Review Article - Interventional Cardiology (2012) Volume 4, Issue 5
Appropriate Use Criteria and percutaneous coronary intervention: measuring patient selection quality
- Corresponding Author:
- Steven M Bradley
VA Eastern Colorado Health Care System
1055 Clermont Street (111B), Denver
CO, USA
Tel: +1 816 932 4581
Fax: +1 816 932 5613
E-mail: Steven.Bradley@va.gov
Abstract
Keywords
appropriate use, coronary artery disease, health services research, hospital, percutaneous coronary intervention, quality of care, utilization
Ensuring the right patient receives the right therapy at the right time is central to high-quality care. In the management of patients with ischemic heart disease, this includes making sure the proper patients are selected for percutaneous coronary intervention (PCI), a procedure performed in approximately 600,000 patients annually in the USA, at a cost of more than US$12 billion [1,101]. High-quality decision-making is of paramount concern, as PCI is associated with risks for both periprocedural complications and longer-term bleeding and stent thrombosis. In some patients, the benefit of PCI may be small and may not justify the risks and costs of treatment. Indeed, the benefit of PCI as an initial strategy for symptom relief of stable angina has been shown to be small, temporary and not cost effective in many individuals, as patients often improve with medications alone [2–5]. Altogether, the prevalence, risks and expense of PCI highlight the need for guidance to define appropriate use, which can ensure that this effective treatment is targeted to those patients who are the most likely to benefit.
Until recently, there was an absence of national standards for measurement of appropriateness and proper patient selection for coronary revascularization. Over the past few years, six major cardiovascular professional organizations collaborated to create Appropriate Use Criteria (AUC) for Coronary Revascularization; a guidelinebased approach for assessing procedural ‘appropriateness’ for a range of clinical scenarios [6,7]. In these AUC, coronary revascularization was considered ‘appropriate’ for a given clinical scenario when the expected benefits, in terms of survival or quality of life, exceeded the potential negative consequences of the procedure and ‘inappropriate’ when the risks were perceived to outweigh the benefits. Therefore, these criteria represent an assessment of PCI quality as it relates to patient selection and the decision to perform PCI.
Assessment of procedural appropriateness using the AUC is rapidly being incorporated into PCI registries and quality improvement programs in the hopes of facilitating high-quality PCI that is both effective and efficient. Given the emerging importance of this quality metric and the considerable debate regarding its merit, this article is designed to clarify what the AUC are (and what they are not), review PCI appropriateness in current practice, discuss opportunities to improve PCI quality through the application of the AUC and ongoing opportunities to refine the AUC.
What the AUC are
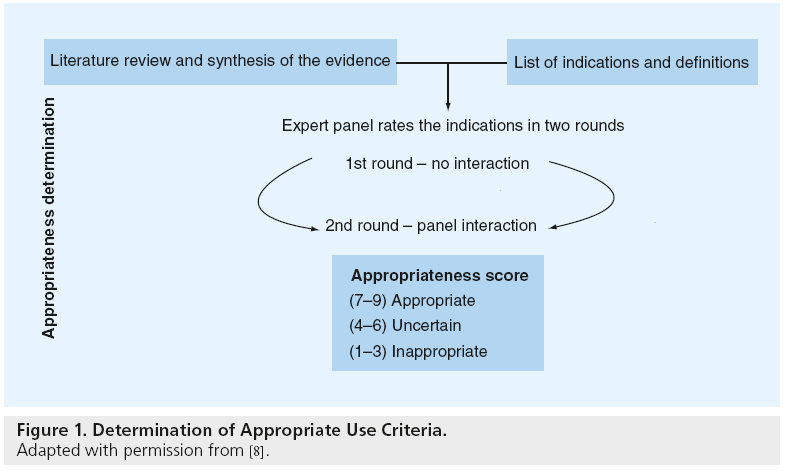
The AUC are practical, quality improvement tools that apply published trial evidence and the generalized recommendations of practice guidelines to specific clinical scenarios likely to be encountered in everyday practice [6,7]. These criteria were developed using a modified Delphi approach (Figure 1) [8]. In this approach, a writing group created prototypical clinical scenarios (n = 198) in which coronary revascularization may be considered. These clinical scenarios were then provided to a 17-member technical panel nominated by national cardiology societies. Members of the technical panel rated the appropriateness of PCI for each clinical scenario based on published trial evidence, practice guidelines and their expert opinion using the following definition of appropriateness:
Figure 1: Determination of Appropriate Use Criteria.
