Research Article - Diabetes Management (2022) Volume 12, Issue 5
Association between triglyceride glucose-body mass index and incident diabetes mellitus in Chinese population: a secondary retrospective analysis
- Corresponding Author:
- Haofei Hu
Department of Nephrology, Shenzhen Second People’s Hospital, Shenzhen 518000, Guangdong Province, China
E-mail: huhaofei0319@126.com
- Corresponding Author:
- Xinyu Wang
Shenzhen University Health Science Center, Shenzhen 518000, Guangdong Province, China
E-mail: wxyhorse@126.com
Received: 09-Oct-2022, Manuscript No. FMDM-22-76782; Editor assigned: 11-Oct-2022, PreQC No. FMDM-22-76782 (PQ); Reviewed: 25-Oct-2022, QC No. FMDM-22-76782; Revised: 31-Oct-2022, Manuscript No. FMDM-22-76782 (R); Published: 07-Nov-2022, DOI: 10.37532/1758-1907.2022.12(5).405-416
Abstract
Background: TyG-BMI is one of the insulin resistance markers. However, few studies have investigated the relationship between TyG-BMI and diabetes. The aim of this study is to investigate the relationship between TyG-BMI and diabetes occurrence in Chinese individuals.
Methods: This retrospective study is a population-based cohort of 204978 non-diabetic individuals using healthy screening program data in China between 2010 and 2016. Cox proportional hazards regression analysis evaluated the Hazard Ratio (HR) and 95% Confidence Interval (95% CI) of the relationship between TyG-BMI and diabetes events. Then the association between TyG-BMI and incident diabetes was further explored by generalized additive models and subgroup analyses.
Results: Our study indicated that a increase in diabetes incidence occurred with the rise of TyG-BMI (HR=1.023, 95% CI (1.022, 1.024) after the adjustment of potential confounders. TyG- BMI has a non-linear relationship with the incidence of diabetes, and the inflection point is 232.416. Subgroup analysis showed that the correlation was stronger in the population aged 20-30 (P for interaction<0.0001, HR 1.029, 95%CI:1.024 to 1.035), and the same trend was found in the following populations: age 30-40(HR=1.032), age 40-50(HR=1.029), HDL (high group) (HR=1.024 ), SBP<140(HR=1.025), DBP<90(HR=1.024), current drinker(HR=1.031), and ever drinker(HR=1.032).
Conclusion: TyG-BMI and incident diabetes had a non-linear positive relationship. Before and after TyG-BMI equals 232.416, the risk of diabetes increased by 2.9% and 1.6%, respectively, when TyG-BMI increased one unit. The findings emphasise the importance of baseline TyG-BMI as a measure for identification of patients at risk of diabetes early and improve their outcomes.
Keywords
■ TyG-BMI
■ incident diabetes
■ nonlinearity
Abbreviations
BMI: Body Mass Index; TyG: Triglycerideglucose Index; TyG-BMI: Triglyceride Glucose-body Mass Index; IR: Insulin Resistance; HRs: Hazard Ratios; 95%CIs: 95% Confidence Intervals; IDF: International Diabetes Federation; DM: Diabetes Mellitus; T2DM: Type 2 Diabetes; TG: Triglyceride; FPG: Fasting Plasma Glucose; LDL-C: Low-Density Lipoprotein Cholesterol; HDL-C: High-density Lipoprotein Cholesterol; TC: Total Cholesterol; BUN: Serum Urea Nitrogen; Scr: Serum Creatinine; AST: Aspartate Aminotransferase; ALT: Alanine Aminotransferase; SBP: Systolic Blood Pressure; DBP: Diastolic Blood Pressure; GAM: Generalized Additive Models; SD: Standard Deviation; IQR: Interquartile Range.
Introduction
Diabetes has become a critical worldwide healthy problem with high prevalence. China has the largest number of patients with diabetes in the world [1]. Diabetes Mellitus (DM) and its complications have resulted in a severe economic burden of mortality and disability. Insulin Resistance (IR) and the consequences of compensatory hyperinsulinemia are vital pathological mechanisms of diabetes mellitus and obesity [2]. Therefore, it is important to recognize IR before clinical manifestations of diabetes.
Recently, as Triglyceride Glucose Body Mass Index (TyG-BMI) combines Triglyceride (TG), Fasting Plasma Glucose (FPG), and obesity status, TyG-BMI has been considered to identify IR more reliably than TyG [3,4]. Triglycerideglucose (TyG) index, which is estimated using the formula Ln (fasting triglycerides (mg/dl) × fasting blood glucose (mg/dl)/2), is an alternative to identify insulin resistance in apparently healthy subjects [5-9]. TyG-BMI=TyG × BMI, which considered BMI on the basis of TyG [3]. Body mass index (BMI) is an easily detectable, inexpensive, and non- invasive measurement parameter closely related to IR. Patients with higher levels of TG, FBG, BMI may progress to diabetes over time [10-12]. Several cross-sectional studies have established the relationship between TyG-BMI and DM. At present, there are few cohort study articles about the relationship between TyG-BMI and diabetes.
In this study, we postulated that the TyG-BMI level might serve as an early predictor of incident DM in China. This study was performed based on a previously published data which identified the association of body mass index and age with incident diabetes [12]. To test our hypothesis, Cox proportional hazards regression analysis was used to evaluate the Hazard Ratio (HR) and 95% Confidence Interval (95% CI) of the relationship between TyG-BMI and diabetes events. Non-linear relationships were assessed using generalized additive models. Subgroup analysis helped comprehend other factors that may affect the relationship between TyG-BMI and diabetes in order to discover potential special populations. Our findings demonstrated that tracking the TyG-BMI level aids in the prediction of a diabetes risk. TyG-BMI should be taken into account for clinicians to plan and initiate the management strategies early to improve outcomes for participants with prediabetes.
Methodology
Methods including study population and design, data collection and measurements, statistical analysis are the same as the preprint of this manuscript [13].
