Research Article - Interventional Cardiology (2022) Volume 14, Issue 2
Association between serum high-density lipoprotein cholesterol concentration and mortality in critically ill patients: A retrospective cohort study
- Corresponding Author:
- Jia Tian
Department of Critical Care,
Hainan General Hospital,
Haikou,
Hainan Province,
China,
E-mail: tianjia123789@163.com
Received date: 04-Mar-2022, Manuscript No. FMIC-22-58788; Editor assigned: 07-Mar-2022, PreQC No. FMIC-22-58788 (PQ); Reviewed date: 21-Mar-2022, QC No. FMIC-22-58788; Revised date: 25-Mar-2022, Manuscript No. FMIC-22-58788 (R); Published date: 01-Apr-2022, DOI: 10.37532/1755- 5310.2022.14(2).481
Abstract
Objective: This research aims to explore the association between serum High-Density Lipoprotein Cholesterol (HDL-C) and mortality in critically ill patients.
Design: This was a retrospective cohort study.
Setting: Data were extracted from an online database named ‘Multiparameter Intelligent Monitoring in Intensive Care III (MIMIC III)’.
Patients and participants: 3384 patients were enrolled, including 967 septic patients and 1380 patients with Acute Kidney Injury (AKI).
Interventions: None.
Main variables of interest: Hospital and ICU mortality, ICU Length of Stay (LOS) and hospital LOS.
Results: Serum HDL-C (42.4 ± 18.0 vs. 45.0 ± 15.2, p<0.001) concentration was significantly lower for non-survivors than survivors. A nonlinear relationship between HDL-C and mortality was found. Low HDL-C was associated with increased hospital and ICU mortality, longer ICU LOS and hospital LOS compared with medium HDL-C level (30-60 mg/dL) for all included patients (p<0.01, all). Multivariate logistic regression models showed only low HDL-C (<30 mg/dL) were associated with increased hospital mortality in all three models (model 1 OR: 1.44, 95% CI 1.05 to 1.97, p=0.022; model 2 OR: 1.51, 95% CI 1.01 to 2.27, p=0.044; model 3 OR: 1.69, 95% CI 1.15 to 2.49, p=0.007). In all models, age, mechanical ventilation use, Sequential Organ Failure Assessment (SOFA) score and Elixhauser comorbidity score were positively associated with mortality.
Conclusion: Low HDL-C is associated with increased mortality in patients who are critically ill.
Keywords
High-density lipoprotein cholesterol • Critically ill • Sepsis • Acute kidney injury
Introduction
Critical illness is preceded by a period of physiological deterioration accompanied by series of internal changes such as systemic inflammatory response, lipid peroxidation, microcirculation disturbances and so on [1]. High-density Lipoprotein Cholesterol (HDL-C), best known for their anti-atherosclerotic effects, also shows pleiotropic properties including anti-inflammatory, anti-oxidant, anti-apoptotic, endothelium protection and Lipopolysaccharide (LPS) neutralization under homeostatic condition or under acute physiologic stress [2]. Studies have shown a predicative value of HDL-C for mortality in the general population [3-5] and patients with specific diseases [6-8]. There is no study illustrating the correlation between HDL-C and mortality in unselected critically ill patients so far. Thus, the prognostic and therapeutic effect of HDL-C on critically ill patients remains unclear. Besides, even though there have been several studies in septic patients [9-11], the results were not consistent and the sample sizes were relatively small. In addition, findings about the interplay of HDL-C and renal function [12] raised our interest in the role of HDL-C in critically ill patients accompanied by Acute Kidney Injury (AKI). So, we conducted a retrospective cohort study to evaluate the association between serum HDL-C and hospital mortality in unselected critically ill patients and further screened septic and AKI patients in the group and investigated the effect of HDL-C on these two subgroups of patients.
