Review Article - Interventional Cardiology (2014) Volume 6, Issue 5
An update on translational and early trials in coronary interventions
- Corresponding Author:
- Richard C Becker
University of Cincinnati Division of Cardiovascular Health & Disease
College of Medicine, 231 Albert Sabin Way, Cincinnati, OH 45267, USA
E-mail: beckerrc@ucmail.uc.edu
Abstract
Translational medicine, which at its core allows discoveries in the laboratory to be applied directly to patients with the ultimate goal of advancing care and changing clinical practice, is well represented within the field of interventional cardiology. Two areas of considerable interest for the scientific and medical communities, stem cell therapy and drug-eluting stents, are examples of fundamentally sound constructs for biologic and device technology respectively, that have experienced widely differing journeys from ‘bench-to-bedside’. Herein we review the progress made in these two areas, focusing on stem cell therapy applied to the settings of acute myocardial infarction and cardiomyopathy and the ever-present concern for clinicians of stent thrombosis following percutaneous coronary intervention.
Keywords
bare metal stent, bioresorbable vascular scaffold, cardiosphere, drug-eluting stent, pluripotent, stem cells, stent thrombosis
Background
Stem cells: definitions & important characteristics
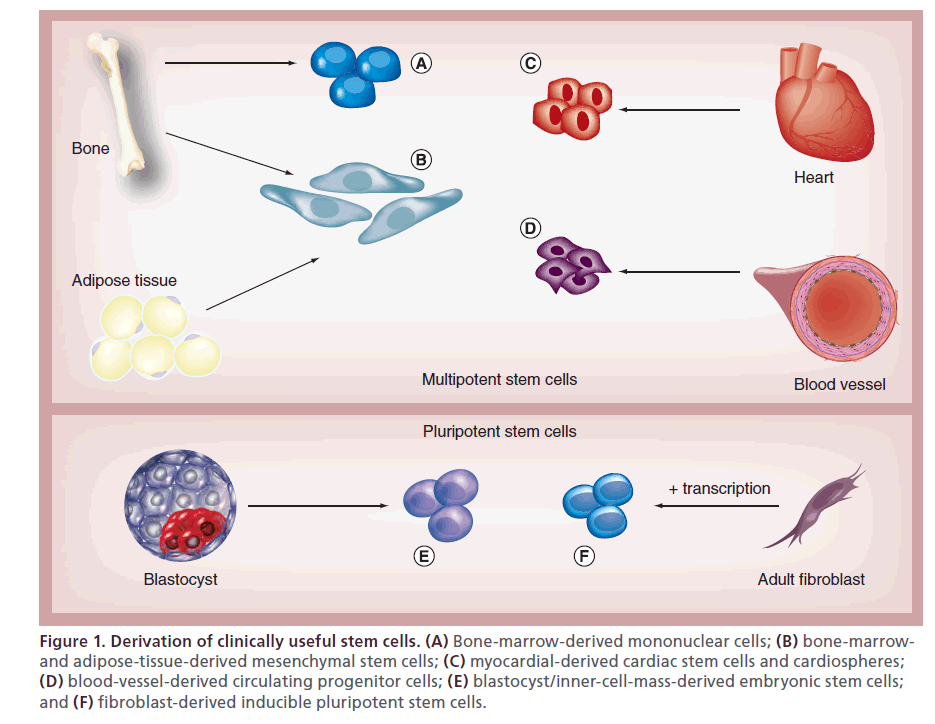
Stem cells are defined by their self-renewal and ability to differentiate into many different cell types – a characteristic known as pluripotency. Embryonic stem cells, derived from the inner cell mass of blastocysts, are pluripotent and able to form functional cardiomyocytes. Another pluripotent cell, avoiding the potential ethical considerations of embryonic stem cells, is the inducible pluripotent stem cell, which is created through overexpression of transcription factors that ‘reprogram’ adult fibroblasts and other adult somatic cells into pluripotent cells [1,2]. Bone-marrow- derived mononuclear cells (BM-MNCs), hematopoietic stem cells, and circulating progenitor cells exert their effects not by transforming to functional cardiomyocytes, but as a result of paracrine or autocrine signaling through cytokines and other factors that, in turn, enhance cardiomyocyte survival and angiogenesis [1,2]. These stem cells are also easily harvested from the bone marrow or peripheral circulation and have been employed in clinical trials.
Cardiac stem cells (CSCs), derived from the atria and ventricular apex, are multipotent stem cells (less differentiation capability than pluripotent stem cells) that can transform to several different types of cardiovascular cells [1,2]. These cells defied the previously held dogma that the heart had only differentiated cells. Cardiospheres are a heterogeneous population of progenitor cells and layers of differentiated cells derived from the myocardium under specific culture techniques that are capable of ‘self renewal’ and the ability to differentiate into cardiomyocytes [2]. Mesenchymal stem cells (MSCs) are distinct multipotent stem cell lines obtained from bone marrow or other mesenchymal tissues such as adipose tissue [1]. These cells can differentiate into myocytes, vascular smooth muscle cells, and endothelial cells; they also are relatively easy to prepare and are the foundation of many recent clinical trials. Skeletal myoblasts are multipotent stem cells that have regenerative capabilities after injury and were employed in early clinical trials; however, these cells do not integrate well with other cells and there was concern that this would cause heterogeneity of dispersion and arrhythmias (Figure 1) [1].
