Review Article - Imaging in Medicine (2013) Volume 5, Issue 2
Automated breast ultrasound: a novel approach to screening women with dense breasts
Megan J Lenihan1 & Rachel F Brem*11Breast Imaging & Interventional Center, Department of Radiology, The George Washington University, 2150 Pennsylvania Avenue, NW, Washington, DC 20037, USA
- Corresponding Author:
- Rachel F Brem
Breast Imaging & Interventional Center
Department of Radiology, The George Washington University
2150 Pennsylvania Avenue, NW, Washington, DC 20037, USA
Tel: +1 202 741 3031
Fax: +1 202 741 3029
E-mail: rbrem@mfa.gwu.edu
Abstract
Keywords
3D sonography ▪ ABUS™ ▪ automated breast ultrasound ▪ breast cancer screening ▪ breast density ▪ breast imaging ▪ cancer detection ▪ ultrasound
Mammography is still the standard in breast cancer detection, and has been shown to reduce breast cancer mortality by as much as 30% by finding small cancers at an early stage [1,2]. However, between 10 and 50% of breast cancers are not visible with mammography [3,4]. Women with dense breast tissue are more likely to have cancers not visible on a mammogram [5]. Not only is cancer less easily seen on mammograms for women with heterogeneously or extremely dense breasts, these women also have a four- to six-times increased risk for breast cancer [6]. Ultrasound (US) has been proven to be a useful adjunct to mammography and it can detect over 40% more cancers than mammography or physical examination alone [7,8]. Standard handheld US screening is limited because it is time consuming for technicians to perform and physicians to review, and it lacks consistency between operators. In response to these issues, automated wholebreast or 3D US has been under development for decades. Recent advances in automated breast US have led to devices that have been shown to be successful in preliminary studies as adjuncts to mammography. These devices provide promising US screens for women with dense tissue.
Breast cancer is the most common cancer in women, detected primarily through mammography [1,9]. Sensitivity of mammograms for the general population ranges from 77.6 to 87.0%, meaning that anywhere from 13.0 to 23.0% of cancers are not detected by mammography [3,7,10]. For women with dense breasts, mammographic sensitivity is even lower [7,8,11–13]. The enhanced clarity provided by full-field digital mammography was thought to improve the ability of radiologists to differentiate these hard-to-see cancers in dense tissue from screen-film mammography. Recent studies, however, have shown that while full-field digital mammography tends to be better at detecting cancers with certain mammographic features, such as small cancers, it has not significantly improved the screening capabilities, as over 30% of cancers are still not visible [14].
Breast density
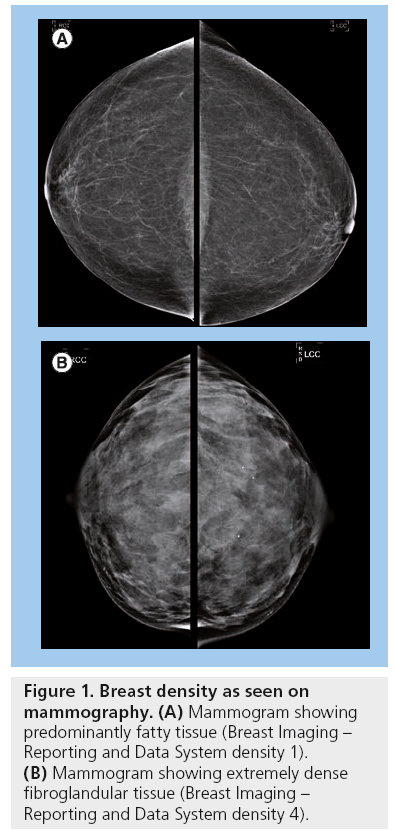
Dense breast tissue is normal and very common; approximately 40% of all women have dense breasts [15]. Breast density is based on the proportion of stromal and epithelial tissue compared with the fibrofatty tissue. The stromal and epithelial tissue appears radiopaque as opposed to the radiolucent fatty tissue (Figure 1). Breast density is classified based on the Breast Imaging–Reporting and Data System (BI-RADS) of the American College of Radiologists, which classifies density into one of four categories. They are: one, almost entirely fatty (0–25% density); two, scattered fibroglandular tissue (25–50% density); three, heterogeneously dense (50–75% density); and four, extremely dense (75–100% density) [16]. A higher risk of breast cancer has been established for those women with BI-RADS 3 and 4 densities [11]. It is also more difficult to image women with dense breasts using mammography.
