Review Article - Interventional Cardiology (2020) Volume 12, Issue 2
Atypical Antipsychotic Medications Disrupt the Cardio-Metabolic and Cardio-Immune Axes
- Corresponding Author:
- Dushad Ram
Department of Biomedical Sciences,
College of Osteopathic Medicine,
University of New England,
Biddeford,
Maine,
USA
E-mail: khouseknecht@une.edu
Received date: March 12, 2020; Accepted date: March 27, 2020; Published date: April 03, 2020
Abstract
Antipsychotic medications, including atypical antipsychotics (AA), are widely prescribed and are associated with significant cardiometabolic side effects. It has been known for some time that AA cause drug-induced tachycardia, weight gain, dyslipidemia and hyperglycemia, and AA use in older adults is associated with increased risk of mortality, largely due to MI or stroke. The pharmacological mechanisms underlying these diverse adverse events are largely unknown. AA drugs are also associated with increased risk of infections, in patients across age ranges and regardless of diagnosis. Emerging evidence indicates that AA medications may have anti-inflammatory and immune dysregulatory properties, which may contribute to medication efficacy for schizophrenia and psychosis and increase susceptibility to infection. The role of antipsychotics in modulating the cardio-immune axis is largely unknown. In this review, we will highlight new data which shed light on potential mechanisms and implications for prescribing and patient care.
Keywords
Antipsychotic • Risperidone • Olanzapine • Cardiac • Immune • Inflammation
Introduction
Prescribing of psychotropic medications is increasing worldwide, and in the United States 1 in 6 adults have been prescribed one or more of these medications [1]. This increase in use is largely due to off- label prescribing for diverse indications, most notably in vulnerable populations including pediatric patients, older adults, and people with neurodevelopmental disorders. Antipsychotic medications were developed and approved by the United States Food and Drug Administration (FDA) for the treatment of psychosis associated schizophrenia and bipolar disorder but are now highly prescribed for non-psychotic conditions. Antipsychotic drugs (APDs), including atypical antipsychotics (AA), are associated with increased mortality and with metabolic and endocrine side effects including rapid weight gain, dyslipidemia, and insulin resistance, all of which contribute to cardio metabolic risk. Antipsychotic use has also been linked to an increased risk of death due to infection. In this review, we will highlight emerging mechanistic evidence linking AA medications to rapid onset dysregulation of immune/inflammatory pathways and implications for cardiac health.
Pharmacology of atypical antipsychotic medications
Antipsychotic medications were originally designed and FDA approved for the treatment of the positive symptoms of schizophrenia and bipolar disorder [2]. First generation, or “typical” antipsychotics (TA; e.g. chlorpromazine, haloperidol) were designed to potently and preferentially antagonize dopamine receptors. Dopaminergic antagonism is thought to be the primary mechanism underlying efficacy for schizophrenia, based upon the dopamine hypothesis that positive symptoms are associated with overactivity in the mesolimbic dopamine pathway. TA are efficacious for the treatment of psychosis, however they are associated with significant side effects including severe and often irreversible extrapyramidal effects. These side effects include rigidity, bradykinesia, dystonias, tremor, akathisia and tardive dyskinesia.
The severity of side effects seen with TA medications led to the development of second generation or more commonly known AA. AA also target dopaminergic receptors, however the pharmacology is distinct from TA in that they display broader affinity for dopamine receptor subtypes, and overall are less potent dopaminergic antagonists than TA. Rational drug design strategies were employed to maintain, yet lessen, dopaminergic antagonism (via partial antagonism, inverse agonism, or partial agonism) in order to maintain antipsychotic efficacy while lessening/ minimizing extrapyramidal side effects. While development of newer AA drugs has been successful in reducing extrapyramidal side effects, the broader pharmacological profile of AA, which includes antagonism of serotonin, histamine, alpha-adrenergic, and muscarinic receptors has resulted in increased metabolic liability (across the drug class) as well as sedation (histamine receptors), hypotension (alpha-adrenergic receptors), and dry mouth/constipation (muscarinic receptors) [3,4].
In summary, AA medications are a class of diverse chemical structures, each with a range of activities at multiple neurotransmitter receptors, including receptors outside of the nervous system. This is important to remember when considering drug associated side effects and their underlying pharmacology. AA broadly antagonize G-protein coupled receptors (GPCRs) including dopamine, serotonin, muscarinic, histamine, and alpha- adrenergic receptors, with varying degrees of potency, resulting in unique efficacy and side effect profiles [5]. However, the broader systemic inhibition of GPCRs is not without consequence, as many cells, including cardiomyocytes [6-9] and immune cells [10,11] express these GPCRs and the effects of their broad inhibition by AA has not been investigated.
Cardio metabolic side effects of AA medications
Atypical antipsychotic medications are associated with a constellation of metabolic and endocrine side effects which increase overall cardiovascular risk. Metabolic side effect profiles vary across the AA class, with the greatest metabolic liability (weight gain, dyslipidemia, hyperglycemia) reported for clozapine and olanzapine, although most drugs in the class, including newer drugs such as aripiprazole, carry some degree of metabolic side effect risk [12,13].
Antipsychotic-induced weight gain
The prescribing of AA continues to expand to new off-label indications, thereby impacting more patients across age ranges [4,14-18]. Among patients with severe mental health disorders, cardiovascular disease is the leading cause of death [19,20]. AA are associated with rapid and significant weight gain, often called antipsychotic-induced weight gain (AIWG), and the resulting obesity and cardiometabolic effects contribute to the 15-20 year decreased life expectancy observed in patients with mental illness [21,22]. These effects are more pronounced in female patients, and pediatric patients are especially susceptible to their adverse cardio -metabolic side effects [23-26]. AA medications cause hyperphagia as a consequence of the dysregulation of dopamine reward pathways, but also via antagonism of histamine and serotonin receptors [27-29].
Beyond appetite control, neurotransmitter receptor antagonism by AA can also directly affect AIWG, for example by the induction of adipogenesis and inhibition of metabolic rate via the antagonism of α1-adrenergic receptors [30]. Increasingly, oral hypoglycemic medications such as metformin and lipid lowering drugs (statins) are currently given concomitantly with AA to manage AIWG associated metabolic side effects, as lifestyle interventions for weight control are proving difficult for some patients with mental illness [30-33]. Cardiometabolic side effects of AA should be closely monitored clinically and the severity of adverse reactions taken into consideration when switching to AA with lower risk profiles.
