Review Article - Interventional Cardiology (2013) Volume 5, Issue 2
Avoiding stent thrombosis: advances in technique, antiplatelet pharmacotherapy and stent design
- Corresponding Author:
- John Coppola
New York University School of Medicine, NY, USA
E-mail: john.coppola1952@yahoo.com
Abstract
Keywords
bioabsorbable stent, clopidogrel, dual antiplatelet therapy, first-generation DES, IVUS, mechanism, OCT, predictor, second-generation DES, stent thrombosis
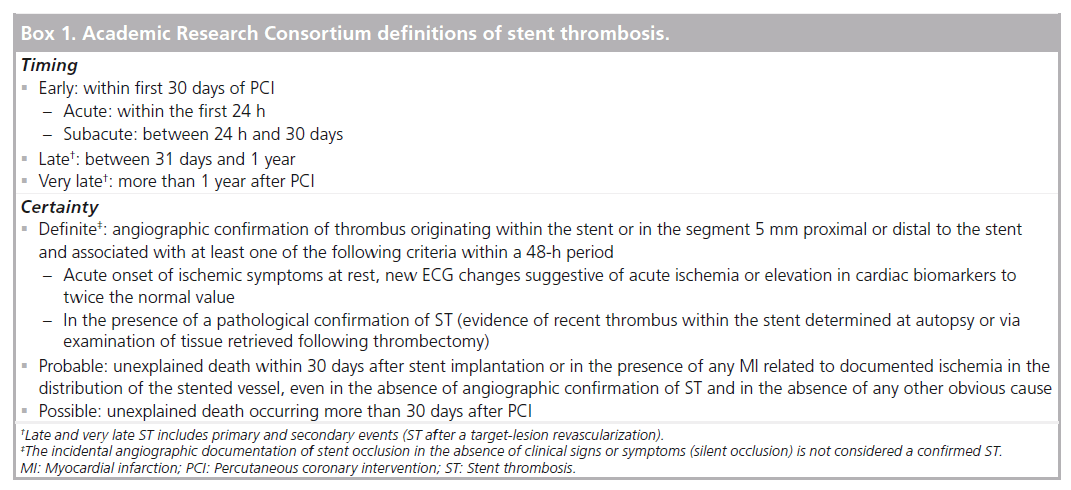
Definitions for stent thrombosis
Historically, stent thrombosis (ST) was proven angiographically by the demonstration of thrombus at the site of a previously placed stent with impaired downstream flow, or inferred clinically in patients with subsequent myocardial infarction (MI), target-lesion revascularization (TLR) or unexplained sudden death. While the angio-graphic definition of ST may underestimate the true incidence by not including patients who fail to survive to angiography, the clinical definition may overestimate the incidence by including events not attributable to ST. Definitions of ST, therefore, require a compromise between sensitivity and specificity. Clinical trials evaluating first-generation bare-metal stents (BMS) defined ST differently, leading to disparities in the reported incidence and making comparisons between studies problematic.
As the development and use of drug-eluting stents (DES) led to a perceived increase in rates of late and very late ST, the lack of uniformity in protocol definitions made comparisons of safety outcomes between different stents more problematic. Even prior to the 2006 European Society of Cardiology meeting that highlighted the issue of ST, a uniform and standardized definition was clearly necessary. To address this need, a group of clinical trialists, members of the US FDA and representatives from the major device manufacturers was assembled, known as the Academic Research Consortium (ARC). The document that resulted from this meeting incorporated terminology to reflect both the timing and the certainty of events and has since become a guide on how multiple clinical end points can be more effectively compared across trials [1].
By establishing uniform criteria for angiographic confirmation and maintaining the principle of intention-to-treat by not excluding patients with interval TLR, this new classification created standardized definitions that reconciled many of the inconsistencies of preceding protocol definitions (Box 1). These new definitions were not, however, without limitations. While the ‘definite’ label is highly specific due to the requirement of evidence of thrombus, the ‘probable’ and ‘possible’ labels allow for increasing levels of sensitivity to capture more events. The inclusion of all unexplained deaths occurring beyond 30 days under the possible label could potentially exaggerate rates of very late ST among patients whose death is without another clear etiology.
Subsequent trials have primarily reported on ARC-definite and/or probable (def/prob) ST rates, although some have advocated for expanded reporting to include ARC-possible events. In an effort to validate ARC definitions of ST among an autopsy registry of subjects with prior coronary stenting, Cutlip et al. suggested that reporting only definite or def/prob events may result in substantial under-reporting of confirmed true ST events that may be captured by the ARC-possible designation [2]. A modification of the possible criteria to designate those events in which sudden cardiac death or acute ischemia was felt to be likely versus unlikely was found to allow for the expected increase in sensitivity by including such events together with def/prob events and with an increase in specificity compared with an inclusion of all possible events. While such criteria have yet to be adopted, the study highlights the ongoing limitations of accurately capturing the true occurrence of ST events.
