Case Report - Interventional Cardiology (2021) Volume 13, Issue 6
Atrial flutter-sign, witness of or reason for congestive heart failure?
- Corresponding Author:
- Karolina Wrona-Kolasa
Department of Cardiology,
Medical University of Silesia,
Katowice,
Poland,
E-mail: ajustoir@gmail.com
Received date: October 01, 2021; Accepted date: October 15 2021; Published date: October 22, 2021
Abstract
A patient with atrial flutter and symptoms of heart failure in NYHA Class III, was admitted with suspicion of tachycardiomyopathy. Diagnostics revealed a different, unexpected reason for congestive heart failure-constrictive pericarditis. During hospitalization, after being carefully prepared, the patient underwent pericardiectomy. As a result of the procedure, clinical improvement with reduction of symptoms of heart failure was achieved (NYHA I).
On the basis of the presented clinical case, we would like to emphasize the importance of an interdisciplinary approach in patient care and the fact that various types of arrhythmias, supraventricular arrhythmias in particular, often have a structural cause, the elimination of which allows to reduce or completely eliminate the reported symptoms.
Keywords
Atrial flutter • Constrictive pericarditis • Pericardial effusion • Chronic heart failure
Background
Constrictive pericarditis (CP) is a relatively rare disease of various etiologies: Idiopathic conditions, tuberculosis, or following radiotherapy or heart surgery [1]. Pericardial fibrosis and calcification lead to compression and diastolic dysfunction of the heart and may cause supraventricular arrhythmias [2].
Case Presentation
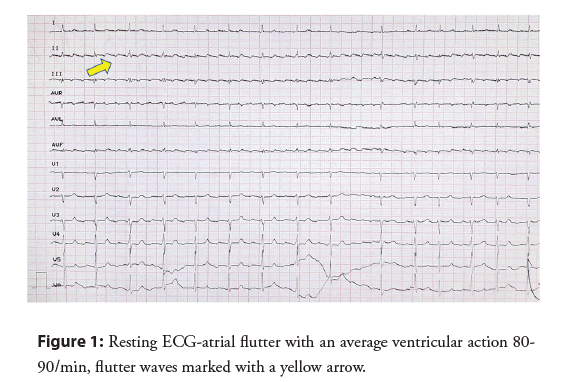
A 65-year-old man with symptoms of heart failure according to New York Heart Association (NYHA) Class III, a history of a persistent Atrial Flutter (AFL) of unknown onset, as well as a history of pulmonary tuberculosis, was admitted with suspicion of tachycardiomyopathy in order to restore sinus rhythm. From the patient’s account the reported symptoms had progressively increased over the past two years. A physical examination revealed signs of right heart failure-increased swelling of the lower limbs, hepatomegaly and jugular veins overflow. A right pleural cavity decompression puncture was performed, obtaining 1500 ml of exudate. On admission, AFL with a QRS complexes rate of 100 bpm was recorded (Figure 1). This is why a baseline diagnosis of tachycardiomyopathy was suspected as a reason for heart failure symptoms.
Laboratory tests showed negative markers of inflammation, euthyroidism, and increased concentration of gamma-glutamyl transferase (193 U/L, normal range <40 U/L). It is well known that right heart failure affects the functioning of the liver in a negative way. Liver dysfunction in the course of heart failure can be manifested by many symptoms, such as: constipation, abdominal distension, nausea, discomfort in the upper right abdominal quadrant. Blood stagnation in the liver with the coexistence of elevated filling pressure of the right heart, leads to an increase in the biochemical markers of cholestasis, including gamma-glutamyl transferase.