Figure 1: Derivation of clinically useful stem cells. (A) Bone-marrow-derived mononuclear cells; (B) bone-marrowand adipose-tissue-derived mesenchymal stem cells; (C) myocardial-derived cardiac stem cells and cardiospheres; (D) blood-vessel-derived circulating progenitor cells; (E) blastocyst/inner-cell-mass-derived embryonic stem cells; and (F) fibroblast-derived inducible pluripotent stem cells.
Advances in stem cell therapy
Stem cell therapy for acute myocardial infarction (AMI) has experienced a protracted course, with early trials oscillating between beneficial effects on global left ventricular function and positive remodeling to no discernible effect compared with placebo. A brief overview of the trials, their designs and outcomes is instructive (Table 1).
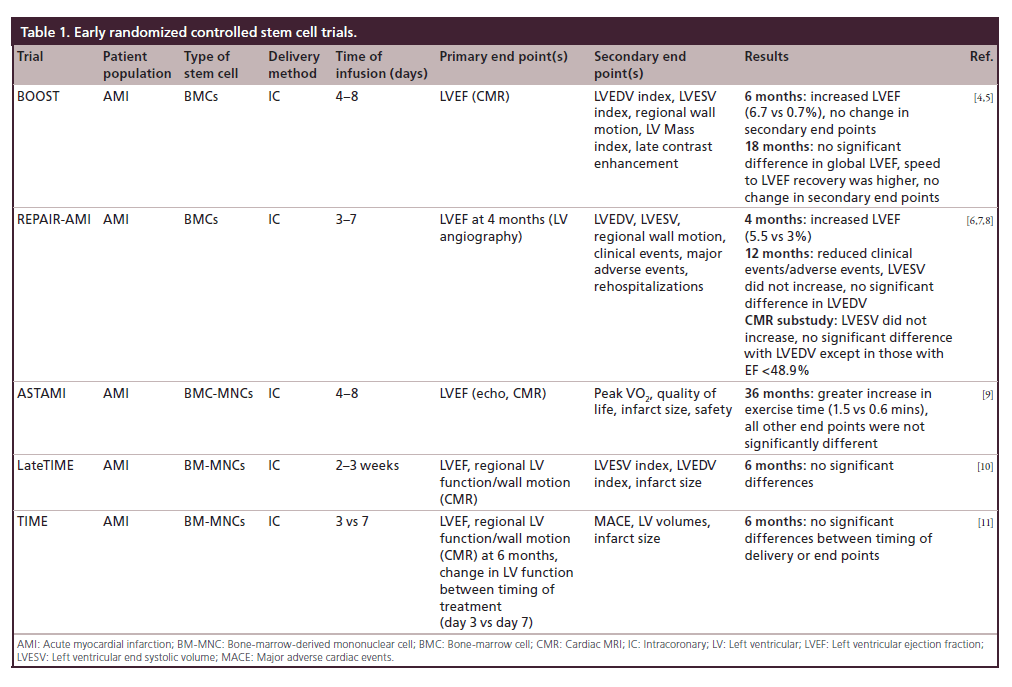
Table 1: Early randomized controlled stem cell trials.
Acute myocardial infarction
TOPCARE-AMI trial showed that intracoronary injection of bone-marrow cells (BMCs) or circulating progenitor cells could be undertaken safely in patients with a first ST-elevation myocardial infarction (STEMI) and yielded an improvement in left ventricular ejection fraction (LVEF) and decreased end systolic volume (ESV); however, it was not a randomized controlled trial [3]. By contrast, the BOOST trial was a randomized controlled trial and showed that infusion of BMCs within 4 to 8 days of STEMI resulted in an improved LVEF at 6 months. At 18 month follow-up there was no difference between groups [4,5]. The REPAIR-AMI trial showed that injection of BMCs 3 to 7 days after revascularization in patients with AMI resulted in improved global ejection fraction (EF) as well as decreased clinical events at 12 months [6,7]. A cardiac magnetic resonance imaging (CMR) substudy performed at 12 months identified an improved EF, though only a small proportion of patients in the overall study cohort underwent MRI [8]. The findings of REPAIR-AMI contrasted to those of the ASTAMI trial, which showed no change in EF, ESV, and end diastolic volume (EDV) by single-photon emission computed tomography (SPECT), echo and CMR at 3 years after intracoronary infusion of BMCs 4 to 8 days after a left anterior descending coronary artery STEMI [9]. Some investigators in the field speculated that differences in cell processing may have been responsible for differing outcomes between the two trials [9].
Late administration
The LateTIME trial reported that intracoronary infusion of BM-MNCs 2–3 weeks after AMI did not cause a change in EF or wall motion at 6 months and was the first trial that delivered a standardized dose of mononuclear cells. This was discovered through the Cardiac Cell Therapy Research Network, a group of five clinical research sites and their satellites, several core laboratories, and a cell processing quality control center, sponsored by the NIH’s National Heart, Blood and Lung Institute [10]. The TIME trial subsequently reported that the timing of infusion (day 3 vs 7) of BM-MNCs had no impact on LV function, volumes or size evaluated by CMR, when compared with placebo at 6 months (Table 1) [11].
