Research Article - Diabetes Management (2018) Volume 8, Issue 4
Anti-hyperglycemic effect of gasca d herbal formulation on alloxan induced diabetic rats
- *Corresponding Author:
- Ibrahim S Ismail
Department of Biochemistry
Federal University Dutse, Nigeria
E-mail: salihu.ibrahim@fud.edu.ng
Abstract
Gasca D herbal formulation is an herbal anti-diabetic medication developed from the combination of Adansonia digitata, Gum Acacia and organic cellulose. Thus, the aim of this study is to evaluate the hypoglycemic potential and to determine hematological indices and biochemical parameters of Gasca D herbal formulation in normal and Alloxan induced diabetic rats. Diabetes was induced by interperitoneal injection of Alloxan monohydrate 150 mg/kg bw followed by a booster dose of 70 mg/kg bw after 48 hours after first induction. Both normal positive (group 2) and diabetic treated (group 4) rats received Gasca D herbal formulation at a dose of 1,000 mg/kg bw for 28 days. Acute toxicity study of Gasca D herbal formulation did not cause any mortality or abnormalities even after 14 days post treatment in rats up to a dose of 5,000 mg/kg bw. The result showed a progressive decrease in both weight and glucose concentration from 1st through the 4th week of the study, with significant decrease in both weight and glucose concentration in the 3rd and 4th week of normal positive control. There is a significant (p<0.001) and progressive reduction in blood glucose concentration in diabetic treated with Gasca D herbal formulation. There was significant increase in PCV, Hb, RBC and WBC in diabetic rats treated with Gasca D herbal formulation. The mean value of Tchol, HDL, LDL and Trig for the lipid parameters and AST and ALT for liver function and Electrolyte, urea and creatinine for kidney function in normal positive and diabetic treated groups was not significantly (p<0.001) different from normal and diabetic control except for urea and potassium in normal positive control group treated with Gasca D herbal formulation. Thus Gasca D herbal formulation can be said to be practically non-toxic herbal formulation category and can therefore be used in the management of diabetes mellitus.
Keywords
diabetes, gasca D herbal, hematological indices, biochemical parameters, toxicity
Introduction
In 2013, the World Health Organization (WHO) launched the WHO Traditional Medicine Strategy 2014-2023 [1] prioritizing that traditional medicine (TM) should be further developed based on research and innovation in line with the “Global Strategy and Plan of Action on Public Health, Innovation and Intellectual Property” adopted at the 61st World Health Assembly in 2008. The main aim is to harness the potential contribution of TM to health, wellness and people-centered health care” and “promote the safe and effective use of TM by regulating, researching and integrating TM products, practitioners and practice into health systems, where appropriate [1]”. The development of a particular TM is a step-by-step process that usually followed the development of new herbal medicine, to validate its safety and efficacy. Herbal medicines are naturally occurring; plant-derived substances with minimal or no industrial processing that have been used to treat illness within local or regional healing practices [2]. The use of herbal medicinal products and supplements has received a tremendous interest and is getting significant attention in global health care. China, India, Nigeria, the United States of America (USA) and WHO have all made substantial research investments in traditional herbal medicines [3]. Many times herbal medicines and related products are introduced into the market without any mandatory safety or toxicological evaluation. These herbal products are continuously made available to consumers without prescription in most cases and the potential hazards in an inferior product are hardly recognized [4]. Despite usage of herbal medicines by approximately 40% of all healthcare services delivered in China and usage of herbal medicine by atleast once by 48%, 70%, 42%, 38%, and 75%, percentage of the populace in Australia, Canada, USA, Belgium, and France respectively [5,6], the problem of safety of herbal remedies continues to remain a major issue of concern. Therefore it is essential to identify the risks associated with the use of herbal medicines, and in this regard, the safety of these products has become an issue of great public health importance [1,7]. The aim of the present study is to determine the safety and efficacy of Gasca D herbal formulation as an alternative medication for the management of diabetes mellitus in wistar albino rats. The research focuses on determining the acute and sub-acute toxicity and effect of the herbal formulation on hematological and biochemical parameters in order to validate its safety in the pre-clinical trial.Given the continued rise in the prevalence of diabetes mellitus worldwide and the attributable obesity burden is likely to continue to increase in the coming years. The estimate given by the International Diabetic Federation (IDF) that 425 million people presently have diabetes worldwide with expected increase to 693 million by the year 2045 [8]. This shows that the disease still remains a serious cause of morbidity and mortality. Diabetes can be defined as a disturbances in glucose and insulin homeostasis, characterized by a deterioration of glucose control, involving impairment in insulin stimulation, secretion and action, resulting in post prandial hyperglycemic rise, which if not properly controlled can put patients at increased risk of developing macrovascular and microvascular complications. Research in the treatment of diabetes has centered on attempts to normalize fasting and postprandial blood glucose levels by aggressively controlling hyperglycemia. Several studies have demonstrated that, the short-term and long-term benefit of improving glycemic control is crucial in delaying the onset of diabetes-related complications [9,10]. Because of the progressive nature of the disease treatment with two or three agents with different modes of action can be required as diabetes advances. However, intensive glycaemic control is not without risks, such as hypoglycaemia, weight gain and possible cardiovascular events and mortality in high-risk individuals. This constitutes the major force for finding alternatives, mainly from plant kingdom that are of less severe or even no side effects.
