Research Article - Neuropsychiatry (2017) Volume 7, Issue 6
Association between Serum Nerve Growth Factor Levels and Depression in Stroke Patients
- Corresponding Author:
- Jincai He
Department of Neurology, the First Affiliated Hospital of Wenzhou Medical University
Wenzhou 325000, Zhejiang Province, P.R. China
Tel: +86 0577 55579363
Fax: +86 0577 55579363
Abstract
Abstract
Background: Previous studies have demonstrated that nerve growth factor (NGF) participates in the development of stroke and depression. However, the interrelationship between serum NGF and post-stroke depression (PSD) has been poorly understood. Therefore, our aim was to determine whether serum NGF is correlated with the development of depression during the acute stage of stroke.
Methods and Findings: In total, 200 patients with acute ischaemic stroke and 100 healthy controls were recruited, and the serum NGF levels were examined within 24 h after admission. We screened for the existence of symptoms of depression using the 17-item Hamilton Depression Scale at one month after
stroke. Sixty-two patients (31.0%) were diagnosed with PSD at one month following stroke. Serum NGF was significantly higher in stroke patients than in normal controls (9.6 ± 3.1 ng/L vs. 7.2 ± 1.5 ng/L; P<0.001), whereas PSD patients had significantly lower NGF than non-PSD patients (8.2 ± 2.3 ng/L vs.10.2 ± 3.2 ng/L; P<0.001). In multivariate analyses, NGF levels (<7.7 ng/L and >11.1 ng/L) were independently associated with the development of PSD [odds
ratio (OR): 2.53; 95% confidence interval (CI): 1.03-6.22, P<0.05 and OR: 0.32; 95% CI: 0.11–0.97, P<0.05, respectively] after adjusting for possible variables.
Conclusions: These findings suggest that lower serum NGF levels might be a risk factor for developing PSD and higher serum levels of NGF might be protective against developing PSD at one month post-stroke.
Keywords
Biochemical marker, Depression, Nerve growth factor, Stroke
Abbreviations
BI: Barthel Index; BMSC: Bone Marrow Mesenchymal Stem Cells; BMI: Body Mass Index; BUN: Urea Nitrogen; CE: Cardio Embolism; CI: Confidence Intervals; CAD: Coronary Artery Disease; CGRP-IR: Calcitonin- Gene-Related Peptide Immune-Reactive; Cr: Creatinine; DSM-IV, Diagnostic And Statistical Manual Of Mental Disorders, 4th Edition; DBP: Diastolic Blood Pressure; ELISA: Enzyme- Linked Immunosorbent Assay; FBG: Fasting Blood Glucose; HAMD: Hamilton Depression Scale; Hba1c: Glycosylated Haemoglobin; HDL: High-Density Lipoprotein; HLP:Hyperlipidaemia; IQR: Interquartile Range; LAA: Large-Artery Atherosclerosis, LDL: Low Density Lipoprotein; Mrs: Modified Rankin Scale; NGF: Nerve Growth Factor; NIHSS: National Institutes Of Health Stroke Scale; OR: Odds Ratios ; PSD: Post-Stroke Depression; TC: Total Cholesterol; TG: Triglyceride; SD: Standard Deviation; SOD: Superoxide Dismutase; SAA: Small-Vessel; SOE: Stroke Of Other Determined Aetiology; SUE: Stroke Of Undetermined Aetiology; SBP: Systolic Blood Pressure
Introduction
Post-stroke depression (PSD) is especially prevalent after stroke, affecting approximately one-third of individuals [1]. Early identification and diagnosis of PSD is very important because it may have severe impacts on the survivors, including poor functional outcomes [2], increased mortality [3] and high risk of recurrent stroke [4]. However, the underlying pathophysiology of PSD still remains unclear today.
Nerve growth factor (NGF) is an indispensable neurotrophic factor involved in several neuronal functions, including neuronal survival, differentiation and assistance in the development and renovation of neurons [5]. Recently, the relationship between NGF concentrations and depression has been well-documented in the literature but there are different conclusions [6]. The majority of evidence has revealed a reduction in serum NGF levels in patients with depressive disorders [7]. Nevertheless, some studies found no difference in NGF concentrations between controls and patients with major depression [8]. The discrepancy may result from the difference in the samples, measurement methods, or time of assessment. Additionally, Reus, et al. [9] found that reduced NGF levels in the rat brain may result in depression-like symptoms. Furthermore, some studies showed that serum NGF levels were significantly normalized after antidepressant treatment [10].
