Research Article - Interventional Cardiology (2023) Volume 15, Issue 1
Aminophylline-mediated cardiotoxicity in zebrafish
- Corresponding Author:
- Qiuxiang Cao State Key Laboratory of Developmental Biology of Freshwater Fish, Hunan Normal University, Changsha, China, E-mail: qxcao@hunnu.edu.cn;
- Xiangding Chen State Key Laboratory of Developmental Biology of Freshwater Fish, Hunan Normal University, Changsha, China, Email: xdchen@hunnu.edu.cn
Received date: 20-Jan-2023, Manuscript No. FMIC-23-87385; Editor assigned: 25-Jan-2023, PreQC No. FMIC-23-87285 (PQ); Reviewed date: 08-Feb-2023, QC No. FMIC-23-87285; Revised date: 15-Feb-2023, Manuscript No. FMIC-23-87285 (R); Published date: 24-Feb-2023, DOI: 10.37532/1755-5310.2023.15 (1). 637
Abstract
Background: Cardiotoxicity is one of the major side effects leading to withdrawal of aminophylline treatment. However, the aminophylline-mediated cardiotoxicity and its underlying mechanisms remain poorly studied. Recently, zebrafish has emerged as a valuable vertebrate animal model to study drug toxicity in vivo.
Methods: Wild-type zebrafish (Danio rerio) embryos were incubated with different concentrations of aminophylline solution, followed by evaluation of their survival rate, hatching rate, malformation rate, body length and Sinus Venous-Bulbus Arteriosus (SV-BA) distance to assess the potential developmental toxicity and cardiotoxicity. In addition, embryonic heart shapes of the Tg (cmlc2: EGFP), vmhcEGFP-KI, Tg (fli1: EGFP) reporter lines were also analyzed at 48 Hours Post-Fertilization (HPF) and 72 hpf, respectively. Furthermore, in situ hybridization was used to evaluate the expression levels of heart marker genes such as cmlc2, amhc, vmhc, and regulatory factors during early heart development such as hand2, nkx2.5, gata4, and valve development-related regulatory factors such as notch1b, has2, bmp4 as well.
Results: After the aminophylline drug treatment at the concentration of 0.2 mg/mL or above, zebrafish embryos exhibited reduced heart rate and increased distance between SV-BA. In addition, blood cells appeared to accumulate in the heart area, leading to pericardial edema and other poisoning phenomena. These aminophylline drug mediated cardiotoxicities were time and dose dependent. In situ hybridization analysis revealed altered expression of the regulatory factor of heart development gene nkx2.5 and valve development-related genes including notch1b, has2, which might convey the aminophylline-mediated cardiotoxicity.
Conclusion: Aminophylline-induced cardiotoxicity may be conveyed by inhibiting the expression of genes related to myocardial differentiation and valve development.
Keywords
Aminophylline • Zebrafish • Drug treatment • Cardiotoxicity
Introduction
Aminophylline is a methylxanthine derivative, which exhibits Aminophylline is a methylxanthine derivative, which exhibits a therapeutic effect on the mitochondrial function of epithelial cells in lung diseases and on acute kidney injury in pediatric patients [1,2]. It also has the effect on heart, which exhibits dilating coronary arteries, increasing blood supply to the myocardium, and strengthening the contractility of the heart [3]. As the therapeutic dose of aminophylline is very close to that of poisoning, multi-organ poisoning such as heart dysfunction and even cardiac arrest can occur when overdosed [4]. It is therefore important to study the mechanism of action of aminophylline on cardiotoxicity, which remains poorly understood [5]. Zebrafish (Danio rerio) is an attractive animal model for studying heart function damage in vivo, as its embryos are transparent in up to 7 Days Post-Fertilization (DPF), making it easy to observe the heart morphology dynamics under optical microscopy [6]. In this study, the effects of aminophylline on zebrafish cardiotoxicity and its underlying mechanisms were explored through using different concentrations of aminophylline solutions to treat zebrafish embryos.
Materials and Methods
Fish
The zebrafish strains used in this study were Wild-Type (WT) Tübingen, Tg (cmlc2: EGFP), Tg(fli1: EGFP), and vmhcEGFPKI. The transgenic strain Tg (cmlc2: EGFP) and knock-in strain (vmhcEGFP-KI) were generated previously [7,8]. All fish were maintained under standard conditions as described [9]. Embryos were raised at 28.5°Cand staged according to time post-fertilization and morphology as described previously [10]. Adult zebrafish were placed in hybridization tanks with one female and two males. With the temperature maintained at 28°C, fish mating was stimulated by lighting after a 10 h dark cycle. All experiments were performed following animal ethics guidelines of the institutions.
TRIzol Reagent and MAXIscript T7 Transcription Kit were purchased from Invitrogen (USA). DIG RNA Labeling Mix (UTP) was purchased from Roche (Switzerland). RNeasy Mini Kit was purchased from Qiagen (Germany). Aminophylline (CAS no. 317-34-0) powder was purchased from Shanghai Acmec Biochemical (China). All other chemicals and reagents were analytical grade.
In vivo heart toxicity in zebrafish
In vivo heart toxicity in zebrafish was measured by a series of physiological tests, such as survival rate, hatching rate, malformation rate, body length and other heart indices. A stock solution of 5 mg/mL of aminophylline was prepared in egg water (15 mm NaCl, 0.5 mm KCl, 1 mm CaCl2, 1 mm MgSO4), stored in 4°C refrigerators. Healthy zebrafish embryos were placed in six-well plate with 40 embryos per well and exposed to different concentrations of aminophylline until analysis at designated time points such as 48, 72 and 96 hpf. The fresh egg water was replaced every 24 h to observe the morphological changes. All tests were independently repeated three times and set a blank control group. Screening concentrations are performed in the initial range of 0.1- 0.8 mg/mL (corresponding to 0.24-1.92 mm) to count survival rate. The hatching and malformation quantities of embryos were recorded at 72 hpf. The body length and SV-BA distance of embryo were measured using a microscope (Leica M205 FA, Germany) at 72 hpf. To measure the heart rates of 48 hpf and 72 hpf embryos, the number of contractions of a beating heart was recorded using a light microscope (Nikon SMZ1270, Japan). Cardiac morphology of different strains of embryos, such as WT, Tg (cmlc2: EGFP) and vmhcEGFP-KI, was determined at 48 hpf and 96 hpf using a fluorescent microscope (Leica M205 FA, Germany). The heart images of Tg (fli1: EGFP) embryos were obtained by a imaging system (Zeiss Celldiscoverer 7, Germany).