Herbal remedies and alternative medicines are used throughout the world and in the past herbs often represented the original sources of most drugs [11,12].Herbal-derived substances remain the basis for a large proportion of the commercial medications used today for the treatment of heart disease, high blood pressure, pain, asthma and other illnesses [13]. For example Biguanides were derived from the guanidine-rich herb Galega officinalis (French lilac), which was used in traditional medicine in Europe [14,15]. Thus medicinal plants are a big reservoir of useful chemical compounds not only as drugs, but also as templates for synthetic analogues.
Gasca D is a 100% organic herbal antidiabetic drug, exclusively produced from the combination of Adansonia Digitata, Gum Acacia and Starch derived from Hyphaenae Thebaica. Adansonia digitata (L.) called the baobab tree, is widespread throughout the hot and drier regions of tropical Africa [16]. Baobab tree has multi-purpose uses and every part of the plant is reported to be useful [17,18]. Baobab leaves, bark, pulp and seeds are used as food and for multiple medicinal purposes in many parts of Africa [19]. Gum Arabic is a dried exudate obtained from the stem and branches of Acacia trees [20], mainly Acacia senegal and Acacia seyal. In modern times, they are used in foods, pharmaceutical, and many other industries [21- 23]. Supplementation of GA in different forms to the normal or diabetic or fed-high fat diet rat/mice significantly reduced blood glucose levels [24,25]. Ingestion of GA was revealed to decrease body mass index (BMI) and percentage of body fat in healthy adult human females [26]. Hyphaene thebaica has an antioxidant activity due to the substantial amount of water-soluble phenolic contents of flavonoids within it. These contents represent conjugates of o-glycosides, which include quercetin, chrysoeriol, luteolin, and isorhamnetin H [27,28]. The plant is known to possess phytochemical constituents with antidiabetic capacities [28]. The fruit shows antimicrobial, anti-inflammatory, and antihypertensive activity with these activities attributed for the presence of flavonoids [29-31].
Materials and methods
▪ Chemicals
Biological kits for estimation of biochemical parameters (liver enzymes, lipid profile, electrolytes, urea and creatinine) were purchased from roche (germany). Alloxan monohydrate was purchased from sigma chemicals (St. Louis, USA). All other chemicals used for this study were of analytical grade.
▪ Plant collection
The leaves, pulp and bark of Adansonia digitata were collected in Kadage community of Ringim local government area (Jigawa state) Nigeria in April 2017.
▪ Extraction procedure
The freshly collected leaves, pulp and bark of Adansonia digitata were shade dried and then coarsely grinded to powder. The coarse powdered leaves and bark were extracted with methanol by cold maceration for 3 days. After decantation and filtering the solvent was removed by distillation over boiling water bath and remaining under reduced pressure. The extracts so obtained were further dried in vacuum desiccators.
▪ Preparation of gasca D
The leaf and bark extract and fruit pulp with gum arabic were mixed together at room temperature to form final formulation called gasca D.
▪ Acute toxicity study
The acute toxicity study for gasca D herbal formulation was performed in wistar albino rats using OECD (Organization of Economic Cooperation and Development) guidelines 423 (Acute toxic class method. In the first phase of acute toxicity study initial dose of (10, 100 and 1,000 mg/kg) was orally administered, followed by second phase where dose of (1,900, 2,600 and 5,000 mg/kg) was orally administered to 3 rats per group. 3 rats were kept as control. gasca D herbal formulation was found safe up to dose of 5,000 mg/kg and no mortality was recorded after 72 hours of observation.
▪ Experimental animals
Healthy male wistar albino rats, weighing 180- 230 grams were obtained from the central animal house, Faculty of Pharmacy, Ahmadu Bello University, Zaria. The animals were fed standard pelleted diet, water ad libitum and kept in 12 hrs light/dark cycle. Twenty rats were divided into four groups of five animals each: group I (control group); group II (control+Gasca D) rats given Gasca D (1,000 mg/kg body wt., orally) for 4 weeks; group III (Diabetic control) rats; group IV (Diabetic + Gasca D) rats given Gasca D (1,000 mg/kg body wt., orally) for 4 weeks.
▪ Induction of diabetes in rats
Diabetes mellitus was induced according to the method of Venugopal et al. [32] with some modification by single intraperitoneal injection of freshly prepared alloxan monohydrate (150 mg/kg/ body weight) followed by a booster dose of 70mg/kg bw after 48 hours. The development of hyperglycemia in rats was confirmed by fasting blood glucose estimation after 4 days of initial injection. The animals that maintained fasting blood glucose higher than 250 mg/dl were considered diabetic and selected for studies.
▪ Blood collection
Blood samples were collected from the animals using orbital techniques for biochemical and haematological analyses. Blood samples for clinical chemistry determination were collected from the retrobulbar plexus of the median canthus of the eye of the rats [33]. A microcapillary tube was carefully inserted into the medial canthus of the eye to puncture the retrobulbar plexus and thus enable outflow of about 2 ml of blood into a clean glass tube. The blood was kept at room temperature for 30 min to clot. Afterwards, the test tubes containing the clotted blood sample were centrifuged at 3,000 rev per min using a table centrifuge. The clear serum supernatant was then carefully aspirated with syringe and needle and stored in a clean sample bottle for the clinical chemistry determinations.