It has long been established that women with predominantly dense breasts are at a greater risk for breast cancer. This was first described by Wolfe in 1976 [17]. As mammography techniques and technology have improved through the decades, Wolfe’s conclusions have been confirmed; studies consistently show a four- to six-fold increase in breast cancer risk for women with 60% or more breast density [18–21]. In comparison with predominantly fatty breasts, there is a 17.8-fold higher likelihood of an interval cancer – that is, a cancer diagnosed within 12 months of a normal mammogram, for women with extremely dense breasts [19].
Part of this increase in interval cancers for women with dense breasts can be attributed to the difficulty in imaging. Glandular breast tissue and cancer, as well as other breast lesions, appear white on a mammogram, making it difficult to discern a lesion from normal dense breast tissue. For this reason it is likely that, in addition to the added risk of breast cancer in women with dense breasts, their cancers are more likely to be missed at their annual mammogram screening than women who do not have dense tissue. This may result in cancers being found at a later stage once the patient notices a lump. Sensitivity of mammography decreases from 87.0% in women with fatty breasts to 62.9% in women with dense breasts [3], which allows for a greater number of missed cancers.
Mammograms are effective breast cancer screenings because they find cancer at an earlier, more treatable stage than if they are found by palpation. Mammograms have been shown to reduce mortality from breast cancer by 30% [1], and only 18% of mammography screen-detected cancers are late-stage cancers, as opposed to 47% of those detected clinically [22]. Therefore, cancers that are missed at the time of mammogram screening are eventually found at a later stage with a worse prognosis. Considering the higher likelihood of an interval cancer occurring in women with dense breasts, and the fact that interval cancers are likely to be larger and found at a later stage, it is important that these women have additional screening options to ensure that cancers are caught at an early and treatable stage.
Alternative screening methods
There has been some success in alternative screening methods. Many women with a very high risk for breast cancer (greater than 20% lifetime risk) due to family or personal history of breast cancer, or a genetic predisposition, opt to undergo MRI or breast-specific g-imaging as an additional screening for cancer [8,23,24]. These examinations, however, are more expensive and more time consuming. Breast-specific g-imaging exposes patients to radiation and requires an injection. MRI also requires an injection and, in addition, can be inaccessible to patients due to obesity or pre-existing conditions, such as renal insufficiency or an implanted device, and is generally not well tolerated by patients [14].
The most reasonable and promising imaging modality to be used for screening women with dense breasts is US. US has long been proven to be a helpful diagnostic tool in breast imaging. On US, breast tissue appears white, but many lesions appear dark gray or black, making it possible for radiologists to distinguish lesions in dense breast tissue. US is most frequently used for the additional visualization of palpable areas and abnormalities seen on a mammogram. It is useful in the differentiation between solid and cystic masses [25].
A number of studies have examined the use of US as a screening adjunct to mammography, particularly for women with dense breasts. In 2002, Kolb et al. found that US was able to detect 42% of cancers that were not detected by mammography in women with BI-RADS density 2–4, increasing the screening sensitivity to 97.3% [7]. These findings were corroborated in the ACRIN 6666 trial, which found an additional 4.2 cancers per 1000 women screened using handheld US after mammography [8,14]. Similar increases in sensitivity have been found when US is used in combination with mammography in women with BI-RADS density 3 or 4 [12].
Many states in the USA are now passing legislation to require physicians to inform their patients of breast density at the time of their mammogram. As mandated by Public Act 09–41, physicians in Connecticut (USA) are among those mandated to inform patients of their breast density. Insurance companies must also cover the cost of US screening for those patients with dense breasts. Hooley et al. studied the implementation of this legislation by following 935 women with normal mammograms and dense breasts who received handheld whole-breast US screenings [26]. Three additional cancers were found in this study due to US screening alone [26,27].