Adapted with permission from [8].
n “Coronary revascularization is appropriate when the expected benefits, in terms of survival or health outcomes (symptoms, functional status, and/or quality of life) exceed the expected negative consequences of the procedure”[6].
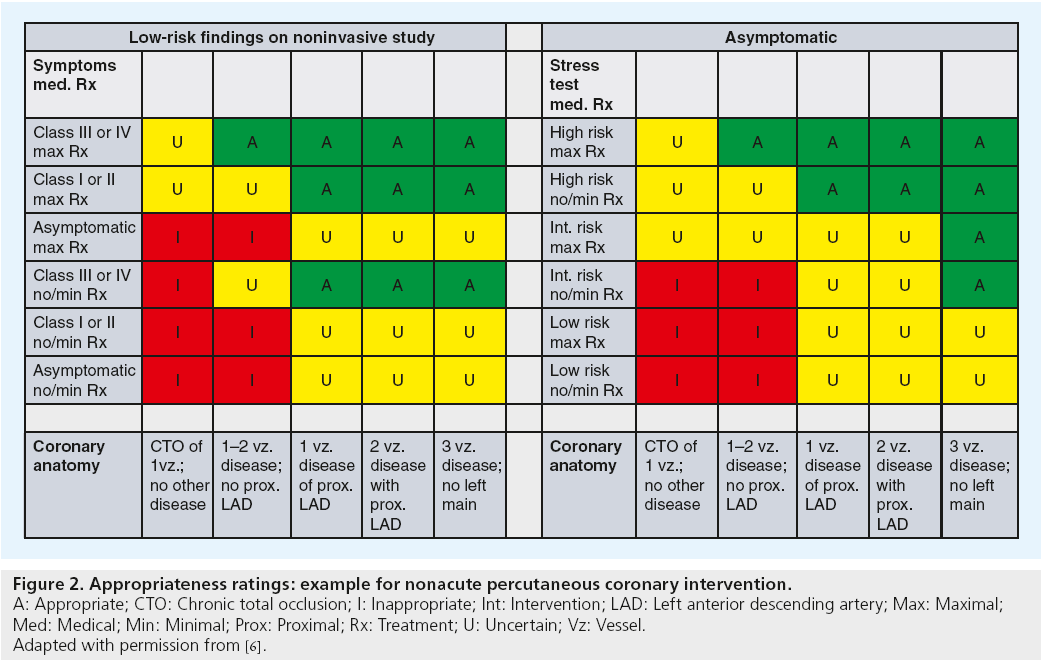
In the first round, the technical panel members individually assigned ratings from 1 (least appropriate) to 9 (most appropriate) for each clinical scenario. The technical panel was then convened for a face-to-face meeting in which they discussed clinical scenarios for which there was a wide variation of appropriateness ratings. A second round of ratings ensued, with each member of the panel again independently assigning appropriateness assessments from 1 to 9. From the ratings of this second round, the median value of the pooled ratings was used to determine the appropriateness classification for each clinical indication. Median values of 7–9 were classified as appropriate, 4–6 as ‘uncertain’, and 1 to 3 as inappropriate. In the published criteria, an appropriate rating represented clinical scenarios where the expected benefits, in terms of survival, symptoms or quality of life, exceeded the expected negative consequences of the procedure; an uncertain rating indicated more research, patient information or both, was required to determine the anticipated balance of potential benefit and risk of revascularization for the clinical scenario; and an inappropriate rating suggested that the risks were perceived to outweigh the benefits of coronary revascularization. Figure 2 provides a sample of appropriateness ratings for nonacute PCI clinical scenarios included in the criteria.
Figure 2: Appropriateness ratings: example for nonacute percutaneous coronary intervention.
A: Appropriate; CTO: Chronic total occlusion; I: Inappropriate; Int: Intervention; LAD: Left anterior descending artery; Max: Maximal;
Med: Medical; Min: Minimal; Prox: Proximal; Rx: Treatment; U: Uncertain; Vz: Vessel.
Adapted with permission from [6].