▪ Hematological assay
Blood samples were collected from rats into heparinized tubes under light ether anesthesia. White blood cells (WBC), red blood cells (RBC), hematocrit (Hct), hemoglobin (Hb), mean cell volume (MCV), mean cell hemoglobin (MCH), and mean cell hemoglobin concentration (MCHC), were measured on Hematology Analyzer (Abacus, Austria).
▪ Biochemical analysis
Serum samples were analyzed for the activities of aspartate aminotransferase (AST), alanine aminotransferase (ALT), cholesterol, total Triglycerides, Bicarbonate, urea and creatinine using a commercially available diagnostic kit from Roche, Germany.
▪ Statistical analysis
Data were analyzed by one-way analysis of variance (ANOVA) followed by Student’s ��-tests using a commercially available statistics software package (SPSS for Windows, V. 20.0) program. Results were presented as means ± SD. �� values<0.05 were regarded as statistically significant.
Results
The result indicated that Gasca D was practically non-toxic herbal drug category, which is safe up to a dose of 5,000 mg/kg b.w. when tested in wistar rats. The results showed no clinical signs and mortality in rats (Table 1).
| Observation | 6h | 12h | 24h | 48h | 72h |
|---|---|---|---|---|---|
| Behavioral patterns | Normal | Normal | Normal | Normal | Normal |
| Condition of fur | Normal | Normal | Normal | Normal | Normal |
| Subcutaneous swelling or lumps | Normal | Normal | Normal | Normal | Normal |
| Nasal bleeding | Normal | Normal | Normal | Normal | Normal |
| paralysis | Normal | Normal | Normal | Normal | Normal |
| Wetness and / or soiling of perineum | Normal | Normal | Normal | Normal | Normal |
| Breathing abnormalities | Normal | Normal | Normal | Normal | Normal |
| Diarrhea | Not | Not | Not | Not | Not |
| observed | observed | observed | observed | observed | |
| Tremors/Corner | Not | Not | Not | Not | Not |
| sitting | observed | observed | observed | observed | observed |
| Mortality | None | None | None | None | None |
Table 1: General appearance and behavioral observations after oral administration Gasca D at 1,000 mg/kg/ bw for 14 days
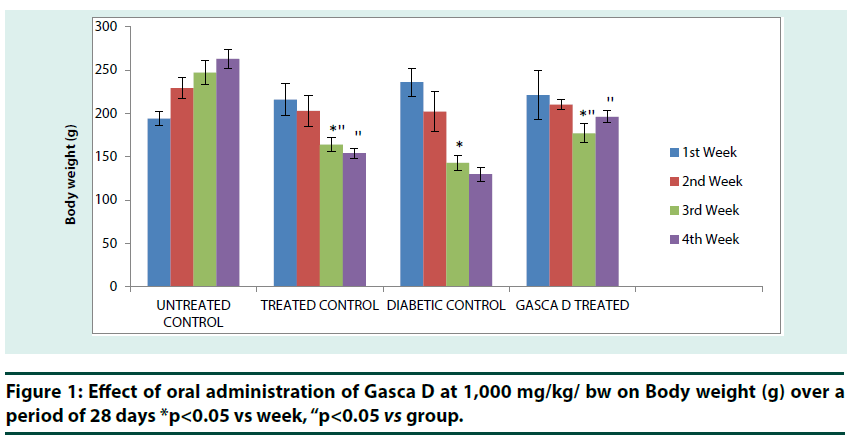
Untreated control group showed a successive increase in body weight from 1st to 4th week. A reverse is the case for (treated control) group that received Gasca D (1,000 mg/kg b.w.) for 4 weeks, the group showed a successive decrease in body weight from 1st to 4th week of treatment, the reduction was significant (p<0.05) from 2nd to 3rd week and the reduction was significant (p<0.05) between (treated control) group in 3rd and 4th week when compared with untreated control (Figure 1). Diabetic control group also showed a successive decrease in body weight from 1st to 4th week with a significant (p<0.05) reduction in body weight from 2nd to 3rd week. However, diabetic rats that received 1,000 mg/ kg b.w dose of Gasca D also showed a significant (p<0.05) reduction in body weight from 2nd to 3rd week, but the body weight increased by the 4th week. Moreover, the results showed that Gasca D treated diabetic rats have significant (p<0.01) increased body weight in 3rd and 4th week when compared with diabetic control (Figure 1).
*p<0.05 vs week, “p<0.05 vs group.