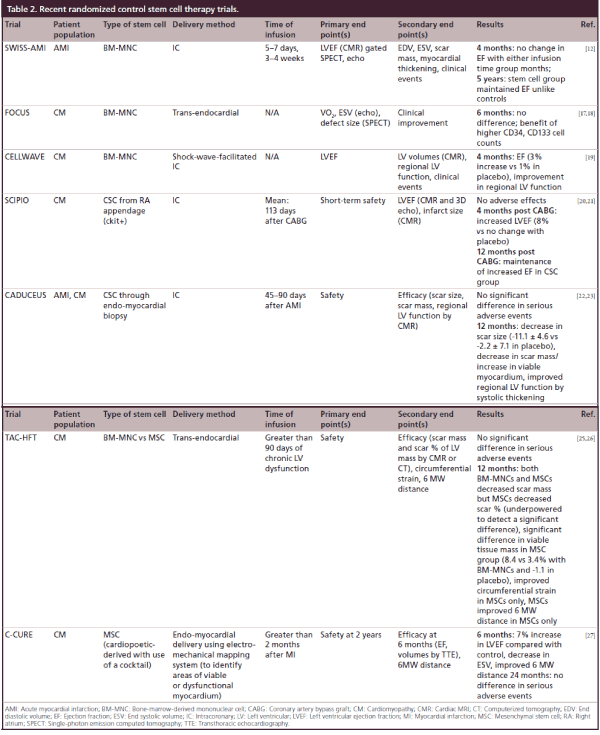
Since an initial series of early trials, additional studies have been undertaken with more consistent and promising outcomes. The Swiss myocardial infarction study set out to compare early infusion (5–7 days) versus late infusion (3–4 weeks) after AMI and found that at 4 months, there was no improvement in LV function compared with placebo as well as between the two infusion groups [12]. This observation was tempered by the 5-year data, which showed that the absolute increase in EF measured by CMR from the infusion group was maintained in the groups that received BM-MNCs and not maintained in the control groups [13].
Pooled data/meta-analyses
Meta-analyses have been done to establish the effect of stem cell therapy in AMI, however they are limited by the heterogeneity of the individual trials as mentioned above with regards to cell dosage, isolation protocols, storage methods and image modalities. Evidence of efficacy with EF has been seen in two recent metaanalyses and a Cochrane review utilizing bone-marrow- derived stem cells [14–16]. The EF improvement has ranged from 2.55 to 3.96% in favor of stem cell therapy at long term follow-up. In addition, ESV, EDV and infarct size decreased at specific time intervals, depending on the individual study [14–16].
Ischemic cardiomyopathy
The FOCUS trial showed that BM-MNCs injected into patients with ischemic cardiomyopathy (EF <45% and angina) resulted in no difference in EF, ESV, myocardial oxygen consumption or reversibility by SPECT compared with controls; however CD34 and CD133 cells were associated with a greater unit increase in EF [17,18]. The CELLWAVE trial used the technique of low energy shockwave to precondition the target tissue by enhancement of secretion of cytokines that increase neovascularization; there a significant increase in EF and wall thickening compared with placebo in patients with baseline EF <50% [19].
The most recent trials have employed autologous CSCs instead of those derived from bone marrow. The SCIPIO trial changed the focus to those with ischemic cardiomyopathy (EF <40%) 4-months post coronary artery bypass graft (CABG) (to allow for any recovery from stunning/hibernation) in a Phase I trial with small patient numbers and showed that CSCs obtained at time of CABG from right atrial appendage could be selected with 4-month data showing an improved EF by 3D echo as well as decreased infarct size by CMR [20]; there was additional increase in EF at 12 months, though not significantly different from 4-month data [21]. The CADUCEUS trial also used autologous CSCs through endomyocardial biopsy through the cardiosphere culture method in patients with ischemic cardiomyopathy (EF = 25–45%) with a recent myocardial infarction (MI; within 4 weeks) and found that at 12 months there was decreased scar size and mass with a corresponding increase in viable myocardium through CMR [22,23].
MSCs were used in the POSEIDON trial comparing allogeneic (‘off the shelf’ MSCs from donors) compared with autologous MSCs. These patients were followed by computed tomography to assess for early enhancement defect as a measure of infarct size, with results showing a decreased early enhancement defect as well as decreased sphericity index (a measure of LV remodeling) with allogeneic and autologous MSCs [24]. This study was limited by lack of a placebo group. The TAC-HFT pioneered the use of a special helical catheter for endocardial delivery of the stem cells rather than intracoronary delivery in patients with EF between 15 and 50% [25,26]. This study compared BMCs versus MSCs and assessed scar mass, with MSCs and BMCs both showing decreased scar mass but with only MSCs showing decreased scar mass as a function of LV mass and increased viability [25,26]. This study was primarily a safety study with end point being adverse effects, and was underpowered. Another trial using MSCs is the C-CURE trial, where MSCs obtained from bone marrow were differentiated to cardiopoeitic stem cells using a cardiogenic cocktail. These cells were injected into the endomyocardium after using an electromechanical mapping system to identify areas of viable and dysfunctional myocardium [27]. This study was in response to studies such as SCIPIO and CADUCEUS, which required the need for an invasive procedure to obtain autologous cardiac stem cells, rather than differentiation prior to injection. While not powered to be an efficacy trial, there was an improved EF and ESV when compared with controls (Table 2) [27]. Trials studying adipose-derived MSCs are currently ongoing.
Table 2: Recent randomized control stem cell therapy trials.
