Research Article - International Journal of Clinical Rheumatology (2018) Volume 13, Issue 1
Association of weight loss with improved disease activity in patients with rheumatoid arthritis: A retrospective analysis using electronic medical record data
- *Corresponding Author:
- Jeffrey A. Sparks
Department of Medicine
Division of Rheumatology
Immunology and Allergy
Brigham and Women’s Hospital
Boston, MA, USA
E-mail: jsparks@bwh.harvard.edu
Abstract
Background: Objective: To evaluate the association between weight loss and rheumatoid arthritis (RA) disease activity.
Methods: We conducted a retrospective cohort study of RA patients seen at routine clinic visits at an academic medical center, 2012-2015. We included patients who had ≥2 clinical disease activity index (CDAI) measures. We identified visits during follow-up where the maximum and minimum weights occurred and defined weight change and CDAI change as the differences of these measures at these visits. We defined disease activity improvement as CDAI decrease of ≥5 and clinically relevant weight loss as ≥5 kg. We performed logistic regression analyses to establish the association between improved disease activity and weight loss and baseline BMI category (≥25 kg/m2 or <25 kg/m2). We built linear regression models to investigate the association between continuous weight loss and CDAI change among patients who were overweight/obese at baseline and who lost weight during follow-up.
Results: We analyzed data from 174 RA patients with a median follow-up of 1.9 years (IQR 1.3-2.4); 117 (67%) were overweight/obese at baseline, and 53 (31%) lost ≥5 kg during follow-up. Patients who were overweight/obese and lost ≥5 kg had three-fold increased odds of disease activity improvement compared to those who did not (OR 3.03, 95%CI 1.18-7.83). Among those who were overweight/obese at baseline, each kilogram weight loss was associated with CDAI improvement of 1.15 (95%CI 0.42-1.88). Our study was limited by using clinical data from a single center without fixed intervals for assessments.
Conclusion: Clinically relevant weight loss (≥5 kg) was associated with improved RA disease activity in the routine clinical setting. Further studies are needed for replication and to evaluate the effect of prospective weight loss interventions on RA disease activity.
Keywords
obesity, rheumatoid arthritis, weight loss, disease activity
Introduction
Obesity, defined by the World Health Organization as body mass index (BMI) ≥30 kg/m2, is an epidemic that currently affects 34.9% of adults in the U.S. [1,2]. Obesity increases the risk of developing chronic diseases such as hypertension, diabetes mellitus, coronary heart disease, and rheumatoid arthritis (RA) [3,4].
The relationship between obesity, weight loss, and disease control has been investigated among patients already diagnosed with chronic diseases. Health benefits of weight loss (ranging from 1.3-6.4 kg) in chronic diseases include improvements in cholesterol levels, decreased risk of cardiovascular events, and improved quality of life [5-7]. Among patients with hypertension, patients that lost ≥5 kg had improved blood pressure [8]. Weight loss of ≥5% was associated with improved glycemic control in patients with diabetes mellitus [9,10]. In patients with osteoarthritis, weight loss of ≥5% was associated with improvement in joint symptoms [11]. Weight loss of ≥5% was also associated with low/remission disease activity in patients with psoriatic arthritis [12]. However, the effect of weight loss on RA disease activity has been less studied. Most prior studies in RA investigating differences in disease activity compared patients with obesity to patients with normal BMI.
Previous studies performed among patients with RA have associated obesity with increased disease activity scores using 28 joints (DAS28) as well as worsened modified health assessment questionnaire (mHAQ). DAS28 is a validated measure of RA disease activity that combines tender and swollen joint count with laboratory measures of inflammation, either erythrocyte sedimentation rate (ESR) or C-reactive protein (CRP) [13]. These serum inflammatory markers are known to be higher in obese individuals [14]. Thus, it is possible that obese patients have increased DAS28 scores on the basis of obesity-related elevations of ESR or CRP independent of RA disease activity [15]. Using an RA disease activity measure that does not include these serum inflammatory markers, such as the clinical disease activity index (CDAI), may therefore be preferred over DAS28 when comparing RA patients with obesity to those without obesity.
The association of obesity with worsened RA outcomes compared to those with normal BMI suggests that weight loss may improve RA disease activity. A prior study reported marked improvements in RA disease activity after bariatric surgery [16]. However, these findings may not apply to other patients with RA in the routine clinical setting, since the magnitude of weight loss after bariatric surgery is greater than would be expected for non-surgical weight loss [16]. Therefore, we aimed to investigate the effect of weight loss on RA disease activity in a routine clinical setting, using the CDAI a disease activity measure that does not rely on serum inflammatory markers. We hypothesized that weight loss would be associated with improved RA disease activity.
Methods
Study population
We identified patients with RA in the electronic medical record at a single academic medical center, Brigham and Women’s Hospital (Boston, Massachusetts), using a previously validated algorithm [17]. Medical record review confirmed RA according to the 2010 ACR/ EULAR classification criteria [18]. All aspects of the study were approved by the Partners HealthCare Institutional Review Board.
Study design
We performed a retrospective cohort study of patients with RA that had at least two CDAI measures obtained during routine clinical care with corresponding weight measures at those clinic visits. CDAI is a measure of RA disease activity that quantifies RA disease activity on a scale of 0-76 (higher score indicating higher disease activity), and is calculated by summing the tender joint count and swollen joint counts of 28 joints as well as physician and patient global assessment on a 0-10 scale [19].
Data collection
Clinic visits for this study were identified as any encounter that had CDAI measured with corresponding weight measures also recorded within one week of that CDAI. Since our goal was to assess both weight and disease activity changes, all patients in this study were required to have at least two clinic visits in order to be included. For patients with more than two clinic visits with CDAI and weight measures, data were collected at each eligible clinic visit. Data on exposures, outcomes, and covariates were collected at each clinic visit using detailed medical record review.