Timing & incidence
Many factors influence the timing and incidence of ST. Early trials reported high rates of acute vessel closure ref lective of the equipment, procedural techniques and pharmacotherapy of the time, all of which have since been greatly refined leading to much lower event rates in contemporary studies. Although the establishment of ARC definitions now allows for more meaningful comparisons of ST between trials, event rates continue to vary based on the study design (i.e., randomized controlled trial [RCT] vs registry analysis), the duration of follow-up and the clinical characteristics of the patient population.
The timing of ST is often the first clue to the underlying mechanism. The majority of events occur within the first 30 days with both BMS and DES, often relating to suboptimal procedural results. Data from over 21,000 patients in the Dutch Stent Thrombosis Registry provide insight into the timing of ST with a 2.1% incidence of definite ST at median follow-up of 31 months [3]. Nearly 75% of these 437 events occurred within the first 30 days (32% acute and 41% subacute) with the remainder occurring with similar frequency during the late period (13%) and very late period (14%). Beyond the early period, the risk for ST varies depending on the type of stent placed with unique risk profiles afforded by each successive generation of stents.
▪ Bare-metal stents
Early investigational BMS were associated with rates of ST and acute vessel closure as high as 16–24% at 30-day follow-up [4,5]. The incorporation of anti-thrombotic therapy and high-pressure inflations to subsequent trials reduced the incidence to below 2% [6]. By 2001, a pooled analysis of six major trials and associated registries reported a 30-day ST rate of 0.9% [7], with the vast majority occurring within the first 48 h. Many of these trials did not report the incidence of ST beyond the early period due to the observation that such events were rarely observed to occur [1]. This attenuated risk results from the vascular response to stent deployment characterized by smooth muscle proliferation and neointimal hyperplasia leading to endothelialization of stent struts.
More recent data, however, suggest that the risks of late and very late ST with BMS are not negligible. The Coronary Angiography and Angioplasty Registery (SCAAR) reported comparable 30-day rates of definite ST (0.6%) to RCTs [8], but more pronounced 1-year (1.2%) and 2-year (1.4%) definite ST rates among this large cohort of unselected patients [9]. A retrospective study of 4503 BMS patients found a cumulative incidence of 0.5% at 30 days, 0.8% at 1 year and 2.0% at 10 years [10]. This greater than twofold increase in events between years 1 and 10 suggests that BMS may not be as safe as many have assumed. Among these patients, clinical restenosis occurred in 18.1% at 10-year follow-up with 2.1% of patients presenting with MI, suggesting that the mechanisms of these late events may be different to those with DES [11].
▪ First-generation DES
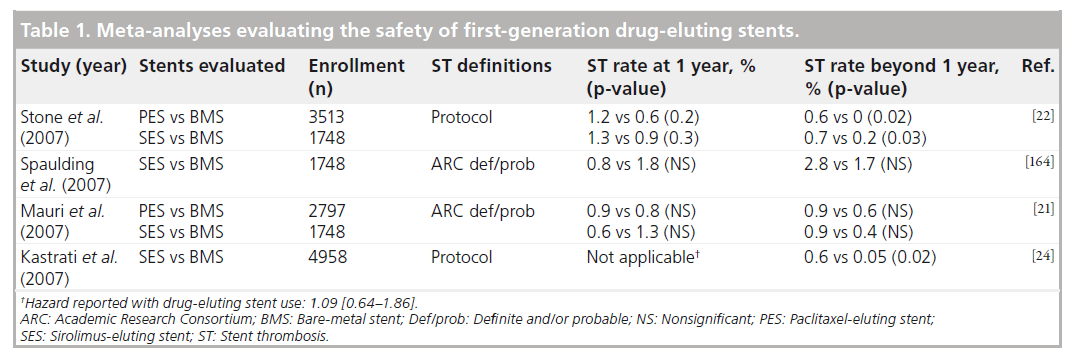
Compared with balloon angioplasty alone, the use of BMS resulted in a significant reduction in both abrupt vessel closure and restenosis at the site of the treated lesion, but at 1 year, revascularization rates remained as high as 21% [12]. This major limitation prompted the development of novel stents coated with durable biocompatible polymers that slowly release drugs capable of inhibiting smooth muscle cell proliferation and migration to the overlying endothelium [13]. In the pivotal SIRIUS trial, the Cypher® sirolimus-eluting stent (SES; Cordis, NJ, USA) was associated with a dramatic reduction in in-stent restenosis and repeat revascularization compared with BMS with low rates of ST observed in both arms (0.4% SES vs 0.8% BMS; p = nonsignificant [NS]) [14]. A similar reduction in revascularization favoring the TAXUS® paclitaxel-eluting stent (PES; Boston Scientific, MA, USA) compared with BMS was demonstrated in the TAXUS-IV trial with no appreciable difference in ST (0.8% PES vs 0.6% BMS; p = NS) [15].
Based on the dramatic reduction in restenosis, the cardiology community praised these first-generation DES as a transforming technology. By the end of 2004, they were used in nearly 80% of coronary interventions in the USA [16]. This early enthusiasm quickly evolved into concern, however, following an FDA advisory alerting physicians of a possible heightened risk associated with DES use based on nearly 300 episodes of reported (60 fatal) ST among SES recipients [17]. Concern intensified following the 2006 European Society of Cardiology Congress during which two meta-analyses reported an increased risk of death associated with DES use compared with BMS [18,19]. Follow-up data from the single-center BASKET–LATE trial also found DES use to be associated with higher rates of death or MI (4.9 vs 1.3%), as well as thrombosis-related clinical events (2.6 vs 1.3%) between 6 and 18 months compared with BMS use [20]. Despite receiving some methodological criticism, these studies had an immediate impact on clinical practice, reflected by a sudden and dramatic decrease in DES use.