Atypical antipsychotic-induced insulin resistance and hyperglycemia
Consumption of AA medications contributes significantly to the development of insulin resistance, hyperglycemia, and increased risk of type-2 diabetes (3-5 fold) [34]. These effects are due to AIWG as well as direct effects on the regulation of glucose metabolism. AA have shown to have dose-dependent effects on whole-body insulin sensitivity and insulin release from pancreatic β-cells [31,32]. AA also directly influence hepatic glucose metabolism, leading to hyperglycemia [22]. The effects of AA on insulin resistance and hyperglycemia are acute in nature, occurring prior to AA-associated weight gain [35]. The indirect effects of AA on hyperglycemia have been attributed to neurotransmitter antagonism in the central nervous system, with increased sympathetic nervous system signaling inducing gluconeogenesis, particularly from the liver [22,34] Concomitant use of metformin with AA appears to be beneficial in moderating weight gain and improving insulin sensitivity [36,37].
Atypical antipsychotics cause dyslipidemia
AA medications can cause dose-dependent dyslipidemia, and hyperlipidemia may be inversely correlated to severity of psychotic symptoms [38] AA increase circulating triglycerides, free fatty acids, LDL, and lipoprotein-associated phospholipase-A2, and reduce HDL [38-41]. AA increase the expression of SREBP1/2 transcription factor proteins, the master regulators of cholesterol biosynthesis, upregulating their many downstream lipogenic gene targets [42,43]. AA also promote resident stem cells to differentiate into adipocyte lineages, as observed with satellite cells in muscle tissues, but potentially in other stem cell depots as well [44]. Although AA are strongly associated with dyslipidemia, the degree of severity varies across the drug class. Thus, it is particularly important to monitor changes in the profile of circulating lipids if patients switch AA drugs [45].
Antipsychotic-induced arrhythmia
Antipsychotic medications carry a class label warning for QT prolongation, and most are known for the potential induction of Torsades de Pointes tachycardia and arrhythmias. Antipsychotics also have the potential for drug associated myocarditis (especially clozapine) and sudden cardiac death, particularly in elderly patients. [46-49]. AA directly affect the electrophysiology of the heart and the cardiac risks are often dose-dependent [50,51]. AA antagonism of cardiomyocyte N+ channels and inwardly rectifying K+ channels are implicated in prolonged QT interval and sudden cardiac death adverse effects, respectively [52]. Clinicians are advised to carefully evaluate patients for predispositions and risks of cardiovascular side effects through history and preventative evaluations, and monitoring of symptoms after prescription. The effectiveness of psychiatric therapy should be weighed together with potential cardiovascular effects for modification of AA treatment regimens. [49,52,53].
Atypical antipsychotics cause autonomic dysfunction
AA medications have significant effects on autonomic nervous system function with impact on insulin resistance and metabolic syndrome including hyperglycemia, non-alcoholic fatty liver disease (NAFLD), obesity, and inflammation pathways [54]. AA effects on cardiac function also occur via instability and dysregulation of autonomic tone, with increased stress on the heart and risk of mortality [55]. The dysregulated autonomic effects can present as reduced heart rate variability, atrial fibrillation, neuroleptic malignant syndrome, sympathovagal imbalance, and sinus tachycardia [56,57]. AA can impair autonomic nervous system function by binding and inhibiting numerous targets in both the central and peripheral nervous systems, including adrenergic and cholinergic receptors, and hERG potassium ion channels [19].
AA have been linked to reduced heart rate variability directly and indirectly via AIWG, and reduced deceleration capacity, increasing the risk for cardiac mortality [58-60]. AA-induced loss of dopaminergic inhibition of the sympathetic nervous system may also be indicated in neuroleptic malignant syndrome [61]. AA-induced autonomic dysregulation is dose-dependent, and the associated increased risk of atrial fibrillation has been linked to AA that possesses high affinity for M2 muscarinic receptors [62].
AA with higher affinity for M2 muscarinic receptors reduce heart rate variability by reducing parasympathetic signaling, exacerbated by anti-cholinergic medications [63]. While evidence indicates AA drugs reduce parasympathetic tone and increase sympathetic tone [20,64,65], countervailing evidence also exists [66,67], illustrating the need for further research to distinguish between these differences. Long-acting injectable AA appear to have a lower impact on autonomic dysregulation and decreased heart rate variability than oral AA, and the impact of AA on autonomic disruptions may be mitigated by angiotensin converting enzyme inhibitors [67,68].
Antipsychotic drugs impact blood pressure
The effects of antipsychotic medications on blood pressure are complex, and the effects are due to their diverse and complex pharmacology. Orthostatic hypotension is a common side effect of antipsychotic medications, believed to be due to robust alpha-adrenergic antagonism [69]. Furthermore, AA associated hypotension is exacerbated by autonomic dysfunction, which is also a risk factor for patients with metabolic syndrome, diabetes, and schizophrenia. Complications of orthostatic hypotension include syncope, stroke, myocardial infarction, and death. Orthostatic hypotension in older adults is also linked to increased risk of falls and fractures in patients taking AA medications [70].
Some antipsychotic medications can also cause hypertension (HTN), the leading cause of major cardiovascular events including stroke, myocardial infarction, and aneurysms. The underlying etiology of HTN is complex, and drug-associated weight gain, dyslipidemia, and insulin resistance can contribute to HTN in patients taking psychiatric medications. Additionally, blood pressure is regulated by dopamine signaling, primarily via dopaminergic receptors in the kidney [71,72]. Briefly, dopamine D1, D3, and D4 receptors interact with the renin-angiotensin system, while D2 and D5 receptors interact with sympathetic nervous system regulation of blood pressure. As antipsychotic medications are potent dopamine receptor antagonists (D2 and/ or D4 antagonists), they are able to negatively impact blood pressure regulation. Among AA medications, clozapine, which antagonizes both dopamine D2 and D4 receptors, has been shown to significantly increase HTN and cardiovascular morbidity [73]. Data on drug-associated HTN are more limited for other AA medications, thus additional studies are needed to further assess the risk of HTN or hypotension across the class of AA medications.
Atypical antipsychotic drugs cause endocrine disruption
AA can also cause endocrine dysregulation including hyperprolactinemia, hypogonadism, and suppression of growth hormone [4,74-78]. Hyperprolactinemia stems from the AA- induced, dopamine-mediated disinhibition of lactotropic neurons of the adenohypophysis, and can have a cascade of effects such as hypogonadism, hypercoagulation, amenorrhea, gynecomastia, and bone loss among others [79-83]. Risperidone has the highest incidence of drug-associated hyperprolactinemia [77], while aripiprazole, especially when administered via long- acting intramuscular injections, appears to have a diminished or negative hyperprolactinemic effect [84-87]. Paliperidone has been reported to have a dose-dependent suppressive effect on the levels of adrenocorticotropin and corticosterone by modulation of the hypothalamic-pituitary axis [88]. Other AA-mediated endocrine effects require further analysis and indicate individual patient monitoring, but cortisol and leptin levels may be directly affected [89-91].
