Mini Review - Interventional Cardiology (2021)
Are the high-risk plaques of no-reflow phenomenon equivalent to vulnerable plaques?
- Corresponding Author:
- Masaaki Okutsu
Department of Cardiovascular Medicine,
NewTokyo Hospital,
Matsudo,
Japan,
E-mail: m-okutsu@shintokyohospital.or.jp
Received date: March 23, 2021 Accepted date: April 06, 2021 Published date: April 13, 2021
Abstract
No-reflow phenomenon during Percutaneous Coronary Intervention (PCI) in Acute Coronary Syndrome (ACS) is associated with some coronary complications such as arrhythmias, heart failure, ventricular remodeling or cardiac death. Therefore, it is very important to predict this phenomenon. The vulnerable plaque, which is almost equivalent to Thin-Cap Fibroatheroma (TCFA), is extrapolated as a predictive factor because the no-reflow phenomenon is caused by the disruption of fibroatheroma (FA). However, these two phenomena have different mechanism of occurrence. In the ACS, it is spontaneous rupture of TCFA. On the other hand, in the no-reflow phenomenon, it is the disruption of fibrous cap by mechanical stimulation such as balloon dilation, subsequent outflow of fragile necrotic core from FA and multiple microvascular obstruction. There are following three major modalities to predict this phenomenon. Intravascular ultrasound can detect whole FA other than calcification but has low resolution to detect fibrous cap. Optical coherence tomography has enough resolution to detect FA but low penetration power to detect whole FA. Cardiac computed tomography angiography can detect whole FA including calcification but has quite low resolution. Therefore, it seems to be necessary to be reconsider whether the theory, that vulnerable plaque is extrapolated as the high-risk plaques of no-reflow phenomenon, is appropriate and which modality is suitable.
Keywords
No-reflow phenomenon • Cardiac computed tomography angiography • Very low attenuation plaque • Intravascular ultrasound • Attenuated plaque
Abbreviations
ACS: Acute Coronary Syndrome; AP: Attenuated Plaque; CAD: Coronary Artery Disease; CCTA: Coronary Computed Tomography Angiography; FA: Fibroatheroma; IVUS: Intravascular Ultrasound; LAP: Low Attenuation Plaque; MVO: Microvascular Obstruction; NC: Necrotic Core; NIRS: Near-Infrared Spectroscopy; OCT: Optical Coherence Tomography; PCI: Percutaneous Coronary Intervention; TCFA: Thin-Cap fibroatheroma; VH: Virtual Histology; v-LAP: very Low Attenuation Plaque.
Introduction
No-reflow phenomenon during Percutaneous Coronary Intervention (PCI), which is more frequent in Acute Coronary Syndrome (ACS) than stable Coronary Artery Disease (CAD), is associated with some coronary complications [1-4]. No-reflow phenomenon in ACS is pathophysiologically caused by distal atherothrombotic embolism, ischemic injury, reperfusion injury and heightened individual susceptibility of coronary microcirculation to injury [5]. In those, the factor of arteriosclerotic embolism can be predicted and this is consistent with in stable CAD. Although the no-reflow phenomenon is rare in stable CAD lesions and there are few major reports, it is one of the complications to be noted. It is extremely important to predict this phenomenon because it may sometimes lead to serious complications such as myocardial infarction [1,4]. The vulnerable plaque is extrapolated as a risk factor because the noreflow phenomenon is caused by the disruption of fibroatheroma (FA), but there seems to be some concerns with this theory. In this review, the risk factors will be reconsidered from the mechanism of occurrence of the no-reflow phenomenon in stable CAD.