■ Study population and design
In this population-based cohort analysis of a medical program established by the Rich Healthcare Group in China between 2010 and 2016, we investigated the effect of TyG- BMI on incident diabetes. We used data from the DATADRYAD website (www.datadryad. org), allowing others to use the database for free. In order to be consistent with the terms of service, this study quoted the data package shared by Chen. The database materials included the following variables: BMI, age, sex, smoking, drinking, family history of diabetes, High-Density Lipoprotein Cholesterol (HDL-C), Total Cholesterol (TC), Low-Density Lipoprotein Cholesterol (LDL-C), TG, Serum urea nitrogen(BUN), FPG, Serum Creatinine (Scr), Alanine Aminotransferase (ALT), Aspartate Aminotransferase (AST), Diastolic Blood Pressure (DBP), Systolic Blood Pressure (SBP), incident diabetes at follow up and follow- up time. The authors claimed that they had given up the copyright of the original paper and related ownership of the database. As for ethics approval, the study was a retrospective analysis approved by the rich healthcare group review committee.
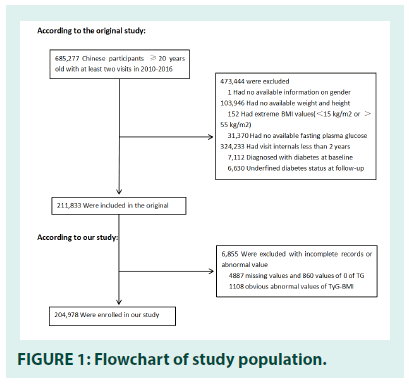
The initial study recruited 685,277 participants ≥ 20 years of age. They conducted at least two visits in 32 locations and 11 cities in China (Shanghai, Nanjing, Beijing, Suzhou, Changzhou, Shenzhen, Guangzhou, Chengdu, Hefei, Nantong, Wuhan) between 2010 and 2016. The data we got has been filtered. Subjects were excluded with the following conditions: (1) missing information about height, weight, fasting plasma glucose, and gender at baseline; (2) extreme BMI values (<15 kg/m2 or >55 kg/ m2); (3) persons were diagnosed with diabetes at baseline and were diagnosed with unclear diabetes status at follow-up; (4) visit intervals less than two years. Finally, in the original study, Ying kept 211,833 participants [12]. Missing values of baseline TG (n=4,887) and zero values of baseline TG(n=860) were excluded from the analysis cohort for further research. And then, TyG-BMI calculation formula was BMI × Ln(fasting triglycerides(mg/dl) × fasting blood glucose(mg/dl)/2). We excluded outliers of TyG- BMI (out of means minus or plus three standard deviation (SD) (n=1,108). Finally, a sum of 204978 participants was selected in our study. FIGURE 1 depicted the participants’ selection process.
■ Data collection and measurements
The original database contained participants’ clinical history and lifestyle factors based on a standardized questionnaire about lifestyle factors, demographic characteristics, family history of chronic disease, and personal medical history. Trained staff measured height, weight, and blood pressure. The body weight was measured in light clothing without shoes and was accurate to 0.1 kg. The measurement height was accurate to 0.1 cm. The body mass index was calculated by dividing the weight in kilograms by the height in meters. Standard mercury sphygmomanometers measured blood pressure. After fasting for at least 10 hours at each visit, fasting venous blood samples were collected. Triglyceride, LDL-C, HDL-C, total cholesterol, and plasma glucose levels were measured on an automatic analyzer (Beckman 5800). The glucose oxidase method was used to determine the plasma glucose level. The TyG index formula was Ln (fasting triglycerides (mg/dl) × fasting blood glucose (mg/dl)/2)). The target-independent variable is TyG-BMI, which equals the BMI × TyG index. The dependent variable is incident diabetes, defined as fasting plasma glucose ≥ 7 mmol/L, and/or self-reported during the follow-up. This is a retrospective cohort study, so it cuts down the possibility of selection bias and observation bias.
■ Statistical analysis
Normally distributed continuous variables were represented by the mean, Standard Deviation (SD), and non-normally distributed continuous variables were replaced by the median of the Interquartile Range (IQR). Categorical variables were expressed as frequencies and percentages. For handling missing values, missing continuous variables were replaced with a mean or median depending on the distribution. Missing categorical variables could be a new categorical group. Stratified by TyG-BMI index quartiles, statistical differences of the groups were described with Kruskal Wallis H (skewed distribution) test, one-way ANOVA (normal distribution), and chi-square test (categorical variables). In order to explore the relationship between TyG-BMI and the incidence of diabetes, univariate and multivariate Cox proportional hazards regression analysis was used to estimate Hazard Ratios (HRs) and 95% Confidence Intervals (CIs). According to the recommendation of the STROBE statement, We used three models: crude model; model I adjust for: Gender, Age, Smoking Status, DBP, SBP, Family History, Drinking Status; model II adjust for: Gender, Age, Smoking Status, DBP, SBP, Family History, Drinking Status, TC, HDL, LDL, AST, Scr, ALT. To ensure the robustness of the data analysis, we conducted a sensitivity analysis. The TyG-BMI was converted into a categorical variable and calculated the P for trend to perform the linear trend tests. The Cox proportional hazards regression model with cubic spline function was used to identify non-linear relationships. In addition, if there was an apparent smooth curve, the recursive method would automatically calculate the inflection point. The associations of TyG-BMI with incident diabetes in subgroups were also studied using a stratified linear regression model and likelihood ratio test to discover corrections and interactions. The subgroups were classified by age (20 to<30, 30 to<40, 40 to<50, 50 to<60, 60 to<70, ≥ 70), gender (male vs. female), HDL( low, middle, high, not recorded), LDL( low, middle, high, not recorded), SBP(<140, ≥ 140 ), DBP(<90, ≥ 90), Smoking status(current smoker, ever smoker, never smoker, not recorded), Drinking status(current drinker, ever drinker, never drinker, not recorded), Family history of diabetes(no, yes). By using the time to the first event for each endpoint, the Kaplan- Meier method was used to compare survival estimates and cumulative event rates.
All analyses were performed with the statistical software package R (http://www.R-project.org, The R Foundation) and Empower-Stats (http:// www.empowerstats.com, X and Y Solutions, Inc., Boston, MA). Significance was accepted at a two-tailed P<0.05.