Immunomodulatory properties of AA medications
Patients with psychiatric illnesses including schizophrenia often have altered immune function profiles [92-95], and the severity of symptoms often correlate with the neuroimmune/ neuroinflammation levels [96]. Animal models, patient case- reports, and ex-vivo/in-vitro studies indicate that multiple AA decrease certain inflammatory cytokines and prostaglandins [97- 99], promote anti-inflammatory pathways, and alter immune cell and immune system phenotypes, all of which may contribute to their efficacy [100-106].
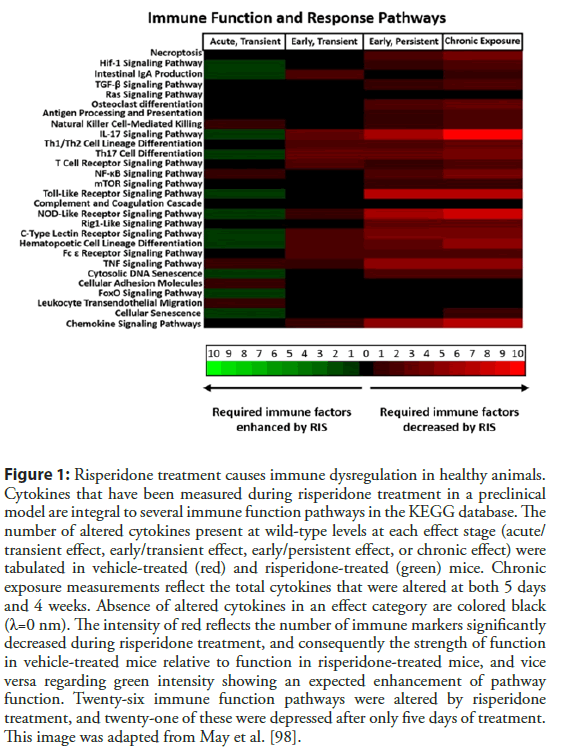
Even with consideration of their obesogenic side-effects taken into account, anti-inflammatory effects of AA can be observed [107]. Direct effects of AA on primary and immune cell lines show suppression of inflammatory cytokine release, and gene expression changes promoting quiescence [108-110]. Depending upon the AA and the patient profile, case reports and preclinical models indicate AA use may be linked to increased infections [98,111,112]. Certain markers of immunosuppression are known side-effects of AA in patients, and they may be indicators of a larger systemic problem of AA use. Agranulocytosis is often an effect associated with clozapine [113]. However, neutropenia, leukopenia, and monocytopenia, in addition to agranulocytosis, have been reported as a result of many other AAs, including aripiprazole, quetiapine, and risperidone [114-120]. In a preclinical murine model of risperidone treatment, AA-induced myeloid dysplasia in the bone marrow, steatosis of the thymus, and global immunosuppression initiated after five days of treatment. Several immune function pathways were severely disrupted, and likely led to the lowered circulating cytokine levels described (Figure 1) [98].
Figure 1: Risperidone treatment causes immune dysregulation in healthy animals. Cytokines that have been measured during risperidone treatment in a preclinical model are integral to several immune function pathways in the KEGG database. The number of altered cytokines present at wild-type levels at each effect stage (acute/ transient effect, early/transient effect, early/persistent effect, or chronic effect) were tabulated in vehicle-treated (red) and risperidone-treated (green) mice. Chronic exposure measurements reflect the total cytokines that were altered at both 5 days and 4 weeks. Absence of altered cytokines in an effect category are colored black (λ=0 nm). The intensity of red reflects the number of immune markers significantly decreased during risperidone treatment, and consequently the strength of function in vehicle-treated mice relative to function in risperidone-treated mice, and vice versa regarding green intensity showing an expected enhancement of pathway function. Twenty-six immune function pathways were altered by risperidone treatment, and twenty-one of these were depressed after only five days of treatment. This image was adapted from May et al. [98].
The cardio-immune axis
There is a lifelong, intimate balance in the cardio-immune axis that maintains cardiac homeostasis, which can damage heart function when there are immune perturbations [121,122]. The interactions between cardiomyocytes, cardiac interstitial cells, and resident cardiac macrophages with peripheral cytokines and immune cell infiltrates that make up the homeostatic physiology are susceptible to drug-induced immunosuppression [123-126]. Long-term AA treatment can lead to drug-induced fevers, sterile myocarditis and/ or cardiomyopathy, which in turn contribute to the arrhythmia and tachycardia discussed above [127,128]. Autopsy findings in AA-treated patients and preclinical models include cardiac fibrosis, hypertrophy, and atherosclerosis [129-131]. Histopathologic findings in the heart include chronic inflammatory lesions featuring eosinophilic infiltrates during clozapine; aripiprazole, quetiapine, and olanzapine treatment [132-135]. The cellular makeup of infiltrates leading to chronic inflammatory pathologies during risperidone or ziprasidone treatment has never been described.
The most extensive body of literature reporting inflammatory and immune dysfunction in patients centers on clozapine, which is considered of limited clinical utility due to its side effect profile. More recent studies describe parallel findings for other, more widely used APDs, however. For example, Kelly et al. reported that the rates of myocarditis, cardiomyopathy, and atherosclerosis were not significantly different between patients treated with clozapine and those treated with risperidone [136]. In addition to clozapine, changes in cytokine levels have now been reported in patients, preclinical models, or in vitro for olanzapine, risperidone, ziprasidone, quetiapine, and aripiprazole, including global immunosuppression during short-term risperidone treatment in a preclinical model [98,137-142].
Proteomic analysis of cardiac tissues during risperidone treatment showed an increase in several proteins with the potential to facilitate inflammation or infiltration of inflammatory cells (i.e., the plasminogen activator receptor PLAUR, Gc globulin, cyclophilin A, alpha 7 integrin, and CD14). Paradoxically, a small number of proteins with similar features (i.e., NOTCH2, DOCK2, and connexin 43) were significantly decreased during treatment, suggesting a complex dynamic attempting to regulate inflammatory processes occurs in the heart [98,143]. This analysis also showed an increase in proteins associated with the pathophysiology of cardiomyopathy (lamin A/C, myosin heavy chain 7, and SLC25A4) and atherosclerosis (apolipoprotein M, afadin, cyclophilin A, and PLAUR) [143]. Taken together, these findings provide insight into potential mechanisms that lead to the clinical findings of myocarditis, cardiomyopathy, myocardial infarction and atherosclerosis in patients treated with APDs over and above their promotion of weight gain.