Patients and Methods
Database introduction
MIMIC-III (Medical Information Mart for Intensive Care III) is a large, freely-available database comprising de-identified health-related data associated with over forty thousand patients who stayed in critical care units of the Beth Israel Deaconess Medical Center between 2001 and 2012 [13]. The database is accessible to researchers who have completed a ‘protecting human subjects’ training. The institutional review boards of the Massachusetts Institute of Technology (Cambridge, MA) and Beth Israel Deaconess Medical Center (Boston, MA) approved the establishment of the database. Requirement for individual patient consent was waived because the project did not impact clinical care and all protected health information was de-identified. Data extraction was performed using PostgreSQL tools V.10.12. GX obtained access to this database (certification number: 35276979) and was responsible for data extraction.
Inclusion and exclusion criteria
The following criteria were used to exclude patients from this analysis: (1) Age lower than 18 years; (2) Having spent less than 24 hours in the Intensive Care Unit (ICU); and (3) Absence of data on the serum HDL-C concentration within 24 hours after ICU admission. If patients who were admitted to the ICU more than once, only their first ICU stay was considered in this study. The first blood sample after ICU admission was used for calculations. Septic and AKI patients were screened for subgroup analysis. The definition of sepsis was adapted from the recommendation in the Surviving Sepsis Campaign 2016. Accordingly, sepsis was defined as the presence of a Sequential Organ Failure Assessment (SOFA) score ≥ 2 within 24 hours after ICU admission, accompanied by at least one infection site [14]. The presence of AKI was defined according to the creatinine-based Kidney Disease Improving Global Outcome (KDIGO) criteria without urine output [15]. Patients meeting one of the following criteria are defined as having AKI:(1) An increase in serum creatinine by ≥ 0.3 mg/dl (≥ 26.5 μmol/l) within 48 hrs; (2) An increase in serum creatinine to ≥ 1.5 times baseline within the previous 7 days.
Data extraction
The following information were extracted: Age, gender, weight, height, race, type of patients admitted to ICU, vasopressor use, Continuous Renal Replacement Therapy (CRRT) use, mechanical ventilation use, sequential organ failure assessment score, Elixhauser comorbidity score [16] and blood lipid indexes (including HDL-C, Total Cholesterol (TC), Low-Density Lipoprotein Cholesterol (LDL-C), Triglyceride (TG)) were extracted from the database. Vasopressor/CRRT/mechanical ventilation use was defined within 24 hours after ICU admission.
Outcome definition
The primary endpoint was hospital mortality, which was defined as death during hospitalization. Secondary endpoints including ICU mortality, ICU Length of Stay (LOS), hospital LOS.
Management of missing data
Variables with missing data are common in the MIMIC III database, as it comprises more than 58000 admissions. For variables included in the current study, the percentage of missing values was less than 5%. For normal distribution variables, we replaced the missing values with their mean values; for non-normal distribution parameters, missing values were replaced by the respective median. For dichotomous variables with less than 5% of missing values, the missing values were not filled.
Statistical analysis
Continuous variables were expressed as mean ± SD or median (Interquartile Range, IQR), as appropriate. The Student’s t-test, Wilcoxon rank-sum test or Kruskal–Wallis test was used as appropriate. Normal distribution was assessed using the Shapiro- Wilk test. Categorical variables are presented as a percentage and compared using the X2 test. The Lowess Smoothing technique was used to explore the crude relationship between serum HDL-C and mortality. An inflection point of HDL-C (at a level of around 30 mg/dL) was detected using the Lowess smoother technique; Thereafter, all the patients were divided into three levels: Those with HDL-C<30 mg/dL (level 1), 30 mg/dL ≤ HDL-C ≤ 60 mg/ dL (level 2) and HDL-C>60 mg/dL (level 3). Logistic regression models were built for all included patients, septic patients and AKI patients using HDL-C as a design variable, with the level 2 (30–60 mg/dL) as the reference group. A stepwise backward elimination method with a significance level of 0.05 was used to build the final models. Variables including age, gender, Body Mass Index (BMI), SOFA score, Elixhauser comorbidity score, mechanical ventilation use, CRRT use and vasopressors use were included in the multivariate logistic regression analyses. Results of the logistic regression model were presented as Odds Ratios (OR) and 95% Confidence Intervals (CI). Multicollinearity was tested using the Variance Inflation Factor (VIF) method, with a VIF ≥5 indicating the presence of multicollinearity. All the logistic regression models underwent a goodness of fit test. A two-tailed test was performed, and p<0.05 was considered statistically significant. All statistical analyses were performed using STATA V.16.0.