While US has been proven to be an effective tool when used in combination with mammography, it does have some drawbacks and difficulties. Many studies have reported higher rates of false positives when US is used for screening [8,14]. With further study of the appearance of benign and malignant lesions as visualized with US, this false-positive rate will likely drop. In addition, US of the whole breast is time consuming to perform with a handheld transducer and is operator-dependent. A typical examination takes 20 min, during which the operator must make frequent adjustments of pressure, gain and patient positioning, and any abnormalities must be identified during the examination [8,14]. As a result, handheld screening USs should be reserved for diagnostic imaging and are not feasible for all patients with dense breasts due to significant workflow problems. Handheld US does not meet the standards for a screening method as defined by WHO [21].
Recent advances: automated breast US
In response to this, new technologies have been developed in order to create a reproducible, comprehensive and efficient US scan of the breast. Most of these technologies are new to the market, but it is an exciting, rapidly developing area that is sure to be beneficial for women with dense breasts. Four devices, the SomoVu Automated Breast US System (ABUS™) by USystems, the Acuson S2000™ Automated Breast Volume Scanner (ABVS) by Siemens, the Automated Whole Breast US (AWBU) by SonoCine, and the 3D Multimodal Ultrasonic Tomography (MUT) show great promise in the emerging field of automated 3D US.
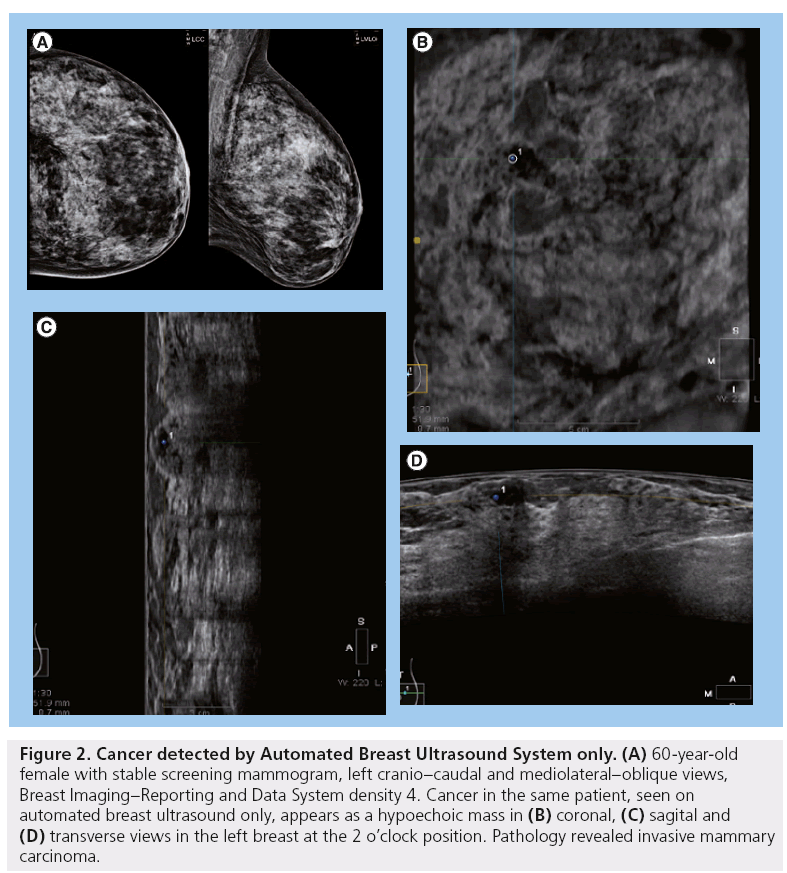
ABUS, developed by USystems and recently acquired by General Electric (CT, USA), has been under study since 2009 in a comprehensive, multicenter clinical trial to compare the sensitivity of mammography and the combination of mammography and ABUS together. So far, of 15,143 women ≥25 years of age with ≥50% density and no symptoms who had the ABUS screening examination in addition to their screening mammogram, 26.8% of all cancers found were seen by ABUS alone and of those, 92.0% were invasive (Figure 2) [28]. Recently, at The Breast Cancer Research Institute, Nova Southeastern University College of Medicine (FL, USA), ABUS, in combination with mammography, detected 12.3 breast cancers per 1000 screening examinations of asymptomatic women with dense tissue, as opposed to 4.6 cancers per 1000 screens detected by mammography alone [29]. The false-positive rate for ABUS is not yet known. ABUS is the only device cleared by the US FDA for screening women with dense breast tissue. The cost for the procedure varies among institutions, although it is typically affordable.