Conclusion & future of stem cell therapy
Early stem cell trials using BM-MNCs have had mixed results with only early data suggesting any improvement (Table 1). Later studies and end points after 12 months have not shown any increase in EF and only maintenance of EF (i.e., lack of negative remodeling) (Table 2). These trials, however, have had smaller patient numbers. Meta-analyses have suggested improvement in LVEF as well decreases in ESV and EDV at long-term follow-up, however there is considerable heterogeneity in the structure of individual trials, making generalization difficult. The BAMI trial (NCT01569178) is a 3000-patient multicenter randomized controlled study that should provide a high powered trial to determine the utility of BM-MNCs in patients with AMI and ischemic cardiomyopathy. The trials that have shown the most promise recently have been ones using endogenous cardiac stem cells and MSCs. One trial comparing BM-MNCs and MSCs suggested an increased benefit of MSCs with regard to the amount of scar [26]. These have also been trials with small patient numbers however, and further research is necessary. Some newer trials are evaluating combinations of cells, with one in a murine model showing the benefits of administration of circulatory angiogenic cells with endogenous CSCs with results showing reduction in scar burden [28]. Another preclinical study in a swine model has shown that administration of human CSCs with human MSCs resulted in decreased scar size, greater than either therapy alone, and improved parameters of systolic and diastolic function [29]. Coadministration of multiple types of cells to support the regeneration of cardiomyocytes, supporting substrates and vasculature makes theoretical sense and may be an area that has promise in future clinical trials.
Timing of administration of stem cells has been addressed in several trials with no difference seen with early or late administration [11,12]. One important question that remains unanswered is the optimal means of delivery. Is it intracoronary, transendocardial or shock-wave-facilitated endomyocardial with electromechanical mapping?
Advances in coronary arterial stents
Because the early experience with balloon angioplasty was characterized by coronary arterial injury, spiral dissections and abrupt vessel closure, bare metal stents (BMS) were developed to establish and maintain vessel patency, reducing the need for emergent coronary bypass grafting. It soon became evident, however, that neointimal, smooth muscle cell, and extracellular matrix hyperplasia with subsequent in-stent stenosis was a common occurrence after BMS placement [30]. In response to an emerging need, drug-eluting stents (DES) employing sirolimus and paclitaxel to interrupt cellular proliferation, were developed [30]. While DES lessened the occurrence of in-stent stenosis, stent thrombosis (ST) emerged as a complication that carried high morbidity and mortality rates. The available evidence suggested strongly that first-generation DES, while lessening smooth muscle cell hyperplasia, impaired endothelialization of the stent struts, creating a nidus for platelet adherence, activation and thrombin generation. The translation-based goal of subsequent generations of DES was to attenuate the localized proliferative response following percutaneous coronary intervention (PCI), while permitting endothelialization and restoration of a natural thromboresistant environment.
Clinical experience
Impact of stent thrombosis
The SYNTAX trial compared CABG and PCI for treatment of patients with either left main or three-vessel obstructive coronary artery disease [31]. The goal of the 5-year follow-up was to confirm the 1- and 3-year results. The end point was the composite rate of major adverse cardiac and cerebrovascular events (MACCE), including all-cause mortality, stroke, MI and repeat revascularization. The SYNTAX score was used to determine anatomic complexity based on number of lesions, tortuosity, lesion length >20 mm, calcification, thrombus and small vessels/diffuse disease. At 5 years, MACCE was higher in the PCI group versus CABG group (37.3 vs 26.9%) along with rates of MI, rates of death or stroke or MI, and repeat revascularization; however all-cause mortality and stroke were not significantly different between the groups [32]. Also, the left main subgroup had similar MACCE in both groups, while the three-vessel subgroup had higher MACCE in the PCI subgroup (37.5 vs 27.2%) [32]. Patients with low SYNTAX scores (0–22) did not have a significant difference in MACCE, while those with high SYNTAX scores (>33) did have a higher MACCE rate with PCI compared with CABG [33]. At 5 years there was no significant difference between ST and graft occlusion (7 vs 6%, respectively); however, there was a higher associated mortality with ST, with the majority of fatal events occurring in patients with ST localized to the left main and proximal left anterior descending artery [33].