Results
The MIMIC III database contains records for 58,976 admissions, of which 12500 were excluded because they were duplications. Of the remaining 46476 admissions, 7965 were excluded because the patients were younger than 18 years old (38511), and 6107 were excluded because of spending less than 24 hours in the ICU (32404). After excluding 29020 patients for lacking data on the blood HDL-C concentration within 24 hours after ICU admission, 3384 admissions were included in this analysis, including 3020 survivors and 364 non-survivors, establishing an initial mortality rate of 10.7%. The mean age was 67.5 ± 15.0 years old, and 2008 patients were male (59.3%). Among all patients 967 septic patients and 1380 AKI patients were screened.
Baseline characteristics of survivors and non-survivors are presented in Table 1. Compared to survivors, non-survivors were older (73.3 ± 14.8 vs. 66.8 ± 14.9, p<0.001) and had more frequent use of vasopressors (37.9% vs. 27.0%, p<0.001), CRRT (3.8% vs. 1.9%, p=0.012) and mechanical ventilation (68.1% vs. 36.3, p<0.001). Serum HDL-C (42.4 ± 18.0 vs. 45.0 ± 15.2, p<0.001), TC (147.7 ± 46.7 vs. 162.5 ± 45.4, p<0.001), LDL-C (78.6 ± 38.1 vs. 89.4 ± 38.1, p<0.001), TG (120.6 ± 71.2 vs. 131.3 ± 77.4, p=0.012) concentration were significantly lower for non-survivors than survivors.
| Variable | Total (n=3384) | Survivors (n=3020) | Non-survivors (n=364) | p value |
|---|---|---|---|---|
| Age (years) | 67.5 ± 15.0 | 66.8 ± 14.9 | 73.3 ± 14.8 | <0.001 |
| Gender, n (%) | <0.001 | |||
| Female | 1376 (40.7) | 1198 (39.7) | 178 (48.9) | |
| Male | 2008 (59.3) | 1822 (60.3) | 186 (51.1) | |
| BMI (kg/m2) | 28.6 ± 10.0 | 28.7 ± 10.3 | 27.3 ± 7.0 | 0.014 |
| Ethnicity, n (%) | 0.251 | |||
| Asian | 70 (2.1) | 63 (2.1) | 7 (1.9) | |
| Black | 227 (6.7) | 207 (6.9) | 20 (5.5) | |
| Hispanic | 88 (2.6) | 81 (2.7) | 7 (1.9) | |
| White | 2269 (67.1) | 2033 (67.3) | 236 (64.8) | |
| Other | 730 (21.6) | 636 (21.1) | 94(25.8) | |
| Admission type, n (%) | 0.59 | |||
| Elective | 89 (2.6) | 82 (2.7) | 7(1.9) | |
| Emergency | 3155 (93.2) | 2815 (93.2) | 340 (93.4) | |
| Urgent | 140 (4.1) | 17 (0.5) | 123 (33.8) | |
| Vasopressors, n (%) | 952 (28.1) | 814 (27.0) | 138 (37.9) | <0.001 |
| CRRT, n (%) | 70 (2.1) | 56 (1.9) | 14 (3.8) | 0.012 |
| Mechanical ventilation, n (%) | 1344 (39.7) | 1096 (36.3) | 248 (68.1) | <0.001 |
| SOFA score | 3 (1-5) | 3 (1-4) | 5 (3-7.5) | <0.001 |
| Elixhauser comorbidity score | 2 (0-7) | 1 (0-6) | 6.5 (0-11.5) | <0.001 |
| Blood lipid results | ||||
| HDL-C (mg/dL) | 44.8 ± 15.5 | 45.0 ± 15.2 | 42.4 ± 18.0 | <0.001 |
| TC (mg/dL) | 160.9 ± 45.7 | 162.5 ± 45.4 | 147.7 ± 46.7 | <0.001 |
| LDL-C (mg/dL) | 88.2 ± 38.3 | 89.4 ± 38.1 | 78.6 ± 38.1 | <0.001 |
| TG (mg/dL) | 131.3 ± 77.4 | 131.3 ± 77.4 | 120.6 ± 71.2 | 0.012 |
Abbreviations: BMI: Body Mass Index; CRRT: Continuous Renal Replacement Therapy; SOFA: Sequential Organ Failure Assessment; HDL-C: High-Dense Lipoprotein Cholesterol; TC: Total Cholesterol; LDL-C: Low-Dense Lipoprotein Cholesterol; TG: Triglyceride
Table 1: Comparisons of demographics between survivors and non-survivors.
