Case Report - Interventional Cardiology (2022) Volume 14, Issue 1
Aortitis, a case report highlighting the need for multidisciplinary and early differential diagnosis
- Corresponding Author:
- Ridah Shaista Shanavas
The Synapse Foundation,
Quthbullapur,
Hyderabad,
TS,
India,
E-mail: ridahsshanavas@gmail.com
Received date: 28-Dec-2021, Manuscript No. FMIC-21-50759; Editor assigned: 29-Dec-2021, PreQC No. FMIC-21-50759 (PQ); Reviewed date: 11-Jan-2022, QC No. FMIC-21-50759; Revised date: 25-Jan-2022, Manuscript No. FMIC-21-50759 (R); Published date: 28-Jan-2022, DOI: 10.37532/1755-5310.2022.14(1).454
Abstract
We present the case of a 28-year-old female patient with a history of Lower Segment Cesarean Section (LSCS) who presented with chest pain, Shortness of Breath (SOB) on exertion, sweating, and uneasiness. She was negative for diabetes, positive for hypertension, and had pedal edema during pregnancy. She had a normal hematology profile except for mild neutrophilic leukocytosis, and Anisopoikilocytosis; Her renal biochemistry and urine pathology profile were normal. The 2D echocardiogram and the doppler study indicated that she had Rheumatic valvular heart disease. The left atrium and ventricle were dilated as expected along with valve issues. Severe Aortic Regurgitation (AR) without Aortic Stenosis (AS) was noted. Mild Mitral Regurgitation (MR) without mitral stenosis was noted. Severe Tricuspid Regurgitation (TR), severe Pulmonary Arterial Hypertension (PAH), tricuspid annulus (2.6 cm), with adequate biventricular function was noted without pericardial effusion. She was advised aortic valve replacement and mitral valve repair or replacement with tricuspid valve repair with ring surgery. However, ring surgery had to be abandoned, after opening the chest, due to severe thickening and inflammation which was seen across the entire aorta. Subsequent Computed Tomography (CT) aortogram findings confirmed aortitis. Aortitis is difficult to diagnose and is usually discovered accidentally and at a much later stage resulting in a poor prognosis. Overall there is no specific preference for age or gender but certain types e.g. Takayasu Arteritis (TA) affects Asian women of reproductive age more commonly. This case and our subsequent research highlight the need to include aortitis as one of the critical complications in high-risk pregnancies especially in the Asian population. Additionally, we believe low pulse or pulseless hypertension should trigger an interdisciplinary holistic patient management for a better prognosis of young female patients.
Keywords
Aortitis • Pregnancy • Hypertension • Aortogram • Valvular disease • Inflammation
Introduction
Aortitis is the term used to describe the inflammation of the aorta, the largest artery in the body. This disorder generally presents in older individuals with pre-existing aortic pathology, such as abdominal aortic aneurysm or atherosclerosis of the abdominal aorta.
Aortitis can result from a variety of causes but can be divided into two main categories: Infectious aortitis, and non-infectious aortitis [1,2].
Infectious aortitis is caused by infections such as Tuberculosis, Syphilis, and Salmonella [3]. Infections do this by triggering non-infectious vasculitis by generating immune complexes or by cross-reactivity [4]. The main treatment for vasculitis is immunosuppressive therapy, which may, in turn, aggravate an active infectious process, thereby, activating an infectious aortitis [4].
Non-infectious aortitis is caused by immunologic conditions and connective tissue disorders. The most common form, aortitis of rheumatologic disorders, is associated with large-vessel vasculitis, Giant Cell Arteritis (GCA), and TA [1,5]. Other rheumatologic conditions that can lead to aortitis include systemic lupus erythematosus, Rheumatoid Arthritis (RA), the HLA-B27– associated spondyloarthropathies, antineutrophil cytoplasmic antibody-associated vasculitis, Crohn’s disease, Behçet’s disease, Marfan’s disease, Cogan syndrome, and Sarcoidosis [2].
Aortitis may also occur in isolation, accompanying idiopathic retroperitoneal fibrosis or an inflamed abdominal aortic aneurysm, or present due to an injury, including trauma [1,4]. Most cases, however, are found to be idiopathic, i.e., they occur due to an unknown cause. In a study done in Denmark, it was found that nearly 75% of patients had idiopathic aortitis while the rest had aortitis associated with diseases known to cause it [1].
