Review Article - Imaging in Medicine (2011) Volume 3, Issue 3
Advances in multiple sclerosis imaging: neurocognitive imaging
Makki A Almuntashri1 & Richard I Aviv†1University of Toronto, University Health Network, Neuroradiology Department, Toronto, ON, Canada
- Corresponding Author:
- Richard I Aviv
Department of Medical imaging
Sunnybrook Health Sciences Center
2075 Bayview Ave., AG31, Toronto
ON M4N 3M5, Canada
Tel: +1 416 480 6100
Fax: +1 416 480 5218
E-mail: richard.aviv@sunnybrook.ca
Abstract
Multiple sclerosis (MS) is the most common inflammatory demyelinating disease of the CNS and the most frequent cause of nontraumatic neurological disability in young and middle-aged adults in North America. MRI plays an important role in diagnosing and monitoring patients with MS; however, current conventional MRI is limited in detecting lesions such as cortical-based plaques. Structural MRI may also be insufficient to explain all neurological presentations in patients with MS, particularly cognitive symptoms. Advanced MRI techniques such as diffusion-weighted imaging, diffusion tensor imaging, functional MRI, magnetization transfer, magnetic resonance (MR) perfusion imaging and MR spectroscopy facilitate a greater understanding of the underlying pathophysiology of MS. This article will provide an overview on the role of imaging in MS, focusing on advanced MR sequences and neurocognitive correlation.
Keywords
advanced MRI ▪ cognitive dysfunction ▪ diffusion tensor imaging ▪ diffusion-weighted imaging ▪ functional MRI ▪ magnetic resonance perfusion ▪ magnetic resonance spectroscopy ▪ magnetization transfer ▪ MRI ▪ multiple sclerosis
Multiple sclerosis (MS) is a neuroinflammatory and neurodegenerative disease [1]. It is the most common inflammatory demyelinating disease of the CNS and the most frequent cause of nontraumatic neurological disability in young and middle-aged adults in North America [2].
Multiple sclerosis is made up of multiple subgroups characterized by their clinical pattern of disease progression: relapsing–remitting MS (RR-MS), secondary progressive MS (SP-MS) and primary progressive MS (PP-MS). RR-MS is characterized by exacerbations of the disease followed by complete or partial remission, and constitutes approximately 80% of the MS population at initial presentation [3,4]. SP-MS is seen in 40% of RR-MS patients after 10 years and 90% after 25 years disease duration. PP-MS represents 5–10% of the MS population; this group is characterized by progressive symptom onset from the disease commencement [5]. Although MS is regarded primarily as a white matter (WM) disease, gray matter (GM) disease burden is increasingly emphasized [6–13]. Recent imaging studies support an interdependent relationship between normal-appearing white matter (NAWM) and GM damage [14]. Demyelinating lesions, metabolic and structural abnormalities are reported in the cerebral cortex, thalamus and basal ganglia (BG) [15–18]. Approximately 26% of lesions occur outside of the WM, with 5% of these reported within the cortex [19]. Other studies have found cortical involvement in 53–80% of cases [11–13]. Several studies have demonstrated that the cortical lesions also involve the juxtacortical region to some extent [10,19]. There is strong evidence that GM demyelination is underestimated by conventional MRI techniques, limiting their use for assessing disease severity [10].
Clinical presentation
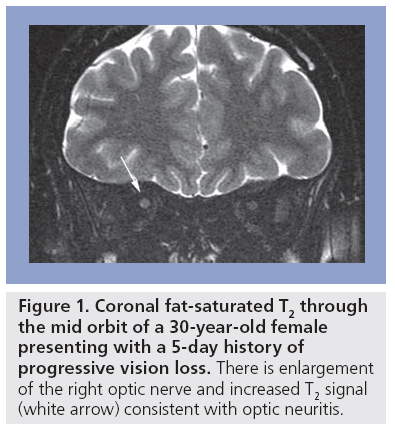
Multiple sclerosis is characterized by wide variability and diversity of symptom presentation, which may be mono- or multi-focal. Visual presentations include optic neuritis (Figure 1) and diplopia. Sensory symptoms include pain, paresthesia and disturbance of micturition, while motor symptoms are also common and include limb weakness and gait disturbance [5].
Figure 1: Coronal fat-saturated T2 through the mid orbit of a 30-year-old female presenting with a 5-day history of progressive vision loss. There is enlargement of the right optic nerve and increased T2 signal (white arrow) consistent with optic neuritis.
Neuropsychiatric abnormalities are frequent symptoms in MS. They are divided into disorders of mood, affect, and behavior and cognitive dysfunction. Depression is the most common pressing clinical problem seen in nearly half of MS patients, which is triple that of the prevalence in the healthy population [20]. Cognitive dysfunction is common, affecting 40–65% of MS patients. Attention, information processing speed, working, semantic and episodic memory, executive function, and verbal and visual–spatial abilities are the predominantly affected cognitive domains [21,22]. MS patients also have difficulty both acquiring and retrieving information [23], and their ability to accurately assess their own memory, a function termed ‘metamemory’, is impaired [24]. Procedural memory on the other hand is unaffected [25]. One of the hallmarks of a ‘subcortical’ dementia is impaired attention and slowness of thinking [26]. The poor performance of MS patients on neuropsychological paradigms such as the Paced Auditory Serial Addition Task (PASAT) and the symbol–digit substitution test illustrates this well [24]. Studies assessing information processing speed have found deficits across all domains in cognitively impaired MS patients [27]. Problems with concept formation and abstract reasoning also occur in MS [28]. An attempt to ascertain the constituent abnormalities in problem solving led Beatty to conclude that the central difficulty in MS patients is their difficulty in identifying concepts rather than perseveration [29]. The functional integrity of the frontal lobes is considered central to these cognitive processes. While injury of the critical links between cortical and subcortical circuits results in memory dysfunction [30], the delineation of widely dispersed neural networks helps explain why patients with nonfrontal pathology may, on occasion, perform poorly as well [27]. Similarly, MS patients have difficulty with verbal fluency measured by the Controlled Oral Word Association Test, which, like problem solving, is also sensitive to frontal function, but not wholly subserved by it [31]. Neuropsychological testing is the most sensitive means of detecting cognitive difficulties, but testing is time consuming, expensive and not always available. Several brief screening instruments have been developed that do not require neuropsychological expertise and can be completed in 30 min or less [21,32]. These tests lack the sensitivity of a complete neuropsychological assessment and their use should therefore be confined to screening patients. The Minimal Assessment of Cognitive Function in MS is a 90-min neurocognitive test, recently proposed by an expert panel for clinical monitoring and research [33]. This comprises of seven tests covering five cognitive domains commonly impaired in MS, including processing speed, memory, executive function, visual–spatial processing and word retrieval. The validity of the Minimal Assessment of Cognitive Function test has recently been confirmed [32,34,35]. MS patients showed significantly lower performance than normal controls in all tests at medium to very large effect sizes.
Cognitive impairment is more prevalent with increasing duration of the disease and is most often seen in patients with SP-MS and RR-MS [6]. Although cognitive impairment in MS is often mild, negative impact on occupational and social life is reported with a decline in performance of everyday tasks.