Figure 2: Cancer detected by Automated Breast Ultrasound System only. (A) 60-year-old female with stable screening mammogram, left cranio–caudal and mediolateral–oblique views, Breast Imaging–Reporting and Data System density 4. Cancer in the same patient, seen on automated breast ultrasound only, appears as a hypoechoic mass in (B) coronal, (C) sagital and (D) transverse views in the left breast at the 2 o’clock position. Pathology revealed invasive mammary carcinoma.
ABUS uses a 14–16 MHz high-frequency ultra-broadband transducer housed in a paddle that is held by the technologist and used to lightly compress the breast against the chest wall. Images are obtained in the transverse view, per standard US, over a 15 × 18 × 5.0 cm area and then stored in a 3D block. A minimum of three views are captured of each breast: anterior–posterior, medial and lateral. A separate view station allows for viewing the images in reconstructed 2-mm coronal or the original transverse views. The size of the coronal sections is large enough to avoid unnecessary slices and a too large file size, but remains small enough to match the size of an individual terminal ductal lobular unit so as not to miss small abnormalities. The axilla of the breast can also be thoroughly imaged with additional views.
ABUS separates image acquisition and interpretation. Unlike handheld US, the entire data set is acquired, and following acquisition is reviewed by the physician, thereby obviating the need to rely on the interpretation of the technologist to determine whether a lesion is present without requiring a physician to perform the US study himself/herself. If a physician were to perform a handheld US examination, he or she may spend 20–30 min scanning a patient; ABUS can be operated by a technologist and the images can be read by a physician in 173.4 s on average [30]. The stereotyped method of image acquisition makes for an easily reproducible examination, and the automation allows for standardization across device operators. Many of the drawbacks of handheld US are resolved by ABUS.
ABUS was designed as a screening tool. In the authors’ practice, ABUS is used solely for screening patients. Additional use of the equipment for US biopsy guidance would hinder patient flow, as fewer patients could be screened. Furthermore, handheld US is better suited and designed for biopsy guidance.
The Acuson S2000 ABVS produced by Siemens is similar in design and operation to the SomoVu ABUS. While less comprehensive research has been carried out on this device, it was successful in detecting 81% of lesions that had previously been seen on mammography, demonstrating that it is reliable for lesions greater than 1.2 cm, of which it detected 92% [31]. This lesion size is larger than cancers reported with ABUS, but additional research is needed to further define the characteristics of cancers detected with ABVS [27].
The AWBU produced by SonoCine takes a slightly different approach to whole-breast US. This device uses a handheld transducer controlled by an automated mechanical arm that moves the transducer in a specific pattern of overlapping longitudinal rows [32]. The AWBU software then creates a cine loop for interpretation, creating the appearance of realtime imaging [32]. In a study of this device used to evaluate 4419 high-risk women at the time of their routine mammograms, AWBU achieved a positive predictive value of 38%, a marked improvement over the 11% positive predictive value of handheld US in the ACRIN 6666 trial [32–34].
A prototype has been under study at the University of Athens (Greece): 3D MUT uses ultrasonic tomography of the pendulant breast in a water bath to create a 3D representation [35]. The device has shown some promise in its ability to correctly classify benign and malignant lesions in a study of 25 patients with lesions ≥0.10 cm [35].
3D automated US is certain to be a significant contributor to the future of breast imaging and early cancer detection. Currently, the primary concern for the use of US to screen women with dense breasts in addition to mammography is a high false-positive rate compared with that of mammography, and the possibility of unnecessary procedures. The ACRIN 6666 trial demonstrated an increase in biopsies as a result of added US screening, although the risk of false positives decreased significantly with additional annual screenings [14]. With further study of lesions and their appearance with 3D automated US, and integration of the examination into annual screenings, the specificity will likely increase and biopsies of benign lesions will decrease.
