Case Report - Interventional Cardiology (2019) Volume 11, Issue 6
An educational operative image of an extreme form of hypoplastic left heart syndrome
- Corresponding Author:
- PM de Siena
IRCCS San Metteo Foundation
Pavia, Italy
E-mail: pietrosc.83@libero.it
Received date: November 19, 2019; Accepted date: November 25, 2019; Published date: December 2, 2019
Abstract
Hypoplastic Left Heart Syndrome (HLHS) represents a rare disease but still common mostly in Middle East and East world population. A large fenotype spectrum, ranging from the less severe mitral and aortic stenosis form to the more severe aortic and mitral atresia one, encompass the disease. We present here a clinical case of aortic and mitral atresia fenotype and show an operative image of the extreme form of that disease. A baby 2 d.o. 2.97 Kg has been admitted with an antenatal diagnosis of Hypoplastic Left Heart Syndrome to our Centre.
Keywords
Hypoplastic left heart syndrome • Aortic stenosis • Ventricular function
Introduction
A gestational echocardiogram, like described in literature, showed aortic and mitral atresia pictures. On the echo repeated at birth, a very large pulmonary artery was depicted, with a very small ascending aorta. The PDA was very large and filling the descending aorta and the aortic arch, tricuspid valve was mildly insufficient, right ventricular function good without RVOTO. Normal coronary pattern. No other anomalous findings were detected. Born in a protective and specialised environment at 36 w.o., the baby was haemodynamically stable immediately at birth and was maintaining a relatively good systemic-pulmonary setting balance with 83% of saturation. After 8 hrs of age, an infusion of alpoprostadil was started on, following severe desaturation and apnea requiring bagging, urgent intubation, mechanical ventilation and paralysis in presence of PDA narrowing. The baby has been stable until the 4th day of age without inotropes [1,2].
On the 4th day after birth, he underwent a Norwood repair by employing the so called “Sano technique” with a 5 mm. RV to PA conduit, used as shunt. The classical “Sano” technique was employed in that case with a distal anastomoses on the RPA, and the chest was left open for 36 hrs. post-procedure by leaving a small liga-clip on the Sano shunt in order to reduce the pulmonary overflow, in view of the pulmonary vascular resistance falling. The liga-clip was then removed at the chest closure in PICU. Patient remained stable with some milrinone drug on board and all the vital parameters in order, compatible with the new univentricular physiological situation. The post-operative course was uneventful and the patient was discharged on the 8th post-operative day in good condition and stable haemodynamical status.
Case Report
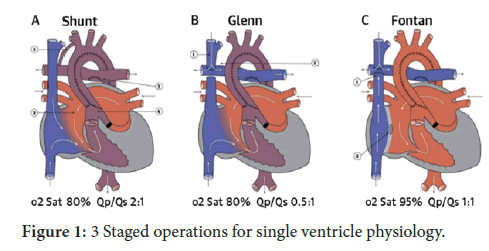
We present the operative picture taken in theatre of the case described, once the sternum and the pericardium have been opened and the thymus glands totally resected. The picture show a huge pulmonary artery compared to a filiform ascending aorta unexpected in comparison to similar cases. The initial good pulmonary/systemic haemodynamical balance, hold at birth, can be explained just right by the PDA filling an enlarged pulmonary artery until the PDA narrowing after some hours after birth. The fenotype of HLHS showed in the picture, represents the more extreme one associated to endocardial fibro-lelastosis on echo (Figure 1). We think that picture would be useful to students and surgeons in training as educational image in order to take into account the surgical scenario faced on live [3-5].
Discussion
This fenotype has to be considered as one as associated to the highest peri-operative mortality and short survival on the long distance , even after the third palliative stage procedure included into the usual treatment pathway.
The second surgical step, planned between 3-6 months of age, to treat an HLHS is a bi caval-pulmonary connection also called “ Glenn procedure” in order to allow a better growth of the pulmonary arterial tree which are not well supported more by the small central Sano shunt applied at birth , and continue on the univentricular haemodynamical pathway, which will divert the venous blood return directly to the lungs , bypassing the RV which is mainly acting as systemic arterial pump chamber connected to the aorta by the Damus-Stansel-Key procedure [6,7]. The completion of the univentricular pathway is represented by the total cavo-pulmonary connection according nowdays to the extra-cardiac conduit by employing the so called “Fontan procedure” between 2-4 years of age.
That final step will be successful only when at least 10 physiological principles will be respected [8].
In some occasions, given by a not extreme fenotype of the disease, like mitral and Aortic stenosis of low grade, it is possible to achieve a biventricular repair, planned in advance after some initial palliative procedures. The chance of a good result is calculated preoperatively by the Rhode score on echo, which takes into consideration different parameters, including the LV form and its volume according to the anatomy, and the presence of endocardium fibro-elastosis disease associated to [9].
The low Rhodes score calculated on echo in this specific case, did not give any indication to a biventricular repair.
The severe uni-ventricular form expressed even by a filiform aorta , in this case , unfortunately carries a poor prognosis on the medium term also due to very high risk of re-coartation of aorta requiring several surgical or interventional procedures , each time demanding and at high mortality risk [7-11].