Role of vascular disease in MS
The underlying etiology of MS is still largely unknown. It is believed that MS is related to an autoimmune process in genetically susceptible individuals [5]; however, there is increasing evidence that microvascular impairment plays a role in MS pathogenesis, contributing to neuroaxonal degeneration [36–38]. Blood–brain barrier disruption is recognized as a crucial step in the evolution of MS [39,40], mediated by inflammatory processes. A recent study using susceptibility- weighted images showed diffuse diminished visibility of WM cerebral vasculature [41]. More controversially, it has recently been suggested that vascular stenosis may in fact be a possible primary event in disease evolution. Impaired outflow is postulated to be responsible for alterations in mural shear stress, resulting in inflammatory activation [42–44]. To date, the work remains controversial; it has not been replicated in other centers [45–47] and no proven link is reported between these apparent underlying vascular stenoses and cognitive impairment.
Diagnostic criteria of MS
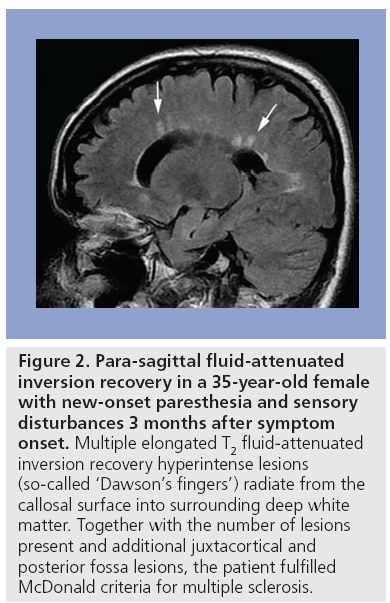
Conventional MRI, in combination with clinical and paraclinical studies, aids the diagnosis of MS and facilitates disease progression monitoring. Guidelines have been proposed to standardize the use of MRI in MS. The McDonald criteria were introduced in 2001 based on dissemination of MS lesions in time and space on conventional MRI (Figure 2), the presence of oligoclonal IgG bands in cerebrospinal fluid (CSF) analysis and visual evoked potential in addition to comprehensive objective clinical assessment [48]. These criteria were revised in 2005 to simplify and clarify issues considered confusing to the clinician, such as the role of spinal cord lesions in MS diagnosis, and to expedite diagnosis while maintaining adequate sensitivity and specificity [49].
Figure 2: Para-sagittal fluid-attenuated inversion recovery in a 35-year-old female with new-onset paresthesia and sensory disturbances 3 months after symptom onset. Multiple elongated T2 fluid-attenuated inversion recovery hyperintense lesions (so-called ‘Dawson’s fingers’) radiate from the callosal surface into surrounding deep white matter. Together with the number of lesions present and additional juxtacortical and posterior fossa lesions, the patient fulfilled McDonald criteria for multiple sclerosis.
GM lesions, cortical atrophy & correlates of cognitive dysfunction
Gray matter lesions are underestimated using conventional MRI. The reasons for this include:
▪ Cortical lesions are less conspicuous on T2-weighted imaging (T2-WI) because of longer lesion relaxation times resulting in poor lesion-to-cortex contrast resolution [9,50];
▪ Partial volume effects from surrounding CSF obscure GM lesions;
▪ Small lesions may be below the spatial resolution of current clinical scanners [51];
▪ GM lesions have a reduced amount of inflammation compared with those located in WM upon pathological study [52].
Higher cortical cellular density may not allow sufficient extracellular space expansion to allow cortical relaxation time increase compared with WM lesions. Attenuating CSF using fluid-attenuated inversion recovery sequence increases MS cortical lesion detection compared with T2-WI [53]. Further improvement is achieved with double inversion recovery imaging [54]. The use of 3D double inversion recovery imaging allows better detection, definition and classification of MS lesions as intracortical, juxtacortical or mixed gray–white matter lesions [50].
Cortical lesion load has been demonstrated to correlate with cognitive impairment [21,55]. Older studies using conventional spin-echo MRI sequences showed moderate correlations between verbal memory, attention, verbal fluency and spatial reasoning, T2-WI hyperintense lesions, total lesion burden and regional lesion volume [56]. Complementing these studies is a recent report showing that the change in T2 hyperintense lesions over 2 years correlated with cognitive performance 13 years later [57]. One study reported a correlation with T2 hyperintense lesions in subcortical GM with neuropsychological dysfunction [34]. Total lesion area was found to correlate with neuropsychological deficits [58]. High lesion volume in the left frontal lobe was found to predict impaired abstract problem solving, memory and word fluency while left parieto-occipital involvement predicted deficits in verbal learning and complex visual-integrative skills [59].
Enhancing lesions on contrast-enhanced short time of repetition spin-echo images (contrast- enhanced T1-WI) were regarded as active lesions. Contrast-enhanced T1-WI were found to detect 85% of all new active lesions compared with 26% observed on long time of repetition spin-echo images [12]. The presence of enhanced lesions was found to correlate with poor PASAT performance, indicating negative impact on cognitive function [60].
In addition to lesion load, cerebral atrophy is considered an imaging hallmark of MS. It is also an important potential marker of cognitive decline [61,62]. Ventricular enlargement, one index of brain atrophy, is associated with abnormalities on various cognitive tests [62,63]. Progressive GM loss has been demonstrated to be important in the context of MS-associated cognitive impairment [64]. A more recent study showed more cortical lesions and more severe cortical atrophy in RR-MS patients with cognitive impairment when compared with cognitively preserved patients [65]. Ventricular dilatation and atrophy of corpus callosum were found to correlate with lower neuropsychological performance [66]. Greater lesion area and significant atrophy of corpus callosum were found in patients with severe cognitive impairment [67]. The performance of MS patients on verbal fluency tasks was found to be strongly affected by the atrophy of anterior corpus callosum, implicating the importance of corpus callosum in interhemispheric transfer of cognitive information [68]. Cross-callosal information flow was found to be reduced in MS patients with corpus callosum atrophy [69].
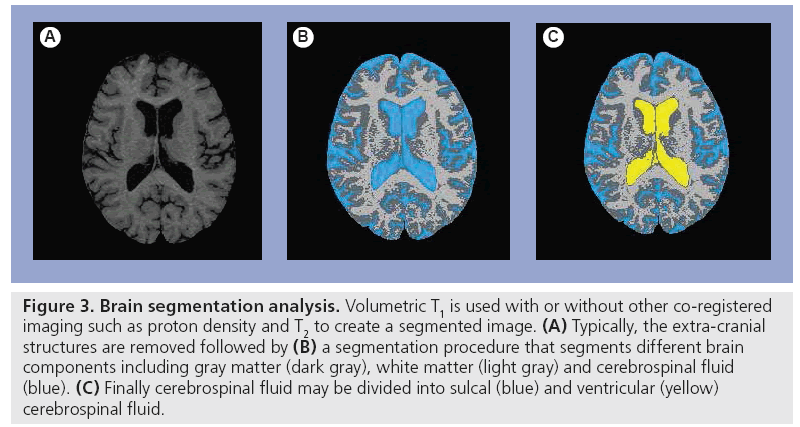
Numerous MRI segmentation techniques exist, facilitating the creation of separate estimations of GM, WM and lesion volume in MS. Discussion of these techniques is beyond the scope of this review but described in other literature [70–72]. In general, these techniques require variable degrees of user input, ranging from completely manual, to semi-automated needing limited user input, to fully automated. The initial processing involved usually requires multisequential high-quality imaging such as proton density T2-WI and 3D-T1 to remove nonbrain tissues (skull, muscle, fat and dura) and divide brain tissues into three main tissue types: GM, WM and CSF (Figure 3), then estimate the volume of the region of interest [70–72]. A study examining the relative contributions of segmented GM and WM volume versus lesion burden to cognitive performance and neuropsychiatric symptoms found a unique association between GM atrophy and verbal memory. By contrast, WM atrophy accounted for significant variance in the performance of mental processing speed and working memory tasks, likely indicating slow communication between brain regions via damaged WM tracts [73].