Stent design & thrombosis
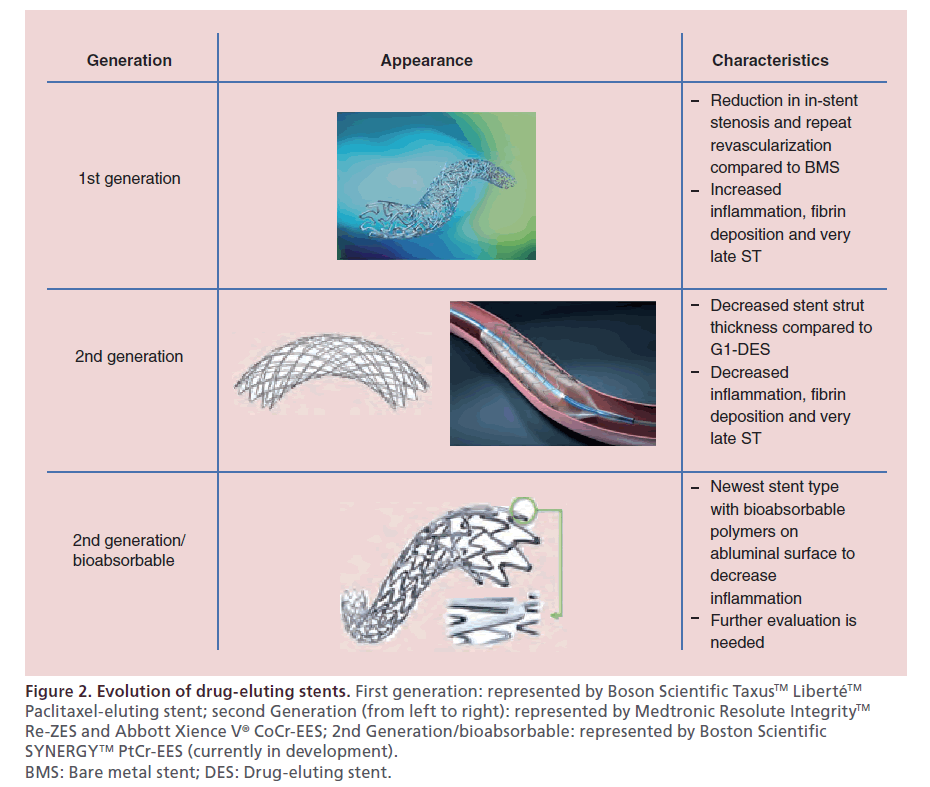
First-generation sirolimus- and paclitaxel-eluting DES (G1-DES) were found to be associated with a heightened risk for late and very late ST resulting in adoption of prolonged dual antiplatelet therapy as the standard of care [34]. Pathologic studies revealed that the durable polymer of G1-DES caused inflammation, fibrin deposition and poor endothelial healing, thus promoting ST [35]. Second-generation DES (G2-DES) were designed to minimize ST with bioabsorbable and biocompatible polymers with thinner strut platforms. These G2-DES include cobalt chromium everolimus-eluting stent (CoCr-EES), phosphorylcholine polymer-based fast-release zotarolimus-eluting stent (PC-ZES), and C10/C19/PVP polymer-based slow-release zotarolimus-eluting stent (Re-ZES) [36]. The latter two use a cobalt chromium stent platform. Newer stents use a platinum–chromium alloy which allows increased radio-opacity and even thinner stent struts, as platinum is denser than cobalt and maintains radial strength [37]. These are the platinum–chromium paclitaxel-eluting stent (PtCr-PES) and the platinum– chromium everolimus-eluting stent (PtCr-EES). The biolimus-eluting stent is among the most recently developed G2-DES that has bioabsorbable polymers. Pathology studies have shown that G2-DES are associated with less inflammation, less fibrin deposition and less ST (Figure 2) [38].
Figure 2: Evolution of drug-eluting stents. First generation: represented by Boson Scientific Taxus™ Liberté™ Paclitaxel-eluting stent; second Generation (from left to right): represented by Medtronic Resolute Integrity™ Re-ZES and Abbott Xience V® CoCr-EES; 2nd Generation/bioabsorbable: represented by Boston Scientific SYNERGY™ PtCr-EES (currently in development). BMS: Bare metal stent; DES: Drug-eluting stent.
Several studies comparing first and second generation DES support a lower risk for ST with G2-DES. A meta-analysis performed by Palmerini et al. found that a G2-DES, specifically the CoCr-EES, was associated with significantly lower composite rates of all-cause death and MI, cardiac death and MI, and ST within one year compared with BMS [39]. Similarly, after 1 year, less ST was observed with CoCr-EES compared with BMS [39]. The observations suggest that G2-DES are associated with a lower rates of ST than G1-DES and BMS. A German registry of 18,334 patients reported that compared with BMS, the odds ratio of having ST with G1-DES was 2.05 [40]. The odds ratio of having ST with G2-DES compared with BMS was 0.82; however, this was not statistically significant. In addition, while ST at 30 days was not significant between BMS, G1-DES and G2-DES, the odds ratio of very late ST (between 1 and 3 years) in G1-DES compared with BMS was 4.72 [40]. The odds ratio of very late ST in G2-DES compared with BMS was 1.01, which was not statistically significant [40]. These findings were similar to those in prior meta-analyses, suggesting that G2-DES have a similar risk of ST to BMS, but significantly less than G1-DES.
The LEADERS trial 5-year data recently showed that a bioabsorbable polymer-based bioliumus-eluting stent (G2-DES) compared with a durable polymer sirolimus- eluting stent (G1-DES) was noninferior for major adverse cardiac event (MACE) rates within 9 months, but there was reduction in very late ST (RR: 0.26), corresponding to a reduction in MI and all cause revascularization [41]. A meta-analysis performed by Palmerini et al. suggested that bioabsorbable polymer-based G2-DES were associated with decreased rates of cardiac death/ MI, MI and target vessel revascularization than BMS; it also had decreased rates of target vessel revascularization compared with PC-ZES but was not different than other G2-DES [42]. In terms of ST, bioabsorbable polymer- based G2-DES had increased 1 year and long-term rates of definite ST compared with CoCr-EES (odds ratio: 2.44) but was not significantly different than other G2-DES [42]. Overall, the data suggest that while bioabsorbable G2-DES are associated with a lower risk of ST compared with BMS, they may not offer a significant advantage over durable polymer-based CoCr-EES.
Conclusion & the future of drug-eluting stents
The DUTCH PEERS trial and the HOST-ASSURE trial have shown noninferiority of the new PtCr-EES to CoCr-ZES with regard to safety and efficacy in all comers requiring PCI [43,44]. Longitudinal stent deformation, a phenomenon described in case reports, is thought to occur from lack of longitudinal stability in thinner strut stents which can cause deformation of the stent in response to guide catheter insertion or balloon inflation [45]. This was seen only in the PtCr-EES group in both DUTCH PEERS and HOST-ASSURE, however there were no clinically significant adverse outcomes. Longitudinal stent deformation will need further evaluation in future trials.