Concurrent development of other technologies may also aid in the accurate identification and diagnosis of breast disease. Computer-aided detection (CAD) has been developed for 3D US volumetric images. One system from the University of Michigan (MI, USA) has found that CAD improves radiologists’ ability to distinguish benign from malignant masses [36]. CAD has also recently been applied to ABUS, and been found to be successful in feature extraction and classification of breast tumors in images generated by ABUS [37]. Elastography may be helpful as a future application of 3D US. It is a measure of the stiffness of tissue computed from US imaging that may help to rule out benign lesions, reducing unnecessary biopsies [38]. The integration of elastography in clinical practice is just beginning and requires significant additional research. As whole-breast US is an emerging technology, additional tools such as Doppler and contrast-enhanced US are being evaluated and integrated.
Overall, the 3D view available through ABUS and similar US screening methods helps radiologists to gain a better understanding of a patient’s breast compared with the 2D mammographic view, especially if that patient has dense breasts that limit the imaging capabilities of mammography. 3D mammography, or tomosynthesis, is another new technology being implemented in many practices across the USA. It has been shown to reduce recall rates for women undergoing screening mammography and increase diagnostic accuracy, but it is unclear as to whether or not it aids radiologists in the detection of cancer in dense breast tissue [39]. Further studies to define the incremental increase in cancer detection in women with dense breasts are still needed.
Conclusion
3D automated breast US is the most promising new technology for the detection of breast cancer in women with dense breasts. There had previously been a lack of options for one of the largest groups at elevated risk for breast cancer, with no reasonable method of screening beyond mammography. 3D screening US will probably fill the void to improve the sensitivity of yearly breast cancer examination for women with dense breasts. There is still more to be explored in these new technologies, including the possible use of CAD and elastography. However, devices such as ABUS are capable of detecting cancers occluded by dense tissue on mammography and give radiologists a more comprehensive image of the breast.
Mammography is effective and sufficient for the majority of the population with predominantly fatty tissue at average risk for breast cancer; it will detect nearly 90% of breast cancers [4]. Women at high risk for breast cancer due to a genetic predisposition or family or personal history of breast cancer typically undergo MRI or breast-specific g-imaging as an additional screening. These screenings are effective and reasonable for this population; they are not, however, reasonable for the 40% of women with dense breast tissue [16]. ABUS is an effective and efficient screening examination for women with dense breast tissue at intermediate risk for breast cancer. With the successful implementation of ABUS, the hope is that the prevalence of interval cancers will decrease and breast cancer will be caught in women with dense breast tissue at an earlier, more treatable stage.
Future perspective
The use of ABUS and similar devices is likely to increase rapidly as more data becomes available, supporting its ability to detect cancers missed by mammography. In 5–10 years, ABUS, as an adjunct to mammography, may well become the standard of care for women with dense breast tissue. Further study on the appearance of benign and malignant lesions on ABUS will lead to more accurate diagnoses of cancer, fewer false positives, less additional imaging and fewer unnecessary biopsies. Eventually, other technologies may be used in combination with whole-breast US, such as CAD and elastography, to enhance the ability to identify and diagnose lesions.
Financial & competing interests disclosure
RF Brem is a consultant for U-Systems Inc., the manufacturer of the SomoVu ABUS™ scanner. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.

References
Papers of special note have been highlighted as:
•of interest
• •of considerable interest
- Tabár L, Vitak B, Chen TH et al. Swedish two-county trial: impact of mammographic screening on breast cancer mortality during 3 decades. Radiology 260(3), 658–663 (2011).
- Duff SW, Tabár L, Chen HH et al. The impact of organized mammography service screening on breast carcinoma mortality in seven Swedish counties. Cancer 95(3), 458–469 (2002).
- Carney PA, Miglioretti DL, Yankaskas BC et al. Individual and combined effects of age, breast density, and hormone replacement therapy use on the accuracy of screening mammography. Ann. Intern. Med. 138(9), 771 (2003).