However, to date, there has not yet been a study exploring serum NGF levels and PSD. Therefore, our aim in this study was to explore the possible association between serum NGF and the development of PSD.
Methods
▪ Study population
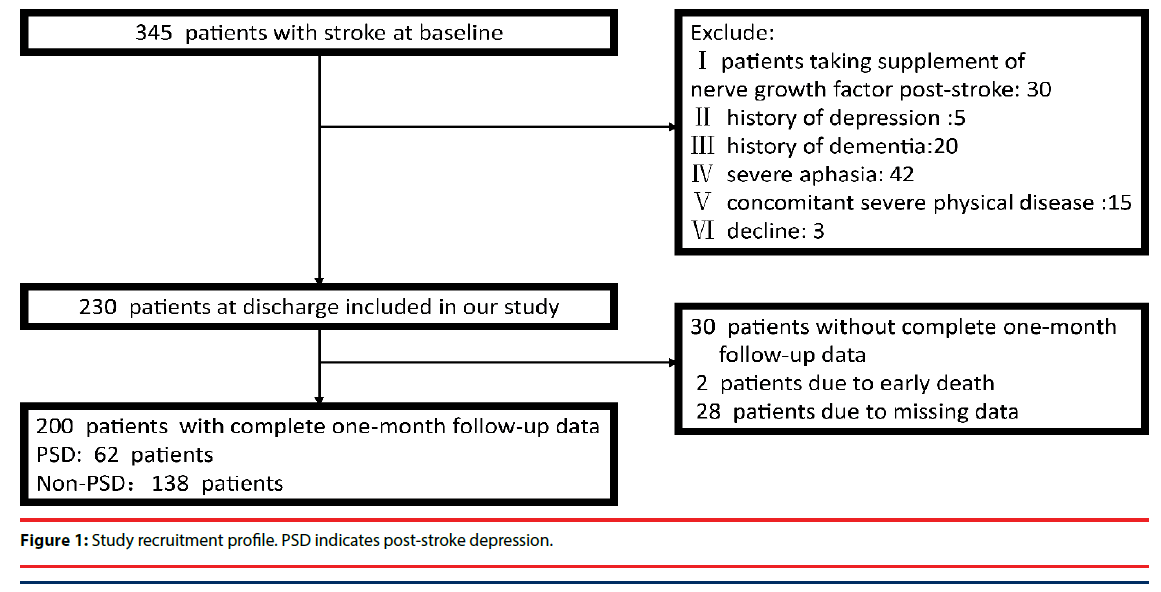
Two-hundred patients with acute ischaemic stroke admitted to the Stroke Unit of the First Affiliated Hospital of Wenzhou Medical University were included in our study between October 2014 and October 2015 and were followed for one month. No patients received antidepressant treatment during the follow-up period. The inclusion criteria were as follows: (I) an age of 18–80 years, (II) the ability and willingness to give informed consent, and (III) patients within the first 7 days of acute ischaemic stroke onset. Exclusion criteria included patients who (I) were taking NGF supplementation; (II) were receiving immunosuppressive or antiinflammatory drugs; (III) had any psychiatric disorder, including a history of depression; (IV) had severe aphasia or dysarthria that prevented us from performing the evaluation; (V) had a history of central nervous system diseases such as dementia, Parkinson’s disease, tumour, trauma or hydrocephalus; or (VI) were lost to follow-up (Figure 1). Additionally, 100 healthy controls were recruited from a health survey conducted at the First Affiliated Hospital of Wenzhou Medical University. The core symptoms of depression were excluded in controls with Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM- IV) and the Subjects were recruited without any personal or familial history of psychiatric illness. Subjects must have had no severe physical diseases, including acute ischaemic stroke. The protocol was approved by the hospital’s Medical Ethics Committee. Written informed consent was obtained from patients or their relatives.