The validity of this approach is evident in the direct comparison of practice guideline recommendations with appropriate use ratings for PCI. Practice guideline class III indications for PCI (i.e., PCI should not be performed since it is not helpful and may be harmful) and clinical scenarios rated as inappropriate by the AUC are in 100% agreement [Brindis R, Pers. Comm.]. The known relationship between practice guideline indication, procedural success and adverse procedural events further support the validity of the AUC [9]. Although AUC are informed by clinical trial evidence and the general recommendations of practice guidelines, the clinical scenarios within the AUC are defined by a limited set of clinical variables. This allows for determination of PCI appropriateness for specific clinical scenarios in a manner that is not feasible within the generalized framework of practice guidelines.
It is important to note the AUC are intended to serve as guides for treatment and quality, and not as absolute mandates for or against treatment. As unique patient factors and preferences are not (and cannot be feasibly) captured in the AUC, there will always be instances in which the AUC rating for PCI may be inappropriate; however, the clinician and patient believe the circumstances justify treatment. Thus, the AUC are not intended to judge or penalize physicians for each ‘inappropriate’ PCI (e.g., deny reimbursement) despite fears to the contrary. Rather, the AUC should be used to examine and reduce practice patterns that exceed the norms of care (e.g., hospitals with very high inappropriate rates compared with the national average).
Appropriateness of PCI in current practice
The first published assessment of PCI appropriateness in the current era came from application of the AUC to the National Cardiovascular Data Registry (NCDR) CathPCI Registry, the largest national registry of diagnostic cardiac catheterization and PCI [10,11]. In this study in which appropriateness assessments were performed for more than 500,000 PCIs at approximately 1000 facilities, the authors found that PCIs performed in acute settings (defined as ST-segment elevation myocardial infarction, non-ST-segment elevation myocardial infarction and unstable angina with high-risk features) was nearly uniformly classified as appropriate [12]. However, among PCIs performed for nonacute indications, 50% were classified as appropriate, 38% as uncertain and 12% as inappropriate. In this analysis, the instances in which PCI was classified as inappropriate in nonacute indications were for clinical scenarios in which the benefit of PCI has not been demonstrated. For example, a majority of patients receiving inappropriate PCI were asymptomatic (53.8%), had low-risk noninvasive stress test findings (71.6%) and a lack of high-risk coronary anatomy findings (94%). Additionally, a trial of adequate medical therapy was not performed in 96% of patients prior to procedures classified as inappropriate.
In addition to suggesting that one in nine PCIs performed for nonacute indications were unlikely to benefit patients, this study found broad variation across hospitals in the proportion of inappropriate PCI for nonacute procedures. The median proportion of inappropriate PCI at the hospital level was 10% and ranged from 0 to 55%. The fact that some hospitals had a high rate of inappropriate PCI suggests potential overuse and an opportunity to improve patient selection at hospitals with high rates of inappropriate PCI.
The findings of this analysis from NCDR were further supported by work conducted independently in Washington and New York States (USA) [13,14]. These analyses found similar or higher proportions of inappropriate PCI for acute and nonacute indications and broad facility level variation in the proportion of inappropriate PCI. Additionally, the study from Washington State specifically addressed PCI appropriateness among facilities that do not participate in the NCDR and suggests that the findings from the NCDR study are broadly applicable to all US hospitals that perform PCI.
In addition to large facility-level variability in appropriateness for nonacute PCI, these studies found that between 30 and 50% of nonacute PCIs lacked sufficiently detailed documentation of noninvasive stress test results to classify procedural appropriateness [12–14]. In a worst-case analysis, in which all nonacute PCIs with missing stress test results were assumed to be low-risk studies, the proportion of inappropriate PCI in the nonacute setting increased from 17 to 38% [13]. In comparison, assuming all nonacute PCIs with missing stress tests were high-risk studies in a best case analysis resulted in 8% of PCI classified as inappropriate. This broad range demonstrates the importance of adequate assessment and documentation of preprocedural evaluation of PCI patients to determine the potential benefit of the procedure.
The large proportion of PCI missing stress test data was accompanied by broad facilitylevel variability in the proportion of PCI with missing stress test data. This resulted in some uncertainty in determining accurate rates of inappropriate PCI at individual facilities due to variation in the proportion of procedures that could not be mapped to the AUC [13]. Improving documentation to justify the clinical indication for treatment is important to allow assessment of broad practice patterns of PCI appropriateness in support of high-quality care. Additionally, future PCI appropriateness studies and quality improvement efforts will need to account for the distribution of PCI that cannot be mapped to the AUC to ensure equitable site to site comparisons.