Figure 1 shows the relationship between serum HDL-C concentration and hospital mortality for all patients/septic patients/AKI patients determined using the Lowess Smoothing technique. All three models yielded non-linear relationships, an arc-shaped relationship between HDL-C and mortality was found for the whole group of patients and the AKI subgroup of patients (Figures 1A-1C) with inflection point at around 30 mg/dL; for septic patients, however, this relationship became ‘U’-shaped with inflection points at around 30 mg/dL and 60 mg/dL, respectively (Figure 1B). Thus, the group with HDL-C of 30-60 mg/dL was used as the reference in all comparisons and logistic regression models.
Figure 1: Association between serum HDL-C concentration and hospital mortality in different patient groups. Non-linear relationships are presented in this figure. An arc-shaped relationship between HDL-C and mortality was found for the whole group of patients and the AKI subgroup of patients with inflection point at around 30mg/ dL; for septic patients, this relationship became ‘U’-shaped with inflection points at around 30mg/dL and 60mg/dL, respectively. Abbreviations: HDL-C: High-Dense Lipoprotein Cholesterol; AKI: Acute Kidney Failure.
Crude outcomes are presented in Figure 2 for the three HDL-C categories. Low HDL-C was associated with increased hospital and ICU mortality, longer ICU LOS and hospital LOS compared with medium HDL-C level for all included patients (p<0.01, all) while the differences between high HDL-C and medium HDL-C group were statistically non-significant. Similar patterns were found among AKI subgroup of patients. For the sepsis subgroup, low HDL-C group also showed higher hospital and ICU mortality rate (p<0.001, p=0.002, respectively); however, low HDL-C group did not show longer ICU LOS or hospital LOS.
Figure 2: Unadjusted outcome comparison between different categories of serum HDL-C concentration among different patient groups. Low HDL-C was associated with
increased hospital and ICU mortality, longer ICU LOS and hospital LOS compared with medium HDL-C level for all included patients while the differences between high
HDL-C and medium HDL-C group were statistically nonsignificant. Similar patterns were found among AKI subgroup of patients. For the sepsis subgroup, low HDL-C
group also showed higher hospital and ICU mortality rate; however, low HDL-C group did not show longer ICU LOS or hospital LOS. Abbreviations: HDL-C: High-
Dense Lipoprotein Cholesterol; ICU: Intensive Care Unit; AKI: Acute Kidney Failure; LOS: Length of Stay; NS: Not Significant. 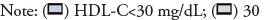
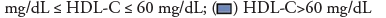
To further explore the effect of HDL-C, multivariate logistic regression models were built with HDL-C as design variable and level 2 (30–60 mg/dL) serving as the reference group. Table 2 shows that after adjusting for covariates, only low HDL-C (<30 mg/dL) was associated with increased hospital mortality in all three models (model 1 OR: 1.44, 95% CI 1.05 to 1.97, p=0.022; model 2 OR: 1.51, 95% CI 1.01 to 2.27, p=0.044; model 3 OR: 1.69, 95% CI 1.15 to 2.49, p=0.007). In all three models, age (OR: 1.03, 1.07, 1.02, p<0.001, p<0.001, p=0.007, respectively), mechanical ventilation use (OR: 3.03, 1.75, 1.72, p<0.001, p=0.004, p=0.003, respectively), SOFA score (OR:1.20, 1.20, 1.18, p<0.001, p<0.001, p<0.001, respectively) and Elixhauser comorbidity score (OR:1.06, 1.04, 1.07, p<0.001, p=0.001, p<0.001, respectively) were positively associated with mortality.