Figure 3: Brain segmentation analysis. Volumetric T1 is used with or without other co-registered imaging such as proton density and T2 to create a segmented image. (A) Typically, the extra-cranial structures are removed followed by (B) a segmentation procedure that segments different brain components including gray matter (dark gray), white matter (light gray) and cerebrospinal fluid (blue). (C) Finally cerebrospinal fluid may be divided into sulcal (blue) and ventricular (yellow) cerebrospinal fluid.
Advanced MRI techniques correlate with cognitive dysfunction
Advanced MRI techniques reveal structural abnormalities beyond the resolution of conventional MRI, and several quantitative MRI studies have displayed similar radiological findings in GM to those described in WM.
■ Magnetization transfer imaging
Magnetization transfer imaging is based on the exchange of magnetization between free water protons and tissue macromolecules. Selective saturation of the bound protons reduces the signal intensity of the whole image. This reduction is expressed as a magnetization transfer ratio (MTR) [74]. Although MTR is slightly reduced by inflammation and edema, profound MTR reduction is described in demyelination. MTR may be used to quantify the degree of tissue integrity disruption in MS. Previous studies have shown correlation between MTR and MS duration, suggesting progressive lesion accumulation. Also described is MTR reduction remote to MS plaque confirming global brain disease including abnormality within normalappearing WM and GM [75]. The NAWM and normal-appearing GM changes may be seen in early RR-MS where minimal disability is manifest clinically [62,76]. MTR is correlated with clinical disability and neuropsychological impairment [75,77] and a histogram analysis of cortical and subcortical brain tissue showed that all histogram-derived measures were significantly different between cognitively impaired and unimpaired MS patients [13].
■ Diffusion-weighted & diffusion tensor imaging
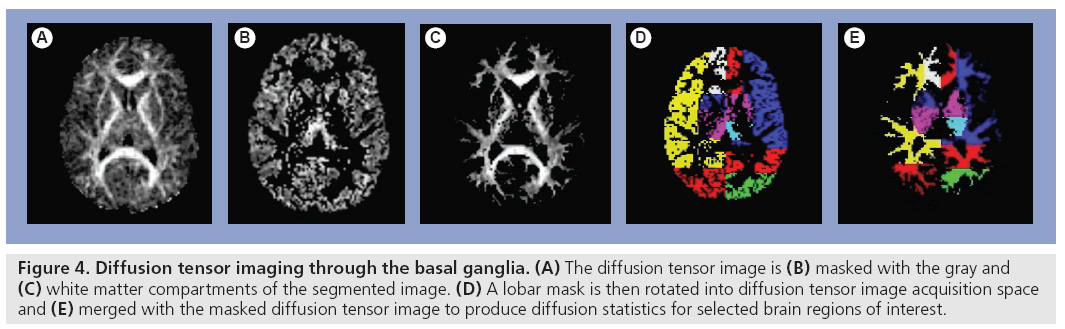
Diffusion is the microscopic random translational motion of molecules. The water molecular diffusion can be measured in vivo using diffusion- weighted imaging (DWI), which considers the magnitude of diffusivity. The motion of molecules is affected by the presence of structural barriers such as WM fibre tracts in the brain; hence, diffusion measurement varies with the direction of measurement. Diffusion is affected by the properties of the medium where molecular motion occurs; therefore, measurement of tissue diffusivity using DWI and diffusion tensor imaging (DTI) (Figure 4) provides information about tissue structures at a microscopic level. Echo planer pulse sequence for isotropically weighted diffusion imaging has been used to measure the mean diffusivity (MD) in lesions larger than 5 mm and NAWM in RR-MS patients, and compared with diffusivity in the WM of a normal control group. Both lesion and NAWM MD were significantly higher than the WM in controls, indicating global tissue disruption in MS patients [78]. DWI studies show a correlation with cognitive dysfunction [15,79]. Fractional anisotropy (FA) provides additional means for quantification of degree of neuronal damage [80,81]. Correlations of MD, FA and cognitive dysfunction are shown in several other diseases including Huntington’s disease, Alzheimer’s and HIV-associated dementia [82–84]. However, reports of DTI correlation with cognitive function in MS are limited. Mesaros found a correlation between cognitive impairment and increased MD in corpus callosum NAWM in benign MS (BMS) [85]. A decreased FA correlated with PASAT, measuring processing speed and working memory. However, Rovaris et al. found no correlation between cognitive function and subcortical/WM DTI metrics in BMS patients [86]. Lin et al. reported significant correlation between corpus callosum apparent diffusion coefficient and PASAT score in RR-MS patients; a moderate correlation between global cognitive impairment and corpus callosum MD was found [87]. Vrenken et al. reported moderate correlation between an MS functional composite score (which included a cognition measure) and tissue damage in NAWM and GM with a higher correlation in SP-MS than RR-MS patients [88].
Figure 4: Diffusion tensor imaging through the basal ganglia. (A) The diffusion tensor image is (B) masked with the gray and (C) white matter compartments of the segmented image. (D) A lobar mask is then rotated into diffusion tensor image acquisition space and (E) merged with the masked diffusion tensor image to produce diffusion statistics for selected brain regions of interest.
A study using a comprehensive, MS-specific neurocognitive tool, the minimal assessment of cognitive function in MS, identified a correlation between PASAT, Benton Visual Retention and California Verbal Learning Test scores and reduced FA in various regions [89]. Both increased and reduced MD and increased FA in the BG and thalamus have been reported [14,15,30,90,91]. A study of 36 patients with early PP-MS found lower FA and higher MD in the NAWM consistent with prior studies; however, significantly increased FA and a trend of MD reduction was reported in the BG when compared with controls [14]. These results were replicated in a second study of 39 patients with various types of MS [90]. Two studies found an increased MD in the BG and one study confirmed increased FA in the thalamus and BG [30]. Finally, Fabiano et al. found a higher apparent diffusion coefficient in the thalami of MS patients [15].
■ Functional MRI
Functional MRI (fMRI) is a physiologic imaging technique that has rapidly evolved and made a major transition from a purely research to a highly useful imaging technique. During the fMRI study, the signal change on echo planar images in response to neural activation is attributable to local change in cerebral deoxyhemoglobin blood level and cerebral blood flow (CBF). Activation of certain brain regions in response to specific tasks increases the local synaptic activity, resulting in increased blood flow, oxygen consumption and deoxyhemoglobin, causing echo planar image signal change [92,93]. The use of blood oxygen level-dependent imaging in fMRI allows assessment of sensorimotor function and visual function, in addition and to more complex functions such as language, memory, reasoning functions and slow processing speeds. The first report of reduced activity in MS patients was described in the visual cortex of a patient with optic neuritis [94]. Abnormal cortical activation was reported in all MS types and related to altered recruitment of regions normally devoted to perform specific tasks, with recruitment of additional areas not typically activated by healthy people for that given task. The recruitment of these new areas is referred to as cortical reorganization and considered to be an adaptive mechanism that likely contributes to limit the functional impact of structural damage in MS [95,96].
