Case Report - International Journal of Clinical Rheumatology (2019) Volume 14, Issue 2
Autoimmune sensory ganglionopathy in primary SjÃÆögrenâÃâ¬Ãâ¢s syndrome treated with combination therapy of cyclophosphamide and rituximab: a case-based review
- Corresponding Author:
- Aadil Al Ghafri
Department of Rheumatology, St. Vincent’s University Hospital, Elm Park, Dublin, Ireland
E-mail: adilalghafri2@gmail.com
Abstract
A 45 year-old male with initial presentation with central nervous system involvement and sensory ganglionopathy, with ultimate diagnosis of primary Sjögren’s syndrome (PSS). Progressive disease was noted despite initial treatment with high-dose steroids and intravenous immunoglobulins (IVIg). He subsequently had an excellent response to combination therapy of intravenous cyclophosphamide (CYC) and rituximab (RTX).
Keywords
Sjögren’s syndrome, rituximab, intravenous cyclophosphamide
Introduction
Severe neurological involvement in patients with primary Sjögren’s syndrome (PSS) is not uncommon. Peripheral neuropathy has been reported in 1.8% to 10% of patients with PSS undergoing electrodiagnostic testing. Sensory ganglionopathy (pure painful sensory or sensory ataxic neuronopathy) is recognized as a characteristic neurological complication of PSS, caused by damage to the sensory neurons of the dorsal root and gasserian ganglia. Other clinical forms of peripheral neuropathy have been reported with PSS, such as axonal polyneuropathy (sensory, motor or sensorimotor), motor neuropathy, small fibre neuropathy, mononeuropathy multiplexmultiplex mono-neuropathy, autonomic neuropathy, demyelinating polyradiculoneuropathy and trigeminal and other cranial neuropathies. We report a case of PSS with initial presentation with neurologic symptoms attributed to central nervous system and sensory ganglionopathy, initially refractory to high dose steroids and IVIg, subsequently treated successfully with combination of intravenous CYC and RTX. To our knowledge, this is the first case report documenting effective treatment of severe neurologic manifestations of PSS with the combination of RTXRituximab and CYCCyclophosphamide.
Case presentation
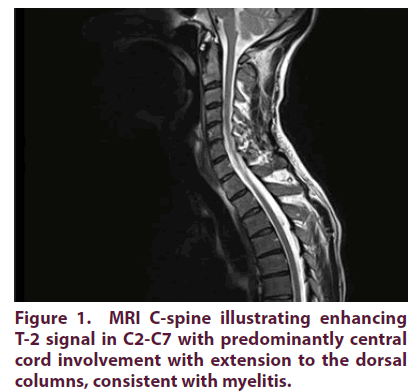
A 45-year-old male with no previous medical background presented with a 3 week history of numbness and paresthesiae of hands and feet, clumsy hands and unsteady gait. He reported a history of progressive foot, followed by arm, weakness [1,2]. Clinical examination revealed no altered mental status with GCS 15/15. His neurological examination showed mild upper limbs pseudoathetosis and lower limbs sensory ataxia with areflexia and no proximal or distal limbs weakness. Nerve Conduction Study (NCS) was consistent with acute sensory neuropathy (affecting mainly upper limbs): non-length dependent sensory neuropathy with diffusely decreased Sensory Nerve Action Potentials (SNAPs), no distal-proximal gradient identified in upper or lower limbs. There were no motor nerve conduction abnormalities seen in both upper and lower limbs. Workup included fasting blood glucose, vitamin B12/B6, microbiological serologic screening for viral (including HIV, HBV and HCV), thyroid function, proteinuria, urea, amyloidosis workup, paraproteinemia, paraneoplastic & vasculitis screen revealed no abnormalities. Additional extensive workup including CSF examination for oligoclonal bands, cytology, and CSF (PCR) viral and bacterial screening showed no abnormalities. Other microbiological serologic screening for bacterial infection (including TB, Borrelia and T-Pallidum) was all negative. Further workup included flow cytometry, tumor markers (included paraneoplastic antibodies), serum immunoglobulin levels and urine and serum protein electrophoresis and negative aquaporin-4, ganglioside and glycine receptor antibodies. The patient was treated initially as acute inflammatory demyelinating polyradiculopathy with 5 days IVIg with slow recovery. After 1 month he reported significant relapse with similar symptoms with additional band like burning chest tightness sensation around rib cage which treated with repeated IVIg course and tapering oral glucocorticoids (GC). Repeated NCS showed progressive findings involving both upper and lower limbs, again with no motor involvement. A month later, he relapsed again and previously reported normal MRI brain revealed T-2 hyper-intensity right thalamic lesions, and MRI C-spine showed worsening T-2 signal in C2-C7 with predominantly central cord involvement with extension to the dorsal columns, consistent with myelitis as illustrated in Figure 1. CT scan revealed no evidence of malignancies in thorax, abdomen or pelvis. US salivary glands showed enlarged sublingual glands and PET CT scan showed bilateral symmetrical diffuse parotid gland uptake. However, although initially equivocal, repeat testing because of mild evolving sicca symptoms showed strongly positive “anti-Ro (anti-SSA) and anti-La (anti-SSB)” antibodies, Schirmer’s test showed positive results and minor salivary gland biopsy revealed interstitial plasmocytic cell infiltration, all consistent with the diagnosis of PSS.