Whole-mount in situ hybridization
In situ hybridization technique allows the sites of expression of particular genes to be detected. As conditions are the same for each probe tested, in situ hybridization allows high throughput analysis of zebrafish gene expression during embryogenesis [11]. Heart marker genes (cmlc2, vmhc, amhc), early heart development regulatory factors (nkx2.5, gata4, hand2) and valve development-related regulatory factors (bmp4, notch1b, has2) were selected to conduct in situ hybridization experiments on control group and experimental group. The antisense RNA probes of above mentioned genes were synthesized using MAXIscript T7 Transcription Kit and DIG RNA labeling mix after Polymerase Chain Reaction (PCR) and were purified with a RNeasy Mini Kit. Whole-mount in situ hybridization was performed as previously described [11]. Then, images were obtained using a microscope (Leica M205 FA, Germany).
The PCR primers used to synthesize RNA probes were designed by primer5 software. Each primer is shown below (5′′3′): cmlc2- ISH-F: GAA CCG GGA TGG AGT TAT CA; cmlc2-ISH-R: GCC CTT AAA CCA AAT GT; vmhc-ISH-F: AGC TCC TCC CCA CAT CTT TT; vmhc-ISH-R: CCT CCC TCT GCT TCT GTT TG; amhc-ISH-F: AGA TCT CAC AGC CA; amhc-ISH-R: CGC TCG CTG ATG T; nkx2.5-ISH-F: ACT TCC ACT CCT TTC TCA GTG C; nkx2.5-ISH-R: TGT TGT AAG TGG ACG TGT CTC; gata4-ISH-F: CCG GTG TCC AGA TTC ACT TT; gata4-ISH-R: CCG ACT GAC CTT CAG AGG A; hand2-ISH-F: CAC CAT GAC GGC TAT TCC TT; hand2-ISH-R: GCG GGA AAT TGC ACA TAA A; bmp4-ISH-F: CAC CAT GAC GGC TAT TCC TT; bmp4-ISH-R: CTG GGT TTC GCT TCA TTT GT; notch1b-ISH-F: CGT TTG ATC ACT TCT GCC AAC A; notch1b-ISH-R: ACT GGC AAT TGT AGG TGT; has2-ISH-F: AGG TCA TGT ACA CCG CCT TC; has2-ISH-R: GAT CCT GCC CTG GTA GAA C.
Statistical analysis
Image J and GraphPad Prism were used for data processing and analysis. Student’s t-test was performed to detect significant differences between two groups. P-values of less than 0.05 were considered to be statistically significant.
Results
Toxicity assessment
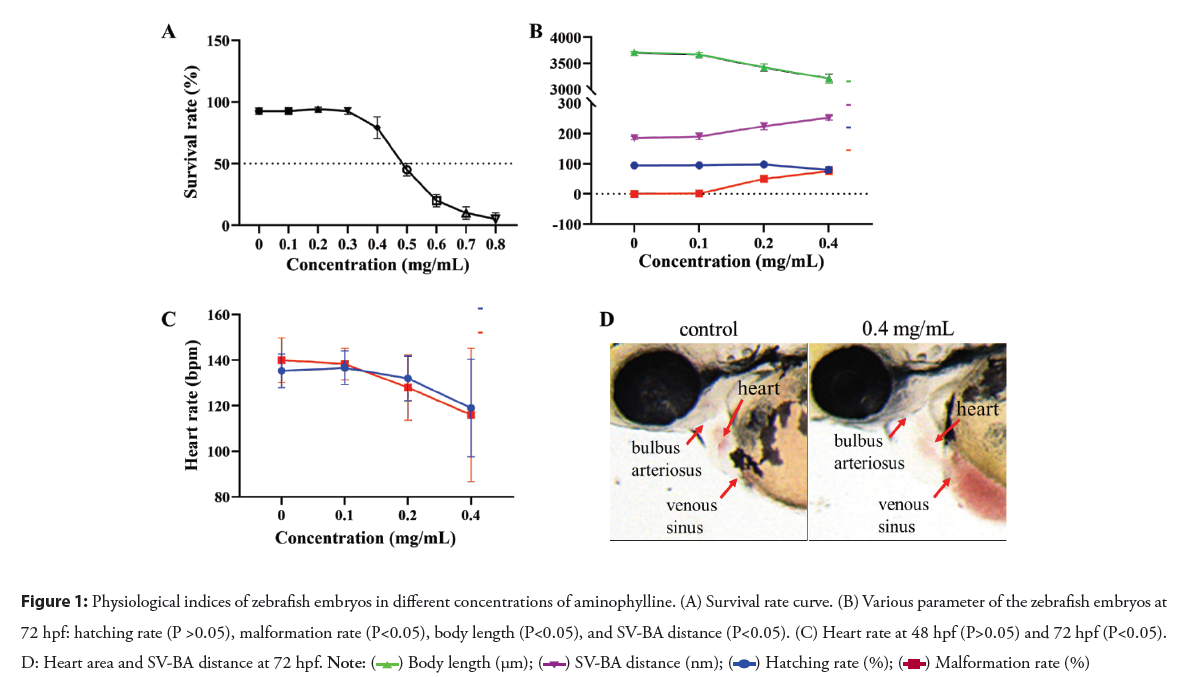
Determination of aminophylline concentration range: As stated in Materials and Methods, the concentration range of aminophylline has been experimentally determined using zebrafish embryos. The toxicity of aminophylline was assessed up to 96 hpf of exposure and half Lethal Concentration (LC50) was calculated. The indicators of lethality were referred to the Embryo Toxicity Test (FET) [12, 13]. As shown in Figure 1: A, newly fertilized embryos (0 hpf) were exposed to 9 different concentrations of aminophylline (0, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8 mg/ mL) until analysis. Decreased survival correlated with increased aminophylline treatment concentrations. Within the range of aminophylline concentration from 0.3 mg/mL to 0.8 mg/mL, the survival rate decreased sharply, indicating that aminophylline has toxic effects on zebrafish embryos which are dose-dependent. According to the dose-response curve fitted with logistic functions and Levenberg-Marquardt algorithms, the regression coefficient was 0.9791. The semi-lethal concentration was 0.4858 mg/mL, and the 95% confidence limits were between 0.4711 to 0.5003 mg/mL. To compare morphological defects, three concentrations (0.1 mg/mL, 0.2 mg/mL, 0.4 mg/mL) below LC50 were selected for toxicity assessment in subsequent experiments.