Post mortem studies of ST have shown lack of neo-intimal stent strut coverage/stent malapposition play a significant role in late ST [46,47]. In addition, patients with impaired endothelial vasomotor function have been shown to have future cardiovascular events. Abnormal vasomotion is seen distal to a deployed DES [48]. Bioresorbable vascular scaffolds (BVS), which are fully biodegradable, offer a potential solution by allowing a temporary scaffolding of the vessel to prevent acute vessel closure as well as offering a transient antiproliferative drug to prevent restenosis, but avoiding caging of the vessel through permanent metallic stents [49]. The ABSORB II trial (NCT01425281) is an ongoing, approximately 500-patient, randomized controlled trial designed to compare the Absorb everolimus-eluting BVS system against the metallic EES with primary end points of vasomotor reactivity and minimum lumen diameter at 2-year follow-up assessed by quantitative coronary angiography [50]. The AIDA trial (NCT01858077) is an even larger randomized, single-blinded noninferiority trial evaluating the Absorb everolimus-eluting BVS system against the metallic EES with primary end point of a composite of cardiac death, MI and target vessel revascularization at 2 years, with a total of 2690 patients to be enrolled [51]. These trials should offer insight on the clinical usefulness of the BVS.
ST is a feared complication of PCI. While several meta-analyses and registries support progress with newer generation DES, head-to-head trials of G2-DES and scaffolds may be required to better determine the optimal stent/scaffold structure to minimize the risk of ST.
Future perspective: translational science & interventional cardiology
Translational science has favorably impacted many fields in medicine, including interventional cardiology. Stem cell therapy following MI and for the treatment of cardiomyopathy is soundly based, but illustrates the complexity of biologics for treating cardiovascular disease and underscores the importance of understanding mechanisms of benefit and applying fundamental principles in early-stage clinical research. The evolution of DES has benefitted greatly from applying translational constructs to structural and functional design.
The field would likely benefit from taking a translational approach to several emerging areas of interest and clinical relevance, including transcatheter aortic valve replacement, complex PCI, and chronic total occlusion PCI. The advent of drug-coated balloons and the next generation of BVS are other areas where a translational approach to research and development will likely have an impact.
Executive summary
Stem cells: definitions & important characteristics
• Pluripotent stem cells can become any other cell or tissue.
• Somatic cells can be ‘reprogrammed’ through use of transcription factors to become pluripotent stem cells.
• Multipotent stem cells can be derived from the heart itself, bone marrow, skeletal muscle and adipose tissue, and can become a limited range of cells within a tissue type.
Advances in stem cell therapy
• Stem cell therapy in the setting of acute myocardial infarction and ischemic cardiomyopathy have had mixed results.
• While individual trials have shown improvement in left ventricular ejection fraction, end-systolic volume, end-diastolic volume, infarct size, and other parameters, lack of standardization makes interpretation of these findings difficult.
• Meta-analyses do suggest improvement in left ventricular ejection fraction, end-systolic volume, end-diastolic volume at long term follow-up in the stem cell group in patients with acute myocardial infarction, however heterogeneity in the trials is a limiting factor.
• Future large volume trials should help determine the efficacy of stem cell therapy in patients with acute myocardial infarction and ischemic cardiomyopathy as well as determining the optimal delivery system.
Advances in coronary arterial stents
• Drug-eluting stents were developed in response to the occurence of in-stent restenosis with bare metal stents.
• G1-DES are at higher risk for late and very late compared with bare metal stents as a result of durable polymers that cause local inflammation.
• G2-DES use bioabsorbable and biocompatible polymers to minimize stent thrombosis.
• Newer stents use different metals such as platinum to allow thinner stent struts but need further evaluation.
• Bioresorbable vascular scaffolds may further minimize the risk of stent thrombosis by decreasing local inflammation with a temporary scaffold that is completely absorbed over time.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- Foldes G, Harding SE. Stem cell therapy to treat heart failure. In: Comprehensive Biotechnology (2nd Edition). Elsevier, London, UK, 407–417 (2011).
- Bernal A, Galvez BG. The potential of stem cells in the treatment of cardiovascular diseases. Stem Cell Rev. Rep. 9, 814–832 (2013).
- Schachinger V, Assmus B, Britten MB et al. Transplanation of progenitor cells and regeneration enhancement in acute myocardial infarction. J. Am. Coll. Cardiol. 44, 1690–1699 (2004).
- Wollert KC, Meyer GP, Lotz J et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet 364, 141–148 (2004).
- Meyer GP, Wollert KC, Lotz J et al. Intracoronary bone marrow cell transfer after myocardial infarction: eighteen month’s follow-up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) trial. Circulation 113, 1287–1294 (2006).
- Schachinger V, Erbs S, Elsasser A et al. Improved clinical outcome after intracoronary administration of bone-marrowderived progenitor cells in acute myocardial infarction: final 1-year results of the REPAIR-AMI trial. Eur. Heart J. 27, 2775–2783 (2006).
- Schachinger V, Erbs S, Elsasser A et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N. Engl. J. Med. 355, 1210–1221 (2006).
- Dill T, Schachinger V, Rolf A et al. Intracoronary administration of bone marrow-derived progenitor cells improves left ventricular function in patients at risk for adverse remodeling after acute ST-segment elevation myocardial infarction: Results of the Reinfusion of Enriched Progenitor cells and Infarct Remodeling in Acute Myocardial Infarction study (REPAIR-AMI) cardiac Magnetic Resonance Imaging substudy. Am. Heart J. 157, 541–547 (2009).