Summary and Implications for Clinical Care
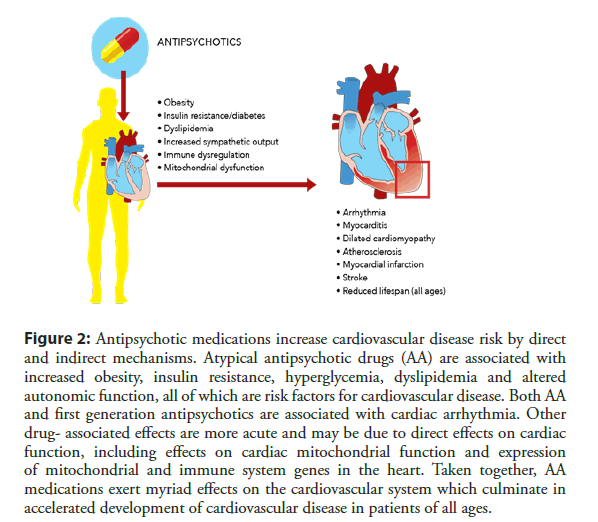
In summary, APD are widely prescribed medications that are associated with significant metabolic, endocrine and cardiovascular side effects (Figure 2).
Figure 2: Antipsychotic medications increase cardiovascular disease risk by direct and indirect mechanisms. Atypical antipsychotic drugs (AA) are associated with increased obesity, insulin resistance, hyperglycemia, dyslipidemia and altered autonomic function, all of which are risk factors for cardiovascular disease. Both AA and first generation antipsychotics are associated with cardiac arrhythmia. Other drug- associated effects are more acute and may be due to direct effects on cardiac function, including effects on cardiac mitochondrial function and expression of mitochondrial and immune system genes in the heart. Taken together, AA medications exert myriad effects on the cardiovascular system which culminate in accelerated development of cardiovascular disease in patients of all ages.
Side effects are especially pronounced in vulnerable populations including children and older adults. Emerging evidence of the anti-inflammatory and immunosuppressive properties of these medications has important implications for antipsychotic efficacy as well as increased susceptibility to infection. Furthermore, preclinical proteomic data indicate that drug associated changes in cardiac proteomic signature relating to mitochondrial and immune functions precede overt cardiac pathology and weight gain, suggesting AA medications accelerate cardiovascular disease directly.
Additional studies are needed to determine the extent to which these effects translate clinically, however clinical evidence is consistent with increased cardiovascular risk for patients prescribed AA. Care should be taken in prescribing AA medications, especially in the case of non-psychotic and vulnerable populations and patient screening protocols for weight gain, hypertension and glycemic endpoints should be followed [144,145]. Furthermore, co-therapies such as metformin should be considered to offset AAassociated insulin resistance and hyperglycemia, known factors which increase cardiovascular disease risk [37]. As psychiatry and cardiovascular disease are both treated with polypharmaceutical therapeutic regimens, it is important for clinicians to be aware of drug interactions between AA medications and medications used to treat cardiovascular disease as interactions can result in changes in pharmacokinetic and/or pharmacodynamic properties [146].
Conclusion
Finally, AA associated immunosuppression may have myriad consequences for patient outcomes including increased susceptibility to infections, impaired adaptive immunity, and impaired surgical outcomes. More data are needed to better understand AA-associated effects on the immune system and the impact on overall patient outcomes.
Conflict of Interest
All authors declare no financial or proprietary conflict of interest related to this study.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit.
References
- Sheehan R, Strydom A, Brown E, et al. Association of Focused Medication Review With Optimization of Psychotropic Drug Prescribing: A Systematic Review and Meta-analysis. JAMA Netw Open. 1(6): e183750 (2018).
- Siafis S, Tzachanis D, Samara M, et al. Antipsychotic Drugs: From Receptor-binding Profiles to Metabolic Side Effects. Curr Neuropharmacol. 16(8): 1210-1223 (2018).
- Brunton LL, Knollmann BrC, Hilal-Dandan R. Goodman & Gilman's the pharmacological basis of therapeutics. Thirteenth edition. ed. New York: McGraw Hill Medical. (2018).
- Ritter J. Rang and Dale's pharmacology. Ninth edition ed. Edinburgh: Elsevier. (2020).
- Kroeze WK, Sassano MF, Huang XP, et al. PRESTO-Tango as an open-source resource for interrogation of the druggable human GPCRome. Nat Struct Mol Biol. 22(5): 362-369 (2015).
- Wang J, Gareri C, Rockman HA. G-Protein-Coupled Receptors in Heart Disease. Circ Res. 123(6): 716-735 (2018).
- Pfleger J, Gresham K, Koch WJ. G protein-coupled receptor kinases as therapeutic targets in the heart. Nat Rev Cardiol. 16: 612-622 (2019).
- Altamish M, Samuel VP, Dahiya R, et al. Molecular signaling of G-protein-coupled receptor in chronic heart failure and associated complications. Drug Dev Res. 81(1): 23-31 (2019).
- De Angelis E, Pecoraro M, Rusciano MR, et al. Cross-talk between neurohormonal pathways and the immune system in heart failure: A review of the literature. Int J Mol Sci. 20(7): 1698 (2019).
- Van der Vorst EPC, Peters LJF, Muller M, et al. G-Protein coupled receptor targeting on myeloid cells in Atherosclerosis. Front Pharmacol. 10: 531 (2019).
- Matt SM, Gaskill PJ. Where Is Dopamine and how do Immune Cells See it? Dopamine-Mediated Immune Cell Function in Health and Disease. J Neuroimmune Pharmacol. (2019).
- Hirsch L, Yang J, Bresee L, et al. Second-generation antipsychotics and metabolic side effects: A systematic review of population-based studies. Drug Saf. 40: 771-781 (2017).
- Pillinger T, McCutcheon RA, Vano L, et al. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: A systematic review and network meta-analysis. Lancet Psychiatry. 7(1): 64-77 (2020).
- Harding R, Peel E. 'He was like a zombie': off-label prescription of antipsychotic drugs in dementia. Med Law Rev. 21(2): 243-277 (2013).
- Daviss WB, Barnett E, Neubacher K, et al. Use of Antipsychotic Medications for Nonpsychotic Children: Risks and Implications for Mental Health Services. Psychiatr Serv. 67(3): 339-341 (2016).
- Ellfolk M, Leinonen MK, Gissler M, et al. Second-generation antipsychotics and pregnancy complications. Eur J Clin Pharmacol. 76: 107-115 (2020).
- Mackie TI, Cook S, Crystal S, et al. Antipsychotic Use Among Youth in Foster Care Enrolled in a Specialized Managed Care Organization Intervention. J Am Acad Child Adolesc Psychiatry. 59(1): 166-176 e3 (2020).
- Kamble P, Sherer J, Chen H, et al. Off-label use of second-generation antipsychotic agents among elderly nursing home residents. Psychiatr Serv. 61(2): 130-136 (2010).
- Leung JY, Barr AM, Procyshyn RM, et al. Cardiovascular side-effects of antipsychotic drugs: the role of the autonomic nervous system. Pharmacol Ther. 135(2): 113-122 (2012).