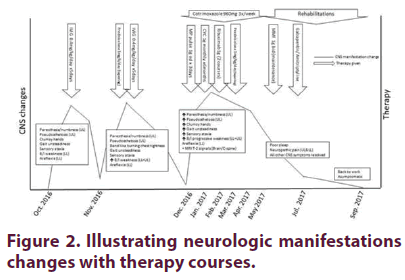
Because of the progressive central nervous system involvement and ganglionopathy despite treatment with IVIg and high-dose steroids, the patient was treated with 1 g methylprednisolone pulse × 3 days, then intravenous CYC (CYCLOPS protocol) over 6 months combined with 1 g RTX (× 2 infusions) along with tapering oral GC course. After clinical neurological improvement, he was switched to maintenance therapy of mycophenolate mofetil (MMF) 1 g BID. Mild persistent neuropathic pain was treated with gabapentin and amitriptyline. Figure 2 illustrates the progress of neurologic manifestations with treatment.
Differential diagnosis
Differential diagnosis of the acquired sensory ganglionopathy are:
• Paraneoplastic related
• Inflammatory (PSS, RA, SLE, AIH)
• Infection related (HIV, EBV, VZV, measles, HTLV-1)
• Medication toxicity/ adverse effects (Platinum based chemotherapy)
• Vitamin toxicity (vitamin B6)
• Idiopathic
Outcome and follow-up
Patient was followed up at interval of 3 months post hospitalization. After period of rehabilitation, the patient had successfully returned to his full-time work with minimal neurological impairment. He remains stable 1 year after initiation of CYC and RTX therapy.
Discussion
Sensory ganglionopathy is produced by posterior spinal root involvement. In PSS, lymphocytic infiltrates of dorsal root and spinal ganglia without vasculitis and degeneration of dorsal root ganglion neuronal cell bodies has been described. Some authors also proposed a role of autoantibodies. To date, there is no consensus about the specific treatment of neurological involvement in PSS. Generally, GC therapy is initiated in patients with either central or peripheral neurologic manifestations. Other immunosuppressive agents that have been studied showed variable results with favourable outcome in CYC and RTX therapy [3-5]. Pereira et al. reported on a series of 13 PSS patients with PSS associated sensory ganglionopathies treated with GC (n=7), MMF (n=7), hydroxychloroquine (n=6), IVIg (n=5), CYC (n=4), and the other patients received other immunosuppressive drugs (n=2). It was concluded that the treatment strategies with GC in association with immunosuppressive drugs, namely MMF, had positive results. In contrast, IVIg had disappointing results [6]. Furthermore, Mekinian et al. have reported efficacy of RTX in PSS with peripheral nervous system involvement (n=17) which included sensory ganglionopathy. Conversely, when the pathogenesis of neurological manifestations is not related to vasculitis, the efficacy of RTX could not be demonstrated. Additional studies are necessary to validate these findings [7,8].
Conclusion
Combined treatment with RTX along with CYC/doxorubicin/vincristine/prednisone (R-CHOP) has been recommended for PSS-associated B cell aggressive non-Hodgkin's lymphomas, under the guidance of an experienced haematologist. The combination of CYC and RTX can be an effective therapy for managing severe refractory central nervous system involvement and sensory ganglionopathy in PSS.
References
- Pavlakis PP, Alexopoulos H, Kosmidis ML et al. Peripheral neuropathies in Sjogren syndrome: a new reappraisal. J. Neurol. Neurosurg. Psychiatry. 82(7), 798-802 (2011).
- Brito-Zerón P, Akasbi M, Bosch X et al. Classification and characterisation of peripheral neuropathies in 102 patients with primary Sjögren's syndrome. Clin. Exp. Rheumatol. 31, 103-110 (2013).
- Ramos-Casals M, Garcia-Hernandez FJ, de Ramon E et al. Off-label use of rituximab in 196 patients with severe, refractory systemic autoimmune diseases. Clin. Exp. Rheumatol. 28, 468-476 (2010).
- Gottenberg JE, Guillevin L, Lambotte O et al. Tolerance and short term efficacy of rituximab in 43 patients with systemic autoimmune diseases. Ann. Rheum. Dis. 64(6), 913-920 (2005).
- Ramos-Casals M, Pilar Brito-Zerón, Albert B et al. Sjögren's syndrome: beyond sicca involvement. Autoimmune. Dis. 116, 45-66 (2011).
- Pereira PR, Viala K, Maisonobe T et al. Sensory Neuronopathy (Sjögren Ganglionopathy): Long-term outcome and treatment response in a series of 13 cases. Medicine (Baltimore). 95(19), e3632 (2016).
- Mekinian A, Ravaud P, Hatron PY et al. Efficacy of rituximab in primary Sjögren’s syndrome with peripheral nervous system involvement: results from the AIR registry. Ann. Rheum. Dis. 71(1), 84-87 (2012).
- Voulgarelis M, Giannouli S, Tzioufas AG, et al. Long term remission of Sjögren's syndrome associated aggressive B cell non-Hodgkin's lymphomas following combined B cell depletion therapy and CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone). Ann. Rheum. Dis. 65(8), 1033-1037 (2006).