Opportunities to improve PCI quality through application of the AUC
Achieving high-quality PCI requires efforts to both minimize the potential for significant complications and maximize the clinical benefit experienced by patients. Historically, measuring the quality of PCI has focused upon processes of care and postprocedural outcomes, such as in-hospital mortality, bleeding and vascular complication rates and the provision of guideline- recommended medications [15]. Despite their importance, these measures fail to assess a key aspect of high-quality care – proper patient selection. The AUC represent an opportunity to measure the quality of patient selection and the decision to perform PCI.
However, it is important to first understand whether hospitals that perform well in traditional metrics of PCI quality are also the same ones with the lowest rate of inappropriate PCI. This was examined in a recent study of more than 200,000 PCIs from nearly 800 NCDR hospitals, which found that there was no relationship between a hospital’s proportion of inappropriate PCI and that hospital’s rate of periprocedural mortality, bleeding or prescription of guideline-recommended medical therapy at discharge [16]. These findings suggest that the AUC for PCI, relative to processes of care and postprocedural outcomes, measure a different and complementary aspect of PCI quality.
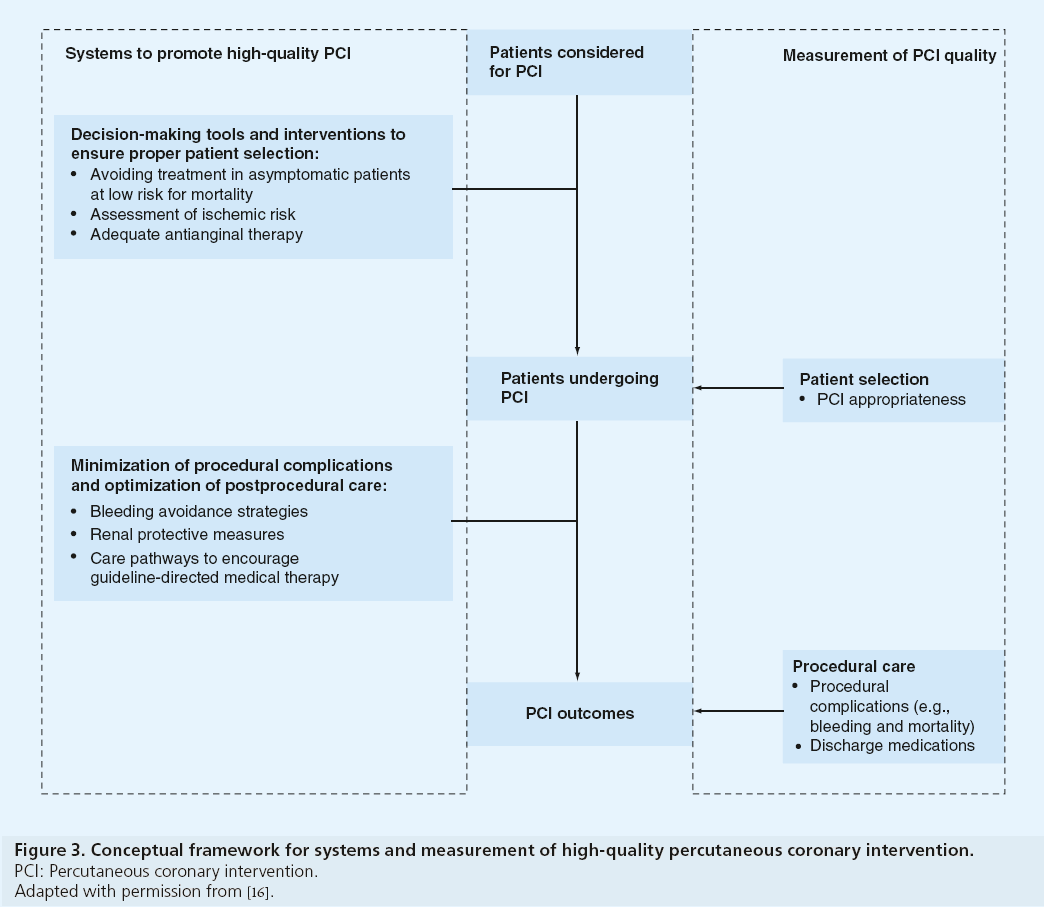
Although appropriateness assessment, processes of care and postprocedural outcomes are all quality measures for PCI, these findings suggest the systems required to improve quality in these domains are likely different (Figure 3). Hospital systems to ensure proper patient selection may include decision-making tools, including shared decision-making with patients to clarify the benefits of revascularization [17,18] and interventions prior to patient arrival in the cardiac catheterization laboratory. Among patients being considered for nonacute PCI, this may include avoidance of revascularization in asymptomatic patients, adequate assessment of ischemia risk and a trial of optimal anti-anginal medications prior to PCI [19,20]. Systems to support patient selection are likely unrelated to procedural systems that minimize complications and promote high-quality postprocedural care. For postprocedural outcomes, these systems may include optimization of bleeding avoidance strategies (e.g., radial access site and bivalirudin), renal protective measures for chronic kidney disease and development of care pathways to improve adherence to guideline-directed medications [21–26]. Future work to explore hospitallevel systems at facilities with low proportions of inappropriate PCI may identify processes that can be implemented at hospitals with high proportions of inappropriate PCI to improve patient selection and the quality of PCI.
