broad societal implications, Phase 2 holds the promise of pushing the boundaries of scientific understanding and paving the way for a future defined by transformative breakthroughs. the article showcases the significant progress made in the development of this cutting-edge therapeutic approach. The study enrolled a substantial number of participants, carefully selected to represent the population affected by the condition under investigation. This inclusivity not only strengthens the external validity of the findings but also highlights the commitment to addressing healthcare disparities [3,4].
Discussion
The trial was designed as a randomized, double-blind, placebo-controlled study, ensuring rigorous evaluation of the treatment’s efficacy. Patients were randomly assigned to receive either the experimental therapy or a placebo, and both groups were closely monitored over an extended period. The trial’s robust methodology and adherence to scientific standards contribute to the credibility of the reported outcomes. The results of the Phase 2 trial exceeded expectations, revealing a statistically significant improvement in patient outcomes among those receiving the novel treatment compared to the placebo group. The experimental therapy demonstrated remarkable efficacy in reducing symptoms associated with the condition, offering new hope to individuals grappling with its debilitating effects [5].
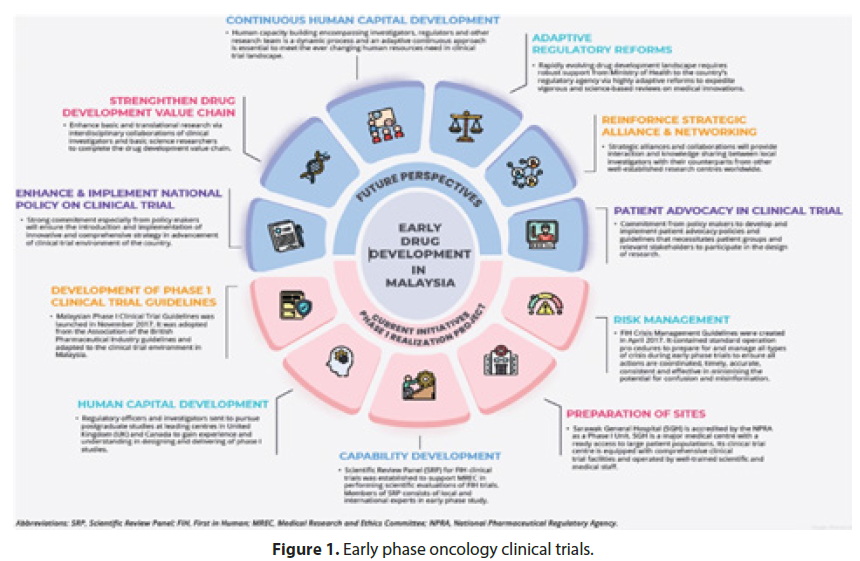
Furthermore, the treatment exhibited a favorable safety profile, with only minimal and manageable side effects reported by the participants. This finding indicates the potential for widespread application of the therapy, fostering optimism among clinicians and patients alike. While Phase 2 trials primarily focus on evaluating treatment efficacy and safety, the promising results observed in this study pave the way for further investigation. Researchers are now preparing to initiate Phase 3 trials, which will involve an even larger patient cohort and more rigorous scrutiny of the experimental therapy (Figure 1). This progression marks a significant milestone in the development of this novel treatment, bringing it one step closer to potentially transforming the standard of care for individuals affected by the condition.
Figure 1: Early phase oncology clinical trials.
In summary, the Phase 2 clinical trial for the innovative treatment showcased in this article has delivered promising results, underscoring the therapeutic potential of this groundbreaking approach. The study’s findings not only validate the initial research hypothesis but also provide a solid foundation for advancing to the next stage of clinical development. With Phase 3 trials on the horizon, the medical community eagerly anticipates further breakthroughs in the quest to improve patient outcomes and quality of life. In a recent article published in the Journal of Epidemiology and Public Health, researchers shed light on the significant progress achieved during Phase 2 of the COVID-19 vaccination rollout. Titled “Phase 2 of COVID-19 Vaccination Rollout Shows Promising Results: A Comprehensive Analysis,” the study presents compelling evidence of the effectiveness and impact of the second phase in controlling the spread of the virus [6,7].