- Wang YC, Chen CY, Kuo TB, et al. Influence of antipsychotic agents on heart rate variability in male WKY rats: implications for cardiovascular safety. Neuropsychobiology. 65(4): 216-26 (2012).
- Brown S. Excess mortality of schizophrenia: A meta-analysis. Br J Psychiatry. 171: 502-508 (1997).
- Grajales D, Ferreira V, Valverde AM. Second-Generation Antipsychotics and Dysregulation of Glucose Metabolism: Beyond Weight Gain. Cells. 8(11): 1336 (2019).
- Castellani LN, Costa-Dookhan KA, McIntyre WB, et al. Preclinical and Clinical Sex Differences in Antipsychotic-Induced Metabolic Disturbances: A Narrative Review of Adiposity and Glucose Metabolism. J Psychiatr Brain Sci. 4: e190013 (2019).
- Sjo CP, Stenstrom AD, Bojesen AB, et al. Development of metabolic syndrome in drug-naive adolescents after 12 months of second-generation antipsychotic treatment. J Child Adolesc Psychopharmacol. 27(10): 884-891 (2017).
- Correll CU, Manu P, Olshanskiy V, et al. Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA. 302(16): 1765-73 (2009).
- Teasdale SB, Ward PB, Jarman R, et al. Is Obesity in young people with psychosis a foregone conclusion? Markedly excessive energy intake is evident soon after antipsychotic initiation. Front Psychiatry. 9: 725 (2018).
- Deng C. Effects of antipsychotic medications on appetite, weight, and insulin resistance. Endocrinol Metab Clin North Am. 42: 545-563 (2013).
- He M, Zhang Q, Deng C, et al. Hypothalamic histamine H1 receptor-AMPK signaling time-dependently mediates olanzapine-induced hyperphagia and weight gain in female rats. Psychoneuroendocrinology. 42: 153-164 (2014).
- Lord CC, Wyler SC, Wan R, et al. The atypical antipsychotic olanzapine causes weight gain by targeting serotonin receptor 2C. J Clin Invest. 127(9): 3402-3406 (2017).
- Aringhieri S, Carli M, Kolachalam S, et al. Molecular targets of atypical antipsychotics: From mechanism of action to clinical differences. Pharmacol Ther. 192: 20-41 (2018).
- Singh R, Bansal Y, Medhi B, et al. Antipsychotics-induced metabolic alterations: Recounting the mechanistic insights, therapeutic targets and pharmacological alternatives. Eur J Pharmacol. 844: 231-240 (2019).
- Holt RIG. Association between antipsychotic medication use and diabetes. Curr Diab Rep. 19(10): 96 (2019).
- Alonso-Pedrero L, Bes-Rastrollo M, Marti A. Effects of antidepressant and antipsychotic use on weight gain: A systematic review. Obes Rev. 20(12): 1680-1690 (2019).
- Kowalchuk C, Castellani LN, Chintoh A, et al. Antipsychotics and glucose metabolism: how brain and body collide. Am J Physiol Endocrinol Metab. 316(1): E1-E15 (2019).
- Houseknecht KL, Robertson AS, Zavadoski W, et al. Acute effects of atypical antipsychotics on whole-body insulin resistance in rats: implications for adverse metabolic effects. Neuropsychopharmacology. 32(2): 289-297 (2007).
- Stroup TS, Gray N. Management of common adverse effects of antipsychotic medications. World Psychiatry. 17(3): 341-356 (2018).
- De Silva VA, Suraweera C, Ratnatunga SS, et al. Metformin in prevention and treatment of antipsychotic induced weight gain: a systematic review and meta-analysis. BMC Psychiatry. 16(1): 341 (2016).
- Kim DD, Barr AM, Fredrikson DH, et al. Association between serum lipids and antipsychotic response in Schizophrenia. Curr Neuropharmacol. 17(9): 852-860 (2019).
- Shen H, Wu D, Wang S, et al. Atypical antipsychotic administration in schizophrenic patients leads to elevated lipoprotein-associated phospholipase A2 levels and increased cardiovascular risk: A retrospective cohort study. Basic Clin Pharmacol Toxicol. 123(6): 756-765 (2018).
- Xu H, Zhuang X. Atypical antipsychotics-induced metabolic syndrome and nonalcoholic fatty liver disease: a critical review. Neuropsychiatr Dis Treat. 15: 2087-2099 (2019).
- Chiliza B, Asmal L, Oosthuizen P, et al. Changes in body mass and metabolic profiles in patients with first-episode schizophrenia treated for 12 months with a first-generation antipsychotic. Eur Psychiatry. 30(2): 277-283 (2015).
- Canfran-Duque A, Casado ME, Pastor O, et al. Atypical antipsychotics alter cholesterol and fatty acid metabolism in vitro. J Lipid Res. 54(2): 310-324 (2013).
- Ferno J, Raeder MB, Vik-Mo AO, et al. Antipsychotic drugs activate SREBP-regulated expression of lipid biosynthetic genes in cultured human glioma cells: a novel mechanism of action? Pharmacogenomics J. 5(5): 298-304 (2005).
- Pavan C, Vindigni V, Michelotto L, et al. Weight gain related to treatment with atypical antipsychotics is due to activation of PKC-beta. Pharmacogenomics J. 10(5): 408-17 (2010).
- Casey DE. Dyslipidemia and atypical antipsychotic drugs. J Clin Psychiatry. 65(18): 27-35 (2004).
- Meyer JM. Pharmacotherapy of Psychosis and Mania. In: LL. Brunton BA. Chabner and Knollmann BC, (eds) Goodman & amp; Gilman's: The Pharmacological Basis of Therapeutics, 12e New York, NY: McGraw-Hill Education. (2015).
- Polcwiartek C, Kragholm K, Schjerning O, et al. Cardiovascular safety of antipsychotics: a clinical overview. Expert Opin Drug Saf. 15(5): 679-88 (2016).
- Danielsson B, Collin J, Jonasdottir Bergman G, et al. Antidepressants and antipsychotics classified with torsades de pointes arrhythmia risk and mortality in older adults-a Swedish nationwide study. Br J Clin Pharmacol. 81(4): 773-83 (2016).
- Manolis TA, Manolis AA, Manolis AS. Cardiovascular Safety of Psychiatric Agents: A Cautionary Tale. Angiology. 70(2): 103-129 (2019).
- Ray WA, Chung CP, Murray KT, et al. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 360: 225-235 (2009).
- Schneeweiss S, Avorn J. Antipsychotic agents and sudden cardiac death--how should we manage the risk? N Engl J Med. 360: 294-6 (2009).
- Woloszyn E, Whig N, Trigoboff E, et al. Cardiac Arrest with Clozapine and Olanzapine: Revealing Long QT Syndrome. Clin Schizophr Relat Psychoses. (2016).