- Taplin SH, Rutter CM, Finder C, Mandelson MT, Houn F, White E. Screening mammography: clinical image quality and the risk of interval breast cancer. AJR Am. J. Roentgenol. 178(4), 797–803 (2002).
- Corsetti V, Houssami N, Ghirardi M et al. Evidence of the effect of adjunct ultrasound screening in women with mammographynegative dense breasts: interval breast cancers at 1 year follow-up. Eur. J. Cancer 47, 1021–1026 (2011).
- Harvey JA, Bovbjerg VE. Quantitative assessment of mammographic breast density: relationship with breast cancer risk. Radiology 230, 29–42 (2004).
- Kolb TM, Lichy J, Newhouse JH. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology 225, 165–175 (2002).
- Berg WA, Blume JD, Cormack JB et al. Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. JAMA 299(18), 2152–2163 (2008).
- Landis SH, Murray T, Bolden S, Wingo PA. Cancer statistics, 1999. CA Cancer J. Clin. 49, 8–31 (1999).
- Huang Y, Kang M, Li H et al. Combined performance of physical examination, mammography, and ultrasonography for breast cancer screening among Chinese women: a follow up study. Curr. Oncol. 19(2), eS22–eS30 (2012).
- Schaefer FK, Waldmann A, Katalinic A et al. Influence of additional breast ultrasound on cancer detection in a cohort study for quality assurance in breast diagnosis – analysis of 102,577 diagnostic procedures. Eur. Radiol. 20, 1085–1092 (2010).
- Kriege M, Brekelmans CT, Boetes C et al. Efficacy of MRI and mammography for breast-cancer screening in women with familial of genetic predisposition. N. Engl. J. Med. 351(5), 427–437 (2004).
- Berg WA, Zhang Z, Lehrer D et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA 307(13), 1394–1404 (2012).
- Hoff SR, Abrahamsen A, Samset JH, Vigeland E, Klepp O, Hofvind S. Breast cancer: missed interval and screening-detected cancer at fullfield digital mammography and screen-film mammography – results from a retrospective review. Radiology 264(2), 378–386 (2012).
- Stomper PC, D’Souza DJ, DiNitto PA, Arredondo MA. Analysis of parenchymal density on mammograms in1353 women 27–79 years old. AJR Am. J. Roentgenol. 167(5), 1261–1265 (1996).
- American College of Radiology. Breast Imaging Reporting and Data System Atlas (BI-RADS Atlas); 4th Edition. American College of Radiology, Reston, VA, USA (2003).
- Wolfe JN. Breast patterns as an index of risk for developing breast cancer. AJR Am. J. Roentgenol. 126(6), 130–137 (1976).
- MacCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol. Biomarkers Prev. 15(6), 1159–1169 (2006).
- Boyd NF, Guo H, Martin LJ et al. Mammographic density and the risk and detection of breast cancer. N. Engl. J. Med. 356(3), 227–236 (2007).
- Chiu SY, Duffy S, Yen AM, Tabár L, Smith RA, Chen HH. Effect of baseline breast density on breast cancer incidence, stage, mortality, and screening parameters: 25-year follow-up of Swedish mammographic screening. Cancer Epidemiol. Biomarkers Prev. 19, 1219–1228 (2010).
- Lander MR, Tabár L. Automated 3-D breast ultrasound as a promising adjunctive screening tool for examining dense breast tissue. Semin. Roentgenol. 46(4), 302–308 (2011).
- Berg WA. Supplemental screening sonography in dense breasts. Radiol. Clin. North Am. 42(5), 845–851 (2004).
- Tadwalker RV, Rapelyea JA, Torrente J et al. Breast-specific gamma imaging as an adjunct modality for the diagnosis of invasive breast cancer with correlation to tumor size and grade. Br. J. Radiol. 85(1014), e212–e216 (2012).
- Brem RF, Rapelyea JA, Zisman J et al. Occult breast cancer: scintimammography with highresolution breast-specific gamma camera in women at high risk for breast cancer. Radiology 237(1), 274–280 (2005).