In the wake of this controversy, multiple independently conducted meta-analyses of RCTs, comparing DES with BMS, were published (Table 1), each demonstrating there to be no significant increase in the overall rates of death or MI among DES recipients [21–24]. Depending on whether protocol definitions of ST were used, some identified a small, but significant, difference in very late ST (~0.2% per year) between groups based on a small number of events occurring beyond the first year [22,24]. A network meta-analysis including 38 trials, 18,023 patients and up to 4-year follow-up data found similar rates of short- and long-term mortality, regardless of the type of stent placed, and no significant differences in the overall risk of definite ST between either DES and BMS [23]. Five-year follow-up from the TAXUS and SIRIUS family of trials have since been reported. Compared with BMS, neither PES nor SES were found to have a statistically significant difference in cumulative def/prob ST, but a trend towards more very late ST with PES compared with BMS (1.4 vs 0.9%; p = 0.18) [25] and with SES compared with BMS (1.4 vs 0.7%; p = 0.22) [26] was found, again corresponding to a 0.2–0.3% rate of very late ST with these first-generation DES.
ST rates reported from registries are slightly higher due to more complex anatomy, medical comorbidities and off-label DES use, compared with clinical trials. Analysis of the Bern–Rotterdam registry revealed late and very late ST rates of 0.4–0.6% per year, persistent for up to 4 years [27,28] with a similar rate of 0.3–0.4% per year reported from the SCAAR registry [8]. SIRTAX–LATE, which randomized 1012 high-risk patients to SES versus PES, found similar overall rates of definite ST (4.6 vs 4.1%; p = NS) between years 1 and 5, translating to a higher (0.65% per year) rate of very late ST that are more consistent with findings from these registries [29].
▪ Second-generation DES
As will be discussed in greater detail, second-generation DES have incorporated multiple design improvements that have resulted in lower rates of ST compared with first-generation DES [9]. No RCTs have directly compared second-generation DES with BMS, but indirect comparisons have suggested that the likelihood of ST associated with some of these new stents is comparable or even lower than BMS [30,31]. Rates of ST also appear to be lower with novel stents utilizing bioabsorbable materials, although these data are currently limited to patients of lower risk and complexity.
Mechanisms of ST
The mechanisms underlying ST are multifactorial and differ based on the timing of stent placement. ST in the early period is often attributable to suboptimal periprocedural results and/or early discontinuation of dual antiplatelet therapy (DAPT), while ST occurring in the late period is more often related to delayed arterial healing caused by a reaction to one ore more stent components. Very late ST can result from the same factors causing late ST and may also be related to late acquired stent malapposition or neoatherosclerosis with plaque rupture. Pathologic studies and in vivo imaging studies utilizing intravascular ultrasound (IVUS) and optical coherence tomography (OCT) have provided unique insight into many of these mechanisms, particularly among recipients of first-generation DES in whom a growing incidence of late and very late ST were increasingly recognized.
▪ Insights from pathologic studies
One of the first case reports described a patient death as a direct result of ST occurring 18 months after being treated with a Cypher DES, Virmani et al. described the findings of an extensive inflammatory infiltration with a predominance of eosinophils, probably caused by a localized hypersensitivity response to the stent polymer [32]. Just prior to this publication, the FDA reported up to 50 possible cases of a generalized hypersensitivity reaction occurring in SES recipients, although many were later attributed to probable reactions to clopidogrel. However, in reviewing 262 cases of possible hypersensitivity reactions from the Research on Adverse Drug Events and Reports (RADAR) project, which investigates causality between therapeutic agents and potentially fatal adverse events, clopidogrel was only implicated in two cases, whereas 17 cases were attributed to the stent itself [33].
In a more robust examination of 14 cases of late ST, DES patients exhibited more evidence of persistent fibrin deposition and incomplete endothelialization compared with matched autopsies of BMS patients, in whom the majority of stent struts have been shown to be endothelialized by 3 months [34]. This delay in arterial healing is now recognized as a probable nidus for thrombosis. Additional risk factors identified in this small series included local hypersensitivity reactions, malapposition, penetration of stent struts into a necrotic core, ostial or bifurcation stenting and early withdrawal of antiplatelet therapy. In another evaluation of patients who had died 30 days after DES placement, Finn et al. identified 28 out of 62 coronary lesions associated with thrombus and confirmed the extent of endothelial coverage to be the best histological predictor of ST [35]. This study also observed a greater degree of nonuniform healing among DES-treated lesions compared with BMS-treated lesions.