Mechanism of No-reflow Phenomenon and Acute Coronary Syndrome
The mechanism of arteriosclerotic embolism is that fibrous cap of FA was disrupted by mechanical stimulation such as balloon dilation or stent implantation, and debris of fragile Necrotic Core (NC) flow out to blood flow from FA and cause Multiple Microvascular Obstruction (MVO) [6,7]. This phenomenon is usually transient and reversible, but prolonged ischemia causes myocardial infarction and leading to an unfavorable prognosis. The myocardial damage depends on the degree of MVO, and there are complete no-reflow phenomenon and incomplete no-reflow phenomenon, and the former tends to be more severe MVO and cause myocardial infarction [3,8]. Among FA, Thin-Cap Fibroatheroma (TCFA) is a vulnerable plaque, which is a high-risk plaque that induces thrombus formation by spontaneous rupture of the fibrous cap and causes ACS [9]. Both no-reflow phenomenon and spontaneous ACS are phenomenon that occur with the injury of the fibrous cap and tend to be regarded as equivalent, but there are some big differences here. One is that no-reflow phenomenon is caused by mechanical disruption of fibrous cap by balloon injury, while ACS is caused due to spontaneous rupture of the fibrous cap. Another is that no-reflow phenomenon is mainly caused by MVO due to outflow of the debris of NC, while ACS is mainly caused by thrombus formation and obstruction due to exposure of necrotic core to blood at the site of ruptured plaque [10]. Therefore, noreflow phenomenon requires the presence of a sufficient amount of fragile and easily flowing out NC, and fibrous cap does not necessarily have to be thin as long as it is mechanically disrupted. On the other hand, ACS requires the presence of thin fibrous cap that causes spontaneous rupture and does not necessarily depend on the quality or burden of atheroma.
Prediction of No-reflow Phenomenon and Acute Coronary Syndrome by Intravascular Imaging
The intravascular imaging is commonly used to evaluate these plaque characters (Table 1). Intravascular ultrasound (IVUS) has good echo penetration, so it is possible to observe the whole plaque, which is suitable for evaluating the quality and quantity of plaque, but the resolution is insufficient for evaluating the thickness of fibrous cap. The presence of Attenuated Plaque (AP) on IVUS is a good predictor of no-reflow phenomenon [11,12]. However, since AP does not always reflect only NC, the positive prediction of no-reflow phenomenon by the presence of AP is not high [13]. Therefore, a special IVUS was devised to improve the diagnostic accuracy of NC. Virtual Histology (VH)-IVUS can detect NC that is difficult to distinguish with gray scale IVUS, and near-infrared spectroscopy (NIRS)-IVUS can quantify the lipid property of plaque. These modalities also have predictive performance of noreflow phenomenon [14-20], but there are no reports that these are superior to gray-scale IVUS, and further verification is needed in the future. Optical Coherence Tomography (OCT) has a higher resolution than IVUS, can measure the thickness of fibrous cap, and can diagnose TCFA. ACS is caused by spontaneous rupture of thin fibrous cap therefor TCFA is considered as a high-risk plaque of future ACS [21,22]. The thickness of fibrous cap is more important than the quality and quantity of atheroma, so evaluation by OCT is extremely rational in ACS prediction. On the other hand, the pathophysiology of no-reflow phenomenon is mainly the mechanical disruption of fibrous cap by balloons and the subsequent outflow of debris from atheroma, therefore, the incidence depends on the presence and amount of lipid-rich NC, which is likely to be an embolic source. Although there is no comparison data with gray-scale IVUS or