Figure 3: Conceptual framework for systems and measurement of high-quality percutaneous coronary intervention.
PCI: Percutaneous coronary intervention.
Adapted with permission from [16].
Opportunities to refine the AUC and assessment of PCI appropriateness
The AUC are a living and dynamic document designed to reflect the current understanding of effective PCI use. As clinical evidence continues to inform the efficacy of PCI in specific clinical scenarios, there will be ongoing opportunities to ensure the AUC reflect this knowledge base. This is highlighted by the update to the AUC for Coronary Revascularization less than 3 years after the original publication date [7]. As an example, this update incorporated information on the use of SYNTAX scoring to understand the balance of appropriateness for PCI and CABG in settings of complex coronary artery disease.
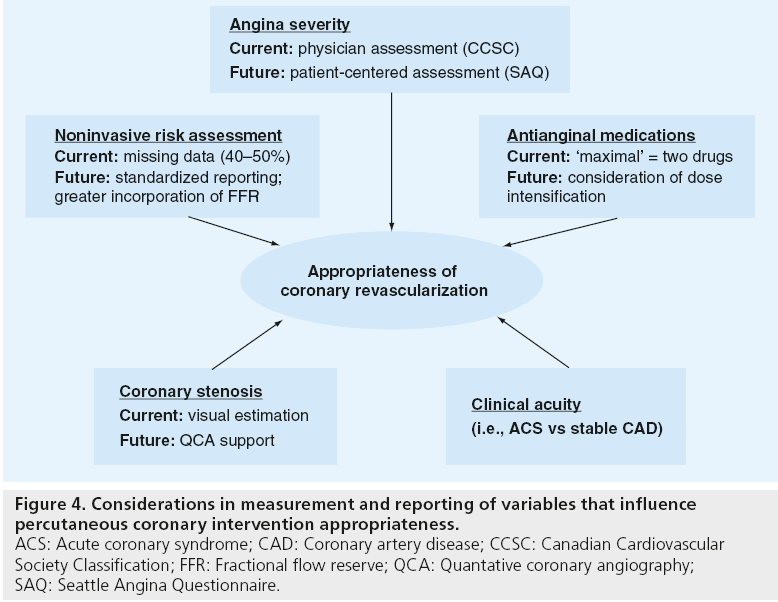
In addition to ensuring the AUC are a current reflection of the evidence base for PCI, there are opportunities to improve the measurement and reporting of the clinical factors that influence appropriateness of PCI for a given scenario (Figure 4). In the example of symptom severity, the AUC are dependent on physician assessment of symptoms as determined by Canadian Cardiovascular Society Classification, a rating system that is known to have substantial deficiencies in inter-rater reliability. Moving toward a patient-centered assessment of angina severity will ensure that the AUC more closely reflect the patient’s perspective in the pursuit of high-quality and efficient care. For instance, the Seattle Angina Questionnaire is the international standard for quantifying disease-specific health outcomes in patients with coronary disease and has very high test–retest reliability (>0.8) [27,28]. Its use in patients’ assessments of symptoms will likely result in a lower likelihood of potential ‘gaming’ by physicians to upgrade symptom severity, as angina is defined by patients’ reported symptoms rather than physician discretion. Similar opportunities exist to improve the measurement of ischemic risk by standardizing the reporting of results of noninvasive stress tests. Lastly, the emerging promise of physiologic assessments of stenosis severity (e.g., fractional flow reserve) and use of quantitative coronary angiography to standardize interpretations of coronary angiography will further improve our ability to ensure proper patient selection for PCI.