▪ Insights from IVUS
IVUS reveals details of lesion morphology beyond those that angiography alone can provide. This that can assist the operator in choosing appropriately sized stents, detecting mechanical problems after stent deployment and understanding mechanisms contributing to ST. IVUS has played an instrumental role in identifying predictors for both early ST due to suboptimal procedural results and late and very late ST due to mechanisms unique to DES.
Incomplete stent apposition (ISA) is characterized by a lack of contact between stent struts and the underlying vessel wall that may be present soon after stent deployment or develop later as a consequence of positive remodeling and/or thrombus resorption [36]. While ISA occurring early after stent placement has not been clearly linked to ST, late-acquired ISA has been identified as an independent risk factor for late and very late ST post-DES [37]. In a series of patients presenting with very late ST who were evaluated with IVUS, ISA was detected more frequently compared with matched controls (77 vs 12%; p < 0.001) [38]. In another series of 30 patients (23 DES and seven BMS), ISA was present exclusively among DES recipients (74 vs 0%) with BMS recipients more commonly exhibiting evidence of disease progression or neointimal rupture within the stent (100 vs 43%) [39]. The etiology of late ISA remains incompletely understood, but pathologic evidence of medial necrosis of the arterial wall is believed to promote focal positive remodeling [40]. Such features may be seen angiographically as either peristent contrast staining or aneurysm formation. Histopathological evaluation of thrombus aspirates from patients presenting with very late ST after DES placement have found eosinophil counts to be three-times higher compared with patients with other causes of acute MI, correlating with the extent of ISA and suggestive of a delayed-type hypersensitivity mechanism, in keeping with pathologic studies [41].
▪ Insights from OCT
While IVUS provides excellent tissue penetration to characterize plaque morphology, OCT offers up to ten-times greater resolution that clearly delineates the interface of the vessel lumen and overlying stent struts, making it an attractive tool for assessing post-percutaneous coronary intervention (PCI) results and complications. The high level of detail provided by OCT allows it to quantify the degree of neointimal stent coverage and detect ISA [42]. In a multimodality imaging study of 18 patients with late ST of DES compared with matched controls, the presence of uncovered stent struts, as assessed by OCT, and positive vessel remodeling, as imaged by IVUS, were associated with late ST [43]. This study also demonstrated the ability of OCT to detect in-stent neoatherosclerosis and plaque rupture as potentially important mechanisms for very late ST occurring in both BMS and DES recipients [43]. OCT can distinguish between red blood cell-rich red thrombi, which appear as high-backscattering protrusions with signal-free shadowing and platelet-rich white thrombi, which appear as low-backscattering structures [44]. However, the strong degree of signal attenuation caused by a red thrombus can limit the ability to assess the underlying stent struts among patients presenting with ST. This presents a practical limitation of using this modality to elucidate the underlying mechanism of ST [42]. Future studies may expand the role of OCT but its role in assessing and reducing ST remains largely investigative at this time.
Predictors of stent thrombois
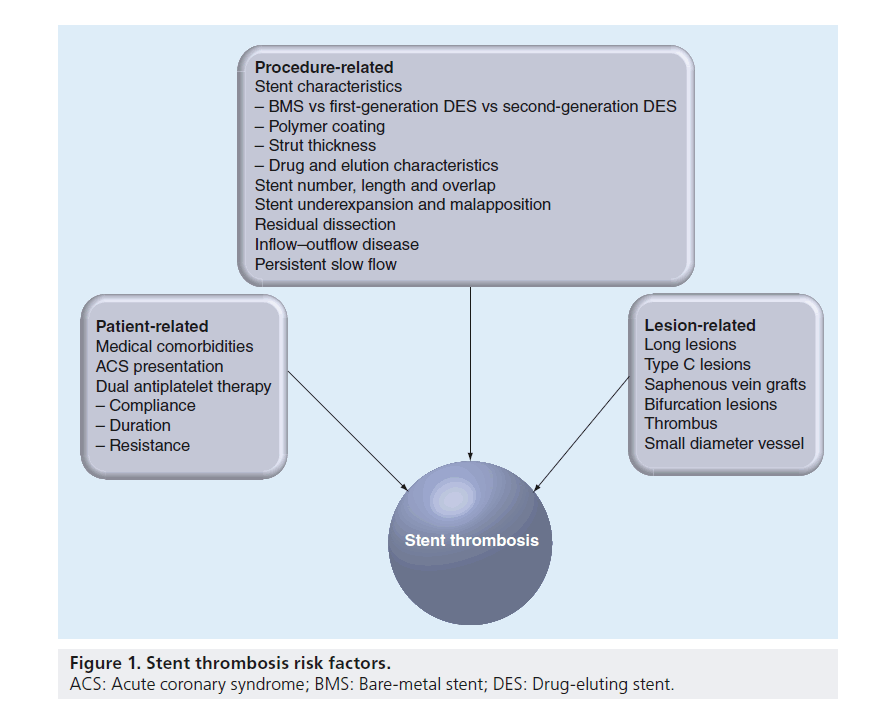
Risk factors for the development of ST are often categorized as being patient related, procedure related and lesion related (Figure 1). The risk of ST increases in accordance with the complexity of each of these features.