▪ Clinical variables
To collect the data of this study, we used standardized questionnaires conducted in faceto- face interviews by trained neurologists. Lesion location was analysed by CT/MRI scans performed within 24 to 72 h after admission. Experienced neurologists evaluated stroke severity using the National Institute of Health Stroke Scale (NIHSS) at the time of admission. The functional outcomes were assessed by the modified Rankin Scale (mRS) and the Barthel Index (BI) at discharge.
▪ Psychological measurement
To evaluate the depressive symptoms, we used the 17-item Hamilton Depression Rating Scale (HAMD) [11] at one month following stroke. Patients were diagnosed with PSD consistent with DSM-IV criteria and a HAMD-17 score >7. Evaluations were performed by trained researchers who were blinded to the laboratory results of stroke patients at one month post-stroke.
▪ Laboratory tests
Blood taken from the antecubital vein was collected during the initial 24 h of admission. Serum NGF levels were measured by enzymelinked immunosorbent assay (ELISA) method according to the manufacturer’s instructions , using a double-antibody sandwich ELISA kit (Labsystems Multiskan MS, Finland). Serum NGF was recorded in ng/L and divided into four quartiles (<7.7 ng/L, 7.7-9.2 ng/L,9.2-11.1 ng/L and >11.1 ng/L), as the raw NGF data were skewed. The number of subjects in each quartile was recorded. The limit of detection was 0.7 ng/L. No significant cross-reactivity or interference was observed.
▪ Statistical analyses
The clinical variables and NGF levels of all subjects were compared using the χ2 test, oneway ANOVA, Student’s t-test, Mann–Whitney U test, and Fisher’s Exact Test, as appropriate. Dunnett’s T3 test was done to further valuate discrepancies in two-group comparisons if a continuous variable showed a significant difference among the groups in the ANOVA. Bonferroni corrections were used for each test to adjust for multiple testing. The quartile of the ranked set of data values was used to divide the data set into four equal groups to better compare the difference in each group between PSD and non-PSD patients. Binary logistic regression was applied to determine the influence of all the factors that were significantly different in the univariate analysis on the development of PSD. The results were expressed as adjusted odds ratios (ORs) with the corresponding 95% confidence intervals (CIs). All statistical analyses were performed using SPSS for Windows, version 22.0 (SPSS Inc., Chicago, IL, USA). Values of p<0.05 were considered to be statistically significant in all tests.
Results
▪ Baseline characteristics of study samples
In total, 345 consecutively admitted patients with acute ischaemic stroke were screened, and 200 individuals were included in our study (Figure 1). There was no difference between the included and the excluded patients in terms of median (IQR) NIHSS score [3(1–4) vs. 3 (2–4); P=0.74] or serum levels of NGF (9.6 ± 3.1 ng/L vs. 10.1 ± 3.2 ng/L; P=0.57). The average age was 62.9 ± 1.0 years and 31% were female.
▪ Main findings
A total of 62 (31%, 42 men, 20 women) patients were diagnosed with a depressive disorder one month after stroke occurrence. The mean ( ± SD) NGF levels for all stroke patients were 9.6 ± 3.1 ng/L; the mean ( ± SD) NGF levels in normal subjects, PSD and non-PSD patients were 7.2 ± 1.5 ng/L, 8.2 ± 2.3 ng/L and 10.2 ± 3.2 ng/L, respectively. Our results demonstrated that the intergroup difference was significant in serum NGF levels at admission (F=41.00, P<0.001). Indeed, serum NGF was significantly higher in non-PSD patients and PSD patients than in normal controls; serum NGF was significantly lower in PSD patients than in non-PSD patients. Additionally, patients with depression poststroke were also more likely to have higher low density lipoprotein (LDL) and have a history of hyperlipidaemia (HLP) (all P<0.1) compared to the non-PSD group. The PSD group also had more severe strokes, poorer functional outcomes, and worse rehabilitation outcomes (Table 1).