| Model 1 | Model 2 | Model 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | OR | 95% CI | p | Variable | OR | 95% CI | p | Variable | OR | 95% CI | p |
| HDL-C (<30) | 1.44 | 1.05-1.97 | 0.022 | HDL-C (<30) | 1.51 | 1.01-2.27 | 0.044 | HDL-C (<30) | 1.69 | 1.15-2.49 | 0.007 |
| HDL-C (30~60) | Ref. | HDL-C (30~60) | Ref. | HDL-C (30~60) | Ref. | ||||||
| HDL-C (>60) | 1.08 | 0.77-1.51 | 0.672 | HDL-C (>60) | 1.17 | 0.71-1.95 | 0.535 | HDL-C (>60) | 0.89 | 0.54-1.45 | 0.631 |
| Age | 1.03 | 1.02-1.04 | 0 | Age | 1.07 | 1.01-1.04 | 0.000 | Age | 1.02 | 1.00-1.03 | 0.007 |
| Gender | 0.74 | 0.58-0.94 | 0.013 | MV | 1.75 | 1.2-2.56 | 0.004 | Gender | 0.66 | 0.47-0.91 | 0.011 |
| Vasopressors | 0.65 | 0.49-0.87 | 0.004 | SOFA | 1.2 | 1.13-1.28 | 0.000 | MV | 1.72 | 1.2-2.46 | 0.003 |
| MV | 3.03 | 2.32-3.96 | 0.000 | ECS | 1.04 | 1.02-1.07 | 0.001 | SOFA | 1.18 | 1.12-1.25 | 0.000 |
| SOFA | 1.2 | 1.15-1.26 | 0.000 | ECS | 1.07 | 1.04-1.09 | 0.000 | ||||
| ECS | 1.06 | 1.04-1.08 | 0.000 | ||||||||
Notes: Model 1 contains information from all 3384 patients and the mean VIF was 3.67. Model 2 contains information from 967 septic patients and the mean VIF was 2.82. Model 3 contains information from 1380 AKI patients and the mean VIF was 4.32. Abbreviations: AKI: Acute Kidney Injury; HDL-C: High-Dense Lipoprotein Cholesterol; MV: Mechanical Ventilation; SOFA: Sequential Organ Failure Assessment; ECS: Elixhauser Comorbidity Score; VIF: Variance Inflation Factor
Table 2: Adjusted ORs using HDL-C as the design variable in different groups of patients.
Discussion
Our results reveal that compared to ICU survivors, non-survivors had lower level of serum lipid, including HDL-C, TC, LDL-C and TG. Low serum HDL-C concentration is associated with increased hospital mortality of patients who are critically ill, presenting as an arc-shaped association with an inflection point at around 30 mg/dL. This pattern was also observed for patients with AKI. For septic patients, we observed a crude U-shaped association between HDL-C and hospital mortality. However, only low HDL-C significantly associated with increased hospital mortality. In addition, age, mechanical ventilation use, SOFA score and Elixhauser comorbidity score were strongly associated with a higher mortality rate in all groups.
First, we observed a significant drop in serum HDL-C, TC, LDL-C and TG concentration in non-survivors compared to survivors, which was also reported in several other studies [6,17]. This reduction could be explained by several hypothesis, including an acute over-consumption of HDL particles, a decrease in liver HDL synthesis, especially in case of hepatic failure, and/or an increased clearance following an upregulation of Scavenger Receptor B1 [18]. For septic patients, redistribution of HDL particles from the intravascular to the extravascular compartment due to inflammation-induced capillary leakage [19], or replacement of Apolipoprotein A-I from HDL particles by Serum Amyloid A (SAA) which accelerates its clearance all contribute to the reducing HDL-C levels [20,21].