Definitions of weight and CDAI changes
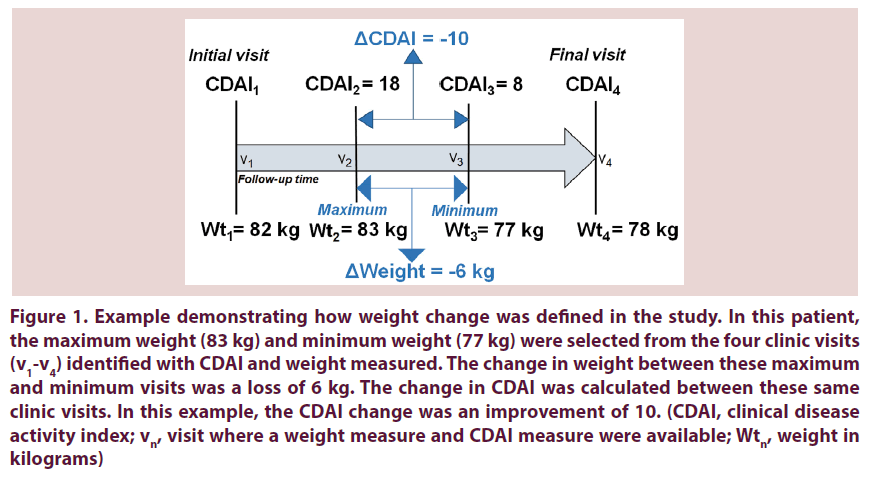
To calculate weight change, we selected the maximum and minimum weights (in kilograms) recorded for each subject among all visit dates that CDAI and weight were measured. We determined which of the maximum and minimum weights occurred first to order them as “initial” or “subsequent”. We calculated weight change as: ΔWeight=Subsequent weight–Initial weight. For example, if the maximum weight during the entire follow-up occurred at the second visit and the minimum weight during the entire follow-up occurred at the third clinic visit, then the weight change was calculated between these two visits. Since the maximum weight was at the initial visit, this patient would have ΔWeight<0 and would be treated as having lost weight. Weight at any other clinic visit besides the maximum and minimum weight visits was not included in the calculations. If all weights were identical, the patient was deemed as having no weight change (ΔWeight=0) and we considered the first and last visits to calculate the corresponding CDAI change. If there were multiple visits with the same minimum or maximum weights, we chose the visits which maximized follow-up duration. We similarly defined the CDAI change as the difference between CDAI scores at the same clinic visits used to define weight change. Figure 1 further illustrates the definition of weight and CDAI change that we used in this study.
Figure 1. Example demonstrating how weight change was defined in the study. In this patient, the maximum weight (83 kg) and minimum weight (77 kg) were selected from the four clinic visits (v1-v4) identified with CDAI and weight measured. The change in weight between these maximum and minimum visits was a loss of 6 kg. The change in CDAI was calculated between these same clinic visits. In this example, the CDAI change was an improvement of 10. (CDAI, clinical disease activity index; vn, visit where a weight measure and CDAI measure were available; Wtn, weight in kilograms)
Categorization of weight loss
Our primary exposure was weight loss ≥5 kg as a binary variable among those who were overweight or obese. We chose this threshold of clinically relevant weight loss due to previously reported health benefits of weight loss above this threshold in other chronic diseases [5,8]. We used the WHO classification of BMI categories for underweight (<18.5 kg/m2), normal weight (18.5 to <25.0 kg/m2), overweight (25.0 to <30 kg/m2), and obese (≥30.0 kg/m2) [20]. As previously described, we used the mode of the height of all measures when calculating BMI since patients may have had slightly different heights recorded at different visits [21]. Since weight loss would typically only be recommended for those who were overweight or obese, we included the BMI category at baseline in our analysis.
Disease activity outcomes
The primary dichotomous outcome was a decrease in CDAI by ≥5 points. Since all measures were obtained through routine clinical care, the treating rheumatologist performed all joint counts and physician global assessments in this study. We chose this dichotomous outcome based on the minimal clinically important difference of CDAI improvement reported in prior literature [22].
Covariates
We collected covariate data at the baseline visit, including sociodemographics, lifestyles, comorbidities, RA characteristics, and medication usage. Sociodemographic covariates included age, sex, race (categorized as white or non-white), education (dichotomized as greater than high school education or high school degree or lower), smoking (dichotomized as ever or never), and BMI categories. We considered the three most prevalent comorbidities recorded: osteoarthritis, hypothyroidism, and hypertension. RA disease-specific characteristics included duration of RA, and RA serologic status (seropositive defined as positive rheumatoid factor [RF] and/or anti-cyclic citrullinated protein [anti-CCP]; seronegative as both RF and anti-CCP negative). Laboratory values included CRP. Medication use included biologic and non-biologic disease-modifying antirheumatic drugs (DMARDs), glucocorticoids, non-steroidal anti-inflammatory drugs, and opioids.
Statistical analysis
We calculated descriptive statistics including frequencies for categorical variables, mean and standard deviation (SD) for continuous variables with normal distributions, and median, range, and interquartile range (IQR) for continuous variables with non-normal distributions. We calculated these statistics for the entire study sample and then stratified by the primary exposure of BMI category and weight loss ≥5 kg. We performed bivariate analyses to examine whether covariates including sex, age, RA duration, smoking status, serologic status, and steroid use were associated with the exposure and outcome. We used t-tests for continuous normally distributed variables, Wilcoxon rank-sum tests for continuous non-normally distributed variables, chi-square tests for categorical variables, and Fisher’s exact tests for categorical variables with small cell sizes. We evaluated these covariates as possible confounders due to their associations with RA disease activity in prior literature [23-27].