Results
■ Baseline characteristics of the study participants
Of the 211,833 subjects recruited in the former study, 204,978 participants were included in the current analysis. The mean age of the population was 42.2 ± 12.7 years old, and 45.17% of participants were women. The average follow- up time was 3.1 ± 0.9 years, and 4093 subjects developed diabetes during the follow-up period.
The mean TyG-BMI was 158.7 ± 32.4, and the mean FPG, BMI, TG were 88.6 ± 10.9 mg/dl, 23.3 ± 3.3 kg/m2 and 24.2 ± 18.6 mg/ dl respectively. Individuals in the highest TyG- BMI group (Q4) were generally older than those in the lowest TyG-BMI group (Q1) and had higher BMI, FPG, TG, SBP, DBP, TC, ALT, SCR values. What’s more, as the value of TyG-BMI increased, the incidence of diabetes gradually increased. (Q1: 0.23% vs. Q2: 0.62% vs. Q3: 1.74% vs. Q4: 5.35%). Compared with the Q1 group, the Q4 group had lower HDL levels, higher AST and LDL levels, higher rates of drinking, smoking, family history (TABLE 1).
| TyG-BMI | Q1(≤ 167.01) | Q2(167.01 to ≤ 191.53) | Q3(191.53 to ≤ 219.26) | Q4(>219.26) | P-value |
|---|---|---|---|---|---|
| Participants | 51245 | 51244 | 51244 | 51245 | |
| Age(years) | 36.65 ± 10.06 | 41.26 ± 12.13 | 44.79 ± 13.08 | 46.16 ± 13.11 | <0.001 |
| BMI(kg/m2) | 19.45 ± 1.36 | 21.98 ± 1.20 | 24.11 ± 1.32 | 27.21 ± 2.14 | <0.001 |
| FPG(mmol/L) | 4.68 ± 0.53 | 4.84 ± 0.55 | 4.98 ± 0.58 | 5.17 ± 0.63 | <0.001 |
| TG(mmol/L) | 0.67 (0.52-0.86) | 0.91 (0.70-1.18) | 1.25 (0.96-1.65) | 1.90 (1.40-2.62) | <0.001 |
| TyG-BMI | 151.98 ± 10.59 | 179.21 ± 7.07 | 204.83 ± 7.94 | 244.14 ± 19.76 | <0.001 |
| SBP(mmHg) | 110.87 ± 13.45 | 116.16 ± 14.96 | 121.63 ± 15.68 | 127.36 ± 16.40 | <0.001 |
| DBP(mmHg) | 69.34 ± 9.12 | 72.00 ± 9.74 | 75.54 ± 10.30 | 79.63 ± 11.02 | <0.001 |
| TC(mmol/L) | 4.36 ± 0.79 | 4.60 ± 0.85 | 4.82 ± 0.88 | 5.05 ± 0.92 | <0.001 |
| ALT(U/L) | 13.00 (10.30,17.20) | 15.90 (12.00-21.90) | 20.00 (15.00-28.60) | 28.00 (19.30-41.70) | <0.001 |
| Scr(umol/L) | 63.93 ± 13.20 | 68.29 ± 15.49 | 72.58 ± 14.84 | 75.36 ± 15.64 | <0.001 |
| Gender | <0.001 | ||||
| Male | 14983 (29.24%) | 24120 (47.07%) | 33452 (65.28%) | 39551 (77.18%) | |
| Female | 36262 (70.76%) | 27124 (52.93%) | 17792 (34.72%) | 11694 (22.82%) | |
| Family history | <0.001 | ||||
| NO | 50327 (98.21%) | 50095 (97.76%) | 50187 (97.94%) | 50179 (97.92%) | |
| YES | 918 (1.79%) | 1149 (2.24%) | 1057 (2.06%) | 1066 (2.08%) | |
| HDL(mmol/L) | <0.001 | ||||
| Low | 4321 (8.43%) | 7255 (14.16%) | 11440 (22.32%) | 15213 (29.69%) | |
| Middle | 8289 (16.18%) | 10160 (19.83%) | 10449 (20.39%) | 9178 (17.91%) | |
| High | 14117 (27.55%) | 11538 (22.52%) | 8407 (16.41%) | 5957 (11.62%) | |
| Not record | 24518 (47.84%) | 22291 (43.50%) | 20948 (40.88%) | 20897 (40.78%) | |
| LDL(mmol/L) | <0.001 | ||||
| Low | 13027 (25.42%) | 10258 (20.02%) | 8164 (15.93%) | 7193 (14.04%) | |
| Middle | 8693 (16.96%) | 10309 (20.12%) | 10299 (20.10%) | 9935 (19.39%) | |
| High | 5080 (9.91%) | 8594 (16.77%) | 12194 (23.80%) | 13712 (26.76%) | |
| Not record | 24445 (47.70%) | 22083 (43.09%) | 20587 (40.17%) | 20405 (39.82%) | |
| AST(U/L) | <0.001 | ||||
| Low | 10327 (20.15%) | 8482 (16.55%) | 6057 (11.82%) | 3722 (7.26%) | |
| Middle | 7088 (13.83%) | 7635 (14.90%) | 7815 (15.25%) | 6243 (12.18%) | |
| High | 3699 (7.22%) | 5490 (10.71%) | 7956 (15.53%) | 11777 (22.98%) | |
| Not record | 30131 (58.80%) | 29637 (57.84%) | 29416 (57.40%) | 29503 (57.57%) | |
| Smoking status | <0.001 | ||||
| Current smoker | 1282 (2.50%) | 2161 (4.22%) | 3248 (6.34%) | 4841 (9.45%) | |
| Ever smoker | 298 (0.58%) | 507 (0.99%) | 768 (1.50%) | 893 (1.74%) | |
| Never smoker | 11551 (22.54%) | 11271 (21.99%) | 11017 (21.50%) | 10307 (20.11%) | |
| Not record | 38114 (74.38%) | 37305 (72.80%) | 36211 (70.66%) | 35204 (68.70%) | |
| Drinking status | <0.001 | ||||
| Current drinker | 108 (0.21%) | 209 (0.41%) | 386 (0.75%) | 615 (1.20%) | |
| Ever drinker | 1059 (2.07%) | 1850 (3.61%) | 2572 (5.02%) | 3234 (6.31%) | |
| Never drinker | 11964 (23.35%) | 11880 (23.18%) | 12075 (23.56%) | 12192 (23.79%) | |
| Not record | 38114 (74.38%) | 37305 (72.80%) | 36211 (70.66%) | 35204 (68.70%) |
TABLE 1: The baseline characteristics of participants.