The correlation of HDL and mortality has not been analyzed in unselected critically ill ICU patients yet. There are several studies investing the relationship between HDL-C and mortality conducted among general population in recent years, most of which revealed J-shaped or U-shaped relationship [3-5,22,23]. For example, Zhong, et al. [3] research demonstrated that in the general population, HDL-C level is associated with all-cause mortality in a J-shaped dose-response manner with the lowest risk observed at HDL-C levels of 54-58 mg/dL ; both extremely high and low HDL-C levels are associated with an increased risk of mortality. Mazidi, et al. [4] reported both extremely low (≤ 30 mg/dL) and extremely high (≥ 100 mg/dL) HDL-C levels were associated with greater risk of mortalities among American adults. Our finding only demonstrated low HDL-C‘s correlation to increased mortality rate. Several factors might have contributed to the differences between our finding and others. On one hand it largely arises from the physiological differences of critical and general population. On the other hand, it might result from the relative low level of HDL-C revealed in our study in critically ill ICU patients (maximal HDL-C is 92 mg/dL). There are limited studies on the correlation of HDL-C and mortality in critically ill patients except sepsis. Degoricija, et al. [24] reported low admission serum levels of HDL3-C are associated with an increased 3-month mortality in acute heart failure patients, whereas total HDL-C and HDL2-C showed no association. Zieliński, et al. [25] reported that baseline HDL-C ≤ 46 mg/dl was independent predictors of 2-year mortality in elderly who underwent Transcatheter Aortic Valve Implantation (TAVI). Karata, et al. [7] reported that serum HDL-C obtained within the first 24 hours of hospital admission was independently reversely correlated with mortality in patients with acute pulmonary embolism. Zhang, et al. [8] reports decreased HDL-C (<1.06 mmol/L) is an independent predictor for in-hospital mortality in acute pancreatitis. It is noticed that most studies revealed a negative effect of low HDL-C concentration on mortality, but due to the diversity of disease categories, sample size and research designs, the cut-off points may vary. In this case, the prognostic value of low HDL-C should be evaluated by clinicians in all critically ill patients.
For septic patients, the correlation between HDL-C and mortality was first reported by Chien, et al. [6]. The low HDL cholesterol level <20 mg/dL reported on day 1 was significantly associated with an increase in mortality and adverse clinical outcomes. Later, two studies reported low level of Apolipoprotein A-I, major constituent of HDL-C, were independent factors to predict mortality in sepsis [9,17]. Recently, Trinder, et al. [26] identified a rare missense variant in CETP (Cholesteryl Ester Transfer Protein gene; rs1800777-A) that was associated with significant reductions in HDL-C levels during sepsis, carriers of which had higher mortality rate compared with noncarriers, indicating a causal effect of reduced HDL levels on decreased sepsis survival. Our findings are in line with the above-mentioned studies, adding a powerful evidence due to its large sample size, demonstrating a protective role of HDL during courses of sepsis..
However, we also discovered an odd rise in mortality in the high HDL-C group though not significant. One explanation might be that some HDL particles become dysfunctional due to size modification and composition change during sepsis as reported in several studies which will lead to worsening outcomes [20,27,28]. Due to the limitation of retrospective study, we are unable to further detect the function of HDL-C in our study. Future studies are needed to illustrate this problem.
For ICU patients with AKI, the impact of HDL-C on mortality has not been reported. In this study, we explored the association between HDL-C and adverse outcome of critically ill patients with AKI. Lower level HDL-C correlated with high hospital mortality rate, ICU mortality rate and longer ICU and hospital LOS. Our findings suggest that HDL-C might be a potential prognostic predictor of critically ill patients with AKI. However, we did not find a protective role of higher HDL-C level in avoiding adverse clinical outcomes. An observation study by Zewinger, et al. [29] also reported in patients with reduced kidney function, higher HDL-C did not associate with lower risk for mortality and there was interaction between HDL-C and estimated Glomerular Filtration Rate (eGFR) in predicting mortality. Although HDL-C confers its renal protection function during courses of AKI by different mechanisms [12], studies have shown patients with renal dysfunction have significant disturbances in HDL composition and function leading to impaired vasoprotective, antioxidative and anti-inflammatory properties [30-33]. Further studies are needed to better characterize HDL dysfunction that is associated with renal dysfunction under acute physiologic stress to identify novel risk factors and therapeutic targets for AKI prevention and treatment in the future.