Figure 1: Effect of oral administration of Gasca D at 1,000 mg/kg/ bw on Body weight (g) over a period of 28 days
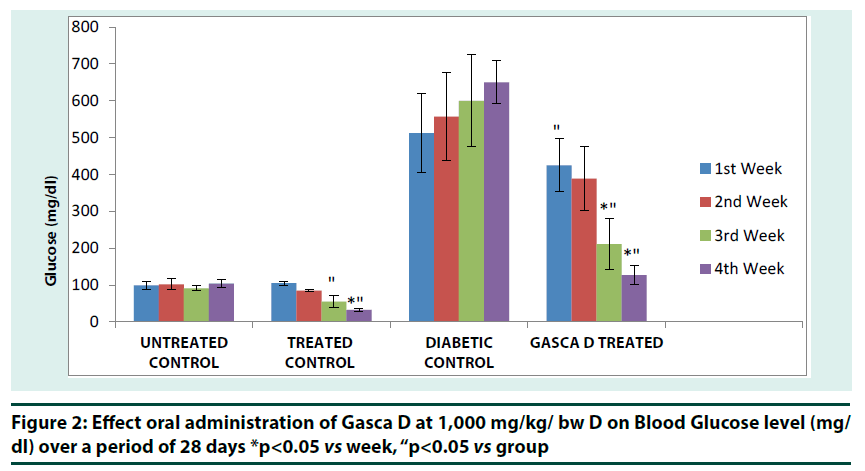
Figure 2
*p<0.05 vs week, “p<0.05 vs group
Figure 2: Effect oral administration of Gasca D at 1,000 mg/kg/ bw D on Blood Glucose level (mg/ dl) over a period of 28 days
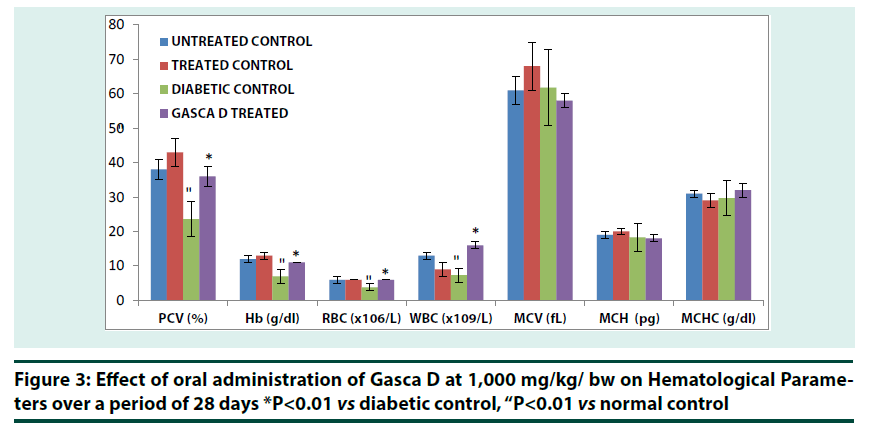
Changes in blood glucose concentration was significant (p<0.01) in treated control group at the 4th week when compared with 1st and 2nd week of treatment. Reduction in blood glucose concentration was also significant (p<0.01) in treated control group at 3rd and 4th week when compared with untreated control (Figure 2). Diabetic group that received Gasca D at 1,000 mg/kg bw showed a significant (p<0.01) reduction in blood glucose concentration at 3rd and 4th week respectively. A significant (p<0.01) reduction was observed in Gasca D treated group when compared with diabetic control group (Figure 2).Gasca D treated diabetic group exhibited significant (��<0.01) increase in PCV, Hb, RBC and WBC compared to Diabetic control (Figure 3). The data showed that diabetes treatment in rats with Gasca D caused a significant increase in PCV, Hb, RBC and WBC counts as compared to diabetic control group. The hematological parameters in normal+Gasca D treated group were similar to untreated control (Figure 3).
*P<0.01 vs diabetic control, “P<0.01 vs normal control
Figure 3: Effect of oral administration of Gasca D at 1,000 mg/kg/ bw on Hematological Parameters over a period of 28 days
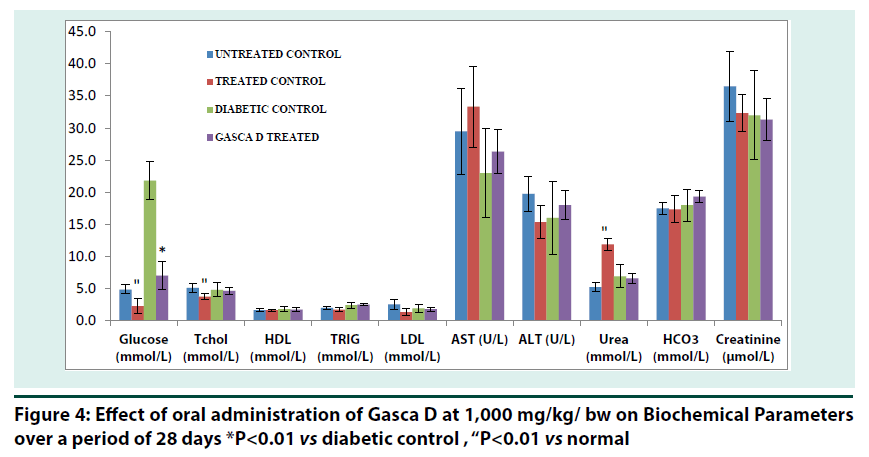
It can be noticed that the changes observed in the two enzyme activities, AST and ALT, are not consistent to indicate hepatic damage, except perhaps for the elevated AST levels in rats, no significant increase was observed in and/ or across the groups (Figure 4). Urea was found to be significantly increased (p<0.01) in treated control compared to normal. In the same vein total cholesterol and glucose were found to significantly (p<0.01) decreased in treated control compared to normal. Blood glucose level was found to decrease significantly (p<0.01) compared to diabetic control (Figure 4).