■ Univariate analysis
The results of the univariate analysis were shown in TABLE 2. TABLE 2 showed that men were more likely to develop diabetes than women, and BMI, SBP, TG, FPG, TyG-BMI, DBP, TC, AST, ALT, LDL, Scr, age, drinking, smoking, and family history were all positively correlated with the incidence of diabetes.
| TyG-BMI | Q1(≤ 167.01) | Q2(167.01 to ≤ 191.53) | Q3(191.53 to ≤ 219.26) | Q4(>219.26) | P-value |
|---|---|---|---|---|---|
| Participants | 51245 | 51244 | 51244 | 51245 | |
| Age(years) | 36.65 ± 10.06 | 41.26 ± 12.13 | 44.79 ± 13.08 | 46.16 ± 13.11 | <0.001 |
| BMI(kg/m2) | 19.45 ± 1.36 | 21.98 ± 1.20 | 24.11 ± 1.32 | 27.21 ± 2.14 | <0.001 |
| FPG(mmol/L) | 4.68 ± 0.53 | 4.84 ± 0.55 | 4.98 ± 0.58 | 5.17 ± 0.63 | <0.001 |
| TG(mmol/L) | 0.67 (0.52-0.86) | 0.91 (0.70-1.18) | 1.25 (0.96-1.65) | 1.90 (1.40-2.62) | <0.001 |
| TyG-BMI | 151.98 ± 10.59 | 179.21 ± 7.07 | 204.83 ± 7.94 | 244.14 ± 19.76 | <0.001 |
| SBP(mmHg) | 110.87 ± 13.45 | 116.16 ± 14.96 | 121.63 ± 15.68 | 127.36 ± 16.40 | <0.001 |
| DBP(mmHg) | 69.34 ± 9.12 | 72.00 ± 9.74 | 75.54 ± 10.30 | 79.63 ± 11.02 | <0.001 |
| TC(mmol/L) | 4.36 ± 0.79 | 4.60 ± 0.85 | 4.82 ± 0.88 | 5.05 ± 0.92 | <0.001 |
| ALT(U/L) | 13.00 (10.30,17.20) | 15.90 (12.00-21.90) | 20.00 (15.00-28.60) | 28.00 (19.30-41.70) | <0.001 |
| Scr(umol/L) | 63.93 ± 13.20 | 68.29 ± 15.49 | 72.58 ± 14.84 | 75.36 ± 15.64 | <0.001 |
| Gender | <0.001 | ||||
| Male | 14983 (29.24%) | 24120 (47.07%) | 33452 (65.28%) | 39551 (77.18%) | |
| Female | 36262 (70.76%) | 27124 (52.93%) | 17792 (34.72%) | 11694 (22.82%) | |
| Family history | <0.001 | ||||
| NO | 50327 (98.21%) | 50095 (97.76%) | 50187 (97.94%) | 50179 (97.92%) | |
| YES | 918 (1.79%) | 1149 (2.24%) | 1057 (2.06%) | 1066 (2.08%) | |
| HDL(mmol/L) | <0.001 | ||||
| Low | 4321 (8.43%) | 7255 (14.16%) | 11440 (22.32%) | 15213 (29.69%) | |
| Middle | 8289 (16.18%) | 10160 (19.83%) | 10449 (20.39%) | 9178 (17.91%) | |
| High | 14117 (27.55%) | 11538 (22.52%) | 8407 (16.41%) | 5957 (11.62%) | |
| Not record | 24518 (47.84%) | 22291 (43.50%) | 20948 (40.88%) | 20897 (40.78%) | |
| LDL(mmol/L) | <0.001 | ||||
| Low | 13027 (25.42%) | 10258 (20.02%) | 8164 (15.93%) | 7193 (14.04%) | |
| Middle | 8693 (16.96%) | 10309 (20.12%) | 10299 (20.10%) | 9935 (19.39%) | |
| High | 5080 (9.91%) | 8594 (16.77%) | 12194 (23.80%) | 13712 (26.76%) | |
| Not record | 24445 (47.70%) | 22083 (43.09%) | 20587 (40.17%) | 20405 (39.82%) | |
| AST(U/L) | <0.001 | ||||
| Low | 10327 (20.15%) | 8482 (16.55%) | 6057 (11.82%) | 3722 (7.26%) | |
| Middle | 7088 (13.83%) | 7635 (14.90%) | 7815 (15.25%) | 6243 (12.18%) | |
| High | 3699 (7.22%) | 5490 (10.71%) | 7956 (15.53%) | 11777 (22.98%) | |
| Not record | 30131 (58.80%) | 29637 (57.84%) | 29416 (57.40%) | 29503 (57.57%) | |
| Smoking status | <0.001 | ||||
| Current smoker | 1282 (2.50%) | 2161 (4.22%) | 3248 (6.34%) | 4841 (9.45%) | |
| Ever smoker | 298 (0.58%) | 507 (0.99%) | 768 (1.50%) | 893 (1.74%) | |
| Never smoker | 11551 (22.54%) | 11271 (21.99%) | 11017 (21.50%) | 10307 (20.11%) | |
| Not record | 38114 (74.38%) | 37305 (72.80%) | 36211 (70.66%) | 35204 (68.70%) | |
| Drinking status | <0.001 | ||||
| Current drinker | 108 (0.21%) | 209 (0.41%) | 386 (0.75%) | 615 (1.20%) | |
| Ever drinker | 1059 (2.07%) | 1850 (3.61%) | 2572 (5.02%) | 3234 (6.31%) | |
| Never drinker | 11964 (23.35%) | 11880 (23.18%) | 12075 (23.56%) | 12192 (23.79%) | |
| Not record | 38114 (74.38%) | 37305 (72.80%) | 36211 (70.66%) | 35204 (68.70%) |
TABLE 2: The results of univariate analysis.