Physiological indices
To detect toxicity of aminophylline, a microscope was used to examine the incubation of eggs in the three experimental concentrations after a total exposure period of 72 h. The number of embryos hatched out of the chorion was recorded. Further, the deformity of embryos was recorded and photographed. According to Figure 1: B, with the increase of aminophylline treatment concentration, the hatching rate of zebrafish embryos decreased, the malformation rate and the SV-BA distance increased significantly, the body length was shortened obviously. The changes in thorax after drug treatment such as heart size, distended thoracic region and hemorrhage (Figure 1: C), indicating that aminophylline plays an important role in the formation and development of heart. All in all, aminophylline has a developmental toxicity to zebrafish embryos.
When a zebrafish embryo develops to 48 hpf, its heart is basically mature, and its heart rate stabilizes. In Figure 1: D, we found that compared to the control group, heart rates in embryos treated with either 0.1 mg/mL or 0.2 mg/mL aminophylline were increased. In contrast, the heart rate in embryos treated with 0.4 mg/mL aminophylline decreased after developed to 48 hpf. When the embryo developed to 72 hpf, the heart rate of the 0.2 and 0.4 mg/mL groups had a downward trend compared to the control group, while the heartbeat of 0.1 mg/mL aminophylline treated embryos increased slightly. Taken together, these results showed that aminophylline has a clear cardiotoxicity side effect to zebrafish embryos as shown in the Figure 1.
Figure 1: Physiological indices of zebrafish embryos in different concentrations of aminophylline. (A) Survival rate curve. (B) Various parameter of the zebrafish embryos at
72 hpf: hatching rate (P >0.05), malformation rate (P<0.05), body length (P<0.05), and SV-BA distance (P<0.05). (C) Heart rate at 48 hpf (P>0.05) and 72 hpf (P<0.05). D: Heart area and SV-BA distance at 72 hpf.
Heart morphology
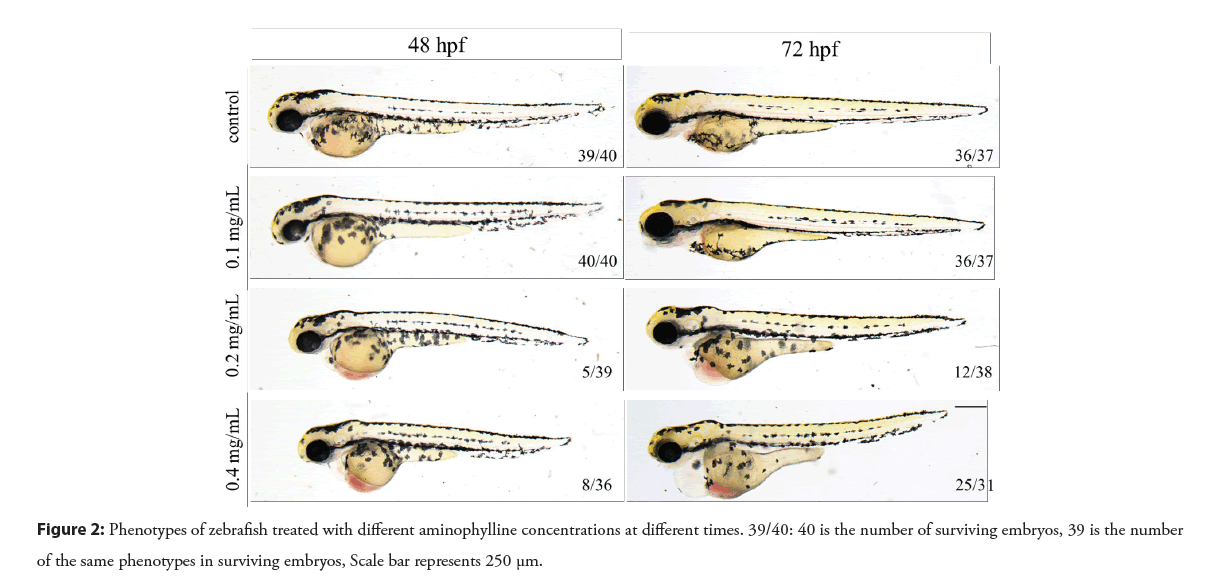
Compared with the control group, the 0.2 and 0.4 mg/mL aminophylline treated embryos showed abnormal phenotypes. After embryos were exposed to aminophylline solution for 48 h, blood cells were accumulated in the heart area as shown in the Figure 2. As the exposure time extended to 72 h, the poisoning effect becomes more severe, mainly manifesting enlarged pericardial cavity, aggregation of blood cells in the heart area, edema of the yolk sac, linearization of the heart, etc. By contrast, the 0.1 mg/ mL aminophylline treated embryos appeared to be similar to the untreated control group.