*P<0.01 vs diabetic control , “P<0.01 vs normal
Figure 4: Effect of oral administration of Gasca D at 1,000 mg/kg/ bw on Biochemical Parameters over a period of 28 days
Electrolytes level did not cause any significant (p<0.01) change in Gasca D treated compared to diabetic control (Table 2). Potassium level decreased significantly (p<0.01) in treated control compared to untreated control (Table 2).
| Na+ (mmol/L) |
K+ (mmol/L) |
Cl- (mmol/L) |
|
|---|---|---|---|
| UNTREATED CONTROL | 141 + 1.2 | *5 + 0.1 | 104.8 + 2.5 |
| TREATED CONTROL | 141.3 + 0.5 | 3.3 + 0.2 | 105.7 + 1.7 |
| DIABETIC CONTROL | 138 + 2 | 5.89 + 0.9 | 98 + 6 |
| GASCA D TREATED | 135 + 2.9 | 6 + 0.0 | 96.7 + 0.9 |
P<0.01 vs normal
Table 2: Effect oral administration Gasca D at 1,000 mg/kg/ bw on Electrolytes Levels over a period of 28 days
Discussion
The reduction in body weight observed in the present study in both normal and diabetic rats that received Gasca D herbal formulation is a good indication of the formulation’s ability to manage diabetic condition. Many studies have reported weight reduction as an essential factor in lifestyle modification strategies for the management of diabetic condition. The weight reduction potential of Gasca D herbal formulation normal rats that received Gasca D may not come as surprise considering the fact that one of the ingredients has been reported in several studies to lower blood cholesterol levels. Gum acacia (GA) a dried exudate obtained from the stems of A. senegal [34], assumed to be an ‘‘inert” substance, has been reported to reduce total serum cholesterol by 6% and 10.4%, respectively when subjects received 25 g/day and 30 g/day of GA for periods of 21 and 30 days [35,36]. It has also been reported that regular intake of 30 gm /day GA for six weeks resulted in significant reduction in BMI and body fat percentage (P<0.0001) [37]. Gum Arabic consumption seems to be an effective dietary strategy to prevent or treat overweight with its several biological mechanisms [38]. There is a significant (p<0.05) improvement in body weights of diabetic rats that received Gasca D herbal formulation in 3rd and 4th week of treatment, indicating a positive response towards good outcome. GA may also play a role in glucose lowering potential of Gasca D herbal formulation. It has been reported that supplementation of GA in different forms to the normal or diabetic or fed-high fat diet rat/mice significantly reduced blood glucose levels [24,25]. The present study showed a significant (p<0.05) reduction in blood glucose concentration in both normal and diabetic rats at 3rd and 4th week of treatment with 1,000 mg/ kg bw Gasca D herbal formulation for 28 days, when compared with normal and diabetic control. There is also a progressive decrease in glucose concentration in Gasca D treated groups from 1st through the 4th week of treatment (Figure 2). Another study has shown that GA inhibits intestinal glucose absorption via interaction with membrane abundance of SGLT1 in mice [38]. Adansonia digitata another ingredient for Gasca D herbal formulation has been reported to lower blood glucose concentration in Streptozocin-Induced Diabetic Wistar Rats [39]. Methanolic fruit pulp extract of Adansonia digitata has been found to reduce blood glucose level at a dose of 300 mg/kg in Alloxan induced diabetic rats [40]. The glucose lowering potential of Gasca D herbal formulation seen in the present study might be the sum of all the effects of the aforementioned ingredients used in the formulation. Administration of Gasca D herbal formulation was associated with changes in both the hematological and biochemical parameters. The results obtained in this study showed significant increase (P<0.05) in PCV, Hb, RBC and WBC in diabetic rats treated with Gasca D herbal formulation at 1,000 mg/kg bw for 28 days. Diabetes mellitus (DM) is a part of metabolic syndrome that comprises dyslipidemia, hypertension, and impaired hematological indices [41]. Several hematological changes affecting the red blood cells (RBCs), white blood cells (WBCs), and the coagulation factors are shown to be directly associated with diabetes mellitus [42]. RBCs, WBCs, and platelet dysfunctions have also been reported to occur in diabetic patients [42,43]. It has been reported that, many patients with diabetes present with reduced hemoglobin (Hb) levels [44]. Another study from National Evaluation of the Frequency of Renal impairment co-existing with Non-insulin dependent diabetes mellitus (NEFRON), showed that the hemoglobin levels of one in seven (14%) patients in general practice were found to be below the normal range [45]. The present study is in support of the previous studies that showed Hb level were found to be lower in diabetic patients. Figure 3 showed the Hb level in the present study to be significantly (p<0.01) lower in diabetic control group when compared with untreated control. Several studies have shown that anemia is a common finding in people with diabetes. Thomas [44] found anemia to be more prevalent and earlier in patients with diabetes than in those with kidney disease from other causes. It is also a common accompaniment to diabetes, particularly in patients with albuminuria or reduced renal function [46]. Third National Health and Nutrition Examination Survey (NHANES-III) showed that diabetic individuals in the general population are nearly twice as likely to have anemia as people without diabetes, but with a similar degree of kidney function impairment [47]. The present study showed the level of PCV to be