Figure 4: Considerations in measurement and reporting of variables that influence percutaneous coronary intervention appropriateness. ACS: Acute coronary syndrome; CAD: Coronary artery disease; CCSC: Canadian Cardiovascular Society Classification; FFR: Fractional flow reserve; QCA: Quantative coronary angiography; SAQ: Seattle Angina Questionnaire.
Finally, the opportunity to refine the AUC to accurately reflect anticipated clinical benefit and procedural risk requires the input and action of individual facilities and providers [29]. In the example of the NCDR, quarterly feedback reports now include benchmarking on PCI appropriateness and include a detailed list of inappropriate cases. This will allow individual facilities to identify reasons for the classification of individual cases as inappropriate and may help providers identify systems to improve preprocedural patient assessment, documentation to support PCI and suggest aspects of the criteria themselves that require review. This feedback loop, together with clinical evidence to further our understanding of effective use of PCI, will ensure that the AUC continue to evolve.
Executive summary
What the Appropriate Use Criteria are
▪ By applying clinical evidence and professional practice guidelines, the Appropriate Use Criteria (AUC) are a guide for proper patient selection for coronary revascularization procedures.
▪ To develop the AUC, common clinical scenarios in which coronary revascularization may be considered were evaluated by a 17-member technical panel nominated by national cardiology societies.
▪ In the published criteria, an ‘appropriate’ rating represented clinical scenarios where the expected benefits exceeded the risks of the procedure; an ‘uncertain’ rating indicated more research, patient information or both was required to determine the balance of potential benefit and risk of revascularization; and an ‘inappropriate’ rating suggested that the risks were perceived to outweigh the benefits of revascularization.
▪ Of note, extenuating patient circumstances virtually ensure that some patients will be incorrectly labeled as inappropriate even when sound clinical judgment would suggest that a procedure is indicated. However, there is not expected to be substantial variation across centers, meaning the substantial differences across centers in the rates of inappropriate procedures likely highlights opportunities to improve patient selection.
▪ The AUC should be used to examine and reduce practice patterns of patient selection that exceed the norms of care and not as absolute mandates for or against treatment for individual patients.
Appropriateness of percutaneous coronary intervention in current practice
▪ Data from a large, national, contemporary registry of percutaneous coronary intervention (PCI) demonstrated procedures performed for acute indications were nearly uniformly appropriate.
▪ Among nonacute PCI, 12% were inappropriate and were performed for clinical scenarios in which the benefit of PCI has not been demonstrated.
▪ Broad variation across hospitals in the proportion of inappropriate PCI for nonacute procedures suggests potential overuse and an opportunity to improve patient selection at hospitals with high rates of inappropriate PCI.
▪ Similar findings of PCI appropriateness and facility level variation were independently demonstrated in a statewide analysis and suggest the generalizability of estimated rates of inappropriate PCI in the USA.
▪ Inadequate documentation to justify the clinical indication for PCI is common and improvements in documentation are necessary to support high-quality care.
Opportunities to improve PCI quality through application of the AUC
▪ Hospitals that perform well in traditional metrics of PCI quality, such as periprocedural mortality, bleeding or provision of guideline-directed medications, are not necessarily the same hospitals that excel in properly patient selection for PCI.
▪ Measurement of both PCI appropriateness and postprocedural complications are important to fully inform PCI quality.
▪ Systems to ensure proper patient selection may include decision-making tools and interventions prior to patient arrival in the cardiac catheterization laboratory.
Opportunities to refine the AUC & assessment of PCI appropriateness
▪ The AUC are designed to reflect the current understanding of effective use of PCI as evidenced by frequent updating of the criteria.