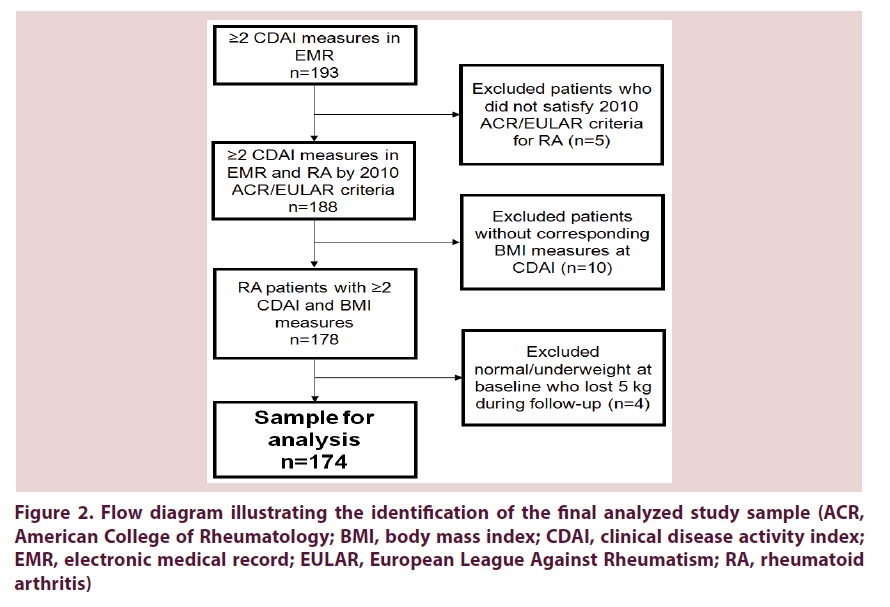
In the primary analysis investigating a threshold of weight loss, we used logistic regression to estimate the odds ratios (OR) and 95% confidence intervals (CI) for the binary outcome of improved disease activity (ΔCDAI ≤ -5 or not) according to BMI category at baseline and weight loss of ≥5 kg. We also included the BMI categories of overweight/obesity and normal weight in our primary analysis, since weight loss would not be recommended to patients with normal or underweight BMI. While we did not have data on the reason for weight loss, our goal was to identify patients who may have lost weight voluntarily. Therefore, we did not analyze four patients who were normal/underweight and lost ≥5 kg, since weight loss would not be recommended for these patients and may have been indicative of pathologic, rather than voluntary, weight loss. The three categories in the primary exposure variable therefore consisted of: BMI ≥25 kg/m2 and did not lose 5 kg; BMI ≥25 kg/m2 and lost ≥5 kg; and BMI <25 kg/m2 and did not lose 5 kg Figure 2.
We initially performed logistic regression analysis without adjustment. In the main analysis using a multivariable logistic regression model, we adjusted for age, sex, and baseline CDAI. Since there were only 10 outcomes in the category of BMI ≥25 kg/m2 and lost ≥5 kg, we were limited in the number of covariates that we could include in a multivariable model. Therefore, we performed sensitivity analyses to examine the possible confounding effect of other variables. In these analyses, we substituted the sex variable with the following possible confounders measured at the initial visit in individual models: steroid use, DMARD use, serologic status, smoking status (ever vs. never), follow-up time, and osteoarthritis. The effect size of the BMI ≥25 kg/m2 and lost ≥5 kg category was similar in all models (each of these models affected the OR by <10%), so we reported the model adjusting for age, sex, and baseline CDAI as the final multivariable model.
As a secondary analysis, we analyzed weight loss in kilograms as a continuous variable, among the subset of patients who were overweight or obese at baseline in order to investigate a dosedependent response of weight loss. In this analysis, we used linear regression to estimate the β coefficient and 95% CI for the association between ΔWeight and ΔCDAI among those who were overweight/obese at baseline and lost any weight during follow-up. In the final multivariable model, we adjusted for age, sex, baseline CDAI, RA duration, smoking (ever vs. never), serologic status, steroid use at baseline, and follow-up time.
Two-sided p values <0.05 were considered statistically significant. All statistical analyses were conducted using SAS software, version 9.4.
Results
We analyzed 174 RA patients that had at least two clinic visits with available CDAI and BMI measures. There were a total of 836 clinic visits, with a median of 5 visits per subject (range: 2-11) over 1.9 years (IQR 1.3-2.4) occurring between March 2012 and May 2015. Mean age at baseline was 60.4 years (standard deviation [SD] 13.2), 85% of patients were female, 85% were white, and 55% had ever smoked. The median time between the clinic visits where the minimum and maximum weights were measured and were used to calculate weight and CDAI change was 1.1 years (IQR 0.7-1.5). The most common comorbidities were osteoarthritis (68%) and hypertension (52%) Table 1. Besides CRP which was missing in 14% of patients, there were no missing data.