The advantage of the present study is the large sample size, which allowed for subgroup analysis and adjustment for confounding factors, but it also has limitations. Firstly, because this is a retrospective research, the association between HDL-C and mortality could only be inferred, further research is needed to establish a definitive causal link. Secondly, the serum concentration of HDL-C can be influenced by many cofounders, such as diabetes, liver function and kinds of drugs. However, due to the nature of retrospective study, these situations cannot be identified in this database. Finally, as mentioned in the patients and methods section, because it is difficult to obtain urine output information in MIMIC III, only the KDIGO criteria of creatinine was used to screen AKI patients in this study, which may lead to some AKI patients not being identified.
Conclusion
Among critically ill patients, a low serum HDL-C (<30 mg/dL) concentration was significantly associated with increased hospital mortality. This was also observed in different disease catagories of critically ill patients with minor differences, for example, AKI and sepsis. The reason why higher HDL-C concentration did not show significant protective role needs further study.
Author’s Contribution
XG, ZYX, HXQ, RW and EN all take responsibility for the integrity of the data, interpretation, and analysis. XG performed the statistical analysis and data synthesis. All authors contributed substantially to the study design, data interpretation, and writing of the manuscript. All authors approved the final version of the manuscript.
Funding
This research was funded by the Health and Family Planning Commission of Hainan Province (CN)(18A200145).
Acknowledgements
Not applicable.
Conflict of Interest
The authors declare that they have no competing interests.
References
- Robertson LC, Al-Haddad M. Recognizing the critically ill patient. Anaesth Intensive Care Med. 14(1): 11-4 (2013).
- Eckardstein AV, Kardassis D. High density lipoproteins. 1st ed. Springer International Publishing. (2015).
- Zhong GC, Huang SQ, Peng Y, et al. HDL-C is associated with mortality from all causes, cardiovascular disease and cancer in a J-shaped dose-response fashion: A pooled analysis of 37 prospective cohort studies. Eur J Prev Cardiol. 27(11): 1187-203 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Mazidi M, Mikhailidis DP, Banach M. Associations between risk of overall mortality, cause-specific mortality and level of inflammatory factors with extremely low and high high-density lipoprotein cholesterol levels among American adults. Int J Cardiol. 276: 242-7 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Oh IH, Hur JK, Ryoo JH, et al. Very high high-density lipoprotein cholesterol is associated with increased all-cause mortality in South Koreans. Atherosclerosis. 283: 43-51 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Chien JY, Jerng JS, Yu CJ, et al. Low serum level of high-density lipoprotein cholesterol is a poor prognostic factor for severe sepsis. Crit Care Med. 33(8): 1688-93 (2005).
[CrossRef] [Google Scholar] [PubMed]
- Karataş MB, Güngör B, İpek G, et al. Association of serum cholesterol levels with short-term mortality in patients with acute pulmonary embolism. Heart Lung Circ. 25(4): 365-70 (2016).
[CrossRef] [Google Scholar] [PubMed]
- Zhang Y, Guo F, Li S, et al. Decreased high density lipoprotein cholesterol is an independent predictor for persistent organ failure, pancreatic necrosis and mortality in acute pancreatitis. Sci Rep. 7(1): 8064 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Tsai MH, Peng YS, Chen YC, et al. Low serum concentration of apolipoprotein A-I is an indicator of poor prognosis in cirrhotic patients with severe sepsis. J Hepatol. 50(5): 906-15 (2009).
[CrossRef] [Google Scholar] [PubMed]
- Liu SH, Liang HY, Li HY, et al. Effect of low high-density lipoprotein levels on mortality of septic patients: A systematic review and meta-analysis of cohort studies. World J Emerg Med. 11(2): 109-16 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Tanaka S, Labreuche J, Drumez E, et al. Low HDL levels in sepsis versus trauma patients in intensive care unit. Ann Intensive Care. 7(1): 60 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Smith LE. High-density lipoproteins and acute kidney injury. Semin Nephrol. 40(2): 232-42 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Johnson AEW, Pollard TJ, Shen L, et al. MIMIC-III, a freely accessible critical care database. Sci Data. (2016).