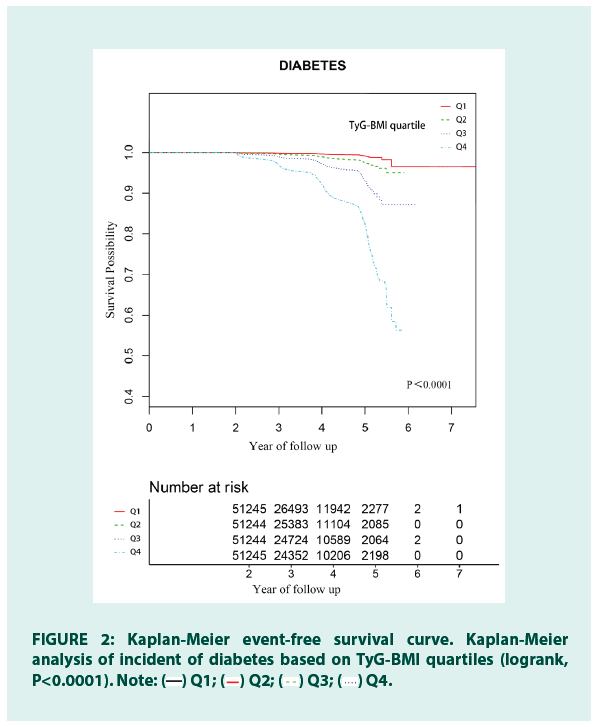
In FIGURE 2, the Kaplan Meier curves of the cumulative risk of diabetes events stratified by TyG-BMI showed that the cumulative risk of diabetes gradually increases with the increase of TyG-BMI. There was a significant difference in the risk of diabetes between the TyG-BMI quartile groups (log-rank test P<0.0001).
Figure 2: Kaplan-Meier event-free survival curve. Kaplan-Meier
analysis of incident of diabetes based on TyG-BMI quartiles (logrank,
P<0.0001). Note:  Q1;
Q1;  Q2;
Q2;  Q3;
Q3;  Q4.
Q4.
■ The multivariate analysis of TyG-BMI with DM risk
We applied Cox proportional hazards models to evaluate group differences in the association between TyG-BMI and incident diabetes, and TABLE 3 showed the unadjusted and adjusted models. In the crude model, TyG-BMI was positively correlated with the incidence of diabetes (HR=1.026, 95% Confidence Interval (CI):1.026 to 1.027, P<0.00001). We could draw the same conclusion in the model I(minimally adjusted model, adjusted DBP, SBP, gender, age, drinking status, smoking status, family history) and model II(fully adjusted model, adjusted DBP, SBP, gender, age, drinking status, smoking status, family history, TC, HDL, LDL, ALT, AST, SCR). Model I (HR=1.022, 95% CI: 1.021 to 1.023, P<0.00001), model II (HR=1.023, 95% CI: 1.022 to 1.024, P<0.00001), respectively. We also performed a sensitivity analysis taking TyG-BMI as a categorical variable (quartile) at the same time and calculating P for trend. The result was consistent with that of TyG-BMI as a continuous variable (trend P<0.00001). In the fully adjusted model (model II), the risk of diabetes in the Q4 group increased by 10.261 times compared to the Q1 group, and the trend in the quartile was significant (trend P<0.00001).
| Variable | Crude model (HR,95%CI,P) | Model I (HR,95%CI,P) | Model II (HR,95%CI,P) |
|---|---|---|---|
| TyG-BMI | 1.026 (1.026, 1.027)<0.00001 | 1.022 (1.021, 1.023)<0.00001 | 1.023 (1.022, 1.024)<0.00001 |
| TyG-BMI(quartile) | |||
| Q1 | Ref | Ref | Ref |
| Q2 | 2.967 (2.405, 3.660)<0.00001 | 2.020 (1.636, 2.495)<0.00001 | 2.049 (1.659, 2.532)<0.00001 |
| Q3 | 8.089 (6.675, 9.801)<0.00001 | 4.195 (3.450, 5.102)<0.00001 | 4.437 (3.644, 5.403)<0.00001 |
| Q4 | 23.876 (19.854, 28.714)<0.00001 | 10.562 (8.730, 12.779)<0.00001 | 11.261 (9.277, 13.668)<0.00001 |
| P for trend | <0.00001 | <0.00001 | <0.00001 |
TABLE 3: Relationship between TyG-BMI and the incident of diabetes in different models.
■ The analyses of the non-linear relationship
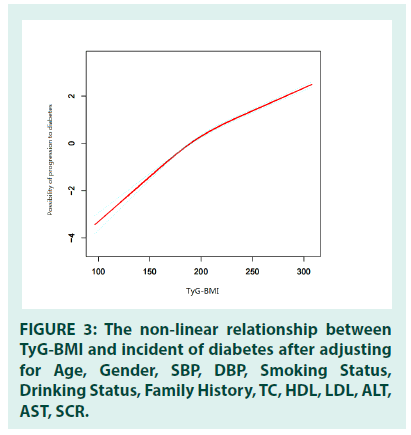
Since TyG-BMI was a continuous variable, we used the Generalized Additive Model (GAM) to identify the nonlinear relationship between TyG- BMI and the incidence of diabetes (adjusted gender, age, DBP, SBP, drinking status, smoking status, family history, TC, HDL, LDL, ALT, AST, Scr). In addition, by using two-piecewise linear regression and recursive algorithm, the inflection point of TyG-BMI was calculated as 232.416. The association between TyG-BMI and incident diabetes was positive on either side of the inflection point. The positive potency was slightly weaker on the right side (HR=1.016, 95%CI: 1.014 to 1.018, P<0.0001) of the inflection point than on the left (HR=1.029, 95%CI: 1.027 to 1.031, P<0.0001) (TABLE 4 and FIGURE 3).
| Incident of diabetes (HR,95%CI, P) | |
|---|---|
| Fitting model by standard linear regression | 1.023 (1.022, 1.024)<0.0001 |
| Fitting model by two-piecewise linear regression | - |
| Inflection point of TyG-BMI | 232.416 |
| <232.416 | 1.029 (1.027, 1.031)<0.0001 |
| >232.416 | 1.016 (1.014, 1.018)<0.0001 |
| P for log likelihood ratio test | <0.001 |
TABLE 4: The result of two-piecewise linear regression model.