In patients with RR-MS, PASAT and recall tests were used to assess multiple cognitive functions including sustained attention, information processing, speed, working memory and memory retrieval. Results showed altered patterns of activation during these tasks. During PASAT, greater activation was seen in MS patients than controls for several brain regions including the inferior and middle frontal gyrus, inferior parietal lobule, supplementary motor area, lateral premotor area, superior and middle temporal gyrus, insula, BG, bilateral thalamus, vermis and brainstem. New areas of recruitment were demonstrated in the anterior cingulate and superior parietal lobule of the right hemisphere. During recall tasks, patients showed similar or greater activation of brain areas compared with healthy controls including the inferior and middle frontal gyrus, superior and middle temporal gyrus, inferior parietal lobule, supplementary motor area, left lateral premotor area, right anterior cingulated, bilateral BG, lateral premotor area and right hemisphere insula. The fMRI activity was greater in patients with better cognitive function, indicating better adaptive properties [97]. Increased activation of specific cortical areas and the cerebellum was seen in BMS during performance of the Stroop task, which might represent preserved adaptive properties [98,99]. Increasing lesion volume was reported to correlate with increased magnitude of brain activation during fMRI in RR-MS patients, which is thought to indicate increased neural recruitment with higher disease burden [100].
A recent study assessed attention and information processing, verbal and visual–spatial memory, abstract reasoning, linguistic abilities, lexical access and spatial cognition in cognitively impaired PP-MS, cognitively preserved patients and a control group. Cognitively impaired patients had increased activations of the secondary sensorimotor cortex, cerebellum and insula. Compared with controls, increased activations of the right precentral gyrus and reduced recruitment of the left prefrontal cortex were found [101].
A study used education and vocabulary knowledge as an estimate of lifetime intellectual enrichment and brain atrophy to estimate MS burden and has shown that brain atrophy negatively impacted verbal learning and memory, while higher lifetime intellectual enrichment lessened this negative impact [102]. When fMRI was used to investigate the relationship between intellectual enrichment and cerebral activity in MS patients, less recruitment of prefrontal cortices was noticed in patients with higher intellectual enrichment, indicating that intellectual enrichment protects against cognitive dysfunction [103,104].
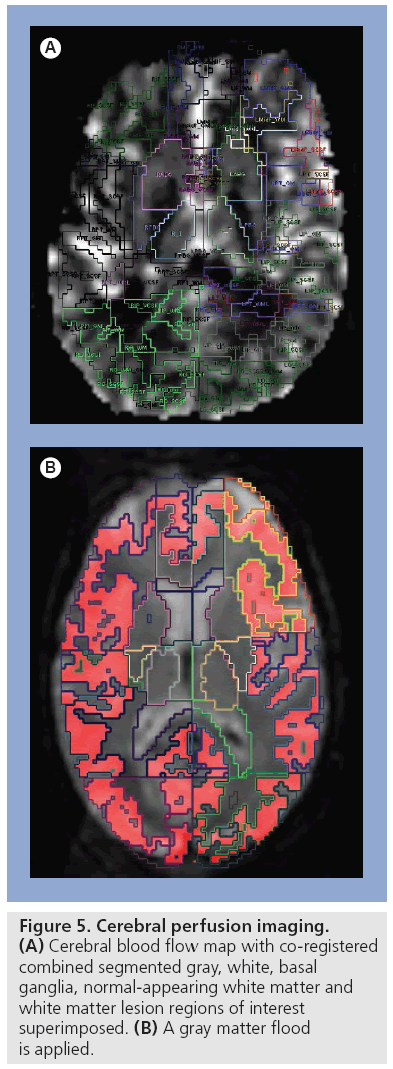
■ Perfusion imaging
Cerebral perfusion is defined as the volume of the blood flowing through a given volume of tissue per unit of time. Dynamic susceptibility contrast MRI is the most commonly used method to investigate hemodynamic changes in tissue after intravenously injected contrast agent (Figure 5). The use of 3 Tesla (3T) MRI in clinical practise facilitates higher spatial resolution, increased signal to noise and shorter acquisition time compared with 1.5T [105]. Cerebral perfusion is, under normal circumstances, coupled to parenchymal metabolism and provides a measure of the extent of neuroaxonal loss or dysfunction considered a crucial factor in irreversible disability in MS patients [106]. Perfusion of MS lesions and NAWM in MS patients has been evaluated on different nuclear medicine and magnetic resonance studies. Hypoperfusion in NAWM has been shown on nuclear medicine studies [107–109]. A study using SPECT demonstrated significantly reduced CBF in frontal GM in PP-MS patients [109]. PET and SPECT studies have shown coupled reduction in WM and cortical/subcortical GM oxygen utilization and blood flow [110]. Magnetic resonance perfusion has distinct advantages over PET and SPECT imaging including shorter scan duration, absence of ionizing radiation and higher resolution. Early dynamic susceptibility contrast MRI perfusion studies focused on MS lesions measuring relative values expressed as a ratio of the contralateral side [111,112]. More recent studies employ absolute perfusion measures but have concentrated largely on WM abnormality. These studies demonstrated reduced CBF in the NAWM of patients with RR-MS presumed secondary to underlying microvascular impairment [113,114]. Elevation of CBF and cerebral blood volume are also reported in NAWM as early as 3 weeks prior to blood–brain barrier breakdown and plaque formation on MRI perfusion [111]. These studies suggest that bolus-tracking perfusion techniques may provide a sensitive measure of disease activity and treatment effect [111]. Few studies have focused on GM or cortical perfusion abnormality, especially utilizing quantitative MRI techniques. Rashid et al. found decreased perfusion in cortical GM, deep GM and subcortical WM regions. Gradation of change was most pronounced in PP-MS and SP-MS subgroups compared with other subgroups, reflecting the severity of neuroaxonal loss; however, no correlation with neurocognition was made [114]. Changes in CBF and cerebral blood volume have been demonstrated to correlate with degree of cognitive impairment [115]. A gradation of cortical and GM hypoperfusion in MRI is previously demonstrated [114] with highest levels in PP-MS, followed by SP-MS and BMS. This gradation mirrors the severity of neuroaxonal loss, highlighting the potential role for perfusion imaging in noninvasive assessment of disease severity.
■ Proton magnetic resonance spectroscopy
Proton magnetic resonance spectroscopy has enabled the measurement of certain metabolites that are affected in a variety of CNS pathologies. These metabolites include N-acetyl aspartate (NAA), choline (Cho), creatine (cr), myoinositol and lipids.