| Characteristic | All patients (n=174) | BMI ≥ 25 kg/m2 and did not lose 5 kg (n=93) | BMI ≥ 25 kg/m2 and lost 5 kg (n=24) | BMI <25 kg/m2 and did not lose 5 kg (n=57) |
|---|---|---|---|---|
| Sociodemographics and Lifestyle | ||||
| Mean age, years (SD) | 60.4 (13.2) | 58.6 (12.9) | 60.7 (12.3) | 63.2 (13.7) |
| Female, no. (%) | 147 (84.5) | 73 (78.5) | 19 (79.2) | 55 (96.5) |
| White, no. (%) | 148 (85.1) | 79 (84.9) | 20 (83.3) | 49 (85.9) |
| >High school education, no. (%) | 97 (55.8) | 49 (52.7) | 11 (45.8) | 37 (64.9) |
| Ever smoker, no. (%) | 96 (55.2) | 52 (55.9) | 16 (66.7) | 28 (49.1) |
| Median BMI, kg/m2 (IQR) | 28.0 (23.8-31.8) | 29.0 (27.2-32.6) | 32.4 (29.4-39.5) | 23.0 (21.3-23.8) |
| BMI category | ||||
| Underweight, no. (%) | 1 (0.6) | 0 (0.0) | 0 (0.0) | 1 (1.8) |
| Normal, no. (%) | 56 (32.2) | 0 (0.0) | 0 (0.0) | 56 (98.2) |
| Overweight no. (%) | 59 (33.9) | 54 (58.1) | 5 (20.8) | 0 (0.0) |
| Obese no. (%) | 58 (33.3) | 39 (41.9) | 19 (79.2) | 0 (0.0) |
| Comorbidities | ||||
| Osteoarthritis, no. (%) | 118 (67.8) | 59 (63.4) | 19 (79.2) | 40 (70.2) |
| Hypertension, no. (%) | 90 (51.7) | 51 (54.8) | 15 (62.5) | 24 (42.1) |
| Hypothyroidism, no. (%) | 32 (18.4) | 16 (17.2) | 4 (16.7) | 12 (21.1) |
| RA characteristics | ||||
| Median RA duration, years (IQR) | 9.8 (4.1-18.6) | 8.3 (3.4-18.4) | 10.8 (6.3-16.0) | 12.2 (5.8-19.4) |
| Median CDAI (IQR) | 10.0 (5.0-18.0) | 10.0 (5.0-15.0) | 16.5 (7.0-24.5) | 11.0 (4.0-22.0) |
| RA disease activity category | ||||
| Remission (CDAI 0-2.8), no. (%) | 12 (6.9) | 4 (4.3) | 0 (0.0) | 8 (14.0) |
| Low (CDAI 2.9-10), no. (%) | 77 (44.3) | 48 (51.6) | 9 (37.5) | 20 (35.0) |
| Moderate (CDAI 10.1-22), no. (%) | 51 (29.3) | 28 (30.1) | 8 (33.3) | 15 (26.3) |
| High (CDAI >22), no. (%) | 34 (19.5) | 13 (14.0) | 7 (29.2) | 14 (24.6) |
| Components of CDAI | ||||
| Mean swollen joint count (SD) | 2.9 (3.5) | 2.6 (3.1) | 3.0 (2.8) | 3.4 (4.2) |
| Mean tender joint count (SD) | 4.4 (5.3) | 3.6 (4.5) | 6.9 (7.5) | 4.5 (5.0) |
| Mean patient global assessment (SD) | 3.8 (2.4) | 3.5 (2.1) | 4.6 (2.7) | 3.9 (2.7) |
| Mean physician global assessment (SD) | 2.6 (1.8) | 2.5 (1.9) | 3.3 (1.8) | 2.5 (1.7) |
| RF positivity, no. (%) | 110 (63.2) | 55 (59.1) | 22 (91.7) | 33 (57.9) |
| Anti-CCP positivity, no. (%) | 82 (47.1) | 48 (51.6) | 12 (50.0) | 22 (38.6) |
| Seropositive (RF or anti-CCP), no. (%) | 136 (78.2) | 73 (78.5) | 22 (91.0) | 41 (71.9) |
| Mean CRP, mg/L (SD)* | 7.1 (10.4) | 7.1 (10.1) | 6.4 (5.8) | 7.2 (12.6) |
| Medications | ||||
| Any DMARD, no. (%) | 151 (86.8) | 84 (90.3) | 20 (80.0) | 47 (82.5) |
| Methotrexate, no. (%) | 86 (49.4) | 51 (54.8) | 13 (54.2) | 22 (38.6) |
| Non-biologic DMARD, no. (%) | 103 (59.2) | 60 (64.5) | 14 (58.3) | 29 (50.9) |
| Biologic DMARD, no. (%) | 106 (60.9) | 56 (60.2) | 17 (70.8) | 33 (57.9) |
| ≥ 2 DMARDs, no. (%) | 64 (36.8) | 37 (39.8) | 11 (45.8) | 16 (28.1) |
| Glucocorticoids, no. (%) | 55 (31.6) | 29 (31.2) | 9 (37.5) | 17 (29.8) |
| NSAIDs, no. (%) | 82 (47.1) | 44 (47.3) | 10 (41.7) | 28 (49.1) |
| Opioids, no. (%) | 21 (12.1) | 14 (15.1) | 4 (16.7) | 3 (5.3) |
*CRP was missing in 25 patients. All other variables had no missing values
Anti-CCP, anti-cyclic citrullinated peptide; BMI, body mass index; CDAI, clinical disease activity index, CRP, C-reactive protein; DMARDs, disease modifying anti-rheumatic drugs; ESR, erythrocyte sedimentation rate; NSAIDs, non-steroidal anti-inflammatory drugs; RA, rheumatoid arthritis; RF, rheumatoid factor.
Table 1. Baseline characteristics of patients according to BMI category and weight loss during follow-up (n=174)
At baseline, the median BMI was 28.0 kg/m2 (IQR 23.8-31.8) and 67% of patients were overweight or obese. The median RA duration was 9.8 years (IQR 4.1-18.6) with 78% being seropositive and 15% having deformities from RA. Seven percent of subjects were in remission, 44% had low disease activity, 29% had moderate activity, and 20% had high disease activity by CDAI at baseline. The median CDAI among those who were normal or underweight (n=53) was 11 (IQR 4-22), while the median CDAI among overweight/obese patients who did not lose 5 kg (n=93) was 10 (IQR 5-15), and 17 (IQR 7-25) for overweight/obese patients who did lose 5 kg (n=24). Within the entire study sample at baseline, 87% were on DMARDs, 59% were on a non-biologic DMARD, 61% were on a biologic DMARD, 47% were on NSAIDs, 12% were on opioids, and 32% were on glucocorticoids.