| Variables | PSD | Non-PSD | Normal controls (n¼100) |
|---|---|---|---|
| (n=62) | (n=138) | (n=100) | |
| Demographic characteristics | |||
| Sex(M/F) | 42/20 | 92/42 | 55/45 |
| Age(years), (mean ±SD) | 62.5 ± 11.0 | 62.1 ± 10.2 | 61.9 ± 6.5 |
| Education (years), (median), (IQR) |
4(0-7) | 3(0-6) | |
| BMI (kg/m2), (mean ±SD) | 24.5 ± 3.3 | 23.7 ± 3.1 | |
| Marital status (married %) | 57(91.9) | 129(93.5) | |
| Lesion location (%) | |||
| Frontal lobe | 12(19.4) | 28(20.3) | |
| Parietal lobe | 7 (11.3) | 12(8.7) | |
| Temporal lobe | 8 (12.9) | 13(9.4) | |
| Occipital lobe | 4 (6.4) | 8(5.8) | |
| Basal ganglia | 16(25.8) | 32(23.2) | |
| Brainstem | 4 (6.4) | 13 (9.4) | |
| Cerebellum | 3 (4.8) | 11 (8.0) | |
| Other | 8(13.0) | 21(15.2) | |
| Vascular risk factors (%) | |||
| History of hypertension | 39(62.9) | 97(70.3) | |
| History of diabetes | 15(24.2) | 30(21.7) | |
| History of hyperlipidemia | 6(9.7) | 5(3.6) | |
| CAD | 1(1.6) | 9(6.5) | |
| History of stroke | 8(5.8) | 11(8.0) | |
| Current smoking | 35(56.5) | 67(48.6) | |
| Current drinking | 21(33.9) | 50(36.2) | |
| Subtype of acute ischaemic | |||
| LAA | 52(83.9) | 103(74.6) | |
| CE | 3(4.84) | 15(10.9) | |
| SAA | 5(8.06) | 10(7.2) | |
| SOE | 1(1.6) | 2(1.4) | |
| SUE | 1(1.6) | 8(5.8) | |
| Neuropsychological function | |||
| NIHSS score (median), (IQR) | a4(2-7) | 2(1-4) | |
| BI score (median), (IQR) | a 65(35-95) | 100(65-100) | |
| mRS score (median), (IQR) | a 3(1-4) | 1(1-3) | |
| Clinical and laboratory variables | |||
| SBP (mm Hg), (mean ±SD) | 156.9 ± 23.8 | 153.3 ± 20.9 | |
| DBP (mm Hg), (mean ±SD) | 83.5 ± 12.7 | 81.5 ± 12.7 | |
| TC(mmol/L), (median), (IQR) | 1.5(1.0-2.3) | 1.6(1.2-2.1) | |
| TG(mmol/L), (mean ±SD) | 5.0 ± 1.2 | 4.9 ± 1.1 | |
| LDL(mmol/L),(mean ±SD) | 3.1 ± 1.1 | 2.9 ± 0.8 | |
| HDL(mmol/L), (median), (IQR) | 1.1(0.9-1.4) | 1.1(0.9-1.3) | |
| FBG(mmol/L), (median), (IQR) | 4.9(4.3-6.0) | 4.9(4.5-6.2) | |
| HbA1c (%),(median),(IQR) | 5.8(5.4-6.5) | 5.9(5.5-6.7) | |
| BUN(mmol/L), (median), (IQR) | 5.2(2.8-54.0) | 4.9(2.6-49.0) | |
| Cr(mmol/L), (median), (IQR) | 70.0(59.5-87.5) | 73.0 (53.5-83.0) | |
| NGF(ng/L),(mean ±SD) | a,b 8.2 ± 2.3 | 10.2 ± 3.2 | 7.2 ± 1.5 |
Values are shown as number (percentage) or as medians (IQR) and means (SD). PSD=post-stroke depression, SD=standard deviation, IQR=interquartile range, BMI=body mass index, CAD=coronary artery disease, LAA=large-artery atherosclerosis, CE=cardio embolism, SAA=small-vessel, SOE=stroke of other determined aetiology, SUE=stroke of undetermined aetiology, mRS=modified Rankin Scale, BI= Barthel Index, NIHSS=National Institutes of Health Stroke Scale, Cr=creatinine, DBP=diastolic blood pressure, FBG=fasting blood glucose, HbA1C (%)= glycosylated haemoglobin, HDL=high-density lipoprotein, LDL=low-density lipoprotein, SBP=systolic blood pressure, TC=total cholesterol, TG=triglyceride, BUN=urea nitrogen.
a P<0.001 compared to non-PSD.
b P<0.05 compared to normal controls.