The prevalence of aortitis varies based on multiple factors such as geography, gender, age, and pre-existing conditions. Aortitis is found to be more common in African a1nd Asian countries as compared to the rest of the countries in the world [6]. It affects both men and women, suggesting that gender may not play a big role [2]. Furthermore, it is found to be present across all age groups with most patients belonging to the older age bracket(s) [2,3]. Nevertheless, nothing conclusive can be said about the prevalence of aortitis based on these factors since it has been insufficiently investigated. This is because most of the data available to us are collected from surgical aortic specimens of symptomatic or critically ill patients in whom aortitis has caused potentially lifethreatening complications, such as aortic aneurysm and dissection. Hence, the inferences cannot be applied to the general population.
The diagnosis of aortitis is quite difficult to establish and is in fact, usually found accidentally when looking for an aortic aneurysm or during a post-op examination [7]. Physicians generally rely on a patient’s clinical symptoms such as back and abdominal pain and exclude other medical conditions to suspect aortitis [8].
Aortitis with underlying inflammatory conditions is expected to have GCA whose diagnosis involves biopsy of a temporal artery. Generally, 2-3 biopsies are needed to confirm as GCA is usually spread across and not all locations may be involved. Takayasu arteritis is associated with low blood pressure and pulse. Additionally, brisk pulse in the legs adds confirmatory evidence for this type of aortitis. Magnetic Resonance Imaging (MRI) and Positron Emission Tomography (PET) are the two imaging tests used to diagnose generalized inflammation of the aorta [8]. The involvement of various arteries and the extent of inflammation helps associate it to specific underlying medical conditions such as RA or syphilis. While all the methods stated above, i.e. biopsy, imaging, and/or pulse are considered to assess the presence of aortitis, blood tests are conducted to understand the associated underlying medical condition and its status.
The clinical symptoms of aortitis include constitutional symptoms, such as fever, fatigue, or malaise caused by systemic inflammation and signs of ischemia due to regional involvement and ostial stenosis of the aortic branches or peripheral embolization. Depending on the location of the aortic involvement, the clinical spectrum of aortitis may include transient ischemic attack, stroke, angina pectoris, myocardial infarction, occlusive disease of the renal, splenic, or mesenteric arteries, and peripheral arterial occlusive disease. Vasculitis of large vessels has the highest morbidity and mortality rate. GCA-associated aortitis is more likely to develop thoracic aneurysms and dissections; this type of aortitis also has a higher mortality rate. Based on the general health of the patients and prognosis of associated medical conditions, the 10-15 year survival rate is more than 60%. Ferfar, et al. have shown that the 5-year event-free survival for patients with GCA, TA, and Isolated Arteritis (IA) was 73%, 67%, and 38%, respectively indicating that management of isolated arteritis is more challenging [9].
The majority of the patients are placed on long-term corticosteroid therapy; some patients with extensive inflammation are additionally treated with immunosuppressive agents such as cyclophosphamide, methotrexate, or mycophenolate mofetil. This helps stop the progression of the disease and prevents the reactivation of the inflammatory cascade. Additionally, patients will need symptomatic management as well as therapies for the underlying medical condition [10].
Case Presentation
A 28-year-old female patient with a history of LSCS (4 months prior to presenting at the hospital) presented with chest pain, SOB on exertion, sweating, and uneasiness. Basic tests such as ECHO, ECG, and CT were done. She was hypertensive but had a normal pulse and did not show raised blood glucose levels. She had a normal hematology profile except for mild neutrophilic leukocytosis, and mild Anisopoikilocytosis. Her renal profile and urine biochemistry were normal and her family history was also non-incidental. High- Resolution Computed Tomography (HRCT) did not show any evidence of pleural effusion but cardiomegaly and pericardial effusion were noted.
The Patient had consulted the cardiology team when she was 8 months pregnant due to SOB. She was negative for diabetes, positive for hypertension, and had pedal edema during pregnancy. She had a normal hematology profile except for mild neutrophilic leukocytosis, and mild Anisopoikilocytosis; Her renal biochemical profile and urine pathology findings were normal.
She was diagnosed with AR and was scheduled for an aortic valve replacement surgery after delivery as AR can be controlled and the team did not want to create complications during delivery.
She continued her treatment under the Gynecologist’s care. However, due to complications, she had to be rushed to the hospital and had to have a Lower Segment Cesarean Section (LSCS) in the 8th month itself. She was seen to have wide pulse pressure potentially due to the AR, and there were complications during her delivery and sadly the fetus was lost. After four months, due to continued shortness of breath, she came back to the Cardiology team as a follow-up.