In normal subjects NAA, used as a neural marker, is the largest peak, resonating at 2.0 ppm. Cho is a precursor of acetylcholine (a vital cell membrane constituent) and phosphatidylcholine is a critical neurotransmitter involved in memory, mood and cognition. Therefore, Cho, with its peak at 3.2 ppm, is used to reflect cell membrane turnover. Myoinositol is a metabolite that is involved in hormonesensitive neuroreceptors (3.56 ppm). In MS patients, the hallmark finding is NAA reduction. Reduction is more prominant in chronic MS plaques, ref lecting axonal loss [116,117]. In acute MS plaques, NAA may temporarily reduce with subsequent restoration following acute-phase resolution. This reversibility indicates that axons have not been permanently damaged, or that the NAA could be related to reversible causes such as edema [118]. Cho and lactate may both be increased in the acute stage of the disease. The presence of free lipid between 1.2 and 1.6 ppm during acute disease is thought to indicate myelin breakdown [119,120]. Elevated myoinositol is additionallysreported in MS plaques [121]. Metabolite alteration is also detected in NAWM beyond lesions [122,123]. Multislice echo planar spectroscopic imaging has been used to measure global brain metabolite changes in cognitively impaired MS patients compared with cognitively intact patients and controls. A correlation between NAA:Cr ratio and cognitive dysfunction factor was found with a significant decrease of the NAA:Cr ratio in cognitive impairment [124]. Expected advances in spectroscopy may provide new opportunities for the detection of additional metabolites that are relevant to MS [125].
Conclusion
Although MS is traditionally considered a WM disease, GM involvement is increasingly recognized, largely due to progress in more physiological or functional MRI techniques. The more widespread brain involvement demonstrated by these techniques may better explain the diversity in clinical symptoms and cognitive impairment. It is anticipated that use of advanced magnetic resonance techniques will continue to contribute to a better understanding of MS etiology and pathophysiology.
Future perspective
Advanced MRI is expected to provide new means for the evaluation of disease burden, previously unmeasured or grossly underestimated with conventional MRI techniques. We expect that these modalities will, in the near future, be included in routine clinical practise and provide useful surrogate markers of disease burden and response to disease-modifying drugs.
Financial & competing interests disclosure
RI Aviv received grant funding from Physician Services Incorporated Foundation and the MS society of Canada. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.

References
Papers of special note have been highlighted as:
• of interest
• • of considerable interest
- Haacke EM, Makki M, Ge Y et al.: Characterizing iron deposition in multiple sclerosis lesions using susceptibility weighted imaging. J. Magn. Reson. Imaging 29(3), 537–544 (2009).
- Beck CA, Metz LM, Svenson LW, Patten SB: Regional variation of multiple sclerosis prevalence in Canada. Mult. Scler. 11(5), 516–519 (2005).
- Weinshenker BG, Bass B, Rice GP et al.: The natural history of multiple sclerosis: a geographically based study. I. Clinical course and disability. Brain 112( Pt 1), 133–146 (1989).
- Weinshenker BG, Bass B, Rice GP et al.: The natural history of multiple sclerosis: a geographically based study. 2. Predictive value of the early clinical course. Brain 112( Pt 6), 1419–1428 (1989).
- Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG: Multiple sclerosis. N. Engl. J. Med. 343(13), 938–952 (2000).
- Filippi M, Horsfield MA, Hajnal JV et al.: Quantitative assessment of magnetic resonance imaging lesion load in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 64(Suppl. 1), S88–S93 (1998).
- Cercignani M, Bozzali M, Iannucci G, Comi G, Filippi M: Magnetisation transfer ratio and mean diffusivity of normal appearing white and grey matter from patients with multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 70(3), 311–317 (2001).
- Bozzali M, Cercignani M, Sormani MP, Comi G, Filippi M: Quantification of brain gray matter damage in different MS phenotypes by use of diffusion-tensor MR imaging. AJNR Am. J. Neuroradiol. 23(6), 985–988 (2002).
- Gawne-Cain ML, O’Riordan JI, Thompson AJ, Moseley IF, Miller DH: Multiple sclerosis lesion detection in the brain: a comparison of fast fluid-attenuated inversion recovery and conventional T2-weighted dual spin echo. Neurology 49(2), 364–370 (1997).
- Kidd D, Barkhof F, Mcconnell R, Algra PR, Allen IV, Revesz T: Cortical lesions in multiple sclerosis. Brain 122(Pt 1), 17–26 (1999).
- Miki Y, Grossman RI, Udupa JK et al.: Isolated U-fiber involvement in MS: preliminary observations. Neurology 50(5), 1301–1306 (1998).
- Miller DH, Barkhof F, Nauta JJ: Gadolinium enhancement increases the sensitivity of MRI in detecting disease activity in multiple sclerosis. Brain 116(Pt 5), 1077–1094 (1993).
- Rovaris M, Filippi M, Minicucci L et al.: Cortical/subcortical disease burden and cognitive impairment in patients with multiple sclerosis. AJNR Am. J. Neuroradiol. 21(2), 402–408 (2000).
- Bodini B, Khaleeli Z, Cercignani M, Miller DH, Thompson AJ, Ciccarelli O: Exploring the relationship between white matter and gray matter damage in early primary progressive multiple sclerosis: an in vivo study with TBSS and VBM. Hum. Brain Mapp. 30(9), 2852–2861 (2009).
- Fabiano AJ, Sharma J, Weinstock- Guttman B et al.: Thalamic involvement in multiple sclerosis: a diffusion-weighted magnetic resonance imaging study. J. Neuroimaging 13(4), 307–314 (2003).
- Bakshi R, Benedict RH, Bermel RA et al.: T2 hypointensity in the deep gray matter of patients with multiple sclerosis: a quantitative magnetic resonance imaging study. Arch. Neurol. 59(1), 62–68 (2002).
- Davies GR, Altmann DR, Rashid W et al.: Emergence of thalamic magnetization transfer ratio abnormality in early relapsing-remitting multiple sclerosis. Mult. Scler. 11(3), 276–281 (2005).
- Inglese M, Liu S, Babb JS, Mannon LJ, Grossman RI, Gonen O: Three-dimensional proton spectroscopy of deep gray matter nuclei in relapsing-remitting MS. Neurology 63(1), 170–172 (2004).
- Brownell B, Hughes JT: The distribution of plaques in the cerebrum in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 25, 315–320 (1962).
- Ghaffar O, Feinstein A: The neuropsychiatry of multiple sclerosis: a review of recent developments. Curr. Opin. Psychiatry 20(3), 278–285 (2007).
- Rao SM, Leo GJ, Bernardin L, Unverzagt F: Cognitive dysfunction in multiple sclerosis. I. Frequency, patterns, and prediction. Neurology 41(5), 685–691 (1991).
- Rao SM, Leo GJ, Ellington L, Nauertz T, Bernardin L, Unverzagt F: Cognitive dysfunction in multiple sclerosis. II. Impact on employment and social functioning. Neurology 41(5), 692–696 (1991).
- Wishart H, Sharpe D: Neuropsychological aspects of multiple sclerosis: a quantitative review. J. Clin. Exp. Neuropsychol. 19(6), 810–824 (1997).
- Beatty WW, Goodkin DE: Screening for cognitive impairment in multiple sclerosis. An evaluation of the Mini-Mental State Examination. Arch. Neurol. 47(3), 297–301 (1990).
- Grafman J, Rao S, Bernardin L, Leo GJ: Automatic memory processes in patients with multiple sclerosis. Arch. Neurol. 48(10), 1072–1075 (1991).
- Cummings JL: Subcortical dementia. Neuropsychology, neuropsychiatry, and pathophysiology. Br. J. Psychiatry 149, 682–697 (1986).