other modalities, TCFA is also considered as a good predictor of no-reflow phenomenon [23-25]. It is true that the thinner fibrous cap is more likely to be disrupted by mechanical stimulation, so the no-reflow phenomenon may be more likely to occur in TCFA, but in theory, that factor is not essential. Furthermore, OCT is not necessarily rational for predicting the no-reflow phenomenon because OCT cannot sufficiently evaluate the NC itself, which is the essence of the pathophysiological condition of the no-reflow phenomenon. In fact, there are reports that TCFA is not an independent risk factor of the no-reflow phenomenon [24]. Coronary Computed Tomography Angiography (CCTA) is capable of tissue diagnosis and three-dimensional volume measurement using CT values, and can predict no-reflow phenomenon [26,27]. In addition, Low Attenuation Plaque (LAP), which means less than 30 HU in CCTA, is regarded as a high-risk plaque, suggesting the existence of TCFA, and is considered to associated with future ACS [28,29]. However, CCTA has a big disadvantage of low resolution, and LAP is an evaluation of atheroma, not fibrous cap. Therefore, it is presumed that the reason why LAP is related to ACS is that the prevalence of TCFA is high in LAP, that is, FA with high lipid component. On the other hand, LAP is also related to the noreflow phenomenon [30]. This indicates that CCTA can detect the presence of highly lipid plaque tissue, that is, the presence of NC. In addition, it has been reported that the volume is also associated with the no-reflow phenomenon, which matches the pathophysiological condition that the no-reflow phenomenon occurs when a high volume of fragile tissue flows out to the distal part and is embolized. However, it should be verified again that LAP (<30 HU), which has been shown to be related to ACS, can be generally extrapolated as predictive factor of the no-reflow phenomenon. Our study showed that very low attenuation plaque (v-LAP), which means less than 0 HU in CCTA, is useful for predicting the no-reflow phenomenon in stable CAD [4]. This suggests that it is important that NC has extremely high lipid component regardless of the thickness of fibrous cap (Figure 1).
| References | Modality | Total number of patients | Diagnosis | Associated factors for prediction of no-reflow phenomenon |
|---|---|---|---|---|
| Okura et al. [11] | IVUS | 110 | ACS | AP |
| Wu et al. [12] | IVUS | 364 | AMI | AP |
| Nakamura et al. [14] | VH-IVUS | 50 | AMI | large plaque burden, “marble”-like image |
| Hong et al. [15] | VH-IVUS | 190 | ACS | large NC, TCFA |
| Ohshima et al. [16] | VH-IVUS | 44 | STEMI | fibrofatty, NC, dense calcium |
| Stone et al. [20] | NIRS-IVUS | 85 | ACS+SAP | LRP, high plaque burden, small cross-sectional |
| area | ||||
| Tanaka et al. [23] | OCT | 83 | NSTEACS | lipid arc |
| Lee et al. [24] | OCT | 135 | UAP+SAP | AP |
| Ikenaga et al. [25] | OCT | 39 | STEMI | length of lipid pool |
| Harigaya et al. [30] | CCTA | 78 | ACS+SAP | LAP |
| Okutsu et al. [4] | IVUS+CCTA | 707 | stable CAD | v-LAP+AP |
Abbreviations: ACS: Acute Coronary Syndrome; AMI: Acute Myocardial Infarction; AP: Attenuated Plaque; CAD: Coronary Artery Disease; CCTA: Coronary Computed Tomography Angiography; IVUS: Intravascular Ultrasound; VH: Virtual Histology; LAP: Low Attenuation Plaque; LRP: Lipid Rich Plaque; NC: Necrotic Core, NIRS: Near-Infrared Spectroscopy; NSTEACS: Non-ST-Elevated Acute Coronary Syndrome; OCT: Optical Coherence Tomography, SAP: Stable Angina Pectoris, STEMI:ST-Elevated Myocardial Infarction, TCFA: Thin-Cap Fibro Atheroma; UAP: Unstable Angina Pectoris; v-LAP: very Low Attenuation Plaque.
Table 1: Major studies for the prediction of no-reflow phenomenon during percutaneous coronary intervention.