Figure 3: The non-linear relationship between TyG-BMI and incident of diabetes after adjusting for Age, Gender, SBP, DBP, Smoking Status, Drinking Status, Family History, TC, HDL, LDL, ALT, AST, SCR.
■ The results of subgroup analyses
TABLE 5 was the subgroup analysis for the association of TyG-BMI with diabetes incidence to explore other risks. The participants were divided into subgroups according to gender, age, HDL, LDL, SBP, DBP, family history of diabetes, drinking status, and smoking status. The association between TyG-BMI and diabetes risk was stable in family history, smoking status, and gender of patients (all P values for interaction>0.05). In contrast, we observed a number of interactions, including age, HDL, LDL, SBP, DBP, Drinking status (all P values of interaction<0.05). The relationship between TyG- BMI and diabetes was stronger in people with age 20-30(HR 1.029, 95%CI :1.024 to 1.035), age 30-40(HR 1.032, 95%CI :1.029 to 1.034), age 40-50(HR 1.029, 95%CI :1.027 to 1.031), HDL(high group) (HR=1.024, 95%CI: 1.022 to 1.026), SBP<140(HR=1.025, 95%CI:1.024 to 1.027), DBP<90(HR=1.024, 95%CI:1.023 to 1.025), current drinker(HR=1.031, 95%CI: 1.022 to 1.041) and ever drinker(HR=1.032, 95%CI: 1.027 to 1.037). In addition, the relationship between TyG-BMI and diabetes risk was weaker in the people with age 60-70 (HR 1.015, 95%CI :1.013 to 1.017), age ≥ 70(HR 1.013, 95%CI :1.011 to 1.016), HDL(low group) (HR=1.020, 95%CI: 1.018 to 1.021), LDL(middle group) (HR=1.022, 95%CI: 1.020 to 1.024), LDL(high group) (HR=1.021, 95%CI: 1.019 to 1.023), SBP ≥ 140(HR=1.017, 95%CI:1.015 to 1.019), DBP ≥ 90(HR=1.018, 95%CI:1.016 to 1.021) and never drinker (HR=1.022, 95%CI: 1.020 to 1.024).
| Characteristic | No. of participants | Effect size(HR,95%CI,P) | P for interaction |
|---|---|---|---|
| Age(years) | <0.0001 | ||
| 20 to<30 | 27301 | 1.029 (1.024, 1.035)<0.0001 | |
| 30 to<40 | 80043 | 1.032 (1.029, 1.034)<0.0001 | |
| 40 to<50 | 43888 | 1.029 (1.027, 1.031)<0.0001 | |
| 50 to<60 | 29252 | 1.023 (1.021, 1.025)<0.0001 | |
| 60 to<70 | 17278 | 1.015 (1.013, 1.017)<0.0001 | |
| ≥ 70 | 7216 | 1.013 (1.011, 1.016)<0.0001 | |
| Gender | 0.9252 | ||
| Male | 112106 | 1.023 (1.022, 1.024) 0.9252 | |
| Female | 92872 | 1.023 (1.021, 1.024) 0.9252 | |
| HDL(mmol/L) | 0.0012 | ||
| Low | 38229 | 1.020 (1.018, 1.021)<0.0001 | |
| Middle | 38076 | 1.023 (1.021, 1.026)<0.0001 | |
| High | 40019 | 1.024 (1.022, 1.026)<0.0001 | |
| Not recorded | 88654 | 1.024 (1.023, 1.026)<0.0001 | |
| LDL(mmol/L) | 0.0222 | ||
| Low | 38642 | 1.023 (1.021, 1.025) <0.0001 | |
| Middle | 39236 | 1.022 (1.020, 1.024) <0.0001 | |
| High | 39580 | 1.021 (1.019, 1.023) <0.0001 | |
| Not recorded | 87520 | 1.024 (1.023, 1.026) <0.0001 | |
| SBP(mmHg) | <0.0001 | ||
| <140 | 185128 | 1.025 (1.024, 1.027)<0.0001 | |
| ≥140 | 20958 | 1.017 (1.015, 1.019)<0.0001 | |
| DBP(mmHg) | <0.0001 | ||
| <90 | 189459 | 1.024 (1.023, 1.025)<0.0001 | |
| ≥ 90 | 16627 | 1.018 (1.016, 1.021)<0.0001 | |
| Smoking status | 0.1151 | ||
| Current smoker | 11532 | 1.025 (1.022, 1.028)<0.0001 | |
| Ever smoker | 2466 | 1.029 (1.021, 1.036)<0.0001 | |
| Never smoker | 44146 | 1.023 (1.021, 1.025)<0.0001 | |
| Not recorded | 146834 | 1.022 (1.021, 1.023)<0.0001 | |
| Drinking status | 0.0002 | ||
| Current drinker | 1318 | 1.031 (1.022, 1.041)<0.0001 | |
| Ever drinker | 8715 | 1.032 (1.027, 1.037)<0.0001 | |
| Never drinker | 48111 | 1.022 (1.020, 1.024)<0.0001 | |
| Not recorded | 146834 | 1.022 (1.021, 1.023)<0.0001 | |
| Family history of diabetes | 0.1543 | <0.0001 | |
| No | 200788 | 1.023 (1.022, 1.024) | <0.0001 |
| Yes | 4190 | 1.020 (1.016, 1.024) | <0.0001 |
TABLE 5: Effect size of TyG-BMI on diabetes in prespecified and exploratory subgroups.
Discussion
Our results suggest that TyG-BMI is associated with diabates. In view of the fact that IR acts as an core agent of T2DM and that diabetes is believed to be an metabolic disorder disease, these findings are not surprising. During 204978 person years of follow-up, we found a positive non-linear relationship between TyG-BMI and diabetes. Results remained essentially the same when we adjusted for gender, age, DBP, TC, LDL, HDL, AST, ALT, Scr, smoking, SBP. The inflection point value was 232.416, which was consistent in the direction before and after the inflection point, but the effect value was not completely consistent (Right (HR: 1.016, 95% CI: 1.014-1.018, P<0.0001), (left (HR: 1.029, 95% CI: 1.027-1.031, P<0.0001)). To be classified as an metabolic disease, the progress of the disorder must be slowed down or prevented by metabolic therapy. Our data indicate that TyG-BMI, as a new IR predictor index, can predict the occurrence of DM and provide important prognostic information.