- Sickles EA, Filly RA, Callen PW. Benign breast lesions: ultrasound detection and diagnosis. Radiology 151(2), 467–470 (1984).
- Hooley RJ, Greenberg KL, Stackhouse RM, Geisel JL, Butler RS, Philpotts LE. Screening US in patients with mammographically dense breasts: initial experience with Connecticut public act 09–41. Radiology 265(1), 59–69 (2012).
- Weigert J, Steenbergen S. The Connecticut experiment: the role of ultrasound in the screening of women with dense breasts. Breast J. 18(6), 717–722 (2012).
- Brem RF, Rapelyea JA, Torrente J, Kann MR. Characteristics cancers detected by 3D Automated Breast Ultrasound (ABUS) in screening mammography with BI-RADS assessment 1 or 2. Presented at: 98th Scientific Assembly and Annual Meeting of the Radiological Society of North America. Chicago, IL, USA, 25–30 November 2012.
- Giuliano V, Giuliano C. Improved breast cancer detection in asymptomatic women using 3D-automated breast ultrasound in mammographically dense breasts. Clin. Imaging doi:10.1016/j.clinimag.2012.09.018 (2012) (Epub ahead of print).
- Brem RF, Rapelyea JA, Torrente J et al. Interpretation time of 3D automated breast ultrasound. Presented at: The American Roentgen Ray Society 2012 Annual Meeting. Vancouver, BC, Canada, 29 April–4 May 2012.
- Shin HJ, Kim HH, Cha JH, Park JH, Lee KE, Kim JH. Automated ultrasound of the breast for diagnosis: interobserver agreement on lesion detection and characterization. AJR Am. J. Roentgenol. 197, 747–754 (2011).
- Kelly KM, Dean J, Comulada WS, Lee S. Breast cancer detection using automated whole breast ultrasound and mammography in radiographically dense breasts. Eur. Radiol. 20, 734–742 (2010).
- Kelly KM, Dean J, Lee S, Comulada WS. Breast cancer detection: radiologists’ performance using mammography with and without automated whole-breast ultrasound. Eur. Radiol. 20, 2557–2564 (2010).
- Kapur A, Carson PL, Eberhard J et al. Combination of digital mammography with semi-automated 3D breast ultrasound. Technol. Cancer Res. Treat. 3(4), 325–334 (2004).
- Zografos G, Koulocheri D, Liakou P et al. Novel technology of multimodal ultrasound tomography detects breast lesions. Eur. Radiol. 23(3), 673–683 (2012).
- Sahiner B, Chan H, Roubidoux MA et al. Malignant and benign breast masses on 3D US volumentric images: effect of computeraided diagnosis on radiologist accuracy. Radiology 242(3), 716–724 (2007).
- Moon WK, Shen Y, Huang C, Chiang L, Chang R. Computer-aided diagnosis for the classification of breast masses in automated ultrasound images. Ultrasound Med. Biol. 37(4), 539–548 (2011).
- Lee SH, Chang JM, Kim WH et al. Differentiation of benign from malignant solid breast masses: comparison of two-dimensional and three-dimensional shear-wave elastography. Eur. Radiol. doi:10.1007/s00330- 012-2686-9 (2012) (Epub ahead of print).
- Rafferty EA, Park JM, Philpotts LE et al. Assessing radiologist performance using combined digital mammography and breast tomosynthesis compared with digital mammography alone: results of a multicenter, multireader trial. Radiology 266(1), 104–113 (2012).
• Explores alternative screening methods for women at elevated risk for breast cancer.
• • Connecticut (USA) legally mandates that doctors inform women of their breast density and that ultrasound screening be covered by insurance companies for women with dense breast tissue. These laws led to the common use of handheld ultrasound as a screening method. This study reports the use of handheld ultrasound as a screening method for women with dense breasts in clinical practice.
• Preliminary results of automated breast ultrasound in screening women with dense breast tissue show that automated breast ultrasound improves cancer detection rate and is a valuable tool for clinical practice.
• First experiences with automated whole-breast ultrasound show improved cancer detection rate and a promising future for this device in clinical screening.