The 2D echocardiogram and the doppler study indicated that she had rheumatic valvular heart disease. The left atrium and ventricle were dilated as expected along with valve issues. Severe AR without AS was noted. Mild MR without mitral stenosis was noted. Severe TR, severe PAH, tricuspid annulus (2.6) with adequate biventricular function was noted without Pericardial effusion. Based on the constellation of findings as shown in Table 1, she was advised to undergo aortic valve replacement and mitral valve repair or replacement with tricuspid valve repair with ring surgery.
| High-Resolution Computed Tomography (HRCT) | |
|---|---|
| Aortic annulus | 1.9 cm |
| Mitral annulus | 2.5 cm |
| Tricuspid annulus | 2.6 cm |
| Left ventricle | Normal |
| Right ventricle | Dysfunction |
| Pericardial effusion | Mild |
| Echocardiogram | |
| Mitral Valve | A2 Prolapse, PML restricted |
| Aortic Valve | Annulus 2.0 cm, Trileaflet NCC prolapse |
| Pulmonary Valve | Normal |
| Tricuspid Annulus | 2.8 cm, ATL Tethered STL restricted |
| Right Atrium (RA) | RA base to apex: 6.2 cm |
| RA area: 22 cm/seq | |
| Right Ventricle (RV) | Basal RV 3.9cm |
| Mid RV 3.6 | |
| RV Diastolic 24 sq. cm | |
| RV Systolic 13 | |
| Left Atrium | 4.1 cm |
| Left Ventricle | Adequate Function |
| Computed Tomography (CT) Aortogram | |
| Aortic Annulus | 22 mm |
| Sinus of Valsalva | 34 mm |
| Sinotubular junction | 27 mm |
| Tubular ascending aorta | 27 mm |
| Arch of aorta | 24 mm |
| Descending thoracic aorta | 20 mm |
Note: A2 in echocardiogram refers to central portion and abbreviations include; PML: Posterior Mitral Leaflet; NCC: Noncoronary Cusps; ATL: Anterior Tricuspid Leaflet: STL: Septal Tricuspid Leaflet; cm: centimeter; sq. cm: square centimeter; seq: sequence; mm: millimeter