Patient-related predictors
▪ Demographics & comorbidities
Among the many risk factors for ST reported in different registries, renal insufficiency [45–48], diabetes mellitus [28,46–51], multivessel coronary artery disease (CAD) [48,51] and left ventricular dysfunction [3,46,50,52] are the most consistently identified comorbidities associated with ST. The DESERT registry, the largest case–control registry of ST to date with over 500 cases of definite late or very late ST, has identified multiple additional independent risk factors including younger age, current smoking and African–American race [53]. Other studies have implicated both cocaine abuse [54] and malignancy [3]. Although many of these risk factors are not readily modifiable, physicians caring for these patients should be aware of the risk they may pose prior to making decisions regarding stent placement in order to maximize the benefit of revascularization and minimize the potential for harm.
▪ Clinical presentation/acute coronary syndrome
Acute coronary syndrome (ACS) is characterized by a state of increased platelet activity that creates an environment in which ST is more prone to occur [7,8,28,45,47,50,55,56]. Findings such as the presence of thrombus at the site of plaque rupture or impaired flow due to distal embolization or spasm may lead to stent undersizing and poor stent expansion at the time of deployment. The penetration of stent struts into the necrotic core of the overlying lesion may also trigger a maladaptive vascular response [57]. Autopsy studies in acute MI have observed substantial delays in culprit site vessel healing among DES recipients characterized pathologically by increased inf lammation, increased fibrin deposition and more uncovered stent struts [58].
Non-ST-elevation–ACS
In the ACUITY trial, which enrolled 13,819 patients with moderate-to-high risk ACS, ST occurred in 1.4% at 30 days [48] and 2.2% by 1 year with similar rates between BMS and DES. These rates are much higher than rates observed in trials conducted in patients with stable CAD [21,22], but are comparable with rates in observational studies that included patients with ACS [27,49]. Subsequent trials evaluating newer P2Y12 inhibitors in a similar population of moderate-to-highrisk ACS patients have also demonstrated elevated ST rates ranging between 2.3 and 2.9% at 12- to 15-month follow-up among patients randomized to clopidogrel, with significant reductions in ST in favor of both prasugrel [59] and ticagrelor [60].
ST-elevation MI
As one might expect, ST rates are even higher among patients who present with ST-elevation MI (STEMI). In the HORIZONS–AMI trial, 3602 patients undergoing primary PCI for acute STEMI were randomized to either unfractionated heparin and glycoprotein IIb/IIIa inhibitors or bivalirudin monotherapy [61]. Among the 3202 patients who received a stent (2261 DES and 861 BMS), def/prob ST occurred in 137 (4.4%) patients with 28 (0.9%) acute, 49 (1.6%) subacute, 32 (1.0%) late and 33 (1.1%) very late [57]. The overall incidence of ST was not affected by the anticoagulation strategy, but acute ST (within 24 h) occurred more often among patients randomized to bivalirudin (1.4 vs 0.3%; p < 0.001), whereas subacute ST occurred more often among those who received unfractionated heparin/glycoprotein IIb/IIIa inhibitors (2.8 vs 4.4%; p < 0.02). ST occurred frequently during the index hospitalization (in over 33%) and was associated with a higher mortality rate compared with events that occurred postdischarge (27.8 vs 10.8%) [62]. The administration of unfractionated heparin prior to randomization and the use of a 600-mg loading dose of clopidogrel (as opposed to 300 mg) were identified as independent predictors of reduced acute and subacute ST, respectively, suggesting that the number of early ST events in this high-risk population may be reduced by these management strategies [57,63].
Prior to the publication of HORIZONS–AMI, the use of BMS over DES was thought to afford greater safety. Although the randomized TYPHOON trial [64] and PASSION trial [65], both comparing DES versus BMS in acute MI, found no differences in rates of ST at 1-year follow-up, other data suggested a higher risk associated with DES use in this population [27]. A subsequent meta-analysis of 13 trials and 7352 STEMI patients (including HORIZONS) found nearly equivalent rates of ST at 1 year, regardless of DES versus BMS (2.7 vs 2.6%, respectively) despite a 56% reduction in target-vessel revascularization (TVR) in favor of DES [66].
Emerging data now suggests that outcomes in STEMI patients may be enhanced with the use of current second-generation and next-generation stents. The EXAMINATION trial, a randomized comparison of everolimus-eluting stents (EES) versus BMS in patients presenting with STEMI, found a reduction in definite ST (0.5 vs 1.9%; p = 0.01) and def/prob ST (0.9 vs 2.6%; p = 0.01) among EES patients [67]. Similarly, the COMFORTABLE–AMI trial, a randomized comparison of a next-generation biolimus-eluting stent (BES) with a biodegradable polymer versus BMS in 1161 patients with STEMI, found a significant reduction in 1-year major adverse cardiac events (8.7 vs 4.3%; hazard ratio [HR]: 0.49 [95% CI: 0.30–30.80]; p = 0.004) in favor of BES driven by a lower risk of target vessel-related reinfarction (0.5 vs 2.7%; p = 0.01) and ischemia-driven TLR (1.6 vs 5.7%; p < 0.001) [68]. Rates of cardiac death and definite ST were not significantly different, although a higher number of ST events occurred among BES patients (12 vs 5; p = 0.10).
