Case Report - Interventional Cardiology (2020) Volume 12, Issue 5
Aortobronchial fistula postcoronary angiography, first reported case in the literature
- Corresponding Author:
- Mohammed Alageel
King Khalid University Hospital
Emergency Medicine, Riyadh, Saudi Arabia
E-mail: moe.alageel@gmail.com
Received Date: July 08, 2020, Accepted Date: July 31, 2020, Published Date: August 07, 2020
Abstract
Percutaneous coronary intervention is a common procedure with well documented complications in the medical literature. Aortobronchial €stulas are a rare entity that requires vigilance to diagnose and prompt treatment. In this article we report on a previously unrecognized complication of percutaneous coronary intervention an aortic pseudoaneurysm, further complicated by infection of the aneurysmal sac and subsequently causing an aortobronchial €stula.
h3>KeywordsAortobronchial fistula; Mycotic aneurysm; Percutaneous coronary intervention; TEVAR
Introduction
Aortobronchial fistula (ABF) is a communication between the thoracic aorta and the adjacent pulmonary parenchyma or tracheobronchial tree. It is a rare, lifethreatening condition that can develop after thoracic aortic interventions. The most common reported causes of ABF in the literature are aortic aneurysms, pseudoaneurysms, traumatic thoracic aorta injuries, penetrating aortic ulcers, and previous thoracic aortic surgery [1]. The diagnosis should be considered in any patient with minor or major hemoptysis and prior history of thoracic aortic operations [2,3].
Percutaneous Coronary Intervention (PCI) is one of the most common medical procedures, with a 140,905 PCIs performed across Canada by 30 cardiac care centers (excluding Quebec) from fiscal years 2013–2014 to 2015–2016 [4]. The most serious complications associated with PCIs include procedural and periprocedural myocardial infarction, stroke, and catheter related vascular complications [5].
To our knowledge, this is the first case of iatrogenic aortic pseudoaneurysm formation post PCI, with subsequent ABF formation due to infection of the of aneurysmal sac ’ ’ mycotic aortic aneurysm’’.
Case Presentation
A 62-year-old woman self-presented to a local tertiary care center emergency department in February 2020. The patient was known to have diabetes, hypertension, and dyslipidemia. In late December 2019, she had undergone therapeutic coronary angiography, for a myocardial infarction, using a femoral insertion site with placement of a single bare metal stent to her right circumflex artery. The procedure was reported to be uneventful and the patient was discharged four days later with a documented ejection fraction of 35%.
The patient reported new onset shortness of breath since the procedure, which was progressive, and required home oxygen to relieve her symptoms. She also reported having new onset chest and back pain, and more recently, the development of hemoptysis and intermittent measured fevers. The patient denied the presence of any other systemic symptoms.
On assessment in the emergency department, the patient was hypoxic on 4 L of oxygen, but not in acute distress. Respiratory auscultation demonstrated crackles in the bases of both lungs. There were no identifiable murmurs. Systemic examination was unremarkable, as was the patient ’ s chest radiography. The initial laboratory investigations were significant for an acute kidney injury, and a drop from the last documented hemoglobin by 30%.
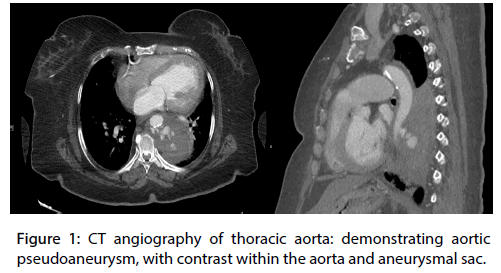
The patient underwent a computed tomography (CT) of the chest with an aortic phase protocol with the primary concern of an aortic dissection. The CT demonstrated a left para-aortic mass with multiple areas of enhancement, suggestive of a pseudoaneurysm and contained rupture (Figure 1).
Figure 1: CT angiography of thoracic aorta: demonstrating aortic pseudoaneurysm, with contrast within the aorta and aneurysmal sac.
Both vascular surgery and cardiothoracic surgery services were asked to evaluate the patient. They agreed that the most likely cause of the patient’s pseudoaneurysm was an iatrogenic injury during recent coronary angiography, yet believed it was less likely that the aneurism itself was infected at presentation.