- Beach SR, Celano CM, Sugrue AM, et al. QT Prolongation, Torsades de Pointes, and Psychotropic Medications: A 5-Year Update. Psychosomatics. 59(2): 105-122 (2018).
- Spallone V. Update on the Impact, Diagnosis and Management of Cardiovascular Autonomic Neuropathy in Diabetes: What Is Defined, What Is New, and What Is Unmet. Diabetes Metab J. 43(1): 3-30 (2019).
- Thayer JF, Yamamoto SS, Brosschot JF. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol. 141(2): 122-131 (2010).
- Chang JS, Yoo CS, Yi SH, et al. Changes in heart rate dynamics of patients with schizophrenia treated with risperidone. Prog Neuropsychopharmacol Biol Psychiatry. 34(6): 924-929 (2010).
- Tagne-Nouemssi AB. Risperidone-associated sinus tachycardia potentiated by paliperidone palmitate in a patient with no prior cardiovascular disease: role of risperidone-related autonomic instability. BMJ Case Rep. (2018).
- Howell S, Yarovova E, Khwanda A, et al. Cardiovascular effects of psychotic illnesses and antipsychotic therapy. Heart. 105(24): 1852-1859 (2019).
- Wang J, Liu YS, Zhu WX, et al. Olanzapine-induced weight gain plays a key role in the potential cardiovascular risk: evidence from heart rate variability analysis. Sci Rep. 4:7394 (2014).
- Birkhofer A, Geissendoerfer J, Alger P, et al. The deceleration capacity - a new measure of heart rate variability evaluated in patients with schizophrenia and antipsychotic treatment. Eur Psychiatry. 28(2): 81-86 (2013).
- Gurrera RJ. Sympathoadrenal hyperactivity and the etiology of neuroleptic malignant syndrome. Am J Psychiatry. 156(2): 169-180 (1999).
- Chou RH, Lo LW, Liou YJ, et al. Antipsychotic treatment is associated with risk of atrial fibrillation: A nationwide nested case-control study. Int J Cardiol. 227: 134-140 (2017).
- Huang WL, Chang LR, Kuo TB, et al. Impact of antipsychotics and anticholinergics on autonomic modulation in patients with schizophrenia. J Clin Psychopharmacol. 33(2): 170-177 (2013).
- Motyl KJ, DeMambro VE, Barlow D, et al. Propranolol Attenuates Risperidone-Induced Trabecular Bone Loss in Female Mice. Endocrinology. 156(7): 2374-2383 (2015).
- Yuen JWY, Kim DD, Procyshyn RM, et al. Clozapine-Induced Cardiovascular Side Effects and Autonomic Dysfunction: A Systematic Review. Front Neurosci. 12: 203 (2018).
- Hattori S, Kishida I, Suda A, et al. Effects of four atypical antipsychotics on autonomic nervous system activity in schizophrenia. Schizophr Res. 193: 134-138 (2018).
- Hattori S, Suda A, Kishida I, et al. Effects of ABCB1 gene polymorphisms on autonomic nervous system activity during atypical antipsychotic treatment in schizophrenia. BMC Psychiatry. 18(1): 231 (2018).
- Suda A, Hattori S, Kishida I, et al. Effects of long-acting injectable antipsychotics versus oral antipsychotics on autonomic nervous system activity in schizophrenic patients. Neuropsychiatr Dis Treat. 14: 2361-2366 (2018).
- Gugger JJ. Antipsychotic pharmacotherapy and orthostatic hypotension: identification and management. CNS Drugs. 25(8): 659-671 (2011).
- Huang AR, Mallet L, Rochefort CM, et al. Medication-related falls in the elderly: causative factors and preventive strategies. Drugs Aging. 29: 359-376 (2012).
- Jose PA, Eisner GM, Felder RA. Role of dopamine receptors in the kidney in the regulation of blood pressure. Curr Opin Nephrol Hypertens. 11(1): 87-92 (2002).
- Jose PA, Soares-da-Silva P, Eisner G, et al. Dopamine and G protein-coupled receptor kinase 4 in the kidney: role in blood pressure regulation. Biochim Biophys Acta. 1802: 1259-67 (2010).
- Gonsai NH, Amin VH, Mendpara CG, et al. Effects of dopamine receptor antagonist antipsychotic therapy on blood pressure. J Clin Pharm Ther. 43(1): 1-7 (2018).
- Park YM, Lee SH, Lee BH, et al. Prolactin and macroprolactin levels in psychiatric patients receiving atypical antipsychotics: A preliminary study. Psychiatry Res. 239: 184-189 (2016).
- Cookson J, Hodgson R, Wildgust HJ. Prolactin, hyperprolactinaemia and antipsychotic treatment: a review and lessons for treatment of early psychosis. J Psychopharmacol. 26(5): 42-51 (2012).
- Bostwick JR, Guthrie SK, Ellingrod VL. Antipsychotic-induced hyperprolactinemia. Pharmacotherapy. 29:64-73 (2009).
- Tewksbury A, Olander A. Management of antipsychotic-induced hyperprolactinemia. Ment Health Clin. 6(4): 185-190 (2016).
- Popovic V, Doknic M, Maric N, et al. Changes in neuroendocrine and metabolic hormones induced by atypical antipsychotics in normal-weight patients with schizophrenia. Neuroendocrinology. 85(4): 249-256 (2007).
- Kaar SJ, Natesan S, McCutcheon R, et al. Antipsychotics: Mechanisms underlying clinical response and side-effects and novel treatment approaches based on pathophysiology. Neuropharmacology. (2019).
- Samadi A, Isikhan SY, Ansari MHK, et al. Effects of clozapine and haloperidol treatment on plasma concentrations of androgen hormones and androgendependent organ changes in rats. Ind J Pharmacol. 51(4): 269-275 (2019).
- Ishioka M, Yasui-Furukori N, Sugawara N, et al. Hyperprolactinemia during antipsychotics treatment increases the level of coagulation markers. Neuropsychiatr Dis Treat. 11: 477-484 (2015).
- Guler G, Kutuk MO, Kara H. Amenorrhea as a Side Effect of Low Dose Aripiprazole: An Adolescent Case. Clin Psychopharmacol Neurosci. 16(3): 343-345 (2018).
- Sakaguchi S, Aizawa K. Galactorrhea Induced by Risperidone. Intern Med. 58(24): 3609-3610 (2019).
- Nasrallah HA, Aquila R, Stanford AD, et al. Metabolic and Endocrine Profiles During 1-Year Treatment of Outpatients with Schizophrenia with Aripiprazole Lauroxil. Psychopharmacol Bull. 47(3): 35-43 (2017).
- Nasrallah HA, Newcomer JW, Risinger R, et al. Effect of Aripiprazole Lauroxil on Metabolic and Endocrine Profiles and Related Safety Considerations Among Patients With Acute Schizophrenia. J Clin Psychiatry. 77(11): 1519-1525 (2016).