▪ Antiplatelet therapy: noncompliance, duration, dose & resistance
Antiplatelet therapy after PCI plays an essential role in the prevention of subsequent ST. Both noncompliance with and resistance to DAPT have been shown to be important risk factors for ST, particularly with first-generation DES. The recommended duration of DAPT has evolved from shorter periods employed in the trials leading to the approval of first-generation DES to longer durations based on the recognition of many events occurring soon after DAPT cessation. The optimal duration continues to be debated due to the ever-changing risks and efficacy associated with newer devices and drugs.
Noncompliance with DAPT
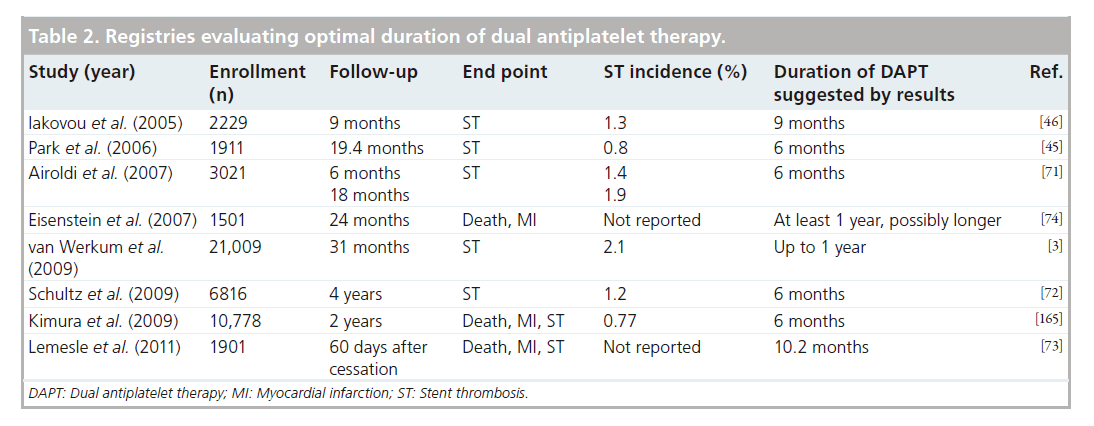
As cases of late ST associated with first-generation DES began to accumulate, multiple studies identified premature cessation of DAPT as an independent predictor for these events. Premature discontinuation of clopidogrel was associated with a greater than 30-fold increase in the risk of ST in a single-center study of 652 SES patients, with nearly 25% of such patients experiencing an event within the first month [69]. Among a cohort of 2229 DES patients, 29 (1.3%) experienced ST by 9 months, with premature discontinuation of DAPT found to be among the most powerful independent predictors of ST to date (HR: 89.8 [95% CI: 29.9–269.6]) [46]. An analysis of the PREMIER registry, which included 500 patients treated with DES during an acute MI, found nearly one in seven was noncompliant with thienopyridine therapy at 30 days [70]. Although ST was not specifically reported, noncompliance with DAPT was associated with higher mortality (7.5 vs 0.7%) during the next 11 months.
Duration & dose of DAPT
Other registries that have correlated early discontinuation of DAPT with ST have suggested that the impact becomes less pronounced as the time after PCI increases (Table 2). In a series of 3021 DES patients, 42 (1.4%) experienced ST by 6 months, with premature DAPT cessation found to be the strongest predictor of ST during this period (HR: 13.7 [95% CI: 4.0–46.7]) [71]. Although 16 additional events occurred beyond this time period, these were not predicted by thienopyridine cessation. A larger cohort of 6816 DES patients also found a strong association between definite ST (0.8% at 1 year and 1.2% at 4 years) and clopidogrel discontinuation that appeared to be confined to the first 6 months of follow-up [72]. An analysis of the Dutch Stent Thrombosis Registry found the lack of clopidogrel use at the time of ST to be the strongest predictor of ST in the first 30 days (HR: 36.5 [95% CI: 8.0–167.5]) with continued, but more modest risk up to and beyond 6 months [3]. Among 1903 DES patients who were stratified into four groups based on duration of clopidogrel therapy until cessation post-PCI (group 1: within 30 days; group 2: 1–6 months; group 3: 6–12 months; and group 4: after 12 months), the composite of death/MI/ST both at 30 days and between 31 and 60 days post-DAPT cessation were indexed [73]. Within the first 30 days postclopidogrel cessation, event rates were higher for group 1 (5.2%) compared with other groups (group 2: 1.2%, group 3: 0.9% and group 4: 0.6%; p = 0.004) with similarly low rates seen in all groups between days 31 and 60. When the time between the index PCI and cessation of clopidogrel was analyzed as a continuous variable, the probability of events occurring within the first 30 days became similar to that observed in the 31–60-day interval following cessation after a minimum of approximately 10.2 months.