Table 1: Clinical and Demographic Characteristics of the Samples under Study.
Additionally, the proportion of serum NGF in the lowest quartile (< 7.7 ng/L) was significantly increased in the PSD patients (x2 =7.01, P=0.008), and the proportion of serum NGF in the highest quartile (>11.1 ng/L) was significantly reduced in the PSD patients (x2=9.00, P=0.003) (Table 2).
| Variables | PSD | Non-PSD | P value |
|---|---|---|---|
| (n=62) | (n=138) | ||
| NGF | 0.007 | ||
| Quartile 1 | 23(37.1) | 27(19.6) | 0.008 |
| Quartile 2 | 16 (25.8) | 34 (24.6) | 0.860 |
| Quartile 3 | 15(24.2) | 34(24.6) | 0.946 |
| Quartile 4 | 7(11.3) | 43(31.2) | 0.003 |
PSD=post-stroke depression, NGF=nerve growth factor.
Table 2: The proportion in NGF level quartiles of patients.
PSD presence was considered a dependent variable and factors with a P-value <0.10 in the univariate analysis were considered independent variables in the logistic analysis. It was found that serum NGF levels (< 7.7 ng/L and >11.1 ng/L) were independently associated with the presence of PSD (OR: 2.53, 95% CI: 1.03–6.22, P=0.043 and OR: 0.32, 95% CI: 0.11–0.97, P=0.044, respectively). Additionally, there was a significant association between the BI scores at discharge and the presence of depression at one month after stroke (OR: 0.97, 95% CI: 0.95– 0.99, P=0.019) (Table 3).
| Variables | OR | 95%CI | P value |
|---|---|---|---|
| NGF | |||
| Quartile 1 | 2.53 | (1.03-6.22) | 0.043 |
| Quartile 4 | 0.32 | (0.11-0.97) | 0.044 |
| Age | 0.220 | ||
| Sex | 0.658 | ||
| LDL | 0.142 | ||
| HLP | 0.068 | ||
| NHISS score | 0.318 | ||
| mRS score | 0.920 | ||
| BI score | 0.98 | (0.95-0.99) | 0.019 |
NGF=nerve growth factor, LDL=low-density lipoprotein, HLP= History of hyperlipidaemia, BI =Barthel Index, mRS=modified Rankin Scale, NIHSS=National Institutes of Health Stroke Scale, OR=odds ratio, CI=confidence interval.
Table 3: Adjusted OR of depression for nerve growth factor levels in stroke patients.
Discussion
To the best of our knowledge, this is the first report to investigate the possible relationship between serum NGF levels and the presence of depression at one month after stroke. Our results indicated that lower serum NGF is an important biological marker of the presence of PSD at one month following stroke.
In the present study, serum levels of NGF within 24 h after admission were found to be significantly higher in stroke patients than in normal subjects, which is in agreement with some previous studies, although some other research has found that serum NGF levels did not differ between stroke patients and controls [12]. The inconsistency may be attributed to the differences in samples, measurement methods, or time of assessment. It has recently been documented that NGF is produced by activated immune cells [13] under inflammatory conditions. As stroke is characterized by an acute pro-inflammatory status [14], our data suggest that increased NGF levels may be due to a response to the pro-inflammatory state seen in stroke patients.
The most important finding of our study is that serum NGF levels were lower in PSD patients who presented with depression within one month following stroke compared to stroke patients without depression. These results are consistent with previous studies that demonstrated lower serum concentrations of NGF in patients with major depression [7].
Previous studies have presented that neuronal loss occurs in the brains of subjects with depression [15]. Recent papers have indicated that NGF plays a vital role in protecting neurons from ischemic induced death after stroke. In stroke, NMDA receptor-mediated excitotoxicity has been demonstrated to be the primary mechanism by which neuronal damage occurs [16]. Additionally, it has been suggested that NGF protects neurons against glutamate-induced cytotoxicity probably by the enhancement of calcium homeostatic mechanisms [17]. A growing body of studies has reported that NGF induces the sprouting of calcitonin-generelated peptide immune-reactive (CGRP-IR) fibres [18], and the protective effect of CGRP on ischaemic brain injury has been observed in previous literature [19].