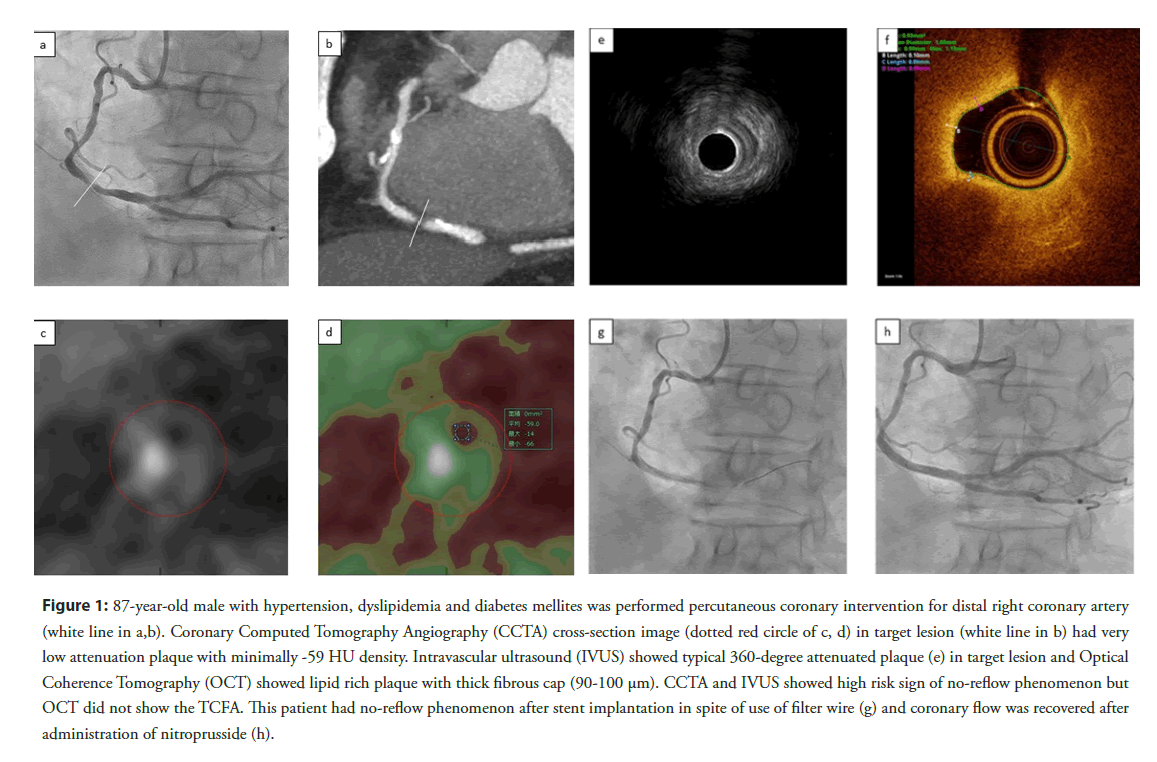
Figure 1: 87-year-old male with hypertension, dyslipidemia and diabetes mellites was performed percutaneous coronary intervention for distal right coronary artery (white line in a,b). Coronary Computed Tomography Angiography (CCTA) cross-section image (dotted red circle of c, d) in target lesion (white line in b) had very low attenuation plaque with minimally -59 HU density. Intravascular ultrasound (IVUS) showed typical 360-degree attenuated plaque (e) in target lesion and Optical Coherence Tomography (OCT) showed lipid rich plaque with thick fibrous cap (90-100 μm). CCTA and IVUS showed high risk sign of no-reflow phenomenon but OCT did not show the TCFA. This patient had no-reflow phenomenon after stent implantation in spite of use of filter wire (g) and coronary flow was recovered after administration of nitroprusside (h).
Discussion and Conclusion
In summary, the high-risk plaque of spontaneous ACS is TCFA, and OCT is a suitable modality to detect it, and the high-risk plaque of no-reflow phenomenon is FA with severe lipid component, that is NC, and IVUS and CCTA are suitable. However, although these factors are statistically significant, they do not necessarily have a useful impact as clinical judgment materials. In our study, we succeeded in improving the predictive performance by using the combination of IVUS and CCTA, which are useful for detecting high-risk plaques of no-reflow phenomenon, and obtained a clinically useful impact. In conclusion, the suitable modalities for pathophysiological predicting the no-reflow phenomenon are IVUS and CT, which can evaluate atheroma. As the cutoff value for predicting the no-reflow phenomenon in CCTA, v-LAP (<0 HU), which has a higher lipid property than LAP (<30 HU) generally used as high-risk plaque, is suitable. In addition, there is a limit to the predictive performance with a single modality, and more accurate predictive performance can be obtained by evaluating with multi-modality.