The subgroup analysis showed that the association between TyG-BMI and diabetes risk were stronger in the following groups: age 20- 30(HR=1.029, 95%CI:1.024 to 1.035), age 30-40(HR=1.032, 95%CI:1.029 to 1.034), age 40-50(HR 1.029, 95%CI:1.027 to 1.031), high HDL(HR=1.024, 95%CI: 1.022 to 1.026 ), SBP<140(HR=1.025, 95%CI:1.024 to 1.027), DBP<90 (HR=1.024, 95%CI:1.023 to 1.025), current drinker(HR=1.031, 95%CI: 1.022 to 1.041), and ever drinker(HR=1.032, 95%CI: 1.027 to 1.037).
The main events in developing type 2 diabetes are decreased β cell function and insulin resistance. Insulin resistance can cause hyperinsulinemia, which in turn can aggravate insulin resistance [2]. It is generally accepted that insulin resistance is closely related to type 2 diabetes risk. Clinically, the gold standard of insulin resistance is the glucose clamp test, which is inconvenient and expensive. Although Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) has a wide range of clinical applications, its application is limited due to its relatively high cost and low repeatability. Simental-Mendía Proposed the concept of the TyG index, which indicated that the TyG index could be used as an alternative index of insulin resistance in healthy subjects. Many studies have shown that the TyG index is a good alternative marker of insulin resistance [4,14-21]. Compared with the HOMA-IR index, the TyG index has higher sensitivity in recognizing insulin resistance [19]. TyG index is regular and easy to get, including FBG and TG, which have been associated with diabetes risk [8,9,18,22]. In addition to the TyG index, the relationship between obesity and diabetes is also well documented. The incidence rate of type 2 diabetes is lower in non-obese patients [23]. BMI is a simple, economical, and helpful indicator of general obesity. A cross- sectional study of the Taiwan population shows that TyG-BMI is an effective marker for early recognition of insulin resistance [3]. A recent study involving 511 participants showed that TyG-BMI is a stronger IR predictor than TyG- WC [3]. In a Nigerian cross-sectional study, in all 473 participants, TyG-BMI shows larger AUC for metabolic syndrome detection (0.838, 95% CI: 0.802–0.870) than TyG index (0.796, 95% CI: 0.757-0.831). After adjusting for age, gender, smoking, DBP and SBP, only the TyG index and TyG-BMI significantly predicted metabolic syndrome in men [24]. Previous studies have examined the association of the IR indicators such as BMI, TyG with DM risk and their performance in DM risk prediction [8,9,18,22,24]. These conclusions are similar to ours. Firstly, consistent with previous studies, TyG-BMI was positively correlated with incident diabetes. Our findings accord with those of three other studies [14-16].Then we further analyzed and discovered the curvilinear relationship. After adjusting for confounding factors in our study, the association between TyG-BMI and incident diabetes was nonlinear. The inflection point of GAM was 232.416 after adjusting for potential confounding factors (gender, age, DBP, LDL, TC, HDL, AST, ALT, Scr, smoking, SBP). We found that the inflection point had a stronger relationship on the left side of the inflection point. Therefore, controlling TyG-BMI is more valuable for reducing diabetes risk under the inflection point.
Our study has some strengths: (1) As far as we know, this is the first study to evaluate the association between TyG-BMI and diabetes events in the Chinese people based on our preprint outcome; (2) Compared with other researches, our sample size is relatively larger, which can better represent the Chinese people; (3) This study was a retrospective cohort study, which reduced selection and observation biases (4) We found the non-linear relationship and made a deeper discussion, and there are also more confounding factors for adjustment; (5) To make the results more robust, TyG-BMI was treated both as a continuous and categorical variable.
The study also has some potential limitations: (1) The data was from the Rich Healthcare Group in China, representing the Chinese population, and couldn’t be extended to other races and particular groups like pregnant women and children. (2) This research was a secondary analysis based on the published data, and variables were limited to the data of the original study. Other important variables such as medication history, hip circumference, hemoglobin A1C, physical activity, dietary factors were not included. (3) The incidence of diabetes may underestimate because of the study’s diabetes definition, which did not conduct a 2-hour oral glucose tolerance test. But for such a large cohort, improving participants’ oral glucose tolerance test is a considerable project. (4) The study did not differentiate diabetes types. But these conclusions may be more appropriate to type 2 diabetes, which accounts for about 90% of diabetic patients. (5) According to TyG-BMI, we only measured it at baseline, not measured over time. We can consider more variables and longer follow-up times in the future and adopt more sophisticated strategies.
Conclusion
The association between TyG-BMI and incident diabetes is positive and nonlinear after correcting the related confounding factors. The inflection point was 232.416. The relationship between TyG-BMI and diabetes is the most significant on the left side of the inflection point. TyG- BMI and incident diabetes had a non-linear positive relationship. Before and after TyG-BMI equals 232.416, the risk of diabetes increased by 2.9% and 1.6%, respectively, when TyG-BMI increased one unit. The findings emphasise the importance of baseline TyG-BMI as a measure for identification of patients at risk of diabetes early and improve their outcomes.
Declarations
■ Ethics approval and consent to participate
In the previously published article, Ying Chen has clearly stated that: the study was conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from all Participants. Original study was approved by the Rich Healthcare Group Review Board, and the information was retrieved retrospectively.
■ Consent for publication
Not applicable.
■ Availability of data and materials
Data can be downloaded from ‘DATADRYAD’ database (www.Datadryad.org).
■ Competing interests
The authors declare that they have no competing interests.
■ Funding
This study was also supported in part by the International Cooperative Research Project of Shenzhen Municipal Science and Technology Innovation Council (accounts GJHZ20180416164814621).
■ Authors’ contributions
Fan Yang and Xiaohan Ding contributed to the study design, analyzed the data and drafted the manuscript. Zhuangsen CHEN, Yan Liao, Miaoling CHEN contributed to review the manuscript and took part in the interpretation and discussion of results. Weili YAO and Qian LIANG approved the final version and oversaw the progress of the project. Xinyu WANG and Haofei HU had full access to all the data in the study and had primary responsibility for the final content. All authors read and approved the final the manuscript.