- Kujala P, Portin R, Revonsuo A, Ruutiainen J: Automatic and controlled information processing in multiple sclerosis. Brain 117(Pt 5), 1115–1126 (1994).
- Heaton RK, Nelson LM, Thompson DS, Burks JS, Franklin GM: Neuropsychological findings in relapsing-remitting and chronicprogressive multiple sclerosis. J. Consult. Clin. Psychol. 53(1), 103–110 (1985).
- Beatty WW, Monson N: Problem solving by patients with multiple sclerosis: comparison of performance on the Wisconsin and California Card Sorting Tests. J. Int. Neuropsychol. Soc. 2(2), 134–140 (1996).
- Tovar-Moll F, Evangelou IE, Chiu AW et al.: Thalamic involvement and its impact on clinical disability in patients with multiple sclerosis: a diffusion tensor imaging study at 3T. AJNR Am. J. Neuroradiol. 30(7), 1380–1386 (2009).
- Basso MR, Beason-Hazen S, Lynn J, Rammohan K, Bornstein RA: Screening for cognitive dysfunction in multiple sclerosis. Arch. Neurol. 53(10), 980–984 (1996).
- Benedict RH, Zivadinov R: Predicting neuropsychological abnormalities in multiple sclerosis. J. Neurol. Sci. 245(1–2), 67–72 (2006).
- Benedict RH, Fischer JS, Archibald CJ et al.: Minimal neuropsychological assessment of MS patients: a consensus approach. Clin. Neuropsychol. 16(3), 381–397 (2002).
- Brass SD, Benedict RH, Weinstock- Guttman B, Munschauer F, Bakshi R: Cognitive impairment is associated with subcortical magnetic resonance imaging grey matter T2 hypointensity in multiple sclerosis. Mult. Scler. 12(4), 437–444 (2006).
- Filippi M: [Magnetic resonance imaging correlates of cognitive dysfunction in patients with multiple sclerosis]. Rev. Neurol. 30(12), 1253–1256 (2000).
- Adams CW, Poston RN, Buk SJ, Sidhu YS, Vipond H: Inflammatory vasculitis in multiple sclerosis. J. Neurol. Sci. 69(3), 269–283 (1985).
- Lassmann H: Hypoxia-like tissue injury as a component of multiple sclerosis lesions. J. Neurol. Sci. 206(2), 187–191 (2003).
- Lightman S, Mcdonald WI, Bird AC et al.: Retinal venous sheathing in optic neuritis. Its significance for the pathogenesis of multiple sclerosis. Brain 110(Pt 2), 405–414 (1987).
- Harris JO, Frank JA, Patronas N, Mcfarlin DE, Mcfarland HF: Serial gadolinium-enhanced magnetic resonance imaging scans in patients with early, relapsing-remitting multiple sclerosis: implications for clinical trials and natural history. Ann. Neurol. 29(5), 548–555 (1991).
- Mcfarland HF, Frank JA, Albert PS et al.: Using gadolinium-enhanced magnetic resonance imaging lesions to monitor disease activity in multiple sclerosis. Ann. Neurol. 32(6), 758–766 (1992).
- Ge Y, Zohrabian VM, Osa EO et al.: Diminished visibility of cerebral venous vasculature in multiple sclerosis by susceptibility-weighted imaging at 3.0 Tesla. J. Magn. Reson. Imaging 29(5), 1190–1194 (2009).
- Zamboni P, Menegatti E, Bartolomei I et al.: Intracranial venous haemodynamics in multiple sclerosis. Curr. Neurovasc. Res. 4(4), 252–258 (2007).
- Zamboni P, Galeotti R, Menegatti E et al.: Chronic cerebrospinal venous insufficiency in patients with multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 80(4), 392–399 (2009).
- Zamboni P, Galeotti R, Menegatti E et al.: A prospective open-label study of endovascular treatment of chronic cerebrospinal venous insufficiency. J. Vasc. Surg. 50(6), 1348–1358 E1–E3 (2009).
- Khan O, Filippi M, Freedman MS et al.: Chronic cerebrospinal venous insufficiency and multiple sclerosis. Ann. Neurol. 67(3), 286–290 (2010).
- Rudick RA: Multiple sclerosis: is multiple sclerosis caused by venous insufficiency? Nat. Rev. Neurol. 6(9), 472–474 (2010).
- Doepp F, Paul F, Valdueza JM, Schmierer K, Schreiber SJ: No cerebrocervical venous congestion in patients with multiple sclerosis. Ann. Neurol. 68(2), 173–183 (2010).
- Mcdonald WI, Compston A, Edan G et al.: Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann. Neurol. 50(1), 121–127 (2001).
- Polman CH, Reingold SC, Edan G et al.: Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann. Neurol. 58(6), 840–846 (2005).
- Geurts JJ, Pouwels PJ, Uitdehaag BM, Polman CH, Barkhof F, Castelijns JA: Intracortical lesions in multiple sclerosis: improved detection with 3D double inversion-recovery MR imaging. Radiology 236(1), 254–260 (2005).
- Bagnato F, Butman JA, Gupta S et al.: In vivo detection of cortical plaques by MR imaging in patients with multiple sclerosis. AJNR Am. J. Neuroradiol. 27(10), 2161–2167 (2006).
- Peterson JW, Bo L, Mork S, Chang A, Trapp BD: Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann. Neurol. 50(3), 389–400 (2001).
- Bakshi R, Ariyaratana S, Benedict RH, Jacobs L: Fluid-attenuated inversion recovery magnetic resonance imaging detects cortical and juxtacortical multiple sclerosis lesions. Arch. Neurol. 58(5), 742–748 (2001).
- Calabrese M, De Stefano N, Atzori M et al.: Detection of cortical inflammatory lesions by double inversion recovery magnetic resonance imaging in patients with multiple sclerosis. Arch. Neurol. 64(10), 1416–1422 (2007).
- Roosendaal SD, Moraal B, Pouwels PJ et al.: Accumulation of cortical lesions in MS: relation with cognitive impairment. Mult. Scler. 15(6), 708–714 (2009).
- Camp SJ, Stevenson VL, Thompson AJ et al.: Cognitive function in primary progressive and transitional progressive multiple sclerosis: a controlled study with MRI correlates. Brain 122(Pt 7), 1341–1348 (1999).
- Rudick RA, Lee JC, Simon J, Fisher E: Significance of T2 lesions in multiple sclerosis: A 13-year longitudinal study. Ann. Neurol. 60(2), 236–242 (2006).
- Rovaris M, Filippi M, Falautano M et al.: Relation between MR abnormalities and patterns of cognitive impairment in multiple sclerosis. Neurology 50(6), 1601–1608 (1998).
- Swirsky-Sacchetti T, Mitchell DR, Seward J et al.: Neuropsychological and structural brain lesions in multiple sclerosis: a regional analysis. Neurology 42(7), 1291–1295 (1992).
- Bellmann-Strobl J, Wuerfel J, Aktas O et al.: Poor PASAT performance correlates with MRI contrast enhancement in multiple sclerosis. Neurology 73(20), 1624–1627 (2009).
- Rovaris M, Comi G, Filippi M: MRI markers of destructive pathology in multiple sclerosis-related cognitive dysfunction. J. Neurol. Sci. 245(1–2), 111–116 (2006).