- Kirino E. Serum prolactin levels and sexual dysfunction in patients with schizophrenia treated with antipsychotics: comparison between aripiprazole and other atypical antipsychotics. Ann Gen Psychiatry. 16: 43 (2017).
- Boggs DL, Ranganathan M, Boggs AA, et al. Treatment of hyperprolactinemia and gynecomastia with adjunctive aripiprazole in 2 men receiving long-acting injectable antipsychotics. Prim Care Companion Cns Disord. 15(4):18 (2013).
- Gupta P, Mohanty B. Atypical antipsychotic drug modulates early life infection induced impairment of hypothalamic-pituitary-adrenal axis: An age related study in mice. Eur J Pharmacol. (2020).
- Hahn MK, Wolever TM, Arenovich T, et al. Acute effects of single-dose olanzapine on metabolic, endocrine, and inflammatory markers in healthy controls. J Clin Psychopharmacol. 33: 740-746 (2013).
- Srisawasdi P, Vanwong N, Hongkaew Y, et al. Impact of risperidone on leptin and insulin in children and adolescents with autistic spectrum disorders. Clin Biochem. 50(12): 678-685 (2017).
- Horska K, Ruda-Kucerova J, Babinska Z, et al. Olanzapine-depot administration induces time-dependent changes in adipose tissue endocrine function in rats. Psychoneuroendocrinology. 73: 177-185 (2016).
- Al-Diwani AAJ, Pollak TA, Irani SR, et al. Psychosis: an autoimmune disease? Immunol. 152(3): 388-401 (2017).
- Garcia-Rizo C, Casanovas M, Fernandez-Egea E, et al. Blood cell count in antipsychotic-naive patients with non-affective psychosis. Early Interv Psychiatry. 13(1): 95-100 (2019).
- Muller N. Inflammation in Schizophrenia: Pathogenetic Aspects and Therapeutic Considerations. Schizophr Bull. 44(5): 973-982 (2018).
- Bocchio-Chiavetto L, Zanardini R, Tosato S, et al. Immune and metabolic alterations in first episode psychosis (FEP) patients. Brain Behav Immun. 70: 315-324 (2018).
- Strzelecki D, Urban-Kowalczyk M, Wysokinski A. Serum levels of interleukin 6 in schizophrenic patients during treatment augmentation with sarcosine (results of the PULSAR study). Hum Psychopharmacol. 33(2): e2652 (2018).
- Dean B, Gibbons A, Gogos A, et al. Studies on Prostaglandin-Endoperoxide Synthase 1: Lower Levels in Schizophrenia and After Treatment with Antipsychotic Drugs in Conjunction with Aspirin. Int J Neuropsychopharmacol. 21(3): 216-225 (2018).
- May M, Beauchemin M, Vary C, et al. The antipsychotic medication, risperidone, causes global immunosuppression in healthy mice. Plos One. 14(6): e0218937 (2019).
- Melbourne JK, Feiner B, Rosen C, et al. Targeting the Immune System with Pharmacotherapy in Schizophrenia. Curr Treat Options Psychiatry. 4(2): 139-151 (2017).
- Azizi E, Zavaran Hosseini A, Soudi S, et al. Alteration of Serum Levels of Cytokines in Schizophrenic Patients before and after Treatment with Risperidone. Iran J Allergy Asthma Immunol. 18(3): 262-268 (2019).
- Ribeiro-Santos R, de Campos-Carli SM, Ferretjans R, et al. The association of cognitive performance and IL-6 levels in schizophrenia is influenced by age and antipsychotic treatment. Nord J Psychiatry. 1(1): 1-7 (2019).
- Noto MN, Maes M, Nunes SOV, et al. Activation of the immune-inflammatory response system and the compensatory immune-regulatory system in antipsychotic naive first episode psychosis. Eur Neuropsychopharmacol. 29(3): 416-431 (2019).
- Stapel B, Sieve I, Falk CS, et al. Second generation atypical antipsychotics olanzapine and aripiprazole reduce expression and secretion of inflammatory cytokines in human immune cells. J Psychiatr Res. 105: 95-102 (2018).
- Romeo B, Brunet-Lecomte M, Martelli C, et al. Kinetics of Cytokine Levels during Antipsychotic Treatment in Schizophrenia: A Meta-Analysis. Int J Neuropsychopharmacol. 21(9): 828-836 (2018).
- Vakilian A, Razavi-Nasab SM, Ravari A, et al. Vitamin B12 in Association with Antipsychotic Drugs Can Modulate the Expression of Pro-/Anti-Inflammatory Cytokines in Alzheimer Disease Patients. Neuroimmunomodulation. 24(6): 310-319 (2017).
- Hung YP, Wang CS, Yen CN, et al. Role of cytokine changes in clozapine-induced fever: A cohort prospective study. Psychiatry Clin Neurosci. 71(6): 395-402 (2017).
- Pisano S, Catone G, Coppola G, et al. Different Immune Signature in Youths Experiencing Antipsychotic-Induced Weight Gain Compared to Untreated Obese Patients. J Child Adolesc Psychopharmacol. 27(9): 844-848 (2017).
- Jeon S, Kim SH, Shin SY, et al. Clozapine reduces Toll-like receptor 4/NF-kappaB-mediated inflammatory responses through inhibition of calcium/calmodulin-dependent Akt activation in microglia. Prog Neuropsychopharmacol Biol Psychiatry. 81: 477-487 (2018).
- Yoo S, Kim MY, Cho JY. Syk and Src-targeted anti-inflammatory activity of aripiprazole, an atypical antipsychotic. Biochem Pharmacol. 148:1-12 (2018).
- Bobermin LD, da Silva A, Souza DO, et al. Differential effects of typical and atypical antipsychotics on astroglial cells in vitro. Int J Dev Neurosci. 69: 1-9 (2018).
- van Strien AM, Souverein PC, Keijsers CJPW, et al. Association Between Urinary Tract Infections and Antipsychotic Drug Use in Older Adults. J Clin Psychopharmacol. 38(4):296-301 (2018).
- Ballard C, Isaacson S, Mills R, et al. Impact of Current Antipsychotic Medications on Comparative Mortality and Adverse Events in People with Parkinson Disease Psychosis. J Am Med Dir Assoc. 16(10): 898.e1-7 (2015).
- Regen F, Herzog I, Hahn E, et al. Clozapine-induced agranulocytosis: Evidence for an immune-mediated mechanism from a patient-specific in-vitro approach. Toxicol Appl Pharmacol. 316:10-16 (2017).
- Pang N, Thrichelvam N, Naing KO. Olanzapine-induced Pancytopenia: A Rare but Worrying Complication. East Asian Arch Psychiatry. 27(1): 35-7 (2017).