- Beitnes JO, Hopp E, Lunde K et al. Long-term results after intracoronary injection of autologous mononuclear bone marrow cells in acute myocardial infarction: the ASTAMI randomised, controlled study. Heart 95, 1983–1989 (2009).
- Traverse JH, Henry TD, Ellis SG, Pepine CJ et al. Effect of intracoronary delivery of autologous bone marrow mononuclear cells 2 to 3 weeks following acute myocardial infarction on left ventricular function. JAMA 306(19), 2110–2119 (2011).
- Traverse JH, Henry TD, Pepine CJ et al. Effect of the use and timing of bone marrow mononuclear cell delivery on left ventricular function after acute myocardial infarction: the TIME randomized trial. JAMA 308(22), 2380–2389 (2012).
- Surder D, Manka R, Cicero VL et al. Intracoronary injection of bone marrow-derived mononuclear cells early or late after acute myocardial infarction: effects on global left ventricular function. Circulation 127, 1968–1979 (2013).
- Moccetti, , Surder D, Klersy C et al. Sustained improvement in left ventricular function after bone-marrow-derived cell therapy in patients with acute ST elevation myocardial infarction. Swiss Med. Wkly 142 (2012).
- Jeevanantham V, Butler M, Saad A, Abdel-Latif A, Zuba- Surma EK, Dawn B. Adult bone marrow cell therapy improves survival and induces long-term improvement in cardiac parameters: a systematic review and meta-analysis. Circulation 126, 551–568 (2012).
- Delewi R, Hirsch A, Tijssen JG et al. Impact of intracoronary bone marrow cell therapy on left ventricular function in the setting of ST-segment elevation myocardial infarction: a collaborative meta-analysis. Eur. Heart J. 35, 989–998 (2014).
- Clifford DM, Fisher SA, Brunskill SJ et al. Stem cell treatment for acute myocardial infarction. Cochrane Library 2, 1–237 (2012).
- Willerson JT, Emerson CP, Ellis SG et al. Intramyocardial injection of autologous bone marrow mononuclear cells for patients with chronic ischemic heart disease and left ventricular dysfunction (First Mononuclear Cells injected in the US [FOCUS]): Rationale and design. Am. Heart J. 160, 215–223 (2010).
- Emerson CP, Willerson JT, Pepine CJ et al. Effect of transendocardial delivery of autologus bone marrow mononuclear cells on functional capacity, left ventricular function, and perfusion in chronic heart failure. JAMA 307(16), 1717–1726 (2012).
- Assmus B, Walter DH, Seeger FH et al. Effect of shock wavefacilitated intracoronary cell therapy on LVEF in patients with chronic heart failure. JAMA 309(15), 1622–1631 (2013).
- Bolli R, Chugh AR, D’Amario D et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised Phase 1 trial. Lancet 378, 1847–1857 (2011).
- Chugh AR, Beache GM, Loughran JH et al. Administration of cardiac stem cells in patients with ischemic cardiomyopathy: the SCIPIO trial: surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation 126, S54–S64 (2012).
- Makkar RR, Smith, RR, Cheng Ke et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised Phase 1 trial. Lancet 379, 895–904 (2012).
- Malliaras K, Makkar RR, Smith RR et al. Intracoronary cardiosphere-derived cells after myocardial infarction. J. Am. Coll. Cardiol. 63, 110–122 (2014).
- Hare JM, Fishman JE, Gerstenblith G et al. Comparsion of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy. JAMA 308(22), 2369–2379 (2012).
- Trachtenberg B, Velazque DL, Williams AR et al. Rationale and design of the Transendocardial Injection of Autologous Human Cells (bone marrow or mesenchymal) in Chronic Ischemic Left Ventricular Dysfunction and Heart Failure Secondary to Myocardial Infarction (TAC-HFT) trial: A randomized, double-blind, placebo-controlled study of safety and efficacy. Am. Heart J. 161, 487–493 (2011).
- Heldman AW, DiFede DL, Fishman JE et al. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy. JAMA 311(1), 62–73 (2014).
- Bartunek J, Behfar A, Dolatabadi D et al. Cardiopoietic stem cell therapy in heart failure. J. Am. Coll. Cardiol. 61, 2329–2338 (2013).
- Latham N, Ye B, Jackson R et al. Human blood and cardiac stem cells synergize to enhance cardiac repair when cotransplanted into ischemic myocardium. Circulation 128, S105–S112 (2013).
- Williams AR, Hatzistergos KE, Addicott B et al. Enhanced effect of combining human cardiac stem cells and bone marrow mesenchymal stem cells to reduce infarct size and to restore cardiac function after myocardial infarction. Circulation 127, 213–223 (2013).
- Garg S, Magro M, Serruys PW. Drug Eluting Stents. Cardiology and Cardiovascular Surgery. Comprehensive Biomaterials. 1st Edition. Ducheyne P (Ed.). Elsevier, Oxford, UK, 427–448 (2011).
- Morice M, Serruys PW, Kappetein P et al. Outcomes in patients with de novo left main disease treated with either percutaneous coronary intervention using paclitaxeleluting stents or coronary artery bypass graft treatment in the synergy between percutaneous coronary intervention with TAXUS and cardiac surgery (SYNTAX) trial. Circulation 121, 2645–2653 (2010).