- Zivadinov R, De Masi R, Nasuelli D et al.: MRI techniques and cognitive impairment in the early phase of relapsing-remitting multiple sclerosis. Neuroradiology 43(4), 272–278 (2001).
- Benedict RH, Carone DA, Bakshi R: Correlating brain atrophy with cognitive dysfunction, mood disturbances, and personality disorder in multiple sclerosis. J. Neuroimaging 14(3 Suppl.), S36–S45 (2004).
- Amato MP, Portaccio E, Goretti B et al.: Association of neocortical volume changes with cognitive deterioration in relapsingremitting multiple sclerosis. Arch. Neurol. 64(8), 1157–1161 (2007).
- Calabrese M, Agosta F, Rinaldi F et al.: Cortical lesions and atrophy associated with cognitive impairment in relapsing-remitting multiple sclerosis. Arch. Neurol. 66(9), 1144–1150 (2009).
- Comi G, Filippi M, Martinelli V et al.: Brain magnetic resonance imaging correlates of cognitive impairment in multiple sclerosis. J. Neurol. Sci. 115(Suppl.), S66–S73 (1993).
- Huber SJ, Bornstein RA, Rammohan KW, Christy JA, Chakeres DW, Mcghee RB: Magnetic resonance imaging correlates of neuropsychological impairment in multiple sclerosis. J. Neuropsychiatry Clin. Neurosci. 4(2), 152–158 (1992).
- Pozzilli C, Bastianello S, Padovani A et al.: Anterior corpus callosum atrophy and verbal fluency in multiple sclerosis. Cortex 27(3), 441–445 (1991).
- Rao SM, Bernardin L, Leo GJ, Ellington L, Ryan SB, Burg LS: Cerebral disconnection in multiple sclerosis. Relationship to atrophy of the corpus callosum. Arch. Neurol. 46(8), 918–920 (1989).
- Kovacevic N, Lobaugh NJ, Bronskill MJ, Levine B, Feinstein A, Black SE: A robust method for extraction and automatic segmentation of brain images. Neuroimage 17(3), 1087–1100 (2002).
- Dade LA, Gao FQ, Kovacevic N et al.: Semiautomatic brain region extraction: a method of parcellating brain regions from structural magnetic resonance images. Neuroimage 22(4), 1492–1502 (2004).
- Clarke LP, Velthuizen RP, Camacho MA et al.: MRI segmentation: methods and applications. Magn. Reson. Imaging 13(3), 343–368 (1995).
- Sanfilipo MP, Benedict RH, Weinstock- Guttman B, Bakshi R: Gray and white matter brain atrophy and neuropsychological impairment in multiple sclerosis. Neurology 66(5), 685–692 (2006).
- Wolff SD, Balaban RS: Magnetization transfer contrast (MTC) and tissue water proton relaxation in vivo. Magn. Reson. Med. 10(1), 135–144 (1989).
- Filippi M, Tortorella C, Rovaris M et al.: Changes in the normal appearing brain tissue and cognitive impairment in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 68(2), 157–161 (2000).
- Davies GR, Ramio-Torrenta L, Hadjiprocopis A et al.: Evidence for grey matter MTR abnormality in minimally disabled patients with early relapsingremitting multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 75(7), 998–1002 (2004).
- Van Buchem MA, Grossman RI, Armstrong C et al.: Correlation of volumetric magnetization transfer imaging with clinical data in MS. Neurology 50(6), 1609–1617 (1998).
- Filippi M, Iannucci G, Cercignani M, Assunta Rocca M, Pratesi A, Comi G: A quantitative study of water diffusion in multiple sclerosis lesions and normalappearing white matter using echo-planar imaging. Arch. Neurol. 57(7), 1017–1021 (2000).
- Benedict RH, Bruce J, Dwyer MG et al.: Diffusion-weighted imaging predicts cognitive impairment in multiple sclerosis. Mult. Scler. 13(6), 722–730 (2007).
- Schiavone F, Charlton RA, Barrick TR, Morris RG, Markus HS: Imaging age-related cognitive decline: A comparison of diffusiontensor and magnetization transfer MRI. J. Magn. Reson. Imaging 29(1), 23–30 (2009).
- Raz E, Cercignani M, Sbardella E et al.: Clinically isolated syndrome suggestive of multiple sclerosis: voxelwise regional investigation of white and gray matter. Radiology 254(1), 227–234 (2010).
- Rosas HD, Tuch DS, Hevelone ND et al.: Diffusion tensor imaging in presymptomatic and early Huntington’s disease: selective white matter pathology and its relationship to clinical measures. Mov. Disord. 21(9), 1317–1325 (2006).
- Huang J, Friedland RP, Auchus AP: Diffusion tensor imaging of normal-appearing white matter in mild cognitive impairment and early Alzheimer disease: preliminary evidence of axonal degeneration in the temporal lobe. AJNR Am. J. Neuroradiol. 28(10), 1943–1948 (2007).
- Chen Y, An H, Zhu H et al.: White matter abnormalities revealed by diffusion tensor imaging in non-demented and demented HIV+ patients. Neuroimage 47(4), 1154–1162 (2009).
- Mesaros S, Rocca MA, Riccitelli G et al.: Corpus callosum damage and cognitive dysfunction in benign MS. Hum. Brain Mapp. 30(8), 2656–2666 (2009).
- Rovaris M, Iannucci G, Falautano M et al.: Cognitive dysfunction in patients with mildly disabling relapsing-remitting multiple sclerosis: an exploratory study with diffusion-tensor MR imaging. J. Neurol. Sci. 195(2), 103–109 (2002).
- Lin X, Tench CR, Morgan PS, Constantinescu CS: Use of combined conventional and quantitative MRI to quantify pathology related to cognitive impairment in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 79(4), 437–441 (2008).
- Vrenken H, Pouwels PJ, Geurts JJ et al.: Altered diffusion-tensor in multiple sclerosis normal-appearing brain tissue: cortical diffusion changes seem related to clinical deterioration. J. Magn. Reson. Imaging 23(5), 628–636 (2006).
- Dineen RA, Vilisaar J, Hlinka J et al.: Disconnection as a mechanism for cognitive dysfunction in multiple sclerosis. Brain 132(Pt 1), 239–249 (2009).
- Ciccarelli O, Werring DJ, Wheeler- Kingshott CA et al.: Investigation of MS normal-appearing brain using diffusiontensor MRI with clinical correlations. Neurology 56(7), 926–933 (2001).
- Filippi M, Bozzali M, Comi G: Magnetization transfer and diffusion-tensor MR imaging of basal ganglia from patients with multiple sclerosis. J. Neurol. Sci. 183(1), 69–72 (2001).
- Ogawa S, Lee TM, Nayak AS, Glynn P: Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magn. Reson. Med. 14(1), 68–78 (1990).
- Ogawa S, Lee TM, Kay AR, Tank DW: Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc. Natl Acad. Sci. USA 87(24), 9868–9872 (1990).
- Belliveau JW, Kennedy DN Jr, Mckinstry RC et al.: Functional mapping of the human visual cortex by magnetic resonance imaging. Science 254(5032), 716–719 (1991).
- Filippi M, Rocca MA: Cortical reorganisation in patients with MS. J. Neurol. Neurosurg. Psychiatry 75(8), 1087–1089 (2004).