significantly (p<0.01) low in diabetic control group when compared with untreated control, confirming previous studies. Anemia has been associated with so many complications in diabetes such as increased risk of background and proliferative retinopathy [48,49], and also a potent risk factor for lower extremity amputation [50]. Treatment of diabetic rats with Gasca D herbal formulation was found to significantly (p<0.01) increased the level of both PCV and Hb level, showing improved treatment outcome of the formulation. Hematological indices are important indicators for the evaluation of variations in size, number, and maturity of different blood cells. Research evidences suggest that hematological indices are altered in patients with T2DM [51]. They are important for the assessment and management of patients with diabetes, especially when it comes to the measurement of glycated hemoglobin (HbA1c). It has been reported that variations in RBC lifespan of this magnitude are sufficient to cause considerable alterations in HbA1c [52]. A study of 40 healthy volunteers reported a mean RBC survival of 123 days, with a standard deviation of 23 days [53]. Diabetes mellitus and hyperglycemia have several additional effects on RBCs [54], besides formation of HbA1c. DM is associated with reduced deformability and changes in mechanical properties of the RBCs [54,55], increased adhesion [56] and increased osmotic fragility [57]. In patients with DM, persistent hyperglycemia exposes RBCs to elevated glucose concentrations, thus resulting in glycation of hemoglobin, prothrombin, fibrinogen, and other proteins involved in clotting mechanisms [58]. Furthermore, some studies have reported that the average lifespan of RBCs is reduced in individuals with DM [59,60]. The present study showed a significant (p<0.01) reduction in the level of RBC in diabetic control group when compared with untreated control (Figure 3) clearly confirming the findings of the previous studies. But diabetic group treated with Gasca D herbal formulation showed a significant (p<0.01) increase in RBC levels, showing a RBC formation enhancing effect of the formulation suggesting a good improvement of RBC values. The present study showed WBC level increased significantly in the diabetic group treated with Gasca D herbal formulation when compared with the diabetic control group. The reason for this variation might be due to the fact that the high WBC count in the diabetes treated group is in keeping with the increased oxidative stress triggered by the high levels of hyperglycemia. Thus, polymorphonuclear and mononuclear WBCs can be activated by AGEs and cytokines in a state of hyperglycemia [61]. Hyperlipidemia is a relatively common problem in patients with poorly controlled diabetes mellitus. In the present study an attempt has been made to study the effect of Gasca D herbal formulation on lipid profile in Alloxan induced diabetic rats. It can be seen that total serum cholesterol level in normal positive control rats that received Gasca D herbal formulation was significantly lower than untreated control. However, serum triglyceride, HDL and LDL level did not show any significant in both the normal positive control and Gasca D treated group following Gasca D herbal treatment. The study also investigated the effect of Gasca D herbal formulation on AST and ALT levels. There is no significant change in AST and ALT levels in serum of both normal positive and diabetic rats that received Gasca D herbal formulation when compared with normal and diabetic control, indicating non- hepatotoxic effects of Gasca D herbal formulation. It is interesting to note that no significant change was observed in the level of kidney function parameters (urea, creatinine, bicarbonate and electrolytes) of normal positive and diabetic rats that received Gasca D herbal formulation when compared with normal and diabetic control. This is a good indication that the formulation does not pose significant danger to kidney. A significant increase in urea and potassium level was observed in normal positive control rats that received Gasca D herbal formulation when compared to untreated control.
Conclusion
The results of the present study showed that Gasca D herbal formulation is practically nontoxic herbal drug category and can thus be used to manage weight, hypoglycemia, anemia and antibody function. The study also demonstrated that Gasca D herbal formulation did not pose significant health risk to liver, kidney and electrolyte. The beneficial effect of Gasca D herbal formulation is attributed to the ingredients used in the formulation which has hypoglycemic and weight reduction potential. Further studies are obviously needed to isolate, characterise and elucidate the active components in principle in the formulation responsible for its hypoglycemic, weight reduction and improvement of some hematological parameters.
Disclosure
The authors report no conflicts of interest in this work.
References
- http://apps.who.int/medicinedocs/documents/s7148e/s7148e.pdf
- Jon C, Ted J. Herbal medicine research and global health: an ethical analysis. Bull. World. Health. Organ. 86(8), 594-599 (2018).
- Willcox M, Bodeker G. Traditional herbal medicines for malaria. BMJ. 329(7475), 1156–1159 (2004).
- Bandaranayake W. Quality control, screening, toxicity and regulation of herbal drugs, in Modern Phytomedicine. Turning Medicinal Plants into Drugs, (2006).
- Foster DF, Phillips RS, Hamel MB, et al. Alternative medicine useinolderAmericans. J. Am.Geriatr.Soc. 2000; 48, 1560–1565.