Table 1: Findings from different imaging studies.
After general anesthesia, the chest was opened for the surgical procedure. However, upon opening the chest, the aorta was found to be thickened and inflamed across the entire length. With possibly reduced lumen, even cannulation was difficult. Aortitis was suspected and the surgical procedure was abandoned. She was referred for primary cardiology care and to a rheumatologist. The patient was advised to undergo Magnetic Resonance (MR) angiography or a Fluorodeoxyglucose (FDG)-Positron Emission Tomography (PET) or CT aortogram. Though she reported to not have any joint pain earlier, she was put on anti-inflammatory and Disease-Modifying Antirheumatic Drugs (DMARDs). The patient returned for a follow-up after being on Azovas 16 mg, Pulmonext 10 mg, and Wysolml 10 mg along with Pan-D 40 mg for 3 months.
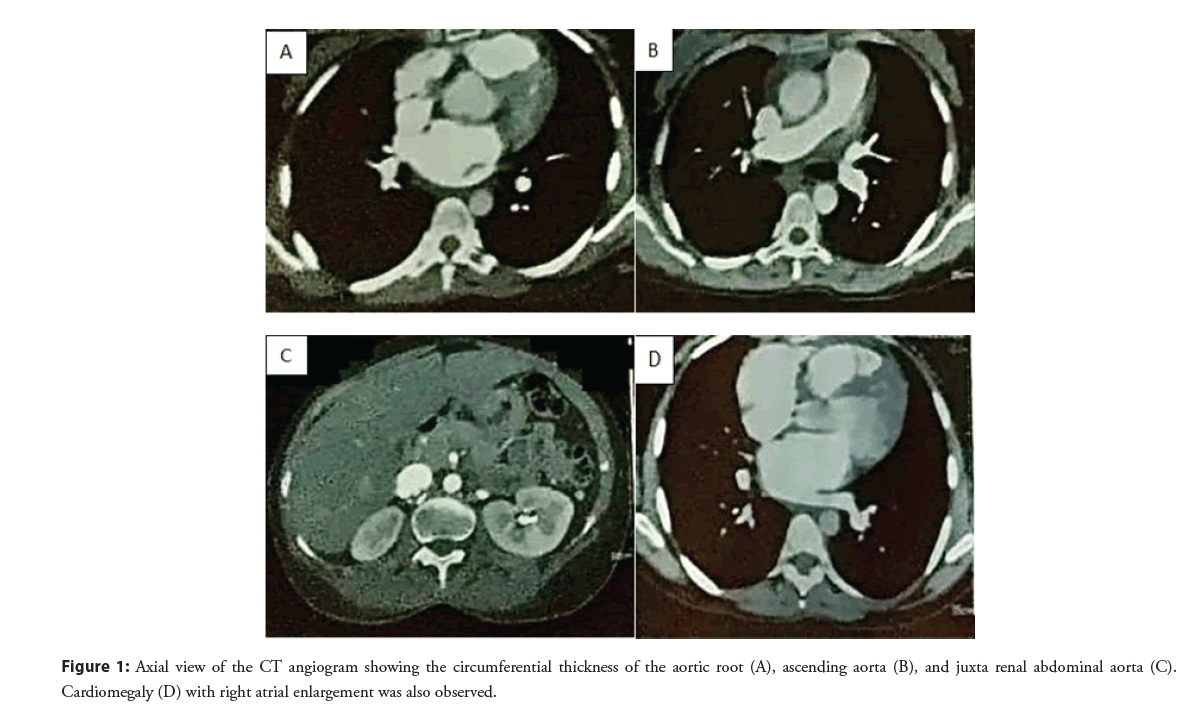
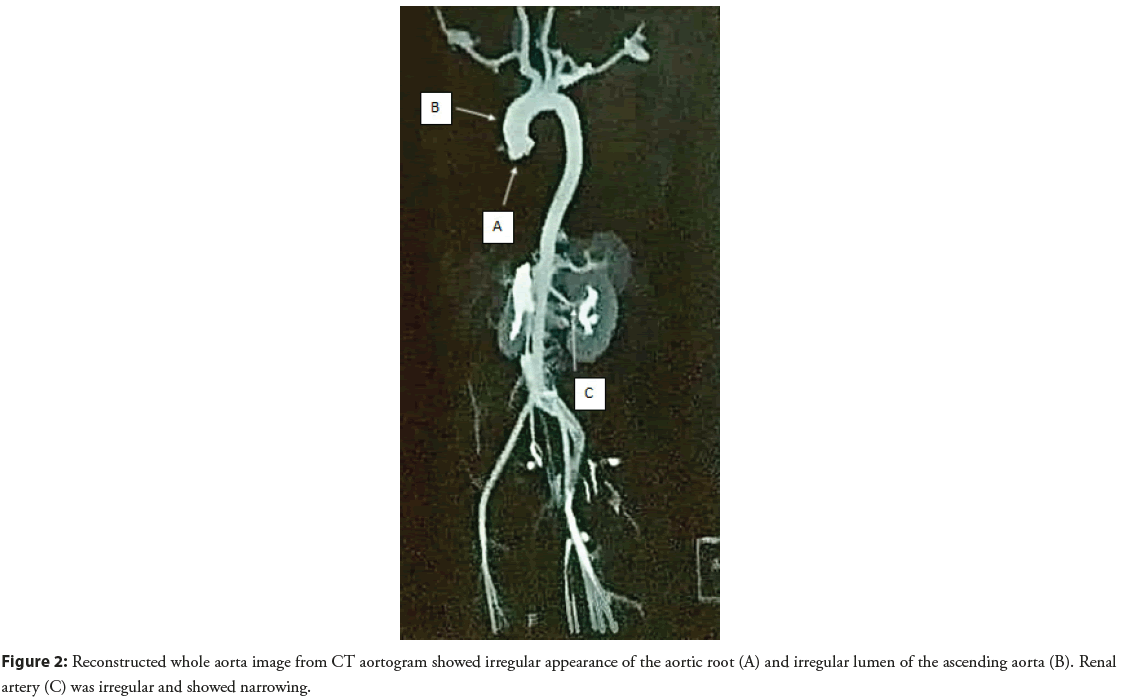
Chest X-ray showed severe cardiomegaly. The subsequent doppler study showed altered results primarily due to underlying cardiogenic causes. CT aortogram (Figure 1) showed a circumferential thickness of the aortic root and ascending aorta; there was no wall enhancement or luminal narrowing. No dissection was seen in the whole of the aorta. The circumferential thickness of Juxtarenal abdominal aorta and proximal renal arteries was noted with 60%- 70% narrowing of the proximal branch of the renal artery (Figure 2). Cardiomegaly with right atrial enlargement was seen. Iliac arteries was normal. There was a focal mild narrowing at the origin of the right coronary. The patient was diagnosed with aortitis with severe AR/MR and PAH and is placed under Rheumatologists care.