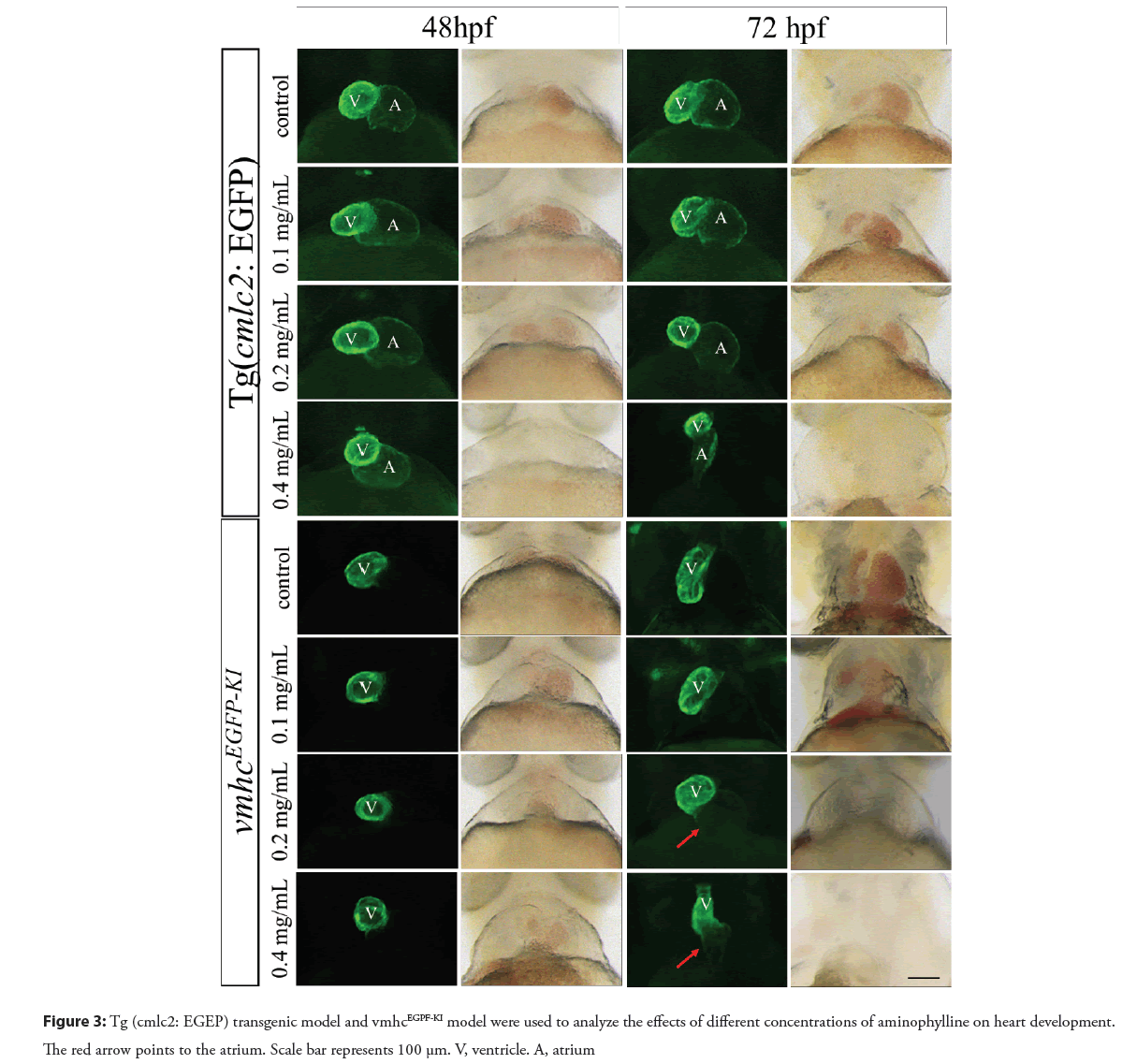
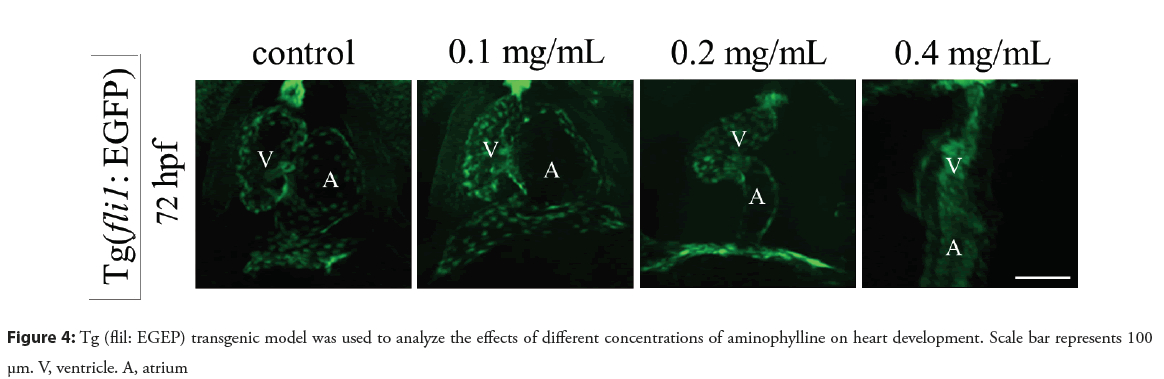
In order to evaluate the toxic effects of aminophylline on the heart development of zebrafish embryos, the Tg (cmlc2: EGFP) and Tg (fli1: EGFP) transgenic models and vmhcEGFP-KI knock-in model were used to detect changes in the heart morphology. As shown in Figure 3, while the heart morphology of the 0.1 mg/mL aminophylline treated embryos appeared to be normal, embryos treated with 0.2 mg/mL or 0.4 mg/mL aminophylline treated manifested abnormal phenotypes such as obvious pericardial edema and abnormal ventricular morphology. Furthermore, cardiac linearization occurred after prolonged aminophylline drug treatment. When zebrafish developed to 72 hpf, the vmhcEGFP-KI model of the 0.2 or 0.4 mg/mL aminophylline treated embryos showed ectopic EGFP expressing at the atrial site, indicating that the expression pattern of vmhc had changed (Figure 3). We thus speculate that the expression of early regulatory factors of heart development (nkx2.5, gata4, etc.) associated with vmhc expression patterns might have changed. The Tg (fl1i: EGFP) transgenic model is a marker strain of vascular endothelial cells, in which EGFP reporter can reflect the distribution of vascular endothelial cells in zebrafish embryos induced by aminophylline. The endothelial cells in the heart of 72 hpf zebrafish embryo were abnormally distributed after 0.2 mg/ml or 0.4 mg/ml aminophylline treatment, and the overall endothelial cell number was also reduced in the ventricle. In addition, abnormal heart morphology and blood reflux were also observed. Together, these results suggested that aminophylline exerts a certain effect on the valve development of zebrafish as shown in the Figure 4.