Ten out of the 24 (42%) of patients who were overweight/obese at baseline and lost above the threshold of ≥5 kg, had a CDAI improvement of ≥5 compared to 18 of the 93 (19%) patients who did not lose ≥5 kg. Overweight/obese patients who lost above the threshold of 5 kg had significantly increased odds of disease activity improvement compared to those who did not lose 5 kg (unadjusted OR 2.74, 95% CI 1.09- 6.91). After adjustment for age, sex, and baseline CDAI, overweight/obese patients that lost ≥5 kg had three-fold increased odds of disease activity improvement (OR 3.03, 95% CI 1.18- 7.83) Table 2 compared to those that did not lose this amount of weight. Results were similar in individual models that adjusted for steroid use, DMARD use, serologic status, smoking, follow-up time, and osteoarthritis instead of sex. Among patients who did not lose 5 kg, those with normal BMI had a trend toward improved CDAI compared to overweight/obese patients (multivariable adjusted OR 1.90, 95% CI 0.88- 4.11).
| Baseline BMI and weight loss | Outcomes / Total in category (n) | % with CDAI improvement ≥ 5 points | Unadjusted OR (95% CI) | Adjusted OR (95% CI)* |
|---|---|---|---|---|
| BMI ≥ 25 kg/m2 and did not lose 5 kg | 18 / 93 | 0.194 | 1.0 (Ref) | 1.0 (Ref) |
| BMI ≥ 25 kg/m2 and lost 5 kg | 45566 | 0.417 | 2.74 (1.09-6.91) | 3.03 (1.18-7.83) |
| BMI <25 kg/m2 and did not lose 5 kg | 19 / 57 | 0.333 | 2.12 (0.99-4.54) | 1.90 (0.88-4.11) |
Weight loss was defined as the difference between the maximum and minimum weights (kg) at routine clinical visits. ΔCDAI was calculated using measures at these corresponding clinic visits and ΔCDAI <-5 was considered a RA disease activity improvement.
Patients who had baseline BMI of <25 kg/m2 and lost 5 kg (n=4) were not analyzed. None of these patients had CDAI improvement ≥ 5 points.
*Adjusted for age, sex, and baseline CDAI.
BMI, body mass index; CDAI, Clinical Disease Activity Index; CI, confidence interval; OR, odds ratio; RA, rheumatoid arthritis.
Table 2. Odds ratios for RA disease activity improvement (≥5 point improvement in CDAI) according to BMI at baseline and significant weight loss (≥5 kg) (n=174).
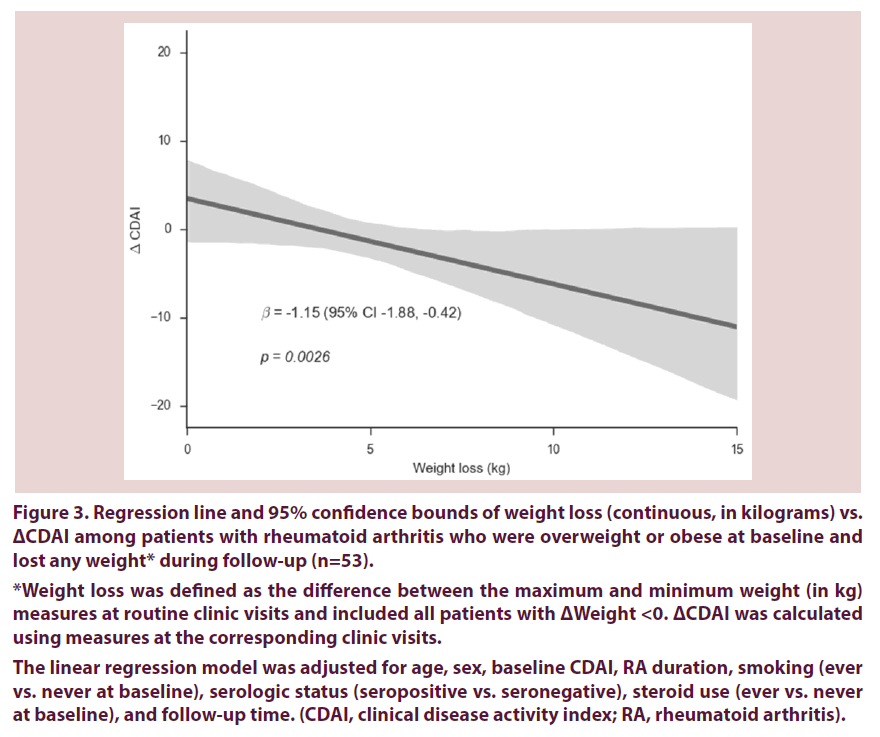
Among the subset of patients who were overweight/obese at baseline and lost any weight during follow-up, there was a dose-dependent response of improved CDAI with weight loss. For every 1 kg of weight lost, CDAI improved by 1.15 points (95% CI 0.42-1.88) Figure 3, after adjusting for age, sex, RA duration, smoking, steroid use, serostatus, and follow-up time.
Figure 3. Regression line and 95% confidence bounds of weight loss (continuous, in kilograms) vs. ΔCDAI among patients with rheumatoid arthritis who were overweight or obese at baseline and lost any weight* during follow-up (n=53).
*Weight loss was defined as the difference between the maximum and minimum weight (in kg) measures at routine clinic visits and included all patients with ΔWeight <0. ΔCDAI was calculated using measures at the corresponding clinic visits.