[CrossRef] [Google Scholar] [PubMed]
- Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 43(3): 304-77 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 120(4): c179-84 (2012).
[CrossRef] [Google Scholar] [PubMed]
- AHRQ. Comorbidity Software, Version 3.7. (2014).
- Barlage S, Gnewuch C, Liebisch G, et al. Changes in HDL-associated apolipoproteins relate to mortality in human sepsis and correlate to monocyte and platelet activation. Intensive Care Med. 35(11): 1877-85 (2009).
[CrossRef] [Google Scholar] [PubMed]
- Pirillo A, Catapano AL, Norata GD. HDL in infectious diseases and sepsis. Handb Exp Pharmacol. 224: 483-508 (2015).
[CrossRef] [Google Scholar] [PubMed]
- van Leeuwen HJ, Heezius EC, Dallinga GM, et al. Lipoprotein metabolism in patients with severe sepsis. Crit Care Med. 31(5): 1359-66 (2003).
[CrossRef] [Google Scholar] [PubMed]
- de la Llera Moya M, McGillicuddy FC, Hinkle CC, et al. Inflammation modulates human HDL composition and function in vivo. Atherosclerosis. 222(2): 390-4 (2012).
[CrossRef] [Google Scholar] [PubMed]
- Choudhury RP, Leyva F. C-Reactive protein, serum amyloid A protein, and coronary events. Circulation. 100(15): e65-6 (1999).
[CrossRef] [Google Scholar] [PubMed]
- Li X, Guan B, Wang Y, et al. Association between high-density lipoprotein cholesterol and all-cause mortality in the general population of northern China. Sci Rep. 9(1): 14426 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Li ZH, Lv YB, Zhong WF, et al. High-density lipoprotein cholesterol and all-cause and cause-specific mortality among the elderly. J Clin Endocrinol Metab. 104(8): 3370-8 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Degoricija V, Potočnjak I, Gastrager M, et al. HDL subclasses and mortality in acute heart failure patients. Clin Chim Acta. 490: 81-7 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Zieliński K, Kalińczuk Ł, Chmielak Z, et al. Additive value of high-density lipoprotein cholesterol and C-Reactive protein level assessment for prediction of 2-year mortality after transcatheter aortic valve implantation. Am J Cardiol. 126: 66-72 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Trinder M, Genga KR, Kong HJ, et al. Cholesteryl ester transfer protein influences high-density lipoprotein levels and survival in sepsis. Am J Respir Crit Care Med. 199(7): 854-62 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Tanaka S, Diallo D, Delbosc S, et al. High-Density Lipoprotein (HDL) particle size and concentration changes in septic shock patients. Ann Intensive Care. 9(1): 68 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Schuchardt M, Prüfer N, Tu Y, et al. Dysfunctional high-density lipoprotein activates toll-like receptors via serum amyloid A in vascular smooth muscle cells. Sci Rep. 9(1): 3421 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Zewinger S, Speer T, Kleber ME, et al. HDL cholesterol is not associated with lower mortality in patients with kidney dysfunction. J Am Soc Nephrol. 25(5): 1073-82 (2014).
[CrossRef] [Google Scholar] [PubMed]
- Moradi H, Pahl MV, Elahimehr R, et al. Impaired antioxidant activity of high-density lipoprotein in chronic kidney disease. Transl Res. 153(2): 77-85 (2009).
[CrossRef] [Google Scholar] [PubMed]
- Holzer M, Birner-Gruenberger R, Stojakovic T, et al. Uremia alters HDL composition and function. J Am Soc Nephrol. 22(9): 1631-41 (2011).
[CrossRef] [Google Scholar] [PubMed]
- Florens N, Calzada C, Lemoine S, et al. CKD increases carbonylation of HDL and is associated with impaired antiaggregant properties. J Am Soc Nephrol. 31(7): 1462-77 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Florens N, Calzada C, Delolme F, et al. Proteomic characterization of high-density lipoprotein particles from non-diabetic hemodialysis patients. Toxins (Basel). 11(11): 671 (2019).
[CrossRef] [Google Scholar] [PubMed]