Figure 4: Tg (flil: EGEP) transgenic model was used to analyze the effects of different concentrations of aminophylline on heart development. Scale bar represents 100 µm. V, ventricle. A, atrium
Spatiotemporal expression of heart-related genes
Through preliminary experiments, we noted obvious cardiac abnormality in embryos treated with 0.4 mg/mL aminophylline, therefore, this concentration was selected as the aminophylline experimental group. Through phenotypic analysis of different EGFP reporter lines, cardiac molecular marker genes including cmlc2, vmhc, amhc, early heart development regulatory factors including nkx2.5, gata4, hand2, and valve development-related regulatory factors such as bmp4, notch1b, has2 were selected for in situ hybridization experiments to determine whether aminophylline-induced heart defects involve in expression changes of these cardiac marker genes.
The expression of heart marker genes
In control embryos, the expression of the heart marker gene cmlc2 was mainly concentrated in the ventricle and was weakly expressed in the atria. In the aminophylline treated embryos, the cmlc2 was up regulated at 48 hpf and 72 hpf, but not 24 hpf as shown in the Figure 5. In control embryos, the vmhc signal was specifically expressed in the ventricle. In the aminophylline treated embryos, the expression of vmhc was downregulated in all stages from 24 hpf to 72 hpf. In addition, ectopic expression of vmhc in the atria was also appreciated, which is consistent with the results obtained in the aminophylline-treated vmhcEGFP-KI knock-in model. These results strongly suggest that aminophylline indeed has an impact on the expression of regulatory factors such as nkx2.5 and gata4 in early heart development. The amhc signal was specifically expressed in the atrium, and there was no difference in the spatiotemporal expression of amhc between the aminophylline experimental group and the control group (Figure 5). These results showed that the defects in zebrafish embryonic heart development caused by aminophylline involve changes in related regulatory factors, and aminophylline had a certain impact on the expression distribution of the heart chamber.