- Felin T, Naveed S, Chaudhary AM. Aripiprazole-Induced Neutropenia: Case Report and Literature Review. J Psychosoc Nurs Ment Health Serv. 56(5): 21-24 (2018).
- Glocker C, Grohmann R, Schulz H. Fatal Agranulocytosis Associated With Quetiapine in Monotherapy: A Case Report. J Clin Psychopharmacol. 37(5): 625-627 (2017).
- Tunsirimas N, Pariwatcharakul P, Choovanichvong S, et al. Clozapine-induced agranulocytosis and leukopenia: Incidence, associated factors, and rate of hematologic adverse-effects monitoring in psychiatric out-patient services in Thailand. Asian J Psychiatr. 41:13-16 (2019).
- Patel R, Lima A, Burke C, et al. Monocytopenia in clozapine-induced agranulocytosis: insights into pathophysiology and treatment. BMJ Case Rep. 12(1): 12 (2019).
- Sood S. Neutropenia with Multiple Antipsychotics Including Dose Dependent Neutropenia with Lurasidone. Clin Psychopharmacol Neurosci. 15: 413-415 (2017).
- Rettenbacher MA, Hofer A, Kemmler G, et al. Neutropenia induced by second generation antipsychotics: a prospective investigation. Pharmacopsychiatry. 43(2): 41-4 (2010).
- Swirski FK, Nahrendorf M. Cardioimmunology: the immune system in cardiac homeostasis and disease. Nat Rev Immunol. 18:733-744 (2018).
- Mann DL. The emerging role of innate immunity in the heart and vascular system: for whom the cell tolls. Circ Res. 108: 1133-1145 (2011).
- Forte E, Furtado MB, Rosenthal N. The interstitium in cardiac repair: role of the immune-stromal cell interplay. Nat Rev Cardiol. 15(10): 601-616 (2018).
- Dick SA, Macklin JA, Nejat S, et al. Self-renewing resident cardiac macrophages limit adverse remodeling following myocardial infarction. Nat Immunol. 20: 29-39 (2019).
- Liao X, Shen Y, Zhang R, Sugi K, et al. Distinct roles of resident and nonresident macrophages in nonischemic cardiomyopathy. Proc Natl Acad Sci U S A. 115(20): E4661-E4669 (2018).
- Ma Y, Mouton AJ and Lindsey ML. Cardiac macrophage biology in the steady-state heart, the aging heart, and following myocardial infarction. Transl Res. 191: 15-28 (2018).
- Ronaldson KJ. Cardiovascular Disease in Clozapine-Treated Patients: Evidence, Mechanisms and Management. CNS Drugs. 31(9): 777-795 (2017).
- Sawicke L, Sturla S. Potentially lethal cardiac side effects caused by psychiatric drugs. Vertex. 19(82): 387-393 (2008).
- Belhani D, Frassati D, Mégard R, et al. Cardiac lesions induced by neuroleptic drugs in the rabbit. Exp Toxicol Pathol. 57(3): 207-212 (2006).
- Coulter DM, Bate A, Meyboom RH, et al. Antipsychotic drugs and heart muscle disorder in international pharmacovigilance: data mining study. BMJ. 322(7296): 1207-1209 (2001).
- Kelly DL, Wehring HJ, Linthicum J, et al. Cardiac-related findings at autopsy in people with severe mental illness treated with clozapine or risperidone. Schizophr Res. 107(3): 134-138 (2009).
- Christoffersen RK, Vestergård LD, Høimark L, et al. Eosinophilic myocarditis and sudden unexpected death in a younger patient treated with antipsychotics. Ugeskr Laeger. 173(44): 2799-2800 (2011).
- Hagiwara H, Fukushima A, Iwano H, et al. Refractory cardiac myocarditis associated with drug rash with eosinophilia and systemic symptoms syndrome due to anti-bipolar disorder drugs: a case report. Eur Heart J Case Rep. 2(4): 100 (2018).
- Kilian JG, Kerr K, Lawrence C, et al. Myocarditis and cardiomyopathy associated with clozapine. Lancet. 354(9193): 1841-1845 (1999).
- Vang T, Rosenzweig M, Bruhn CH, et al. Eosinophilic myocarditis during treatment with olanzapine - report of two possible cases. BMC Psychiatry. 16: 70 (2016).
- Kelly DL, Wehring HJ, Linthicum J, et al. Cardiac-related findings at autopsy in people with severe mental illness treated with clozapine or risperidone. Schizophr Res. 107(3):134-138 (2009).
- Drzyzga L, Obuchowicz E, Marcinowska A et al. Cytokines in schizophrenia and the effects of antipsychotic drugs. Brain Behav Immun. 20(6): 532-545 (2006).
- Duarte T, Barbisan F, do Prado-Lima PAS, et al. Ziprasidone, a second-generation antipsychotic drug, triggers a macrophage inflammatory response in vitro. Cytokine. 106: 101-107 (2018).
- Juncal-Ruiz M, Riesco-Dávila L, Ortiz-García V, et al. Comparison of the anti-inflammatory effect of aripiprazole and risperidone in 75 drug-naïve first episode psychosis individuals: A 3 months randomized study. Schizophr Res. 202: 226-233 (2018).
- Speen AM, Hoffman JR, Kim HH, et al. Small Molecule Antipsychotic Aripiprazole Potentiates Ozone-Induced Inflammation in Airway Epithelium. Chem Res Toxicol. 32(10):1997-2005 (2019).
- Sárvári AK., Veréb Z, Uray IP, et al. Atypical antipsychotics induce both proinflammatory and adipogenic gene expression in human adipocytes in vitro. Biochem Biophys Res Commun. 450(4):1383-1389 (2014).
- Zhu S, Shi R, Li V, et al. Quetiapine attenuates glial activation and proinflammatory cytokines in APP/PS1 transgenic mice via inhibition of nuclear factor-κB pathway. Int J Neuropsychopharmacol. 18(3): 24: (2014).
- Beauchemin M, Geguchadze R, Guntur AR, et al. Exploring mechanisms of increased cardiovascular disease risk with antipsychotic medications: Risperidone alters the cardiac proteomic signature in mice. Pharmacol Res. 152: 104589 (2020).
- American Diabetes A, American Psychiatric A. American Association of Clinical E and North American Association for the Study of O. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 27: 596-601 (2004).
- Morrato EH, Newcomer JW, Kamat S, et al. Metabolic screening after the American Diabetes Association's consensus statement on antipsychotic drugs and diabetes. Diabetes Care. 32: 1037-42 (2009).
- Siwek M, Woron J, Gorostowicz A, et al. Adverse effects of interactions between antipsychotics and medications used in the treatment of cardiovascular disorders. Pharmacol Rep. (2020).