▪ Efforts to verify the accuracy of key variables used in adjudicating PCI appropriateness, such as angina severity, are important to ensure that the AUC continue to represent accurate assessment of patient selection.
Conclusion
The AUC for Coronary Revascularization represent an important step forward in ensuring high-quality PCI by assessing the quality of patient selection and the decision to perform PCI. The AUC complement traditional PCI quality metrics, which have focused on periprocedural processes of care and outcomes. Together, these quality measures ensure that patients who undergo PCI have a high likelihood of benefitting from the procedure and minimize periprocedural risks.
Future perspective
National and state registries have demonstrated broad hospital-level variability in PCI appropriateness for nonacute indications. These findings highlight an important opportunity to improve patient selection, particularly at hospitals with high rates of inappropriate PCI. By avoiding potentially unnecessary PCIs in which clinical benefit is not expected, use of the AUC are envisioned to reduce procedural complications and costs to patients. To facilitate their ultimate adoption in routine practice, efforts are needed to verify the accuracy of key variables in the AUC, such as symptom severity, and ensure that the AUC are responsive to emerging clinical trial evidence.
Financial and competing interests disclosure
SM Bradley is supported by a Career Development Grant Award (CDA2 10-199) from VA Health Services Research and Development. PS Chan is supported by a Career Development Grant Award (K23HL102224) from the NHLBI. JA Spertus is supported by a Clinical and Translational Science Award (1UL1RR033179). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- Roger VL, Go AS, Lloyd-Jones DM et al. Heart disease and stroke statistics – 2012 update: a report from the American Heart Association. Circulation 125(1), e2–e220 (2012).
- Boden WE, O’Rourke RA, Teo KK et al. Optimal medical therapy with or without PCI for stable coronary disease. N. Engl. J. Med. 356(15), 1503–1516 (2007).
- Boden WE, O’Rourke RA, Teo KK et al. Impact of optimal medical therapy with or without percutaneous coronary intervention on long-term cardiovascular end points in patients with stable coronary artery disease (from the COURAGE Trial). Am. J. Cardiol. 104(1), 1–4 (2009).
- Weintraub WS, Spertus JA, Kolm P et al. Effect of PCI on quality of life in patients with stable coronary disease. N. Engl. J. Med. 359(7), 677–687 (2008).
- Weintraub WS, Boden WE, Zhang Z et al. Cost-effectiveness of percutaneous coronary intervention in optimally treated stable coronary patients. Circ. Cardiovasc. Qual. Outcomes 1(1), 12–20 (2008).
- Patel MR, Dehmer GJ, Hirshfeld JW, Smith PK, Spertus JA. ACCF/SCAI/STS/AATS/ AHA/ASNC 2009 appropriateness criteria for coronary revascularization: a report by the American College of Cardiology foundation appropriateness criteria task force, society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons, American association for Thoracic Surgery, American Heart Association, and the American Society of Nuclear Cardiology Endorsed by the American Society of Echocardiography, the Heart Failure Society of America, and the Society of Cardiovascular Computed Tomography. J. Am. Coll. Cardiol. 53(6), 530–553 (2009).
- Patel MR, Dehmer GJ, Hirshfeld JW, Smith PK, Spertus JA. ACCF/SCAI/STS/AATS/ AHA/ASNC/HFSA/SCCT 2012 Appropriate Use Criteria for coronary revascularization focused update. J. Am. Coll. Cardiol. 59(22), 1995–2027 (2012).
- Patel MR, Spertus JA, Brindis RG et al. ACCF proposed method for evaluating the appropriateness of cardiovascular imaging. J. Am. Coll. Cardiol. 46(8), 1606–1613 (2005).
- Anderson HV, Shaw RE, Brindis RG et al. Relationship between procedure indications and outcomes of percutaneous coronary interventions by American College of Cardiology/American Heart Association task force guidelines. Circulation 112(18), 2786–2791 (2005).
- Brindis RG, Fitzgerald S, Anderson HV, Shaw RE, Weintraub WS, Williams JF. The American College of Cardiology-National Cardiovascular Data Registry (ACC-NCDR): building a national clinical data repository. J. Am. Coll. Cardiol. 37(8), 2240–2245 (2001).
- Weintraub WS, McKay CR, Riner RN et al. The American College of Cardiology National database: progress and challenges. American College of Cardiology Database Committee. J. Am. Coll. Cardiol. 29(2), 459–465 (1997).