Figure 5: The expression of heart marker genes at 24 hpf, 48 hpf and 72 hpf after 0.4 mg/mL of aminophylline exposure. (A) In situ hybridization images of cmcl2, vmhc and amhc. (B) Grayscale analysis of each gene. ‘ns’ means no significant differences (P>0.05); ‘*’ means significant differences (P<0.05). 
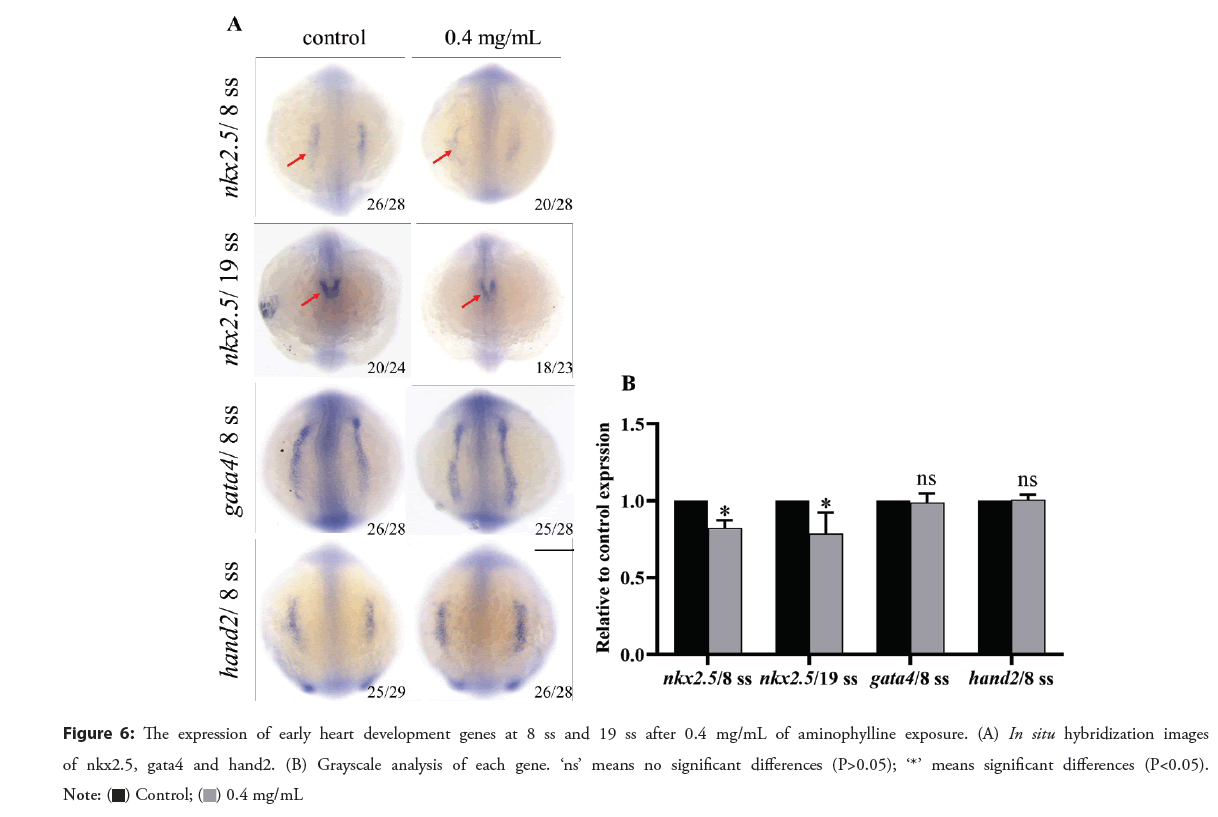
The expression of early heart development genes
According to Figure 6, in control embryos, the nkx2.5 expression is concentrated in the side plate mesoderm at 8 ss (8-somite) stage. At 19 ss stage, nkx2.5 was expressed on the back, which was horseshoe- shaped. In contrast, in the aminophylline treated embryos, the expression of nkx2.5 in 8 ss and 19 ss was downregulated. Since nkx2.5 is a key transcription factor for myocardial differentiation, we thus speculate downregulation of nkx2.5 may be related to heart defects caused by aminophylline. The expression of gata4 and hand2 in the 8 ss aminophylline experimental group appeared to be similar to that in the control group, indicating that the cardiac abnormality caused by aminophylline treatment may not involve changes in the expression levels of regulatory factors such asgata4 and hand2.
Figure 6: The expression of early heart development genes at 8 ss and 19 ss after 0.4 mg/mL of aminophylline exposure. (A) In situ hybridization images
of nkx2.5, gata4 and hand2. (B) Grayscale analysis of each gene. ‘ns’ means no significant differences (P>0.05); ‘*’ means significant differences (P<0.05). 
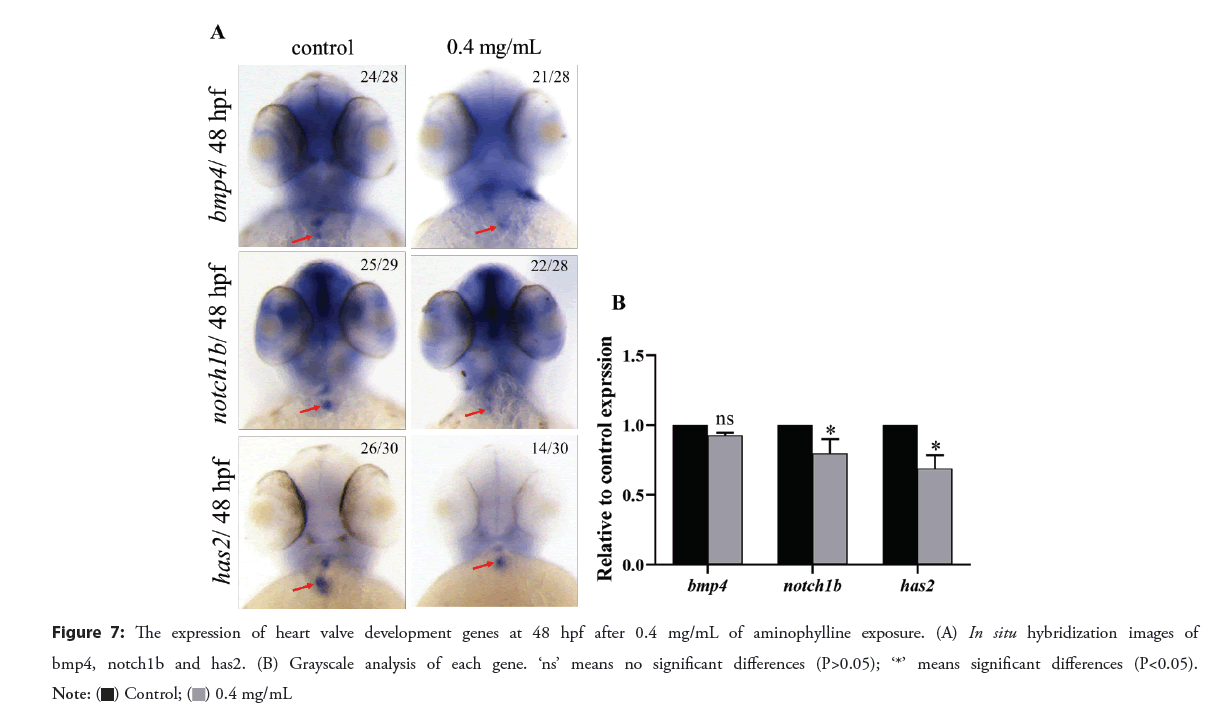
The expression of heart valve development genes
In control embryos at 48 hpf, the bmp4 is specifically expressed in cardiomyocytes, while the notch1b and has2 genes are specifically expressed in endothelial cells at the atrioventricular junction. Compared with the control group, while the expression of bmp4 in the aminophylline experimental group was similar, the expression of notch1b was downregulated, and the expression of has2 was down regulated with additional loss of the outflow channel position signal as shown in the Figure 7. These results showed that aminophylline affected the gene expression of endothelial cells at the valve. Therefore, we speculate that changes in the expression patterns of notch1b and has2 genes in the endocardial layer of the aminophylline experimental group might have caused valve malformation due to lack of the necessary molecular basis, which may be the underlying mechanism of blood reflux.