In HLHS mortality is in any case very high on the long term, even after a successful third stage palliation procedure [12-15].
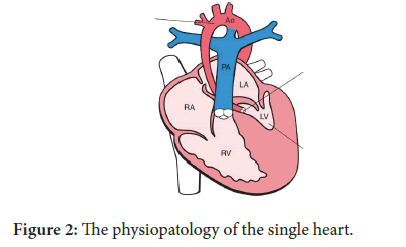
The LV itself and all the components of the LV are severely underdeveloped here [12]. The presence of a solitary RV, often associated to a tricuspid insufficiency does not guarantee, on the long term, a proper survival [13]. The presence of a more or less RV development , contractility and anatomy is still debatable in regards to the long term survival but seems now quite clear that the RV function and anatomy are two of factors of paramount importance for the long term survival up to the youth (Figure 2). The physiopatology of the single heart has been very well studied over the last years, and certainly that has contributed to a better post-operative and long term survival.
Even considering that, the real final and definitive conclusion in the treatment of an HLHS is represented by the heart transplant [15].
Also this procedure is charged of high peri-operative mortality, following the complex and sick multi-organ failure of patients accepted into the heart waiting list. That does not help the choice of those candidates in the waiting list, when an heart is available for transplant, if we consider the shortness of organs like we are facing nowdays. A debate on the transplantability of the Fontan patients is for that reason currently on going. The psychological impact on the adult patients survived to the long term Fontan procedure but going towards the darkness about their future needs also to be taken to the long term Fontan procedure but going towards the darkness about their future needs also to be taken into consideration.
Conclusion
It is still debatable whether or not to intervene at all on babies born with HLHS or it would be better interrupt the pregnancy in Countries at least where that is legally allowed.
References
- Bravo-Valenzuela NJ, Peixoto AB, Araujo Júnior E, et al. Prenatal diagnosis of congenital heart disease: A review of current knowledge. Indian Heart J. 2018;70:150-164.
- Crucean A, Alqahtani A, Barron DJ, et al. Re-evaluation of hypoplastic left heart syndrome from a developmental and morphological perspective. Orphanet J Rare Dis. 2017;12:138.
- Newland DP, Poh CL, Zannino D, et al. The impact of morphological characteristics on late outcomes in patients born with hypoplastic left heart syndrome. Eur J Cardiothorac Surg. 2019;56:557-563.
- Plymale JM, Frommelt PC, Nugent M, et al. The Infant with Aortic Arch Hypoplasia and Small Left Heart Structures: Echocardiographic Indices of Mitral and Aortic Hypoplasia Predicting Successful Biventricular Repair. Pediatr Cardiol. 2017;38:1296-1304.
- Abarbanell G, Border WL, Schlosser B, et al. Preoperative echocardiographic measures in interrupted aortic arch: Which ones best predict surgical approach and outcome? Congenit Heart Dis. 2018;13:476-482.
- Linda Edwards, Kevin P Morris, Ameen Siddiqui, et al. Norwood procedure for hypoplastic left heart syndrome: BT shunt or RV‐PA conduit? Arch. Disease Child Fetal Neonatal. 2007;92:210–214.
- Sakurai T, Rogers V, Stickley J, et al. Single-center experience of arch reconstruction in the setting of Norwood operation. Ann. Thorac Surg. 2012;94:1534-9.
- Hosein RB, Clarke AJ, McGuirk SP, et al. Factors influencing early and late outcome following the Fontan procedure in the current era. The 'Two Commandments'? Eur J Cardiothorac Surg. 2007;31:344-52.
- Tani LY, Minich LL, Pagotto LT, et al. Left heart hypoplasia and neonatal aortic arch obstruction: is the Rhodes left ventricular adequacy score applicable? J Thorac Cardiovasc Surg. 1999;118:81-6.
- Mart CR, Eckhauser AW. Development of an echocardiographic scoring system to predict biventricular repair in neonatal hypoplastic left heart complex. Pediatr Cardiol. 2014;35:1456-66.
- Szypulski A, Rai V, Sacharczuk J, et al. Risk factors for recoarctation of aorta after Norwood procedure in patients with hypoplastic left heart syndrome. Folia Med Cracov. 2018;58:11-21.
- Bjurbom M, Iyengar AJ, Moenkemeyer F, et al. Evolution of Left Ventricular Size in Late Survivors of Surgery for Hypoplastic Left Heart Syndrome. Ann Thorac Surg. 2017;104:926-931.
- Petko C, Möller P, Hoffmann U, et al. Comprehensive evaluation of right ventricular function in children with different anatomical subtypes of hypoplastic left heart syndrome after Fontan surgery. Int J Cardiol. 2011;150:45-9.
- Walsh MA, McCrindle BW, Dipchand A, et al. Left ventricular morphology influences mortality after the Norwood operation. Heart. 2009;95:1238-44.
- Roeleveld PP, Axelrod DM, Klugman D, et al. Hypoplastic left heart syndrome: from fetus to Fontan. Cardiol Young. 2018;28:1275-1288.