- Audoin B, Au Duong MV, Ranjeva JP et al.: Magnetic resonance study of the influence of tissue damage and cortical reorganization on PASAT performance at the earliest stage of multiple sclerosis. Hum. Brain Mapp. 24(3), 216–228 (2005).
- Mainero C, Caramia F, Pozzilli C et al.: fMRI evidence of brain reorganization during attention and memory tasks in multiple sclerosis. Neuroimage 21(3), 858–867 (2004).
- Rocca MA, Valsasina P, Ceccarelli A et al.: Structural and functional MRI correlates of Stroop control in benign MS. Hum. Brain Mapp. 30(1), 276–290 (2009).
- Rocca MA, Ceccarelli A, Rodegher M et al.: Preserved brain adaptive properties in patients with benign multiple sclerosis. Neurology 74(2), 142–149 (2010).
- Bobholz JA, Rao SM, Lobeck L et al.: fMRI study of episodic memory in relapsingremitting MS: correlation with T2 lesion volume. Neurology 67(9), 1640–1645 (2006).
- Rocca MA, Riccitelli G, Rodegher M et al.: Functional MR imaging correlates of neuropsychological impairment in primaryprogressive multiple sclerosis. AJNR Am. J. Neuroradiol. 31(7), 1240–1246 (2010).
- Sumowski JF, Wylie GR, Chiaravalloti N, Deluca J: Intellectual enrichment lessens the effect of brain atrophy on learning and memory in multiple sclerosis. Neurology 74(24), 1942–1945 (2010).
- Sumowski JF, Wylie GR, Deluca J, Chiaravalloti N: Intellectual enrichment is linked to cerebral efficiency in multiple sclerosis: functional magnetic resonance imaging evidence for cognitive reserve. Brain 133(Pt 2), 362–374 (2010).
- Sumowski JF, Chiaravalloti N, Deluca J: Cognitive reserve protects against cognitive dysfunction in multiple sclerosis. J. Clin. Exp. Neuropsychol. 31(8), 913–926 (2009).
- Wuerfel J, Paul F, Zipp F: Cerebral blood perfusion changes in multiple sclerosis. J. Neurol. Sci. 259(1–2), 16–20 (2007).
- De Stefano N, Iannucci G, Sormani MP et al.: MR correlates of cerebral atrophy in patients with multiple sclerosis. J. Neurol. 249(8), 1072–1077 (2002).
- Pozzilli C, Passafiume D, Bernardi S et al.: SPECT, MRI and cognitive functions in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 54(2), 110–115 (1991).
- Brooks DJ, Leenders KL, Head G, Marshall J, Legg NJ, Jones T: Studies on regional cerebral oxygen utilisation and cognitive function in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 47(11), 1182–1191 (1984).
- Lycke J, Wikkelso C, Bergh AC, Jacobsson L, Andersen O: Regional cerebral blood flow in multiple sclerosis measured by single photon emission tomography with technetium-99m hexamethylpropyleneamine oxime. Eur. Neurol. 33(2), 163–167 (1993).
- Paulesu E, Perani D, Fazio F et al.: Functional basis of memory impairment in multiple sclerosis: a [18F]FDG PET study. Neuroimage 4(2), 87–96 (1996).
- Wuerfel J, Bellmann-Strobl J, Brunecker P et al.: Changes in cerebral perfusion precede plaque formation in multiple sclerosis: a longitudinal perfusion MRI study. Brain 127(Pt 1), 111–119 (2004).
- Haselhorst R, Kappos L, Bilecen D et al.: Dynamic susceptibility contrast MR imaging of plaque development in multiple sclerosis: application of an extended blood-brain barrier leakage correction. J. Magn. Reson. Imaging 11(5), 495–505 (2000).
- Law M, Saindane AM, Ge Y et al.: Microvascular abnormality in relapsingremitting multiple sclerosis: perfusion MR imaging findings in normal-appearing white matter. Radiology 231(3), 645–652 (2004).
- Rashid W, Parkes LM, Ingle GT et al.: Abnormalities of cerebral perfusion in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 75(9), 1288–1293 (2004).
- Inglese M, Adhya S, Johnson G et al.: Perfusion magnetic resonance imaging correlates of neuropsychological impairment in multiple sclerosis. J. Cereb. Blood Flow Metab. 28(1), 164–171 (2008).
- Davie CA, Hawkins CP, Barker GJ et al.: Serial proton magnetic resonance spectroscopy in acute multiple sclerosis lesions. Brain 117(Pt 1), 49–58 (1994).
- Arnold DL, Matthews PM, Francis GS, O’Connor J, Antel JP: Proton magnetic resonance spectroscopic imaging for metabolic characterization of demyelinating plaques. Ann. Neurol. 31(3), 235–241 (1992).
- De Stefano N, Matthews PM, Arnold DL: Reversible decreases in N-acetylaspartate after acute brain injury. Magn. Reson. Med. 34(5), 721–727 (1995).
- Wolinsky JS, Narayana PA, Fenstermacher MJ: Proton magnetic resonance spectroscopy in multiple sclerosis. Neurology 40(11), 1764–1769 (1990).
- Kapeller P, Ropele S, Enzinger C et al.: Discrimination of white matter lesions and multiple sclerosis plaques by short echo quantitative 1H-magnetic resonance spectroscopy. J. Neurol. 252(10), 1229–1234 (2005).
- Bitsch A, Bruhn H, Vougioukas V et al.: Inflammatory CNS demyelination: histopathologic correlation with in vivo quantitative proton MR spectroscopy. AJNR Am. J. Neuroradiol. 20(9), 1619–1627 (1999).
- Narayana PA, Doyle TJ, Lai D, Wolinsky JS: Serial proton magnetic resonance spectroscopic imaging, contrast-enhanced magnetic resonance imaging, and quantitative lesion volumetry in multiple sclerosis. Ann. Neurol. 43(1), 56–71 (1998).
- Tartaglia MC, Narayanan S, De Stefano N et al.: Choline is increased in pre-lesional normal appearing white matter in multiple sclerosis. J. Neurol. 249(10), 1382–1390 (2002).
- Mathiesen HK, Jonsson A, Tscherning T et al.: Correlation of global N-acetyl aspartate with cognitive impairment in multiple sclerosis. Arch. Neurol. 63(4), 533–536 (2006).
- Bakshi R, Thompson AJ, Rocca MA et al.: MRI in multiple sclerosis: current status and future prospects. Lancet Neurol. 7(7), 615–625 (2008).
• Discusses gray matter involvement in patients with multiple sclerosis (MS).
• Discusses cognitive impairment in MS.
• Discusses cognitive impairment in MS.
• Discusses the theory of underlying vascular insufficiency in patients with MS.
• Discusses the theory of underlying vascular insufficiency in patients with MS.
• Discusses functional MRI techniques in MS.
• Studies diffusion changes in MS and correlation to cognitive dysfunction.
• Studies diffusion changes in MS and correlation to cognitive dysfunction.
• Studies diffusion changes in MS and correlation to cognitive dysfunction.
• Discusses functional MRI techniques in MS.
• Discusses functional MRI techniques in MS.
• Studies magnetic resonance perfusion change correlation with neuropsychological impairment.
• • Studies magnetic resonance perfusion change correlation with neuropsychological impairment.
• Discusses magnetic resonance spectroscopy in MS and correlation with cognition.