Recent studies have found that impairment of neurogenesis in the brain is related to the presence of depression [20], and the promotion of neurogenesis in the hippocampus may be an effective antidepressant treatment, possibly because of the increased ability of the brain to respond or adapt to aversive stimuli [21]. Many studies have indicated that NGF is important in neurogenesis after ischaemia [22]. A study observed that a daily application of 100 μl NGF in rats via intranasal administration during the first 6 days after ischaemia significantly enhanced neurogenesis [23]. Another study showed that NGF promoted the differentiation of bone marrow mesenchymal stem cells (BMSCs) into neuro-like cells and increased expression of synaptophysin in the ischaemic brain compared with non-treated animals [24].
Furthermore, a growing body of research suggests that oxidative stress plays an important role in the pathophysiology of major depressive disorder. Oxidative stress occurs due to overproduction of free radicals during stroke and leads to progressive tissue damage and cellular death [25]. Interestingly, it has been suggested that NGF protects cells from oxidative stresstriggered death [26]. A preclinical experiment showed that NGF-transgenic (t g) mice have an intrinsic resistance to oxidative stress due to their high concentration of basal copper zinc superoxide dismutase (SOD) and glutathione transferase, leading to the reduction of ischaemic damage in NGF-t g mice [27]. Taken together, NGF may influence the development of PSD by inhibiting oxidative stress. A conclusion might be drawn that the mechanism linking NGF to PSD involves the aforementioned factors.
However, our finding that some patients with elevated serum NGF levels also had depression symptoms indicated that the NGF serum concentrations in PSD patients were not sufficiently high enough to prevent neuronal cell loss and promote neurogenesis as well as alleviate the oxidative stress. Additionally, it is pertinent to mention that the reduction in NGF levels was observed in depression-related brain regions in PSD patients [28] although the serum NGF was increased compared to controls in our study. Stroke results in inflammation [14] and recent research has indicated that NGF peripheral levels increase in response to pro-inflammatory stimuli [29] despite the reduction in NGF levels in depression-related brain regions, which leads to the development of depression. As such, stroke might interfere with the role of NGF in the pathogenesis of depression via the inflammation that occurs after stroke. Generally, the reasons mentioned above might explain the phenomenon that the PSD patients with increased serum NGF still have depressive symptoms post-stroke. However, the exact pathophysiology needs to be further explored.
We also found that lower serum NGF levels might be a risk factor for developing PSD and higher serum levels of NGF might be protective against developing PSD. The results seem to strengthen the suggestion that NGF plays a key role in the pathophysiology of PSD during the acute stage of ischaemic stroke.
In our study, 31.0% of stroke patients were diagnosed with depression at one month poststroke, which is consistent with earlier studies [1]. Our results also demonstrated that physical disability (measured by BI) at discharge was independently associated with PSD, which was consistent with the findings of previous studies [30]. We did not detect any significant association between the development of PSD and other controversial variables, such as female sex [31,32] or age [32,33].
However, it is worth to note that although depressive symptoms resemble those of other types of depression, there are still some differences between PSD patients and depressed Patients without stroke. Stroke Patients with depression have greater cognitive impairment, greater physical impairment and experience more sleep disturbances, vegetative symptoms, and social withdrawal, than do depressed Patients without stroke [34]. Moreover, the patients with PSD usually have a later age at onset than the depressed patients without stroke. As a result, the mechanism of NGF in PSD patients may partly differ from depression without stroke. But we would like to note that animal research was not carried out in our study. Moreover, to date, there has never been a preclinical study exploring the association between NGF and PSD. Thus, we will investigate the exact mechanism about NGF in PSD through an animal model of PSD in a future study.
This study has several limitations. First, we excluded patients with both severe aphasia and serious conditions, which might make us, underestimate the actual incidence of PSD. Second, the serum NGF levels were tested only once, and the delay between stroke onset and blood collection was not confirmed. Further studies are needed to control the time between stroke onset and blood collection and evaluate how NGF levels change with time post-stroke and whether levels drawn at later time points can offer more prognostic information. Third, the HAMD scale has not been measured in control group, and except for gender, age and serum NGF levels, the other data of the control group has not been collected during the study. Fourth, the size of the subjects was not enough which might make the 95% CI limits are very close to 1 in the result of multiple regression analysis. Finally, another limitation is the short follow-up period, which did not allow us to observe the effects of lengthy institutionalization on post-stroke depression. We plan to address these limitations in future work.
