Opinion Article - Clinical Investigation (2019) Volume 9, Issue 1
Assessing the turn over time of critical value in hospitals
- *Corresponding Author:
- Chandraja CV
Cuba Sarada Krishna Homoeopathic Medical College Nagercoil, Tamil Nadu, India.
E-mail: chandrajarathish@gmail.com
Submitted: 11 December 2018; Accepted: 31 December 2018; Published online: 07 January 2019
Abstract
Introduction
Critical values are important information required for the immediate health care of the patient by health care providers. Accreditation bodies require standardized laboratories to establish critical values [1-3]. Critical values first described by Dr. George Lundberg [1]. Critical value reporting is crucial for patient safety, standardization of this practice would be beneficial. The College of American Pathologists has created studies that have defined some of the procedural parameters by which critical values operate in multiple laboratories [3]. In a voluntary study of 163 laboratories, about one third used published literature sources, one third used non laboratories medical staff recommendations and one third used other sources such as internal studies, inter laboratory comparisons or manufactures recommendations. Critical values are reported for low and high critical value for a value for a selected analyst list [4].
Recent studies provide guide new guideline for managing critical values so that they best contribute to patient safety clinical staff and hospital administration can aid in during a list that clearly identifies life-threatening condition [2,4]. The present study was conducted in Sarada Krishna Homoeopathic Medical College Kulasekharam, Kanyakumari district, Tamil Nadu, India during Jan 2016 to June 2017, for a period of 18 months in the Biochemistry and hematology laboratories for a process of quality control and internal auditing.
An acceptable time interval between test completion and caregiver notification may be 15 to 30 minutes. In one study, most inpatient results were reported in a median time interval of 4 minutes, the outpatient time interval may be longer, give the requirement to identify a physician on call or call service. Finally, it is important to stay informed regarding the evolution of patient safety.
Lab Investigations Conducted in Clinical Laboratory Hold their Importance as it Ensures
• Accurate diseases diagnosis
• Disease intensity/ stage of infection or disease
• Patient response to specific treatment
• Lab values are very important in the determination of disease severity, drug doses and in follow up
Policies Adopted to Improve Services
• The abnormal results of the patient will be intimated to the concerned consultant (Physician and nurse) [2,3]
• In case the consultant is not reachable the result will be brought into the notice of the Medical Officer on duty [5,6]
• Special Registers are maintained and authorized by the concerned Physician and Head of service/Department/ Unit
• Investigation request received from critical care areas of the hospital like Emergency, ICU would be given priority
Improving the Work Flow in Clinical Laboratory
Three areas during analysis could improve critical value reporting is:
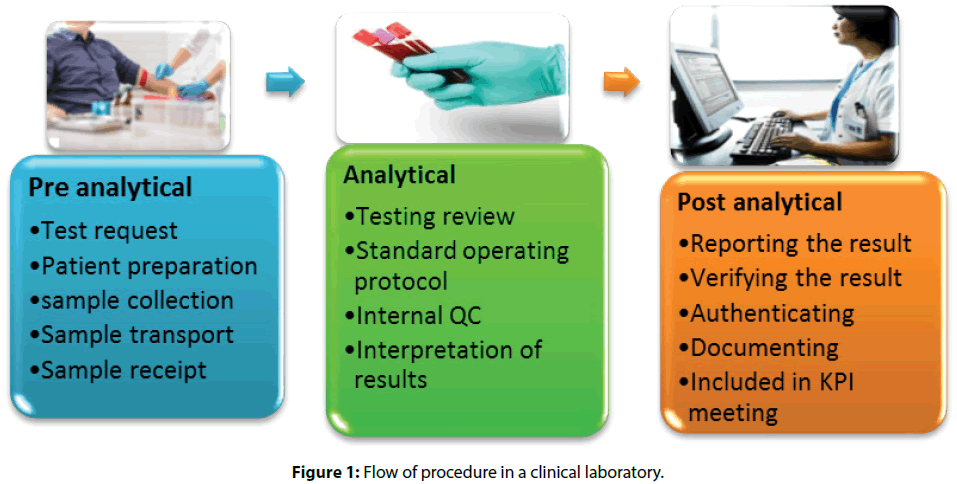
• Pre-analytical services where requisition form for the test is sent, Patient is prepared followed by sample collection, transport, and receipt of sample to the technician
• In Analytical services, the investigation is reviewed based on standard operating protocol, compared with Internal QC and results were interpreted
• Post-analytical services include Reporting of the result, result in verification by Authenticated officials and documenting data is pre-structured formats or registers, subsequently presented in KPI meeting [7]
The test result with critical values will be immediately reported. The laboratory has established a 5-minute Turn Around Time (TAT) from the verified critical result to the notification. This will be audited on a monthly basis and submitted to Quality Assurance for evaluation. Given result demands a read-back of “what was written down” with name and details like UIN (Unique Identification Number) of the patients clearly before notifying the results [8]. Flagging each test results with color codes is found effective and easy during reference. Laboratory technicians in the clinical laboratory are regularly given follow-ups and training to follow the procedure [3,9] (Figure 1).
During the period of 18 months, Jan 2016 to June 2017 the Biochemistry and hematology laboratories reported 516 critical values, out of 42,673 test results. Therefore, critical values reporting were done at a rate of 0.012% of the total test results reported. The reporting time different for each clinical investigation. The mean time taken to communicate individual critical-value result was found to be 8 to 10 minutes till documenting in the case of inpatients, it may take more than twice that 15-20 minutes for outpatient results. The callback was done for 87 cases, which were done during an emergency, others were reported by the Laboratory technicians in person to the respective physician or nurse. Flagging was introduced after evaluation from March 2017 onwards.
Conclusion
Critical values were established with an individual institution’s needs and requirements. This survey helped to establish a list of frequently reported critical values. Thus the need for critical value reporting is inevitable for patient care and safety, therefore standardization of this practice on a regular basis would be significant. Policy improvement is an important to step in the Quality improvement process. Critical value reporting system needs quicker and effective methods for call back system. Laboratory Information System (LIS) must be developed for quicker turn over time and also use communication markers like flagging by adopting red/yellow/green alert systems, highlighting techniques and a quick reference section in Medical records also.
References
- Lundberg GD. When to panic over abnormal values. MLO Med Lab Obs 4: 47-54 (1972).
- Lundberg GD. Critical (panic) value notification: An established laboratory practice policy (parameter). JAMA 263: 709 (1990).
- www.jointcommission.org/NR/rdonlyres/6447E5C3-E9EF-4431-885A-7FCF624DD73A/0/July2010NPSGs_Scoring_LAB.pdf
- Catrou P. How critical are critical values? Am J Clin Path 108: 245-246 (1997).
- Kost G. Critical limits for urgent clinician notification at US medical centers. JAMA 263: 704-707 (1990).
- Dighe A, Lewandrowski K. Avoiding errors in clinical laboratory critical value reporting. Legal Medicine 42-49 (2007).
- Plebani M, Lippi G. Improving the post-analytical phase. Clin Chem Lab Med 48: 435-436 (2010).
- Valenstein P. Critical communication. Clin Chem 56: 334-335 (2010).
- Royal College of Pathologists. Out-of-hours reporting of markedly abnormal laboratory test result to primary care: Advice to pathologists and those that work in laboratory medicine (2007).