Discussion
Studies have shown that the pharmacological responses of mammals and zebrafish to known cardiotoxins are very similar [14]. It is also an important reason why zebrafish have been used to explore the toxicological response and mechanism of drugs in recent years. The drug aminophylline has been clinically proven to restore the electrical activity of the heart in cases of slow- constricting cardiac arrest, thus has a potential side effect to cause sinus tachycardia [15]. Adverse reactions or poisoning symptoms may occur when overdosed. In this study, the overall survival rate, hatching rate, heart rate and body length were largely reduced by increased doses of aminophylline treatment. In contrast, the embryonic malformation rate and SV-BA distance were increased. As heart rate is an important variable to reflect heart function, changes of heart rate can be used to assess the cardiotoxic effects of aminophylline [16]. Along with the slowing of heart rate, the increased SV-BA distance, blood cell aggregation, pericardial edema, and cardiac linearization, we concluded that aminophylline can lead to developmental toxicity and cardiotoxicity to zebrafish embryos in a dose-dependent manner.
The heart is the first organ that forms and functions during embryonic development. Heart development is a complex and orderly process of tissue morphogenesis and remodeling, which is regulated by a complex genes and transcription factors of heart development. These genes and transcription factors control the structure and morphology of the heart [17]. Tg (cmlc2: EGFP) transgenic model, vmhcEGFP-KI knock-in model and Tg (fli1: EGFP) transgenic model were treated with different concentrations of aminophylline. We found that 48 hpf and 72 hpf zebrafish embryos were accompanied by different degrees of abnormal heart phenotypes, and the severity of abnormal phenotypes were concentration dependent. Interestingly, a weak expression of EGFP was found in the atrial site in 0.2 mg/mL or 0.4 mg/mL aminophylline treated embryos at 72 hpf, indicating that the chamber-specific expression of the vmhc in the aminophylline experimental group may be affected. The results of in situ hybridization experiments support the above findings. As expression pattern of vmhc is closely related to the early regulatory factors of heart development such as nkx2.5, gata4, and etc., aminophylline may have an impact on that [18, 19]. Results from in situ hybridization of nkx2.5, gata4 and hand2 revealed that when zebrafish developed to 8 ss and 19 ss, the expression of nkx2.5 in the aminophylline experimental group was significantly downregulated compared to the control group. Since nkx2.5 is a key transcription factor for myocardial differentiation which contributes to the refinement of ventricular muscle cells and enhances the development process of the heart [20, 21]. It is reasonable for us to speculate that the downregulation of transcription factor nkx2.5 might convey to abnormal differentiation of ventricular cardiomyocytes, resulting in abnormal heart function. Meanwhile, abnormal differentiation of cardiomyocytes may also be the cause of ectopic expression of vmhc.
Blood reflux, a common feature of incomplete valve closure, was observed in some aminophylline treated embryos in the Tg (fli1: EGFP) transgenic model. We thus reasoned that aminophylline might have an effect on the valve development. The morphogenesis of heart valves depends on the signaling molecules of the myocardial layer in the atrioventricular canal and on the response of the cells of the endocardial layer to the signals of the myocardial layer [22, 23]. Mutations in the notch1b can cause atrophy of the endocardium of the heart [24]. Mice with has2 gene knockout showed backward development and blood reflux [25]. A mild decrease in bmp4 expression can cause partial defects in the atrioventricular septum [26]. Through in situ hybridization of valve development-related regulatory factors (bmp4, notch1b, has2), we found that when zebrafish developed to 48 hpf, the expression of the endothelial cell marker factor notch1b and has2 at the valve was significantly down regulated. The expression level of has2 was also reduced accompanied by the loss of outflow tract location signal. We thus speculate that down regulation of notch1b and has2 genes in the endocardial layer might have caused valve malformation, which might be the underlying mechanism of blood reflux.
Conclusion
In summary, here we utilized the zebrafish embryonic animal model to study the potential side effects aminophylline exposure on early heart development and malformations. Mechanically, aminophylline-induced heart developmental defects might involve in down regulation of key transcription factor for myocardial differentiation nkx2.5, resulting in changes in the specific expression of vmhc. This pathway may also involve changes in certain regulatory factors, resulting in down regulation of endothelial cell molecular markers such as notch1b and has2 at the heart valve.
Limitations
The specific mechanism of action is still uncertain. Further experiments through performing cardiac dissection of zebrafish embryos treated with aminophylline for transcriptome analysis and Real-time quantitative Polymerase Chain Reaction (qPCR) verification of in situ hybridization results are needed.
Acknowledgments
We thank all the members of the Zebrafish Genetics Laboratory in the Hunan Normal University for their assistance.
Authors’ Contributions
Xiangding Chen, Qiuxiang Cao and Yun Deng designed the study; Donghua Huang, Hui Cao and Qiuxiang Cao performed the experiments and carried out the analysis; Hui Cao wrote the first draft of the manuscript; Qiuxiang Cao, Yun Deng and Xiangding Chen supervised the study and edited the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by the National Health Commission Key Laboratory of Birth Defects Research, Prevention and Treatment Project (Hunan Provincial Maternal and Child Health Care Hospital) (NO. KF2021003).
Availability of Data and Materials
All original (deidentified) data and materials are available upon request from the corresponding author.
Ethics Approval and Consent to Participate
The Biomedical Research ethics committee of Hunan Normal University approved this study. Animal study protocols were approved by the Institutional Animal Care and Use Committee of Hunan Normal University.
Competing Interests
The authors declare that they have no competing interests.
References
- Razi W, Haque AU, Sadiq H, et al. Safety and efficacy of aminophylline in intrathecal methotrexate-related neurological toxicity in large pediatric oncology centre. J Coll Physicians Surg Pak. 8: 481-484 (2021).