Figure 1: Axial view of the CT angiogram showing the circumferential thickness of the aortic root (A), ascending aorta (B), and juxta renal abdominal aorta (C). Cardiomegaly (D) with right atrial enlargement was also observed.
Specifically, TA could be considered as the final diagnosis as the patient was female, Asian and middle-aged, all the risk factors published for TA in literature.
When following up with the patient, she was found to be in adequate condition and following her normal routine. She is asymptomatic. Her LV function is, also, improving. She is continuing to be on steroids to decrease the inflammatory process. Her inflammation per hs CRP and blood picture is being followed routinely.
Results and Discussion
The case presented here was that of a female patient in her late twenties and one who had a LSCS just four months before her first visit to a cardiologist. The patient did not recollect any symptoms associated with infections known to cause aortitis. Additionally, her symptoms included chest pain, shortness of breath, and fatigue. Accordingly, physicians’ focused on PAH and valvular disease states, both of which are inflammatory disease symptoms. Additionally, the blood tests were normal.
Subsequent echocardiogram, doppler study, and eventually CT aortogram confirmed the inflammation of the aorta and other arteries, especially the renal arteries. The extent of wall thickening and the broader arterial involvement was indicative of the inflammatory cause.
The time taken and core focus given to her cardiology complications including the opening of the chest for the valve repair highlights the gap in the current diagnosis and management of this disease even when cardiologists are involved. It draws our attention to the need for more interdisciplinary evaluation especially in cases such as these where such a drastic progression of her medical state from gestational pedal edema and hypertension to postnatal PAH is seen.
It has been shown that the physiologic hemodynamics of pregnancy (increased cardiac output, blood volume, and heart rate) and hormonal changes, in conjunction with multiple heritable aortopathies, significantly increase the chances of aortic aneurysms and dissections during pregnancy [11]. In this case, even though the patient did not have any aneurysms or dissection, the severity of the valvular heart disease is indicative of potentially accelerated deterioration with pregnancy. Additionally, pregnancy has been specifically seen to be conducive to TA [12].
Hypertension is fairly common due to reduction in elasticity and narrowing of the arteries, besides abnormalities in the functioning of the aortic and carotid baroreceptors; it should be prudently contained during pregnancy, as severe renovascular hypertension, cardiac involvement, or pulmonary hypertension is associated with poor feto-maternal prognosis [13]. Blood pressure in such patients should be also measured in the lower extremity to pick up blood pressure discrepancies. Besides, pulselessness of unilateral or bilateral radial arteries and vascular bruit should be seen in all cases of hypertension [14].
Additionally, chest pain in pregnant or post-natal women should be taken seriously. Though heightened cardiology complications have been documented, an assumed higher risk to fetus or mother deters many physicians from taking up differential diagnoses. Valvular stenosis or regurgitation, congenital heart defects (atrial or ventricular septal defects), and aortic root disease can be diagnosed using echocardiography. Exercise stress tests are not considered safe, but a 12 lead ECG is normally taken in pregnant women with chest pain and hence, in such cases, the reading should be carefully correlated with clinical symptoms, by the treating physician, against the backdrop of detailed medical and family history [15].
TA is observed more frequently in patients of Asian or Indian descent and is more common in women than in men (1.6:1) with a mean age of onset of approximately 30 years. The survival rates are poor-69% and 36% respectively after 5 and 10 years if two complications are involved. However with one or no complications the survival rates are close to 100%. Hence it’s crucial that in spite of the absence of specific direct measures, red flags as summarized by Nazareth and Mason are watched out even as the community understands and creates awareness and guidelines to diagnose and manage this condition [16].
Although the European Society for Cardiology (ESC) and the National Institution for Health and Care Excellence (NICE) has laid out the protocol for management of cardiovascular complications during pregnancy, aortitis is not recognized as an important medical condition in these protocols [17,18]. TA is a rare form of aortitis but is mostly seen in women of Asian descent especially during their childbearing age. Hence, careful follow-up of common clinical symptoms such as hypertension and chest pain, throughout the gestational period, at least, will be critical to prevent the progression of TA. Ideally, the increased risk of aneurysms and dissections in patients prone to aortitis requires correction of cardiovascular complications prior to pregnancy; however, knowing that many of these have delayed diagnosis, an interdisciplinary collaboration of obstetricians, cardiologists and rheumatologists can improve maternal and fetal prognosis. We propose that a low pulse or pulseless hypertension should be treated as a trigger for such interventions.