The linear regression model was adjusted for age, sex, baseline CDAI, RA duration, smoking (ever vs. never at baseline), serologic status (seropositive vs. seronegative), steroid use (ever vs. never at baseline), and follow-up time. (CDAI, clinical disease activity index; RA, rheumatoid arthritis).
Discussion
Among patients with RA seen in routine clinical care, we found that weight loss beyond a threshold of 5 kg was associated with a threefold increased odds of reduced disease activity compared to overweight/obese patients that did not reach this threshold of weight loss. We further observed a dose-dependent response of weight loss and reduced disease activity amongst the subset of patients that were overweight/ obese and lost any weight during follow-up. These results suggest that losing weight may improve RA disease activity. Counseling RA obese patients on the benefits of weight loss on the potential for improving disease activity may provide an additional tool for RA management to clinicians.
Prior studies have associated obesity with worsened RA outcomes compared to patients with either normal or non-obese BMI. A French study found that obese patients were 83% less likely to attain a DAS28 decrease of ≥1.2 after 6 months of follow-up [28]. In the Better Anti-Rheumatic Farmacotherapy (BARFOT) study, obesity was associated with 50% lower odds of sustained remission by DAS28 (OR 0.51, 95% CI 0.32-0.84) and worse mHAQ scores compared to non-obese patients [13]. In a meta-analysis of four previous studies, obese patients had a mean difference in DAS of 0.14 (95% CI 0.01-0.27) compared to patients with normal BMI [29]. In the Epidemiologic Investigation for RA, obese patients were less likely to be in remission by DAS28 compared to RA patients with normal BMI (OR 0.58, 95% CI 0.37-0.92) [30]. Similar results reporting lower odds for remission/low disease activity for obese patients compared to patients with normal weight were found in another study performed in the United Kingdom (OR 0.44, 95% CI 0.22-0.88) [31]. In another meta-analysis of four previous studies, RA patients who were obese were 47% less likely to be in remission/low disease activity by DAS28 (OR 0.53, 95% CI 0.41, 0.69) [32]. Most recently, in a meta-analysis of 10 prior studies, patients with obesity were 42% less likely to achieve minimal disease activity compared to patients with normal BMI (OR 0.58, 95% CI 0.40-0.85) [33]. In a study performed in the Netherlands, continuous BMI was positively correlated with DAS28 (r=0.34, p=0.001) [34]. In another Dutch study, obese/overweight patients had higher tender and swollen joint counts than patients with normal BMI after one year of follow-up [35]. In a meta-analysis of two studies, RA patients with obesity had worse HAQ scores compared to patients with normal BMI (mean difference 0.10, 95% CI 0.01-0.19) [32].
Our results expand upon this prior literature that mostly focused on comparing BMI categories of obese and normal. Similar to this prior literature, we observed a trend towards improved disease activity for patients with normal weight compared to overweight/obese patients who did not lose weight [35-37]. Additionally, we observed that patients who were overweight/ obese at the baseline visit had higher CDAI measures compared to normal weight patients [29]. Unlike prior studies, we aimed to investigate the effect of weight loss on disease activity, rather than static categories of BMI. We found that weight loss beyond a threshold of 5 kg was significantly associated with improved disease activity. We also included the BMI categories of overweight/obesity and normal weight in our primary analysis, since weight loss would not be recommended for patients with normal or underweight BMI. The threshold of 5 kg that we investigated has been described as an obtainable goal through diet and exercise and also improves other chronic disease outcomes [38,39]. In the secondary analysis, we found a dose-dependent response of weight loss with improved disease activity, suggesting that weight loss beyond 5 kg may provide further improvement in RA disease activity.
While the association between obesity and worse RA outcomes is well described, few previous studies have investigated the relationship between weight change and RA disease activity. A prior study by our group investigated the effect of weight loss on RA measures after bariatric surgery [16]. This retrospective cohort identified 53 subjects with RA who underwent bariatric surgery. Twelve months after weight loss surgery, patients lost a mean of 41.0 kg (SD 17.3), and 68% were in remission compared to only 26% at baseline (p<0.01). Another study prospectively followed a cohort of 19 patients with RA who lost a mean of 4.5 kg due to nonsurgical interventions and found a significant improvement in physical function [40]. In BARFOT, there was no association between weight gain during follow-up and RA disease activity, but the association of weight loss with disease activity was not reported [13].
It is possible that the findings of our study may not be generalizable to other populations since the study was performed a single site. However, characteristics of our study sample are similar to other established RA cohorts and the data were collected through routine clinical care [41,42].
Since our study was a retrospective cohort study, we used data already collected and the reason for weight loss was unknown. However, our results suggesting improved outcomes for patients with normal BMI compared to obese patients are consistent with prior literature. More patients with obesity had osteoarthritis in our study than those with normal BMI, also consistent with prior literature [43,44]. However, it is possible that improvements in pain and function from osteoarthritis after weight loss may have contributed to our findings. While we did not utilize DAS28 since this measure includes inflammatory markers that might be higher in patients with obesity, it is possible that subjective components of CDAI, such as joint tenderness, might also be biased. Therefore, even validated disease activity measures such as CDAI may not truly reflect biologic RA disease activity. We designed our study based on when CDAI was measured in the routine clinical setting, as opposed to having a standard interval for CDAI measurement. To standardize follow-up and define weight loss, we identified the maximum and minimum weights during follow-up. However, it is possible that this definition of weight loss may have influenced our results. Glucocorticoid use is associated with weight gain, leflunomide may be associated with weight loss, while other DMARDs had relatively less impact on weight change [45]. However, glucocorticoid use was similar across exposure groups and was adjusted for in the analysis using weight loss as a continuous variable so is likely to influence our results. Few patients started on leflunomide during this study, so this is unlikely to explain our results. Since weight was measured in routine clinical care, it is possible that seasonal factors such as weight of clothes and mood changes might have affected our results. However, median follow-up was 1.1 years, so seasonal differences would be less likely to be important in this length of follow-up. While our goal was to capture voluntary weight loss in the routine clinical testing, we were unable to measure whether patients were actively participating in dietary or physical activity programs to lower weight. Finally, while we aimed to investigate the effect of weight loss on RA disease activity, it is possible that the converse may be responsible for the association we report. Patients with improved RA disease activity may be more likely to lose weight through mechanisms such as improved quality of life, less pain, increased physical activity, and healthier diet. Prospective weight loss intervention studies are needed to definitively establish the causal role of weight loss and disease activity.