References
- Nakazawa G, Tanabe K, Onuma Y, et al. Efficacy of culprit plaque assessment by 64-slice multidetector computed tomography to predict transient no-reflow phenomenon during percutaneous coronary intervention. Am Heart J. 155(6): 1150-1157 (2008).
- Kodama T, Kondo T, Oida A, et al. Computed tomographic angiography-verified plaque characteristics and slow-flow phenomenon during percutaneous coronary intervention. JACC Cardiovasc Interv. 5(6): 636-643 (2012).
- de Waha S, Patel MR, Granger CB, et al. Relationship between microvascular obstruction and adverse events following primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: an individual patient data pooled analysis from seven randomized trials. Eur Heart J. 38(47): 3502-3510 (2017).
- Okutsu M, Horio T, Tanaka H, et al. Predictive performance of dual modality of computed tomography angiography and intravascular ultrasound for no-reflow phenomenon after percutaneous coronary stenting in stable coronary artery disease. Heart Vessels. 33(10): 1121-1128 (2018).
- Niccoli G, Burzotta F, Galiuto L, et al. Myocardial no-reflow in humans. J Am Coll Cardiol. 54(4): 281-292 (2009).
- Jaffe R, Charron T, Puley G, et al. Microvascular obstruction and the no-reflow phenomenon after percutaneous coronary intervention. Circulation. 117(24): 3152-3156 (2008).
- Niccoli G, Scalone G, Lerman A, et al. Coronary microvascular obstruction in acute myocardial infarction. Eur Heart J. 37(13): 1024-33 (2016).
- Reffelmann T, Hale SL, Li G, et al. Relationship between no reflow and infarct size as influenced by the duration of ischemia and reperfusion. Am J Physiol Heart Circ Physiol. 282(2): H766-H772 (2002).
- Burke AP, Farb A, Malcom GT, et al. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. N Engl J Med. 336(18): 1276-1282 (1997).
- Jaffe R, Dick A, Strauss BH. Prevention and treatment of microvascular obstruction-related myocardial injury and coronary no-reflow following percutaneous coronary intervention: A systematic approach. JACC Cardiovasc Interv. 3(7): 695-704 (2010).
- Okura H, Taguchi H, Kubo T, et al. Atherosclerotic plaque with ultrasonic attenuation affects coronary reflow and infarct size in patients with acute coronary syndrome: An intravascular ultrasound study. Circ J. 71(5): 648-653 (2007).
- Wu X, Mintz GS, Xu K, et al. The relationship between attenuated plaque identified by intravascular ultrasound and no-reflow after stenting in acute myocardial infarction: the HORIZONS-AMI (Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction) trial. JACC Cardiovasc Interv. 4(5): 495-502 (2011).
- Pu J, Mintz GS, Biro S, et al. Insights into echo-attenuated plaques, echolucent plaques, and plaques with spotty calcification: Novel findings from comparisons among intravascular ultrasound, near-infrared spectroscopy, and pathological histology in 2,294 human coronary artery segments. J Am Coll Cardiol. 63(21): 2220-2233 (2014).
- Nakamura T, Kubo N, Ako J, et al. Angiographic no-reflow phenomenon and plaque characteristics by virtual histology intravascular ultrasound in patients with acute myocardial infarction. J Interv Cardiol. 20(5): 335-339 (2007).