In conclusion, despite these limitations, our findings demonstrated that lower serum NGF levels might be a risk factor for developing PSD and higher serum levels of NGF might be protective against developing PSD at one month post-stroke, which suggests that this biomarker might be used as a novel marker for PSD at one month post-stroke. Further investigations are essential to confirm the dynamic changes in serum NGF along with the disease course and to determine this relationship between serum and cortical NGF concentrations, which may provide a new solution for PSD.
Acknowledgements
Authors Jincai He, Zhuoying Zhu, Chaowen Wu designed the study and wrote the protocol. Authors Zhihua Liu, Qiongzhang Wang, Kai Zhao, Xiaoqian Luan, Yan Cai conducted literature searches and provided summaries of previous research studies. Author Zhuoying Zhu conducted the statistical analysis. Author Zhuoying Zhu wrote the first draft of the manuscript and all authors contributed to and have approved the final manuscript.
Funding
This research was supported by a grant from Wenzhou Municipal Sci-Tech Bureau Program (Y20160002) and National Key Technology Research and Development Program of the Ministry of Science and Technology of China (grant number: 2015BAI13B01). We are greatly indebted to the staff and to the patients with stroke for their contributions during this study.
Conflicts of interest
All authors declare that they have no conflicts of interest.
References
- Hackett ML, Yapa C, Parag V, et al. Frequency of depression after stroke: a systematic review of observational studies. Stroke 36(6), 1330-1340 (2005).
- Schulz R, Beach SR, Ives DG, et al. Association between depression and mortality in older adults: the Cardiovascular Health Study. Arch. Intern. Med 160(12), 1761-1768 (2000).
- House A, Knapp P, Bamford J, et al. Mortality at 12 and 24 months after stroke may be associated with depressive symptoms at 1 month. Stroke 32(3), 696-701 (2001).
- Yuan HW, Wang CX, Zhang N, et al. Poststroke depression and risk of recurrent stroke at 1 year in a Chinese cohort study. PLoS. One 7(10), e46906 (2012).
- Mufson EJ, Kroin JS, Sendera TJ, et al. Distribution and retrograde transport of trophic factors in the central nervous system: functional implications for the treatment of neurodegenerative diseases. Prog. Neurobiol 57(4), 451-484 (1999).
- Chen YW, Lin PY, Tu KY, et al. Significantly lower nerve growth factor levels in patients with major depressive disorder than in healthy subjects: a meta-analysis and systematic review. Neuropsychiatr. Dis. Treat 11(1), 925-933 (2015).
- Wiener CD, de Mello Ferreira S, Pedrotti Moreira F, et al. Serum levels of nerve growth factor (NGF) in patients with major depression disorder and suicide risk. J. Affect. Disord 184(1), 245-248 (2015).
- Kheirouri S, Noorazar SG, Alizadeh M, et al. Elevated Brain-Derived Neurotrophic Factor Correlates Negatively with Severity and Duration of Major Depressive Episodes. Cogn. Behav. Neurol 29(1), 24-31 (2016).
- Reus GZ, Stringari RB, Ribeiro KF, et al. Maternal deprivation induces depressive-like behaviour and alters neurotrophin levels in the rat brain. Neurochem. Res 36(3), 460-466 (2011).
- Bilgen AE, Bozkurt Zincir S, Zincir S, et al. Effects of electroconvulsive therapy on serum levels of brain-derived neurotrophic factor and nerve growth factor in treatment resistant major depression. Brain. Res. Bull 104(1), 82-87 (2014).
- Hamilton M. A rating scale for depression. J. Neurol. Neurosurg. Psych 23(1), 56-62 (1960).
- Stanzani L, Zoia C, Sala G, et al. Nerve growth factor and transforming growth factor-beta serum levels in acute stroke patients. Possible involvement of neurotrophins in cerebrovascular disease. Cerebrovas. Dis 12(3), 240-244 (2001).
- Bonini S, Rasi G, Bracci-Laudiero ML, et al. Nerve growth factor: neurotrophin or cytokine? Int. Arch. Allergy. Immunol 131(2), 80-84 (2003).