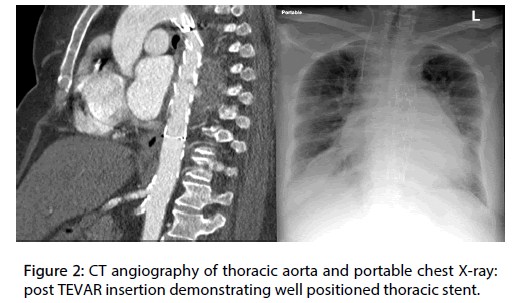
The patient was admitted and urgently underwent successful thoracic endovascular graft repair (TEVAR) using a Medtronic (Valiant™ thoracic stent graft with the Captivia ™) uneventfully, without consideration for eventual open repair. The patient was transferred to the floor in a stable condition (Figure 2). During her stay, the patient continued to demonstrate fever spikes with an associated bacteremia and persistently positive blood cultures, growing methicillin sensitive staphylococcus aureus (MSSA), despite initial treatment with broad spectrum empiric piperacillintazobactam and vancomycin combination, and eventual sensitivity selective stepdown to cloxacillin. Repeat CT of chest, abdomen, and pelvis, attempting to identify an occult source of infection, demonstrated a well-positioned thoracic stent with no leak and no infected collections, but multiple splenic infarcts were identified. This clinical presentation was suggestive of an endovascular infection, which was likely the cause of the patient’s progression of symptoms after the initial iatrogenic injury.
Figure 2: CT angiography of thoracic aorta and portable chest X-ray: post TEVAR insertion demonstrating well positioned thoracic stent.
The patient began to improve clinically, but on the 10th day of admission, she suffered a sudden cardiac arrest of unclear etiology for which she could not be resuscitated. No autopsy was performed.
Discussion
ABFs are thought to be uniformly fatal if not diagnosed and treated [1,3]. The diagnosis should be considered based on a high index of suspicion in the context of a patient with treated thoracic pathology. CT angiography is the diagnostic modality of choice, due its availability and accuracy [6]. Survival has been estimated at 76%, based on earlier findings using an open repair approach [7]. More recent literature, however, establishes TEVAR as the treatment of choice for this condition [8]. Although, there is a concern of persistent infection and the use of endovascular repair, if the cause of the ABF was an infected aneurism [9].
Vascular complications post PCI are not infrequent and include: access site hematomas, retroperitoneal hematomas, pseudoaneurysm of access artery, arteriovenous fistula, and arterial dissection and/or occlusion [10]. Incidence of these complications ranges from 1.5% to 9%, with femoral pseudoaneurysm formation estimated at 3.42% in case series [11,12].
In this case report, the presumed cause of the aneurysmal sac is an iatrogenic injury during the PCI. Another plausible sequence is an intimal injury and seeding of aorta with an infectious organism, causing an infectious arteritis, which could have progressed to an aneurysmal sac and subsequently fistulized to the bronchus. Due to a lack of imaging immediately post procedure it would be impossible to be certain. We note that the reported rate of PCI related persistent bacteremia, of which infectious arteritis is a small subset of, is 0.64% in the literature, suggesting the second possibility as less likely [13].
Infective, commonly referred to as “ mycotic ” aortic aneurysms (MAA) are localized, irreversible vascular dilations, caused by weakening and destruction of the vessel wall by an invasive organism resulting in an infective arteritis [14]. Although the exact diagnostic criteria are not well defined in the literature, it is likely a combination of four criteria: clinical presentation, laboratory findings, radiological findings and intraoperative findings [15]. MAA causing an ABF have been documented in up to 23% of patients with established aortic aneurysms. The gold standard for treatment of MAA is surgical resection, debridement and revascularization [16]. Repair using TEVAR has shown favorable outcomes [17].
The relationship of infection of the aorta causing ABF has been well established in the medical literature, with infection of the aortic aneurism or prosthetic grafts cited as being the most significant factor related to mortality in ABF, in a recent review [18]. Multiple infectious organisms have been implicated including gram positive, gram negative, anaerobes, mycobacterium as well fungal species [1,19-21].
In this specific case, the persistent bacteremia post intervention was likely due to an infection of the endograft (TEVAR), a conservative antibiotic only approach was attempted to treat the infection. It is important to note that more recent medical literature has demonstrated a higher mortality rate in cases of infected TEVAR treated conservatively, when compared to surgical intervention [22].
Learning Objective
• Aortobronchial fistulas is a rare but critical diagnosis that should be considered in any patient presenting with hemoptysis and a history of previous aortic intervention.
• Vascular complication related to percutaneous coronary interventions is frequent. Most common are access site hematomas, retroperitoneal hematomas, pseudoaneurysm of access artery, arteriovenous and arterial dissection and/or occlusion.