Acknowledgement
Not applicable.
References
- Czech MP. Insulin Action and Resistance in Obesity and Type 2 Diabetes. Nat Med. 23(7):804-14(2017).
- Er LK, Wu S, Chou HH, et al. Triglyceride Glucose-body Mass Index Is a Simple and Clinically Useful Surrogate Marker for Insulin Resistance in Nondiabetic Individuals. PLoS One. 11(3):e0149731 (2016).
- Zeng ZY, Liu SX, Xu H, et al. Association of Triglyceride Glucose Index and Its Combination of Obesity Indices with Prehypertension in Lean Individuals: A Cross‐sectional Study of Chinese Adults. J Clin Hypertens. 22(6):1025-32 (2020).
- Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The Product of Fasting Glucose and Triglycerides as Surrogate for Identifying Insulin Resistance in Apparently Healthy Subjects. Metab Syndr Relat Disord. 6(4):299-304 (2008).
- Lee SH, Kwon HS, Park YM, et al. Predicting the Development of Diabetes Using the Product of Triglycerides and Glucose: The Chungju Metabolic Disease Cohort (Cmc) Study. PLoS One. 9(2):e90430 (2014).
- Lee JW, Lim NK, Park HY. The Product of Fasting Plasma Glucose and Triglycerides Improves Risk Prediction of Type 2 Diabetes in Middle-aged Koreans. BMC Endocr Disord. 18(1):1-0 (2018).
- Low S, Khoo KC, Irwan B, et al. The role of triglyceride glucose index in development of Type 2 diabetes mellitus. Diabetes Res Clin Pract. 143:43-9 (2018).
- Li X, Li G, Cheng T, et al. Association Between Triglyceride-glucose Index and Risk of Incident Diabetes: A Secondary Analysis Based on a Chinese Cohort Study. Lipids Health Dis. 19(1):236 (2020).
- Mackey RH, Mora S, Bertoni AG, et al. Lipoprotein Particles and Incident Type 2 Diabetes in the Multi-ethnic Study of Atherosclerosis. Diabetes care. 38(4):628-36 (2015).
- Balkau B, Soulimane S, Lange C, et al. Are the Same Clinical Risk Factors Relevant for Incident Diabetes Defined by Treatment, Fasting Plasma Glucose, and Hba1c?. Diabetes Care. 34(4):957-9 (2011).
- Chen Y, Zhang XP, Yuan J, et al. Association of Body Mass Index and Age with Incident Diabetes in Chinese Adults: A Population-based Cohort Study. BMJ open. 8(9):e021768 (2018).
- Fan Yang XDZC. Association of Triglyceride Glucose-Body Mass Index and Incident Diabetes Mellitus: A Secondary Retrospective Analysis Based On a Chinese Cohort Study. BMJ open. 9(2):1-6 (2021).
- Wang X, Liu J, Cheng Z, et al. Triglyceride Glucose-body Mass Index and the Risk of Diabetes: A General Population-based Cohort Study. Lipids Health Dis. 20(1):1-0 (2021).
- Zheng S, Shi S, Ren X, et al. Triglyceride Glucose-waist Circumference, a Novel and Effective Predictor of Diabetes in First-degree Relatives of Type 2 Diabetes Patients: Cross-sectional and Prospective Cohort Study. J Transl Med. 14(1):260 (2016).
- Ramírez-Vélez R, Pérez-Sousa MÁ, González-Ruíz K, et al. Obesity-and Lipid-related Parameters in the Identification of Older Adults with a High Risk of Prediabetes According to the American Diabetes Association: An Analysis of the 2015 Health, Well-being, and Aging Study. Nutrients. 11(11):2654 (2019).
- Alejandro EU, Gregg B, Blandino-Rosano M, et al. Natural History of Β-cell Adaptation and Failure in Type 2 Diabetes. Mol Aspects Med. 42:19-41 (2015).
- Zhang M, Wang B, Liu Yet al. Cumulative Increased Risk of Incident Type 2 Diabetes Mellitus with Increasing Triglyceride Glucose Index in Normal-weight People: The Rural Chinese Cohort Study. Cardiovasc Diabetol. 16(1):1-1 (2017).
- Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, et al. The Product of Triglycerides and Glucose, a Simple Measure of Insulin Sensitivity. Comparison with the Euglycemic Hyperinsulinemic Clamp. J Clin Endocrinol Metab. 95(7):3347-51 (2010).
- Guerrero-Romero F, Villalobos-Molina R, Jiménez-Flores JR, et al. Fasting Triglycerides and Glucose Index as a Diagnostic Test for Insulin Resistance in Young Adults. Arch Med Res. 47(5):382-7(2016).
- Mohd Nor NS, Lee S, Bacha F, et al. Triglyceride Glucose Index as a Surrogate Measure of Insulin Sensitivity in Obese Adolescents with Normoglycemia, Prediabetes, and Type 2 Diabetes Mellitus: Comparison with the Hyperinsulinemic–euglycemic Clamp. Pediatr Diabetes. 17(6):458-65(2016).
- Wang B, Zhang M, Liu Y, et al. Utility of Three Novel Insulin Resistance‐related Lipid Indices for Predicting Type 2 Diabetes Mellitus among People with Normal Fasting Glucose in Rural China. J Diabetes. 10(8):641-52 (2018).
- Abdullah A, Peeters A, de Courten M, et al. The Magnitude of Association between Overweight and Obesity and the Risk of Diabetes: A Meta-analysis of Prospective Cohort Studies. Diabetes Res Clin Pract. 89(3):309-19 (2010).
- Raimi TH, Dele-Ojo BF, Dada SA, et al. Triglyceride-glucose Index and Related Parameters Predicted Metabolic Syndrome in Nigerians. Metab Syndr Relat Disord. 19(2):76-82 (2021).
- Teufel F, Seiglie JA, Geldsetzer P, et al. Body-mass Index and Diabetes Risk in 57 Low-income and Middle-income Countries: A Cross-sectional Study of Nationally Representative, Individual-level Data in 685 616 Adults. Lancet. 398(10296):238-48 (2021).