In conclusion, we demonstrated that weight loss above a threshold of 5 kg in overweight/ obese patients was associated with a significant improvement in RA disease activity. Additionally, we found a dose-dependent response between weight loss and improved disease activity among RA patients who were overweight or obese suggesting additional benefit in disease activity for weight loss beyond 5 kg. These findings suggest that weight loss may have a role in the non-pharmacologic management to improve RA disease activity specifically for overweight or obese RA patients. Further research is needed to replicate these findings in other populations and to prospectively investigate the effect of weight loss interventions on RA disease outcomes.
Funding
This work was supported by the Rheumatology Research Foundation Scientist Development Award (Dr. Sparks) and the Student Achievement Award (Mr. Kreps) and the National Institute of Arthritis and Musculoskeletal and Skin Diseases (grant numbers K24 AR052403, K24 AR057827, P60 AR047782, L30 AR066953, R01 AR049880, P30 AR070253, P30 AR072577, and K23 AR069688). The funders had no role in study design, data collection, analysis, decision to publish, or preparation of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard University, its affiliated academic health care centers, or the National Institutes of Health.
Acknowledgements
The authors would like to thank the Section of Clinical Sciences of the Division of Rheumatology, Immunology and Allergy at Brigham and Women’s Hospital for helpful guidance.
References
- Ravussin E, Swinburn BA. Pathophysiology of obesity. Lancet. 340(8816), 404–8 (1992).
- Ogden CL, Carroll MD, Kit BK et al. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 311(8), 806–14 (2014).
- National Task Force on the Prevention and Treatment of Obesity: Overweight, obesity, and health risk. Archives of Internal Medicine. 160(7), 898–904 (2000).
- Qin B, Yang M, Fu H et al. Body mass index and the risk of rheumatoid arthritis: a systematic review and dose–response meta–analysis. Arthritis. Res. Ther. 17, 86 (2015).
- Felix HC, West DS. Effectiveness of weight loss interventions for obese older adults. Am. J. Health. Promot. 27(3), 191–9 (2013).
- Whelton PK, Appel LJ, Espeland MA et al. Sodium reduction and weight loss in the treatment of hypertension in older persons: a randomized controlled trial of nonpharmacologic interventions in the elderly (TONE). TONE Collaborative Research Group. JAMA. 279(11), 839–46 (1998).
- Morey MC, Snyder DC, Sloane R et al. Effects of home–based diet and exercise on functional outcomes among older, overweight long–term cancer survivors: RENEW: a randomized controlled trial. JAMA. 301(18), 1883–91 (2009).
- Neter JE, Stam BE, Kok FJ et al. Influence of weight reduction on blood pressure: a meta–analysis of randomized controlled trials. Hypertension. 42(5), 878–84 (2003).
- Goldstein DJ. Beneficial health effects of modest weight loss. Int. J. Obes. Relat. Metab. Disord. 16(6), 397–415 (1992).
- Williamson DF, Thompson TJ, Thun M et al. Intentional weight loss and mortality among overweight individuals with diabetes. Diabetes. Care. 23(10), 1499–504 (2000).
- Christensen R, Bartels EM, Astrup A et al. Effect of weight reduction in obese patients diagnosed with knee osteoarthritis: a systematic review and meta–analysis. Ann. Rheum. Dis. 66(4), 433–9 (2007).
- Di Minno MN, Peluso R, Iervolino S et al. Weight loss and achievement of minimal disease activity in patients with psoriatic arthritis starting treatment with tumour necrosis factor alpha blockers. Ann Rheum Dis. 73(6), 1157–62 (2014).
- Ajeganova S, Andersson ML, Hafstrom I et al. Association of obesity with worse disease severity in rheumatoid arthritis as well as with comorbidities: a long–term followup from disease onset. Arthritis. Care. Res (Hoboken). 65(1), 78–87 (2013).
- Visser M, Bouter LM, McQuillan GM et al. Elevated C–reactive protein levels in overweight and obese adults. JAMA. 282(22), 2131–5 (1999).
- Sparks CR, Moots RJ, Goodson NJ. OP0196 Obesity and Disease Activity in A Large International Rheumatoid Arthritis Cohort. Annals of the Rheumatic Diseases. 73(Suppl 2), 136–7 (2014).
- Sparks JA, Halperin F, Karlson JC et al. Impact of Bariatric Surgery on Patients With Rheumatoid Arthritis. Arthritis. Care. Res. (Hoboken). 67(12), 1619–26 (2015).
- Liao KP, Cai T, Gainer V et al. Electronic medical records for discovery research in rheumatoid arthritis. Arthritis. Care. Res (Hoboken). 62(8), 1120–7 (2010).
- Aletaha D, Neogi T, Silman AJ et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis. Rheum. 62(9), 2569–81 (2010).
- Anderson J, Caplan L, Yazdany J et al. Rheumatoid arthritis disease activity measures: American College of Rheumatology recommendations for use in clinical practice. Arthritis. Care. Res (Hoboken). 64(5), 640–7 (2012).