Conclusion
ABF is a rare but critical diagnosis that should be considered in any patient presenting with hemoptysis and a history of previous aortic intervention. In this case report, we discuss a unique cause of aortic injury due to percutaneous coronary angiography and further complicated by another unique and serious disease, an MAA.
Competing interests
None
Conflicts of interests
The authors report no conflict of interests.
Author contributions
MA drafted the manuscript in entirety and the performed the literature search.
Acknowledgement
Dr. B. Altassan provided the medical imaging and helped collect patient pertinent data.
References
- Liu SF, Chen YC, Lin MC, et al. Thoracic aortic aneurysm with aortobronchial fistula: A thirteen-year experience. Hear Lung J Acute Crit Care. 33(2): 119-123 (2004).
- Favre JP, Gournier JP, Adham M, et al. Aortobronchial fistula: Report of three cases and review of the literature. Surgery. 115(2): 264-270 (1994).
- MacIntosh EL, Parrott JCW, Unruh HW. Fistulas between the aorta and traceobronchial tree. Ann Thorac Surg. 51(3): 515-519 (1991).
- Cardiac Care Quality Indicators Report. (2017).
- Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/ SCAI guideline for percutaneous coronary intervention a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 124(23): e574–e651 (2011).
- Foster CL, Kalbhen CL, Demos TC, et al. Aortobronchial fistula occurring after coarctation repair: Findings on aortography, helical CT, and CT angiography. Am J Roentgenol. 171(2): 401-402 (1998).
- Favre JP, Gournier JP, Adham M, et al. Aortobronchial fistula: report of three cases and review of the literature. Surgery. 115(2): 264-270 (1994).
- Bailey CJ, Force S, Milner R, et al. Thoracic endovascular repair as a safe management strategy for aortobronchial fistulas. J Vasc Surg. 53(5): 1202-1209 (2011).
- Eldien AS, Deo S, Nichols FC, et al. A bad experience with endovascular treatment of an aortobronchial fistula. Ann Thorac Surg. 93(2): 650-651 (2012).
- Koreny M, Riedmüller E, Nikfardjam M, et al. Arterial Puncture Closing Devices Compared with Standard Manual Compression after Cardiac Catheterization: Systematic Review and Meta-analysis. J Am Med Assoc. 291(3): 350-357 (2004).
- Nasser TK, Mohler ER, Wilensky RL, et al. Peripheral vascular complications following coronary interventional procedures. Clin Cardiol. 18(11): 609-614 (1995).
- Kassem HH, Elmahdy MF, Ewis EB, et al. Incidence and predictors of post-catheterization femoral artery pseudoaneurysms. Egypt Hear J. 65(3): 213-221 (2013).
- Samore MH, Wessolossky MA, Lewis SM, et al. Frequency, risk factors, and outcome for bacteremia after percutaneous transluminal coronary angioplasty. Am J Cardiol. 79(7): 873-877 (1997).
- Fisk M, Peck LF, Miyagi K, et al. Case report Mycotic aneurysms: a case report, clinical review and novel imaging strategy Case report. Q J Med. 105: 181-188 (2012).
- Sörelius K, di Summa PG. On the Diagnosis of Mycotic Aortic Aneurysms. Clin Med Insights Cardiol. ( 2018).
- Sörelius K, Mani K, Björck M, et al. Endovascular treatment of mycotic aortic aneurysms: a European multicenter study. Circulation. 130(24): 2136-2142 (2014).
- Lepidi S. Is TEVAR a Safe Approach for the Treatment of Mycotic Thoracic Aortic Aneurysms? Eur J Vasc Endovasc Surg. 57(2): 247 (2019).
- Yuan SM. Aortobronchial fistula. Gen Thorac Cardiovasc Surg. 68(2): 93-101 (2020).
- Islam S, Williams DM, Teitelbaum DH. Aortobronchial fistula from invasive Aspergillus infection of the lung: An endovascular approach to repair. J Pediatr Surg. 40(12): e19-e22 (2005).
- Giglio P, Patel VI. Successful management of infected thoracoabdominal graft and aortobronchial fistula using a hybrid approach. J Vasc Surg Cases. 1(4): 239-241 (2015).
- Lauk O, Fulchini R, Hasse BK, et al. Aortobronchial fistula and Listeria endograft infection after repeated T/EVAR: A rare combination. BMJ Case Rep. 13(3): 229924 (2020).
- Li LH, Chan CY, Cheng SW, et al. Current Evidence on Management of Aortic Stent-graft Infection: A Systematic Review and Meta-Analysis. Surg. 51: 306-313 (2018).