- Chan PS, Patel MR, Klein LW et al. Appropriateness of percutaneous coronary intervention. JAMA 306(1), 53–61 (2011).
- Bradley SM, Maynard C, Bryson CL. Appropriateness of percuteneous coronary interventions performed in Washington State. Circ. Cardiovasc. Qual. Outcomes 5(4), 445–453 (2012).
- Hannan EL, Cozzens K, Samadashvili Z et al. Appropriateness of coronary revascularization for patients without acute coronary syndromes. J. Am. Coll. Cardiol. 59(21), 1870–1876 (2012).
- Frey P, Connors A, Resnic FS. Quality measurement and improvement in the cardiac catheterization laboratory. Circulation 125(4), 615–619 (2012).
- Bradley SM, Chan PS, Spertus JA et al. Hospital PCI appropriateness and in-hospital procedural outcomes: insights from the NCDR. Circ. Cardiovasc. Qual. Outcomes 5(3), 290–297 (2012).
- Rothberg MB, Sivalingam SK, Ashraf J et al. Patients’ and cardiologists’ perceptions of the benefits of percutaneous coronary intervention for stable coronary disease. Ann. Intern. Med. 153(5), 307–313 (2010).
- Lee JH, Chuu K, Spertus J et al. Patients overestimate the potential benefits of elective percutaneous coronary intervention. Mo. Med. 109(1), 79–84 (2012).
- Borden WB, Redberg RF, Mushlin AI, Dai D, Kaltenbach LA, Spertus JA. Patterns and intensity of medical therapy in patients undergoing percutaneous coronary intervention. JAMA 305(18), 1882–1889 (2011).
- Ahmed B, Dauerman HL, Piper WD et al. Recent changes in practice of elective percutaneous coronary intervention for stable angina. Circ. Cardiovasc. Qual. Outcomes 4(3), 300–305 (2011).
- Jolly SS, Amlani S, Hamon M, Yusuf S, Mehta SR. Radial versus femoral access for coronary angiography or intervention and the impact on major bleeding and ischemic events: a systematic review and meta-analysis of randomized trials. Am. Heart J. 157(1), 132–140 (2009).
- Marso SP, Amin AP, House JA et al. Association between use of bleeding avoidance strategies and risk of periprocedural bleeding among patients undergoing percutaneous coronary intervention. JAMA 303(21), 2156–2164 (2010).
- Rao SV, Ou FS, Wang TY et al. Trends in the prevalence and outcomes of radial and femoral approaches to percutaneous coronary intervention: a report from the National Cardiovascular Data Registry. JACC Cardiovasc. Interv. 1(4), 379–386 (2008).
- Lincoff AM, Bittl JA, Harrington RA et al. Bivalirudin and provisional glycoprotein IIb/ IIIa blockade compared with heparin and planned glycoprotein IIb/IIIa blockade during percutaneous coronary intervention: REPLACE-2 randomized trial. JAMA 289(7), 853–863 (2003).
- Stacul F, Adam A, Becker CR et al. Strategies to reduce the risk of contrast-induced nephropathy. Am. J. Cardiol. 98(6A), 59K–77K (2006).
- Marenzi G, Assanelli E, Campodonico J et al. Contrast volume during primary percutaneous coronary intervention and subsequent contrast-induced nephropathy and mortality. Ann. Intern. Med. 150(3), 170–177 (2009).
- Spertus JA, Winder JA, Dewhurst TA et al. Development and evaluation of the Seattle Angina questionnaire: a new functional status measure for coronary artery disease. J. Am. Coll. Cardiol. 25(2), 333–341 (1995).
- Spertus JA, Jones P, McDonell M, Fan V, Fihn SD. Health status predicts long-term outcome in outpatients with coronary disease. Circulation 106(1), 43–49 (2002).
- Marso SP, Teirstein PS, Kereiakes DJ, Moses J, Lasala J, Grantham JA. Percutaneous coronary intervention use in the United States defining measures of appropriateness. JACC Cardiovasc. Interv. 5(2), 229–235 (2012).
▪ Website
- Healthcare costs and utilization project. Agency for Healthcare Research and Quality 2011. http://hcupnet.ahrq.gov (Accessed 19 February 2010)