- Nguyen DM, El–Serag HB. The epidemiology of obesity. Gastroenterol. Clin. North. Am. 39(1), 1–7 (2010).
- Baker JF, Billig E, Michaud K et al. Weight Loss, the Obesity Paradox, and the Risk of Death in Rheumatoid Arthritis. Arthritis. Rheumatol. 67(7), 1711–7 (2015).
- Curtis JR, Yang S, Chen L et al. Determining the Minimally Important Difference in the Clinical Disease Activity Index for Improvement and Worsening in Early Rheumatoid Arthritis Patients. Arthritis. Care. Res (Hoboken). 67(10), 1345–53 (2015).
- Sokka T, Toloza S, Cutolo M et al. Women, men, and rheumatoid arthritis: analyses of disease activity, disease characteristics, and treatments in the QUEST–RA study. Arthritis. Res. Ther. 11(1), R7 (2009).
- Ruban TN, Jacob B, Pope JE et al. The influence of age at disease onset on disease activity and disability: results from the Ontario Best Practices Research Initiative. Clin. Rheumatol. 35(3), 759–63 (2016).
- Madsen SG, Danneskiold–Samsoe B, Stockmarr A et al. Correlations between fatigue and disease duration, disease activity, and pain in patients with rheumatoid arthritis: a systematic review. Scand. J. Rheumatol. 45(4), 255–61 (2016).
- Serdaroglu M, Cakirbay H, Deger O et al. The association of anti–CCP antibodies with disease activity in rheumatoid arthritis. Rheumatol. Int. 28(10), 965–70 (2008).
- Haraoui B, Jovaisas A, Bensen WG et al. Use of corticosteroids in patients with rheumatoid arthritis treated with infliximab: treatment implications based on a real–world Canadian population. RMD Open. 1(1):e000078 (2015).
- Ottaviani S, Gardette A, Tubach F et al. Body mass index and response to infliximab in rheumatoid arthritis. Clin. Exp. Rheumatol. 33(4), 478–83 (2015).
- Stavropoulos–Kalinoglou A, Metsios GS, Panoulas VF et al. Underweight and obese states both associate with worse disease activity and physical function in patients with established rheumatoid arthritis. Clin. Rheumatol. 28(4), 439–44 (2009).
- Sandberg ME, Bengtsson C, Kallberg H et al. Overweight decreases the chance of achieving good response and low disease activity in early rheumatoid arthritis. Ann. Rheum. Dis. 73(11), 2029–33 (2014).
- Ellerby N, Mattey DL, Packham J et al. Obesity and comorbidity are independently associated with a failure to achieve remission in patients with established rheumatoid arthritis. Ann. Rheum. Dis. 73(11), e74 (2014).
- Liu Y, Hazlewood GS, Kaplan GG et al. The Impact of Obesity on Remission and Disease Activity in Rheumatoid Arthritis: A Systematic Review and Meta–Analysis. Arthritis. Care. Res (Hoboken). 69(2), 157–165 (2017).
- Lupoli R, Pizzicato P, Scalera A et al. Impact of body weight on the achievement of minimal disease activity in patients with rheumatic diseases: a systematic review and meta–analysis. Arthritis. Res. Ther. 18(1), 297 (2016).
- Klaasen R, Wijbrandts CA, Gerlag DM et al. Body mass index and clinical response to infliximab in rheumatoid arthritis. Arthritis. Rheum. 63(2), 359–64 (2011).
- Heimans L, van den Broek M, le Cessie S et al. Association of high body mass index with decreased treatment response to combination therapy in recent–onset rheumatoid arthritis patients. Arthritis. Care. Res (Hoboken). 65(8), 1235–42 (2013).
- Gremese E, Carletto A, Padovan M et al. Obesity and reduction of the response rate to anti–tumor necrosis factor alpha in rheumatoid arthritis: an approach to a personalized medicine. Arthritis. Care. Res (Hoboken). 65(1), 94–100 (2013).
- George MD, Baker JF. The Obesity Epidemic and Consequences for Rheumatoid Arthritis Care. Curr. Rheumatol. Rep. 18(1), 6 (2016).
- Bray GA. Lifestyle and pharmacological approaches to weight loss: efficacy and safety. J. Clin. Endocrinol. Metab. 93(11 Suppl 1), S81–8 (2008).
- Sacks FM, Bray GA, Carey VJ et al. Comparison of weight–loss diets with different compositions of fat, protein, and carbohydrates. N. Engl. J. Med. 360(9), 859–73 (2009).
- Engelhart M, Kondrup J, Hoie LH et al. Weight reduction in obese patients with rheumatoid arthritis, with preservation of body cell mass and improvement of physical fitness. Clin. Exp. Rheumatol. 14(3), 289–93 (1996).
- Furst DE, Pangan AL, Harrold LR et al. Greater likelihood of remission in rheumatoid arthritis patients treated earlier in the disease course: results from the Consortium of Rheumatology Researchers of North America registry. Arthritis. Care. Res (Hoboken). 63(6), 856–64 (2011).
- Prince FH, Bykerk VP, Shadick NA et al. Sustained rheumatoid arthritis remission is uncommon in clinical practice. Arthritis. Res. Ther. 14(2), R68 (2012).
- Suri P, Morgenroth DC, Hunter DJ. Epidemiology of osteoarthritis and associated comorbidities. PM R. 4(5 Suppl), S10–9 (2012).
- Felson DT, Anderson JJ, Naimark A et al. Obesity and knee osteoarthritis. The Framingham Study. Ann. Intern. Med. 109(1), 18–24 (1998).
- Baker JF, Sauer BC, Cannon GW et al. Changes in Body Mass Related to the Initiation of Disease–Modifying Therapies in Rheumatoid Arthritis. Arthritis. Rheumatol. 68(8), 1818–27 (2016).