- http://apps.who.int/medicinedocs/en/d/Js2297e/
- http://apps.who.int/iris/bitstream/handle/10665/43229/9241593237.pdf?sequence=1&isAllowed=y
- http://www.diabetesatlas.org/
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 352(9131), 837–853 (1998).
- Holman R, Paul S, Bethel M et al. A 10‑year follow‑up of intensive glucose control in type 2 diabetes. N. Engl. J. Med. 359(15), 1577–1589 (2008).
- Cooper E. Drug discovery, CAM and natural products. Evid. Based. Complement. Altern. Med. 1(3), 215–217 (2004).
- Tsao J, Zeltzer L. Complementary and alternative medicine approaches for pediatric pain: a review of the state-of-the-science. Evid. Based. Complement. Altern. Med. 2(4), 149–59 (2005).
- Bashar S, Hassan A and Omar S. Tradition and Perspectives of Arab Herbal Medicine. Evid. Based. Complement. Alternat. Med. 2(4), 475–479 (2005).
- Krentz A and Bailey C. Oral antidiabetic agents: current role in type 2 diabetes mellitus. Drugs. 65(3), 385–411(2005).
- Bailey C and Turner R. Metformin. N. Engl. J. Med. 334 (9), 574–579 (1996) .
- FAO/WHO Traditional food plants. FAO Food and Nutrition paper 42,(1988).
- Igboeli L, Addy E, Salami L. Effects of some processing techniques on the antinutrient contents of baobab seeds (Adansonia digitata). Biores. Technol. 59, 29–31 (1997).
- Gebauer J, El-Siddig K, Ebert G. Baobab (Adansonia digitata L.): A review on a multipurpose tree with promising future in the Sudan. Gartenbauwissenschaft, 67(4), 155–160 (2002).
- Diop A, Sakho M, Dornier M et al. Le baobab africain (Adansonia digitata L.): principales caractéristiques et utilisations. Fruits. 61(1), 55–69 (2005).
- Phillips G, Williams P. Tree exudate gums: natural and versatile food additives and ingredients. Food. Ingred. Anal. Int. 23,26–28 (2001).
- Glicksman M, Line Back D, Ingett J et al. Gum and stabilizers for the food industry, Vol 2. Tergamon Press, Oxford.
- Chikamai B, Casadei E, Coppen J et al. A review of production, markets and quality control of gum Arabic in Africa. FAO. 191 (1996).
- Rodge A, Sonkamble S, Salve R et al. Effect of hydrocolloid (guar gum) incorporation on the quality characteristics of bread. J. Food. Process. Technol. 3(2),136 (2012).
- Schneeman B. Dietary fiber: comments on interpreting recent research. J. Am. Diet. Assoc. 87,1163 (1987).
- Ahmed A, Fedail J, Musa H. Gum Arabic extracts protect against hepatic oxidative stress in alloxan induced diabetes in rats. Pathophysiology. 22 (4), 189–194 (2015).
- Babiker R, Merghani T, Elmusharaf K et al. Effects of gum Arabic ingestion on body mass index and body fat percentage in healthy adult females: two-arm randomized, placebo controlled, double-blind trial. Nutr. J. 11,111 (2012).
- Abdel-Rahim E, El-Beltagi H, Fayed A. Comparative studies on the influences of Juniperus phoenicea and Hyphaena thebaica as hypoglycemic factors in diabetic rats. J. Advanc. Food. Scien. 33(3), 128–132 (2011).
- Hsu B, Coupar I, Ng K. Antioxidant activity of hot water extract from the fruit of the Doum palm, Hyphaene thebaica. Food. Chem. 98, 317–328 (2006).
- Irobi O, Adedayo O. Antifungal activity of aqueous extract of dormant fruits of Hyphaene thebaica (Palmae). Pharmaceut. Biol. 37(2),114–117 (1999).
- Elegami A, Almagboul A, Omer M et al. Sudanese plants used in folkloric medicine: screening for antibacterial activity. Part X. Fitoterapia. 72(7), 810–817 (2001).
- Eltayeb E, Abdalla A, Eltohami M. Anti-inflammatory study and phytochemical analysis of Hyphaene thebaica (Doum plant). Planta. Med. 75(09), 1076 (2009).
- Prince P, Menon V, Pari L. Hypoglycemic activities of Syzigium cumini seeds; effect on lipid peroxidation in alloxan diabetic rats. J. Ethnopharmacol. 61(1),1–7 (1998).
- Parasuraman S, Raveendran R, Kesavan R. Blood sample collection in small laboratory animals. J. Pharmacol. Pharmacother. 1(2), 87–93 (2010).
- FAO (Rome). Gum Arabic. Food and Nutrition Paper, No. 52, (1999).
- Ross A, Eastwood M, Brydon W et al. A study of the effects of dietary gum arabic in humans. Am. J. Clin. Nutr. 37(3), 368–375 (1983).
- Sharma R. Hypocholesterolaemic effect of gum acacia in men. Nutr. Res. 1321–1326 (1985).
- Chandalia M, Garg A, Lutjohann D, et al. Beneficial effects of high dietary fiber intake in patients with type 2 diabetes mellitus. N. Engl. J. Med. 342(19),1392–1398 (2000).