In contrast to these studies, other registries have suggested benefit from extended durations of DAPT. In BASKET–LATE, 746 patients were randomized to DES versus BMS with DAPT for 6 months post-PCI followed by aspirin (ASA) monotherapy [20]. Although the 30-day rate of composite death and MI was lower for the DES group (2.0 vs 4.7%, p = 0.05), late ST occurred twice as frequently (2.6 vs 1.3%). Similarly, in an observational study of 4666 patients utilizing the Duke database, extended use of clopidogrel at 6, 12 and 24 months in DES patients was associated with lower rates of death and MI at all time intervals, a finding not observed in patients treated with BMS [74].
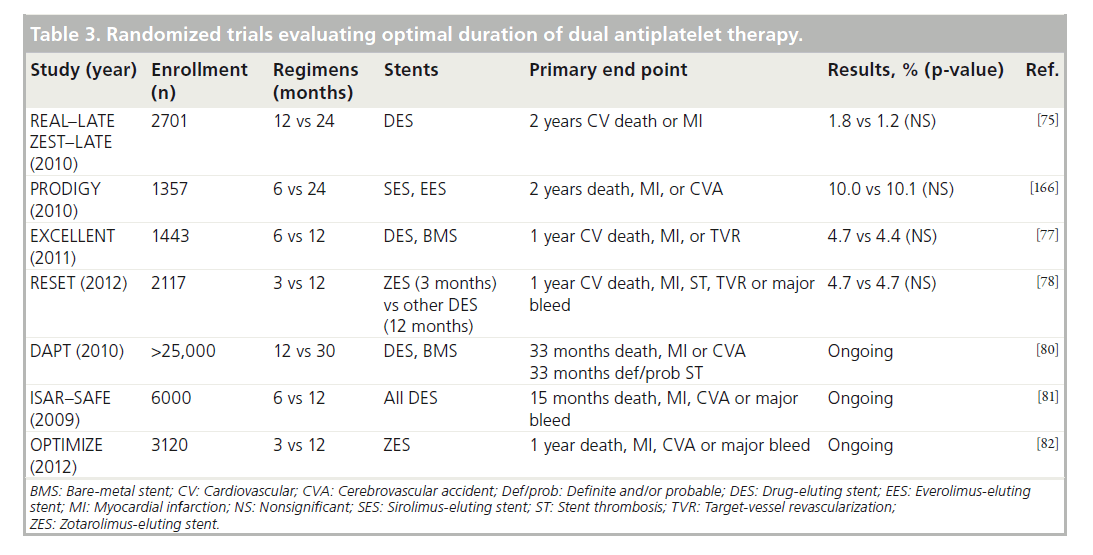
Given these conflicting findings, RCTs have been conducted to test the hypothesis that extended regimens of DAPT may reduce ischemic events (Table 3). Although these trials have important differences with regards to the patient population, the duration of DAPT and the clinical end points, those that have been published to date (REAL–LATE and ZEST–LATE [75], PRODIGY [76], EXCELLENT [77] and RESET [78]) found no significant difference in outcomes between shorter and extended durations of DAPT. In fact, the PRODIGY study reported a significant increase in major bleeding associated with prolonged DAPT to 24 months (3.5 vs 7.4%; p = 0.0002) [79]. While none of these were adequately powered to individually determine if ischemic end points may be lowered with extended DAPT, some found that shorter durations of DAPT following placement of second-generation DES are not associated with an appreciable increase in ischemic events, suggesting that these devices may be safer than first-generation stents [78].
The use of differing durations and different stents adds to the difficulty of directly comparing results. Nevertheless, as a whole they signal that the risk-to-benefit ratio does not favor extended therapy, particularly when considering the elevated bleeding risks of prolonged DAPT. Fortunately, results from larger trials are forth-coming and may provide answers. The largest of these, the DAPT study, recently completed enrollment of over 25,000 patients across a broad spectrum of clinical conditions and stents with planned randomization to 12 versus 30 months of DAPT [80]. Other studies currently enrolling include ISAR–SAFE [81] and OPTIMIZE [82], which are comparing shorter durations of DAPT post-PCI with a DES to the currently recommended duration of 1 year.
The optimal dose of ASA and clopidogrel after PCI has also been debated. A 600-mg loading dose of clopidogrel provides more potent, rapid and uniform platelet inhibition compared with a 300-mg dose, establishing it as the recommended loading dose (along with ASA 325 mg) prior to PCI in most patients [83]. Varying durations of higher maintenance doses of both clopidogrel and ASA have been used after these loading doses, primarily to reduce the risk of subacute ST. CURRENT–OASIS-7 sought to determine the comparable efficacy and safety of different post-PCI DAPT regimens in the first 7 days on short- and long-term outcomes [84]. A total of 25,087 patients with ACS, of whom 70% underwent PCI, were randomized in a 2 × 2 factorial design to higher versus lower dose ASA (325 vs 81 mg) and/or double-versus standard-dose clopidogrel (600-mg load followed by 75 mg twice daily vs 300-mg load followed 75 mg daily). While the primary end point of composite death, MI or stroke did not differ between higher versus lower ASA, or double versus standard clopidogrel strategies in the overall population, a 46% reduction in definite ST (0.7 vs 1.3%; p = 0.0001) in favor of double-dose clopidogrel was observed in the PCI-subgroup translating into a 14% reduction in the primary end point, albeit at the expense of a 41% increase in major bleeding (1.6 vs 1.1%; p = 0.001) [85]. Although this trial has to be viewed as showing no efficacy in favor of extended durations of higher dosages of ASA and clopidogrel, the ability to reduce ST is an important finding that may allow for targeted therapy among populations at lower risk for bleeding.