- Hong YJ, Jeong MH, Choi YH, et al. Impact of plaque components on no-reflow phenomenon after stent deployment in patients with acute coronary syndrome: A virtual histology-intravascular ultrasound analysis. Eur Heart J. 32(16): 2059-2066 (2011).
- Ohshima K, Ikeda S, Watanabe K, et al. Relationship between plaque composition and no-reflow phenomenon following primary angioplasty in patients with ST-segment elevation myocardial infarction-analysis with virtual histology intravascular ultrasound. J Cardiol. 54(2): 205-213 (2009).
- Schultz CJ, Serruys PW, van der Ent M, et al. First-in-man clinical use of combined near-infrared spectroscopy and intravascular ultrasound: A potential key to predict distal embolization and no-reflow? J Am Coll Cardiol. 56(4): 314 (2010).
- Goldstein JA, Grines C, Fischell T, et al. Coronary embolization following balloon dilation of lipid-core plaques. JACC Cardiovasc Imaging. 2(12): 1420-1424 (2009).
- Brilakis ES, Abdel-Karim AR, Papayannis AC, et al. Embolic protection device utilization during stenting of native coronary artery lesions with large lipid core plaques as detected by near-infrared spectroscopy. Catheter Cardiovasc Interv. 80(7): 1157-1162 (2012).
- Stone GW, Maehara A, Muller JE, et al. Plaque characterization to inform the prediction and prevention of periprocedural myocardial infarction during percutaneous coronary iIntervention: The CANARY Trial (Coronary Assessment by Near-infrared of Atherosclerotic Rupture-prone Yellow). JACC Cardiovasc Interv. 8(7): 927-936 (2015).
- Stone GW, Maehara A, Lansky AJ, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 364(3): 226-235 (2011).
- Calvert PA, Obaid DR, O'Sullivan M, et al. Association between IVUS findings and adverse outcomes in patients with coronary artery disease: The VIVA (VH-IVUS in Vulnerable Atherosclerosis) Study. JACC Cardiovasc Imaging. 4(8): 894-901 (2011).
- Tanaka A, Imanishi T, Kitabata H, et al. Lipid-rich plaque and myocardial perfusion after successful stenting in patients with non-ST-segment elevation acute coronary syndrome: An optical coherence tomography study. Eur Heart J. 30 (11): 1348-1355 (2009).
- Lee T, Kakuta T, Yonetsu T, et al. Assessment of echo-attenuated plaque by optical coherence tomography and its impact on post-procedural creatine kinase-myocardial band elevation in elective stent implantation. JACC Cardiovasc Interv. 4(5): 483-491 (2011).
- Ikenaga H, Ishihara M, Inoue I, et al. Longitudinal extent of lipid pool assessed by optical coherence tomography predicts microvascular no-reflow after primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. J Cardiol. 62(2): 71-76 (2013).
- Schroeder S, Kopp AF, Baumbach A, et al. Noninvasive detection and evaluation of atherosclerotic coronary plaques with multislice computed tomography. J Am Coll Cardiol. 37(5): 1430-1435 (2001).
- Motoyama S, Kondo T, Anno H, et al. Atherosclerotic plaque characterization by 0.5-mm-slice multislice computed tomographic imaging. Circ J. 71(3): 363-366 (2007).
- Motoyama S, Ito H, Sarai M, et al. Plaque characterization by coronary computed tomography angiography and the likelihood of acute coronary events in mid-term follow-up. J Am Coll Cardiol. 66(4): 337-346 (2015).
- Yang DH, Kang SJ, Koo HJ, et al. Coronary CT angiography characteristics of OCT-defined thin-cap fibroatheroma: a section-to-section comparison study. Eur Radiol. 28(2): 833-843 (2018).
- Harigaya H, Motoyama S, Sarai M, et al. Prediction of the no-reflow phenomenon during percutaneous coronary intervention using coronary computed tomography angiography. Heart Vessels. 26(4): 363-369 (2011).