- Anrather J, Iadecola C. Inflammation and Stroke: An Overview. Neurotherapeutics 13(4), 661-670 (2016).
- Lee AL, Ogle WO, Sapolsky RM. Stress and depression: possible links to neuron death in the hippocampus. Bipolar. Disord 4(2), 117-128 (2002).
- Li V, Wang YT. Molecular mechanisms of NMDA receptor-mediated excitotoxicity: implications for neuroprotective therapeutics for stroke. Neural. Regen. Res 11(11), 1752-1753 (2016).
- Cunha GM, Moraes RA, Moraes GA, et al. Nerve growth factor, ganglioside and vitamin E reverse glutamate cytotoxicity in hippocampal cells. Eur. J. Pharmacol 367(1), 107-112 (1999).
- Chaudhry N, de Silva U, Smith GM. Cell adhesion molecule L1 modulates nerve-growth-factor-induced CGRP-IR fiber sprouting. Exp. Neurol 202(1), 238-249 (2006).
- Holland JP, Sydserff SG, Taylor WA, et al. Calcitonin gene-related peptide reduces brain injury in a rat model of focal cerebral ischemia. Stroke 25(10), 2055-2058 (1994).
- Elizalde N, Garcia-Garcia AL, Totterdell S, et al. Sustained stress-induced changes in mice as a model for chronic depression. Psychopharmacology(Berl) 210(3), 393-406 (2010).
- DeCarolis NA, Eisch AJ. Hippocampal neurogenesis as a target for the treatment of mental illness: a critical evaluation. Neuropharmacology 58(6), 884-893 (2010).
- Yin X, Meng F, Wei W, et al. Role of mouse nerve growth factor in neural recovery following hypoxic-ischemic brain damage. Int. J. Clin. Exp. Med 6(10), 951-955 (2013).
- Zhu W, Cheng S, Xu G, et al. Intranasal nerve growth factor enhances striatal neurogenesis in adult rats with focal cerebral ischemia. Drug. Delivery 18(5), 338-343 (2011).
- Ding J, Cheng Y, Gao S, et al. Effects of nerve growth factor and Noggin-modified bone marrow stromal cells on stroke in rats. J. Neurosci. Res 89(2), 222-230 (2011).
- Ng F, Berk M, Dean O, et al. Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int. J. Neuropsychopharmacol 11(6), 851-876 (2008).
- Satoh T, Sakai N, Enokido Y, et al. Free radical-independent protection by nerve growth factor and Bcl-2 of PC12 cells from hydrogen peroxide-triggered apoptosis. J. Biochem 120(3), 540-546 (1996).
- Guegan C, Ceballos-Picot I, Chevalier E, et al. Reduction of ischemic damage in NGF-transgenic mice: correlation with enhancement of antioxidant enzyme activities. Neurobiol. Dis 6(3), 180-189 (1999).
- Y Li,Chun P, W Wang, et al. Frequency of depression after stroke: a systematic review of observational studies. Chin. J. Geriat. Heart. Brain. Vessel. Dis 17(1), 1305-1309 (2015).
- Takei Y, Laskey R. Interpreting crosstalk between TNF-alpha and NGF: potential implications for disease. Trends. Mol. Med 14(9), 381-388 (2008).
- Zhang WN, Pan YH, Wang XY, et al. A prospective study of the incidence and correlated factors of post-stroke depression in China. PLoS. One 8(11), e78981 (2013).
- Poynter B, Shuman M, Diaz-Granados N, et al. Sex differences in the prevalence of post-stroke depression: a systematic review. Psychosomatics 50(6), 563-569 (2009).
- Seifert CL, Poppert H, Sander D, et al. Depressive symptoms and the risk of ischemic stroke in the elderly-influence of age and sex. PLoS. One 7(11), e50803 (2012).
- Jorgensen TS, Wium-Andersen IK, Wium-Andersen MK, et al. Incidence of Depression after Stroke, and Associated Risk Factors and Mortality Outcomes, in a Large Cohort of Danish Patients. JAMA. Psychiatry 73(10), 1032-1040 (2016).
- Esparrago Llorca G, Castilla-Guerra L, Fernandez Moreno MC, et al. Post-stroke depression: an update. Neurologia 30(1), 23-31 (2015).