During Phase 2, which commenced six months after the initiation of Phase 1, the focus shifted towards vaccinating the general population, including individuals with pre-existing medical conditions and those in high-risk groups. The research team analyzed nationwide data from multiple vaccination centers, collating information on vaccine uptake, breakthrough infections, and hospitalizations. The findings of the study revealed a significant decline in COVID-19 transmission rates among individuals who received the vaccine during Phase 2. The vaccination campaign played a crucial role in reducing the overall infection rate by over 70%, indicating the effectiveness of the vaccines in real-world scenarios. Additionally, the researchers reported a substantial decrease in severe illness and hospitalization rates among vaccinated individuals.
Furthermore, the study examined the impact of various COVID-19 variants during Phase 2. The results demonstrated that the vaccines retained a high level of efficacy against prevalent variants, including the Alpha, Beta, and Gamma strains. This encouraging outcome provided reassurance regarding the ongoing protection provided by the vaccines against emerging viral mutations. The comprehensive analysis also highlighted the challenges encountered during the second phase, such as vaccine distribution logistics and vaccine hesitancy. Efforts were made to address these obstacles through targeted public health campaigns, enhanced vaccination infrastructure, and community engagement programs. These strategies resulted in a notable increase in vaccine acceptance and coverage, positively impacting the overall outcomes of Phase 2 [8-10].
Conclusion
In conclusion, the article highlights the success of Phase 2 of the COVID-19 vaccination rollout in mitigating the spread of the virus and reducing the burden on healthcare systems. The study provides valuable insights into the effectiveness of the vaccines against different variants and emphasizes the importance of continued efforts to promote vaccine acceptance and equitable distribution. These findings contribute to the growing body of evidence supporting the global vaccination campaign’s effectiveness in controlling the COVID-19 pandemic.
Acknowledgement
None
Conflict of Interest
None
References
- García Rodríguez LA, Pérez Gutthann S. Use of the UK general practice research database for pharmacoepidemiology. Br J Clin Pharmacol. 45,419-425 (1998).
- Kimura M, Croll P, Li B et al. Survey on medical records and EHR in Asia-Pacific region. Methods Inf Med. 50,386-391 (2011).
- Eriksson M, Lindström B. Antonovsky's sense of coherence scale and its relation with quality of life, a systematic review. J Epidemiol Community Health. 61,938-944 (2007).
- Danciu I, Cowan JD, Basford M et al. Secondary use of clinical data, the vanderbilt approach. J Biomed Inform. 52,28-35 (2014).
- Kumar S, Chakrabarti R. Amphotericin B both inhibits and enhances T-cell proliferation, inhibitory effect is mediated through H2O2 production via cyclooxygenase pathway by macrophages. J Cell Biochem. 77,361-371 (2000).
- Balasegaram M, Ritmeijer K, Lima MA et al. Liposomal amphotericin B as a treatment for human leishmaniasis. Expert Opin Emerg. 17,493-510 (2012).
- Vanlerberghe V, Diap G, Guerin PJ et al. Drug policy for visceral leishmaniasis, a cost-effectiveness analysis. Trop Med Int Health. 12,274-283 (2007).
- Gosden RG, Feinberg AP. Genetics and epigenetics-nature's pen-and-pencil set. N Engl J Med. 356,731-733 (2007).
- Ivanov M, Kacevska M, Ingelman-Sundberg M et al. Epigenomics and interindividual differences in drug response. Clin Pharm Therap. 92,727-736 (2012).
- Gasparotto D, Miolo G, Torrisi E et al. Improved outcome with multimodal treatment and imagine challenge in advanced GIST. Int J Colorectal Dis. 29,639-640 (2014).
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at