- Nasir O, Artunc F, Wang K et al. Down regulation of mouse intestinal Na(+)-coupled glucose transporter SGLT1 by Gum Arabic (Acacia Senegal). Cell Physiol Biochem 25(2–3), 203–210 (2010).
- Tanko Y, Yerima M, Mahdi M et al. Hypoglycemic activity of methanolic stem bark of Adansonnia digitata extract on blood glucose levels of streptozocin-induced diabetic Wistar rats. Int. J. Appl. Res. Nat. Prod. 1(2), 32–36 (2008).
- Mohammad Y and Yakubu H. Hypoglycemic Activity of Methanolic Fruit Pulp Extract of Adansonia digitata on Blood Glucose Levels of Alloxan Induced Diabetic Rats. Int. J. Ani.Vete. Adv. 5(3), 108–113 (2013).
- Gundogan K, Bayram F, Capak M. Prevalence of metabolic syndrome in the Mediterranean region of Turkey: evaluation of hypertension, diabetes mellitus, obesity, and dyslipidemia. Metab. Syndr. Relat. Disord. 57 (1),427–434 (2009).
- Mirza S, Hossain M, Mathews C. Type 2-diabetes is associated with elevated levels of TNF-alpha, IL-6 and adiponectin and low levels of leptin in a population of Mexican American: a cross-sectional study. Cytokine. 57(1),136–142 (2012).
- Gkrania-Klotsas E, Ye Z, Cooper A et al. Differential white blood cell count and type 2 diabetes: systematic review and meta-analysis of cross-sectional and prospective studies. PLoS One. 5(10), e13405 (2010).
- Thomas M. Anemia in diabetes: marker or mediator of microvascular disease? Nat. Clinl. Prace. Nephrol. 3(1), 20–30 (2007).
- Thomas M, Weekes A, Broadley O. The burden of chronic kidney disease in Australian patients with type 2 diabetes (the NEFRON study). Med. J. Aust. 185(3), 140–144(2006).
- Thomas M, Macisaac R, Tsalamandris C. Unrecognized Anemia in Patients With Diabetes: A cross-sectional survey. Diabetes. Care. 26(4), 1164–1169 (2003).
- Astor B, Muntner P, Levin A. Association of kidney function with anemia: the Third National Health and Nutrition Examination Survey (1988–1994). Arch. Intern. Med. 162(12), 1401–1408(2002).
- Davis MD. Risk factors for high-risk proliferative diabetic retinopathy and severe visual loss: Early Treatment Diabetic Retinopathy Study Report #18. Invest. Ophthalmol. Vis. Sci. 39(2), 233–252 (1998).
- Qiao Q, Keinanen-Kiukaanniemi S, Laara E. The relationship between hemoglobin levels and diabetic retinopathy. J. Clin. Epidemiol. 50(2), 153–158 (1997).
- Akanji A, Famuyiwa O, Adetuyibi A. Factors influencing the outcome of treatment of foot lesions in Nigerian patients with diabetes mellitus. Q. J. Med. 73( 271), 1005–1014 (1989).
- Dallatu M, Anaja P, Bilbis L. Antioxidant micronutrient potentials in strengthening the antioxidant defense in alloxan-induced diabetic rats. Niger. J. Pharm. Sci. 8(1), 89–94 (2010).
- Cohen R, Franco R, Khera P. Red cell life span heterogeneity in hematologically normal people is sufficient to alter HbA1c. Blood 112(110), 4284–4291 (2008).
- Furne J, Springfield J, Ho S. Simplification of the end-alveolar carbon monoxide technique to assess erythrocyte survival. J. Lab. Clin. Med. 142(1), 52–57 (2003).
- Singh D, Winocour P, Farrington K. Erythropoietic stress and anemia in diabetes mellitus. Nat. Rev. Endocrinol. 5, 204–210 (2009).
- Singh M, Shin S. Changes in erythrocyte aggregation and deformability in diabetes mellitus: a brief review. Indian. J. Exp.Biol. 47(1), 7–15 (2009).
- Grossin N, Wautier M, Wautier J. Red blood cell adhesion in diabetes mellitus is mediated by advanced glycation end product receptor and is modulated by nitric oxide. Biorheology. 46(1), 63–72 (2009).
- Kowluru R, Bitensky M, Kowluru A et al. Reversible sodium pump defect and swelling in the diabetic rat erythrocyte: effects on filterability and implications for microangiopathy. Proc. Natl. Acad. Sci. USA. 86(9), 3327–3331(1989).
- Selvin E, Michael W, Steffes M et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N. Engl. J. Med. 362, 800–811(2010).
- Virtue M, Furne J, Nuttall F et al. Relationship between GHb concentration and erythrocyte survival determined from breath carbon monoxide concentration. Diabetes. Care. 27(4), 931–935 (2004).
- Venerando B, Fiorilli A, Croci G et al. Acidic and neutral sialidase in the erythrocyte membrane of type 2 diabetic patients. Blood. 99 (3), 1064–1070 (2002).
- Chung F, Shin S, Tsai J et al. Peripheral total and differential leucocyte count in diabetic nephropathy. Diabetes. Care. 28 (7), 1710–1717 (2005).