[CrossRef] [GoogleScholar] [PubMed]
- Albert S. Aminophylline toxicity. Pediatr Clin North Am. 34(1): 61-73 (1987).
[CrossRef] [GoogleScholar] [PubMed]
- Cooney L, Sinha I, Hawcutt D, et al. Aminophylline dosage in asthma exacerbations in children: a systematic review. PLoS One. 11(8): e0159965 (2016).
[CrossRef] [GoogleScholar] [PubMed]
- RuDusky BM. Aminophylline: exploring cardiovascular benefits versus medical malcontent. Angiology. 56(3): 295-304 (2005).
[CrossRef] [GoogleScholar] [PubMed]
- Zafar Gondal A, Zulfiqar H. Aminophylline. (2022).
- Lal H, Kolaja KL, Force T, et al. Cancer genetics and the cardiotoxicity of the therapeutics. J Am Coll Cardiol. 61(3): 267-274 (2013).
[CrossRef] [GoogleScholar] [PubMed]
- Peng XY, Chen TF, Huang T, et al. Construction and assessment of heart-specific green fluorescence zebrafish line. Yi Chuan. 35(4): 511-518 (2013).
- Ouyang S, Qin WM, Niu YJ, et al. An egfp knock-in zebrafish experimental model used in evaluation of the amantadine drug safety during early cardiogenesis. Front Cardiovasc Med. 5: 715 (2022).
[CrossRef] [GoogleScholar] [PubMed]
- Westerfield M. The zebrafish book: a guide for the laboratory use of zebrafish (Brachydanio rerio). (1995).
- Kimmel CB, Ballard WW, Kimmel SR, et al. Stages of embryonic development of the zebrafish. Dev Dyn. 203(3): 253-310 (1995).
[CrossRef] [GoogleScholar] [PubMed]
- Thisse C, Thisse B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat Protoc. 3(1): 59-69 (2008).
[CrossRef] [GoogleScholar] [PubMed]
- Braunbeck T, Kais B, Lammer E, et al. The Fish Embryo Test (FET): Origin, applications, and future. Environ Sci Pollut Res Int. 22: 16247-16261 (2015).
[CrossRef] [GoogleScholar] [PubMed]
- Lammer E, Carr GJ, Wendler K, et al. Is the Fish Embryo Toxicity test (FET) with the zebrafish (Danio rerio) a potential alternative for the fish acute toxicity test?. Comp Biochem Physiol C Toxicol Pharmacol. 149(2): 196-209 (2009).
[CrossRef] [GoogleScholar] [PubMed]
- Basnet RM, Guarienti M, Memo M, et al. Zebrafish embryo as an in vivo model for behavioral and pharmacological characterization of methylxanthine drugs. Int J Mol Sci. 18(3) (2017).
[CrossRef] [GoogleScholar] [PubMed]
- van Beynum IM, Kapusta L, Bakker MK, et al. Protective effect of periconceptional folic acid supplements on the risk of congenital heart defects: a registry-based case-control study in the northern Netherlands. Eur Heart J. 31(4): 464-471 (2010).
[CrossRef] [GoogleScholar] [PubMed]
- Suvarchala G, Philip GH. Toxicity of 3, 5, 6-trichloro-2-pyridinol tested at multiple stages of zebrafish (Danio rerio) development. Environ Sci Pollut Res Int 15: 15515-15523 (2016).
[CrossRef] [GoogleScholar] [PubMed]
- Olson EN. Gene regulatory networks in the evolution and development of the heart. Science. 313(5795): 1922-1927 (2006).
[CrossRef] [GoogleScholar] [PubMed]
- Targoff KL, Schell T, Yelon D, et al. Nkx genes regulate heart tube extension and exert differential effects on ventricular and atrial cell number. Dev Biol. 322(2): 314-321 (2008).
[CrossRef] [GoogleScholar] [PubMed]
- Park JS, Kim HS, Kim JD, et al. Isolation of a ventricle‐specific promoter for the zebrafish Ventricular Myosin Heavy Chain (VMCH) gene and its regulation by GATA factors during embryonic heart development. Dev Dyn. 238(6): 1574-1581 (2009).
[CrossRef] [GoogleScholar] [PubMed]
- Huang M, Jiao J, Wang J, et al. Characterization of acrylamide-induced oxidative stress and cardiovascular toxicity in zebrafish embryos. J Hazard Mater. 347: 451-460 (2018).
[CrossRef] [GoogleScholar] [PubMed]
- Sun G, Liu K. Developmental toxicity and cardiac effects of butyl benzyl phthalate in zebrafish embryos. Aquat Toxicol.192: 165-170 (2017).
[CrossRef] [GoogleScholar] [PubMed]
- Wagner M, Siddiqui MA. Signal transduction in early heart development (II): ventricular chamber specification, trabeculation, and heart valve formation. Exp Biol Med (Maywood). 232(7): 866-880 (2007).
[GoogleScholar] [PubMed]
- Person AD, Klewer SE, Runyan RB, et al. Cell biology of cardiac cushion development. Int Rev Cytol. 243: 287-335 (2005).
[CrossRef] [GoogleScholar] [PubMed]
- Dontu G, Jackson KW, McNicholas E, et al. Role of Notch signaling in cell-fate determination of human mammary stem/progenitor cells. Breast Cancer Res. 6(6): R605-R615 (2004).
[CrossRef] [GoogleScholar] [PubMed]
- Camenisch TD, Spicer AP, Brehm-Gibson T, et al. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J Clin Invest. 106(3): 349-360 (2000).
[CrossRef] [GoogleScholar] [PubMed]
- Jiao K, Kulessa H, Tompkins K, et al. An essential role of Bmp4 in the atrioventricular septation of the mouse heart. Genes Dev. 17(19): 2362-2367 (2003).
[CrossRef] [GoogleScholar] [PubMed]