Conclusion
CT aortogram helped us reach the final conclusion though AR and absence of pulse delayed the entire diagnosis. Aortitis is a rare medical condition caused due to infectious, inflammatory, or idiopathic causes. Though debilitating, with a high morbidity and mortality rate, the condition is usually ignored or has delayed diagnosis and is usually found accidentally during cardiovascular checks. In certain cases such as pregnancy, the risk of complications is much higher and hence we propose that hypertension with low or no pulse should be taken seriously. Pedal edema, PAH, and any cardiology-associated symptoms such as chest pain should be followed through carefully not just in peri but also in the postnatal phase. The physiological changes and their implications on individuals prone to such asymptomatic but highly morbid conditions highlight the need of involving a multidisciplinary team of cardiologists, rheumatologists, and gynecologists during both the peri and postpartum phases for better prognosis.
References
- Gornik HL, Creager MA. Aortitis. Circulation. 117(23): 3039-3051 (2008).
[CrossRef] [Google Scholar] (All versions)
- Aortitis.Wikipedia, the Free Encyclopedia.
- Bossone E, Pluchinotta FR, Andreas M, et al. Aortitis. Vasc Pharmacol. 80: 1-10 (2016).
[CrossRef] [Google Scholar] (All versions)
- Aortitis. Medscape. (2021).
- Kinare SG. Aortitis in early life in India and its association with tuberculosis. J Pathol. 100(1): 69-76 (1970).
[CrossRef] [Google Scholar] [PubMed]
- Tyagi S, Safal S, Tyagi D, et al. Aortitis and aortic aneurysm in systemic vasculitis. Indian J Thorac Cardiovasc Surg. 35(2): 47-56 (2019).
[CrossRef] [Google Scholar] (All versions) [PubMed]
- Scheel AK, Meller J, Vosshenrich R, et al. Diagnosis and follow up of aortitis in the elderly. Ann Rheumat Dis. 63(11): 1507-1510 (2004).
[CrossRef] [Google Scholar] [PubMed]
- Plowman RS, Javidan-Nejad C, Raptis CA, et al. Imaging of pregnancy-related vascular complications. Radiographics. 37(4): 1270-1289 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Ferfar Y, Morinet S, Espitia O, et al. Long-term outcome and prognosis factors of isolated aortitis. Circulation. 142(1): 92-94 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Shchetynska-Marinova T, Amendt K, Sadick M, et al. Aortitis: An interdisciplinary challenge. In Vivo. 35(1): 41-52 (2021). [CrossRef] [Google Scholar] (All versions)
- Smok DA. Aortopathy in pregnancy. Semin Perinatol. 38(5): 295-303 (2014).
[CrossRef] [Google Scholar] (All versions) [PubMed]
- Matsumura A, Moriwaki R, Numano F. Pregnancy in Takayasu arteritis from the view of internal medicine. Heart Vessels. 7: 120-124 (1992).
[CrossRef] [Google Scholar] [PubMed]
- Lindheimer MD, Taler SJ, Cunningham FG, et al. ASH position paper: Hypertension in pregnancy. J Clin Hypertens (Greenwich). 11(4): 214-225 (2009).
[CrossRef] [Google Scholar] [PubMed]
- Hahn D, Thomson PD, Kala U, et al. A review of Takayasu's arteritis in children in gauteng, South Africa. Pediatr Nephrol. 12(8): 668-675 (1998).
[CrossRef] [Google Scholar] [PubMed]
- European Society of Gynecology (ESG), Association for European Paediatric Cardiology (AEPC), German Society for Gender Medicine (DGesGM), et al. ESC Guidelines on the management of cardiovascular diseases during pregnancy: The Task force on the management of cardiovascular diseases during pregnancy of the European Society of Cardiology (ESC). Eur Heart J. 32(24): 3147-3197 (2011).
- Nazareth R, Mason JC. Takayasu arteritis: Severe consequences of delayed diagnosis. QJM. 104(9): 797-800 (2011).
[CrossRef] [Google Scholar] [PubMed]
- Overview: Intrapartum care for women with existing medical conditions or obstetric complications and their babies: Guidance. (2019).
- Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, et al. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy: The task force for the management of cardiovascular diseases during pregnancy of the European Society of Cardiology (ESC). Eur Heart J. 39(34): 3165-3241 (2018).
[CrossRef]