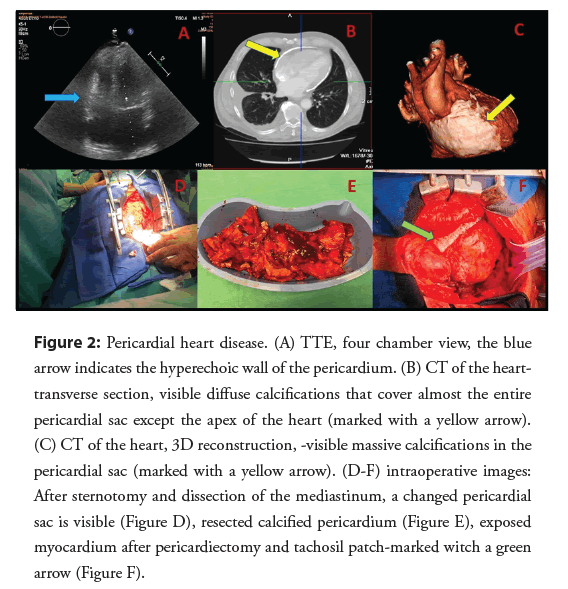
Transthoracic echocardiography revealed mild dilation of the left atrium, standard dimensions of the heart chambers, a tendency towards generalized left ventricular hypokinesis-ejection fraction approximately 40%, no valvular defects, and additionally, thickening and significant hyperechogenicity of the pericardium and pleural fluid up to 9 cm (Figure 2A). Due to the suspicion of pericardial heart disease, the diagnosis was extended by the use of computed tomography, showing lamellar calcifications in the pericardial zone-almost surrounding the heart (Figures 2B and 2C). A diagnosis of constrictive pericarditis was made. Due to the patient’s age and the associated risk of coronary heart disease it was vital to rule out significant atherosclerotic changes in the coronary arteries prior to the surgery. A coronary angiography did not show any pathology in the coronary arteries. Before being qualified for an urgent pericardiectomy, the patient was also consulted within the heart team. During the operation, all calcification of the pericardium was removed without damaging the phrenic nerves (Figures 2D and 2E). Bleeding from the epicardium of the right ventricle was stopped with a Tachosil patch (Figure 2F). No complications were observed in the peri-and post-procedure periods.
Figure 2: Pericardial heart disease. (A) TTE, four chamber view, the blue arrow indicates the hyperechoic wall of the pericardium. (B) CT of the hearttransverse section, visible diffuse calcifications that cover almost the entire pericardial sac except the apex of the heart (marked with a yellow arrow). (C) CT of the heart, 3D reconstruction, -visible massive calcifications in the pericardial sac (marked with a yellow arrow). (D-F) intraoperative images: After sternotomy and dissection of the mediastinum, a changed pericardial sac is visible (Figure D), resected calcified pericardium (Figure E), exposed myocardium after pericardiectomy and tachosil patch-marked witch a green arrow (Figure F).
Follow-up
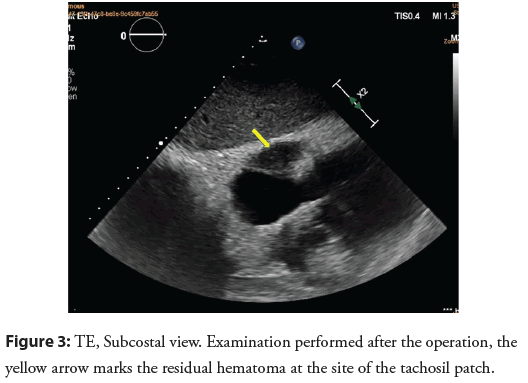
The patient experienced no symptoms (NYHA I) during a 3-month observation. After surgery, arrhythmia was still present, recognized as permanent atrial flutter with optimal rate control. In a transthoracic echocardiography (Figure 3) performed after removal of the calcified pericardium, which surrounded the greater part of the heart muscle like an armor and constituted a mechanical barrier to full diastole, normalization of the left ventricular ejection fraction was observed. In the figure, the yellow arrow marks the residual hematoma at the site of application of the tachosil patch. The patient refused to be treated with the rhythm control strategy (cardioversion/ablation) due to complete improvement of symptoms and good exercise tolerance. The patient receives outpatient care.