- Mohr FW, Morice M, Kappetein AP et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet 381, 629–638 (2013).
- Farooq V, Serruys PW, Zhang Y et al. Short-term and long-term clinical impact of stent thrombosis and graft occlusion in the SYNTAX trial at 5 years. J. Am. Coll. Cardiol. 62, 2360–2369 (2013).
- Wenaweser P, Daemen J, Zwahlen M et al. Incidence and correlates of drug-eluting stent thrombosis in routine clinical practice. 4-year results from a large 2-institutional cohort study. J. Am. Coll. Cardiol. 52, 1134–1140 (2008).
- Finn AV, Nakazawa G, Joner M et al. Vascular responses to drug eluting stents: importance of delayed healing. Arterioslcer. Thromb. Vasc. Biol. 27, 1500–1510 (2007).
- Palmerini T, Biondi-Zoccai G, Riva DD et al. Stent thrombosis with drug-eluting stents. J. Am. Coll. Cardiol. 62, 1915–1921 (2013).
- Garg S, Magro M, Serruys PW. Drug eluting stents. Comprehensive Biomaterials. 6, 427–448 (2011).
- Otsuka F, Vorpahl M, Nakano M et al. Pathology of second-generation everolimus-eluting stents versus firstgeneration sirolimus- and paclitaxel-eluting stents in humans. Circulation 129(2), 211–223 (2014).
- Palmerini T, Biondi-Zoccai G, Riva DD et al. Clinical outcomes with drug-eluting and bare-metal stents in patients with ST-segment elevation myocardial infarction. J. Am. Coll. Cardiol. 62, 496–504 (2013).
- Tada T, Byrne RA, Simunovic I et al. Risk of stent thrombosis among bare-metal stents, first-generation drugeluting stents, and second-generation drug-eluting stents. JACC Cardiovasc. Interv. 6, 1267–1274 (2013).
- Serruys PW, Farooq V, Kalesan B et al. Improved safety and reduction in stent thrombosis associated with biodegradable polymer-based biolimus-eluting stents versus durable polymer-based sirolimus-eluting stents in patients with coronary artery disease. JACC Cardiovasc. Interv. 6, 777–789 (2013).
- Palmerini T, Biondi-Zoccai G, Riva DD et al. Clinical outcomes with bioabsorbable polymer- versus durable polymber-based drug-eluting and bare-metal stents. J. Am. Coll. Cardiol. 63, 299–307 (2014).
- Von Birgelen C, Sen H, Lam MK et al. Third-generation zotarolimus-eluting and everolimus-eluting stents in all-comer patients requiring a percutaneous coronary intervention (DUTCH PEERS): a randomised, single-blind, multicentre, non-inferiority trial. Lancet 383, 413–423 (2014).
- Park KW, Kang SH, Kang HJ et al. A randomized comparison of platinum chromium-based everolimuseluting stents versus cobalt chromium-based zotarolimuseluting stents in all-comers receiving percutaneous coronary intervention. J. Am. Coll. Cardiol. 63(25), 2805–2816 (2014).
- Abdel-Wahab M, Sulimov DS, Kassner G, Geist V, Toelg R, Richardt G. Longitudinal deformation of contemporary coronary stents: an integrated analysis of clinical experience and observations from the bench. J. Interv. Cardiol. 25(6), 576–585 (2012).
- Joner M, Finn AV, Farb A et al. Pathology of drug-eluting stents in humans. J. Am. Coll. Cardiol. 48(1), 193–202 (2006).
- Finn AV, Joner M, Nakazawa G et al. Pathological correlates of late drug-eluting stent thrombosis: strut coverage as a marker of endothelialization. Circulation 115, 2435–2441 (2007).
- Kitta Y, Obata J, Nakamura T et al. Persistent impairment of endothelial vasomotor function has a negative impact on outcome in patients with coronary artery disease. J. Am. Coll. Cardiol. 53(4), 323–330 (2009).
- Onuma Y, Serruys PW. Bioresorbable scaffold: the advent of a new era in percutaneous coronary and peripheral revascularization? Circulation 123, 779–797 (2011).
- Diletti R, Serruys PW, Farooq V et al. ABSORB II randomized controlled trial. A clinical evaluation to compare the safety, efficacy, and performance of the Absorb everolimus-eluting bioresorbable vascular scaffold system against the XIENCE everolimus-eluting coronary stent system in the treatment of subjects with ischemic heart disease caused by de novo native coronary artery lesions: Rationale and study design. Am. Heart J. 164(5), 654–663 (2012).
- Woudstra P, Grundeken MJ, Kraak RP et al. Amsterdam Investigator-initiateD Absorb strategy all-comers trial (AIDA): a clinical evaluation comparing the efficacy and performance of ABSORB everolimus-eluting bioresorbable vascular scaffold strategy vs the XIENCE family (XIENCE PRIME or XIENCE Xpedition) everolimus-eluting coronary stent strategy in the treatment of coronary lesions in consecutive all-comers: rationale and study design. Am. Heart J. 167, 133–140 (2014).
•• Good overview of the types of stem cells, advantages of the different cell types and stem cell delivery approaches.
• Demonstrates the overall benefit of bone marrow-derived stem cells in the setting of a stent thrombosis-elevation myocardial infarction, including data from 16 studies.
•• Discusses the need for coronary stents and their development through multiple generations.
•• Discusses the phenomenon of stent thrombosis and its prevention through drug-eluting stent development.