Results and Discussion
Our case shows very good long-term results of surgical treatment in a patient with constrictive pericarditis, the clinical manifestation of which was, among others, atrial flutter. The above-presented case proves the importance of thorough and meticulous diagnostics. Tchoracic echocardiography is the first step in the diagnosis of constrictive pericarditis. However, due to the limitations of acoustic windows by the bones of the chest and the air, this method does not allow for full imaging of the pericardium. Additionally, the experience of the echocardiographist has a great influence on the quality of the examination. Therefore, the diagnosis of constrictive pericarditis should be completed with computed tomography or magnetic resonance imaging [3]. Both of the above diagnostic methods allow to obtain a perfect overall picture of the pathologically thickened pericardium. It is also a confirmation of the diagnosis and allows for precise planning of the cardiac surgery. A seemingly common arrhythmia such as AFL turned out to be only a symptom of another serious disease, not the root cause of the problem itself. According to Talreja, et al. [4] atrial arrhythmias occur in more than 20% of patients with constrictive pericarditis. Busch, analyzing 97 patients treated for constrictive pericarditis, showed that the results of surgical treatment were dependent on the patient’s baseline condition and optimal intervention time. Among the factors increasing the risk of early mortality, he considered: reduced left ventricular ejection fraction and right ventricular dilatation. Coronary artery disease, chronic obstructive pulmonary disease and renal insufficiency significantly influenced the increase in late mortality [5]. Despite the high risk (6%-18%), pericardiectomy is a recognized and acceptable causal treatment of patients with constrictive pericarditis [5,6].
Conclusion
We believe that this example may provide a new insight into the understanding of atrial flutter and other supraventricular arrhythmias, which are often a manifestation of other cardiological diseases, and which affects the selection of appropriate treatment. In the presented case, the surgical treatment resulted in a dramatic improvement in the patient’s clinical condition to such an extent that despite the persistent atrial flutter in the control electrocardiograms, he gave consent neither to the proposed electrical cardioversion procedure nor cavotricuspid isthmus ablation. Despite our desire to restore the sinus rhythm, we were obliged to abide by the patient’s refusal to consent and consolidate the arrhythmia.
Ethical Statement
The patient has given their written informed consent to publish their case, including publication of images.
Conflict of Interest
The authors have no conflicts of interest to declare.
Funding Sources
N/A
Author Contributions
All authors made substantial contributions to the conception or design of the work as well as to the acquisition, analysis, or interpretation of data for the work; participated in drafting the work or revising it critically for important intellectual content; approved the final version to be published; and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Peset AM, Martí V, Cardona M, et al. Outcome of pericardiectomy for chronic constrictive pericarditis. Rev Esp Cardiol. 60(10): 1097-1101 (2007).
- Pittaway JF, Presern E, Rathod VS, et al. Atrial flutter ablation in a case of diuretic resistant constrictive pericarditis. Indian Heart J. 67(4): 377-380 (2015).
- Raissuni Z, Lachhab A, Haddour L, et al. The role of multimodality imaging in the diagnosis of constrictive pericarditis. Ann Cardiol Angeiol. 63(1): 32-39 (2014).
- Talreja DR, Edwards WD, Danielson GK, et al. Constrictive pericarditis in 26 patients with histologically normal pericardial thickness. Circulation. 108: 1852-1857 (2003).
- Busch C, Penov K, Amorim PA, et al. Risk factors for mortality after pericardiectomy for chronic constrictive pericarditis in a large single-centre cohort. Eur J Cardiothorac Surg. 48(6): e110-116 (2015).
- Bertog SC, Thambidorai SK, Parakh K, et al. Constrictive pericarditis: Etiology and cause-specific survival after pericardiectomy. J Am Coll Cardiol. 43(8): 1445-1452 (2004).