Clopidogrel resistance & the role of platelet function testing
While noncompliance with DAPT appears to be responsible for many ST events, variability in response to clopidogrel may contribute to ischemic events among patients adhering to therapy [86]. Clopidogrel requires activation before inhibiting platelet P2Y12 receptors. Variability in intestinal absorption, drug interactions affecting hepatic cytochromes and genetic polymorphisms of genes encoding CYP450 isoenzymes are among the more studied mechanisms that contribute to the variable pharmacologic action of clopidogrel. Up to onethird of the population may exhibit significant hyporesponse to clopidogrel, reflected by high on-treatment platelet reactivity (HTPR) [87]. HTPR is an independent risk factor for the occurrence of ischemic events after PCI [86]. A pooled analysis of 20 trials and over 9000 patients found HTPR to increase the risk for ischemic events by fivefold and ST by fourfold [88]. Several studies have used receiver–operator curve analysis to define a threshold value of HTPR to identify thrombotic risk in patients undergoing PCI. Among those utilizing the more commonly used VerifyNow assay (Accumetrics, CA, USA), threshold values greater than 230–240 P2Y12 reaction units (PRU) have been shown to be the best predictors of subsequent ischemic events [89–91].
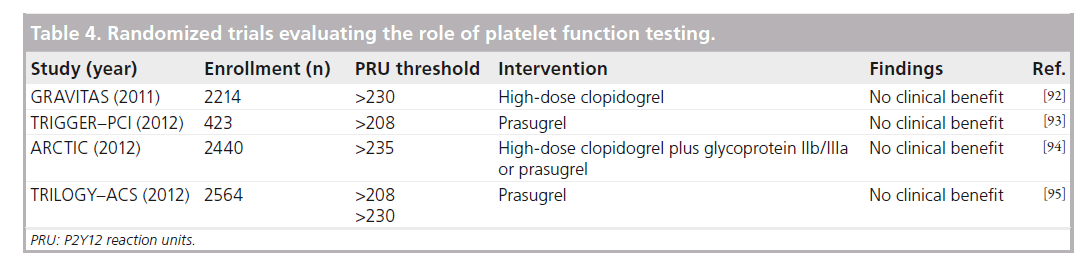
Despite the correlation between HTPR and ischemic events, the role of platelet function testing (PFT) in guiding the management of patients after PCI remains to be determined. Several studies have prospectively evaluated the role of PFT in guiding subsequent antiplatelet therapy (Table 4). The GRAVITAS trial compared standard-dose (300-mg load and 75-mg maintenance) versus high-dose (600-mg load and 150-mg maintenance) clopidogrel in patients with HTPR (PRU: >230) after elective PCI and found no reduction in the composite of cardiovascular death, MI and ST at 6 months (2.3 vs 2.3%) [92]. Despite a significant decrease in the proportion of patients with persistent HTPR at 30 days in the high-dose group, only a modest reduction in PRU levels was observed. Whether or not a more potent antiplatelet agent may improve outcomes in such a population was tested in TRIGGER–PCI, which randomized 423 patients with HTPR (PRU: >208) 1 day after elective PCI following a 600-mg loading dose of clopidogrel to continued clopidogrel versus prasugrel [93]. Prasugrel was shown to be very effective at lowering PRU levels but the extremely low event rate observed in both arms (only one periprocedural MI; no ST events), likely due to the low-risk population and high use of next-generation EES (~50%), prompted early termination of the study due to futility. Consistent with these findings, the ARCTIC trial prospectively tested a strategy of tailored antiplatelet therapy both before and after PCI based on results of PFT, but found no difference in clinical outcomes [94]. Similarly disappointing results in the medically managed ACS population were also reported from the TRILOGY–ACS substudy examining the role of PFT [95]. Although associated with lower PRU versus clopidogrel, no correlation between ischemic outcomes and platelet reactivity was present at 30-month follow-up. The 1-year follow-up results from the prospective observational ADAPT–DES study of patients undergoing PFT with VerifyNow before and after DAPT loading were also recently reported. Among the 70 patients (0.84%) who experienced def/prob ST by 1 year, HTPR (PRU: >208) was shown to be an independent predictor of MI and ST, but was also protective against major bleeding, thus it was not associated with a mortality reduction [96].
A loss-of-function polymorphism in the CYP2C19 gene has been associated with decreased activation of clopidogrel, a reduced antiplatelet effect and increased cardiovascular events in patients receiving clopidogrel [97]. A meta-analysis of nine studies evaluating the impact of CYP2C19 genotype on clinical events demonstrated an increased risk of ST in carriers of both one (HR: 2.67 [95% CI: 1.69–64.22]) and two (HR: 3.97 [95% CI: 1.75–79.02]) reduced-function alleles compared with noncarriers, identifying nearly 30% of patients as potentially less protected on clopidogrel therapy [98]. The role of genetic testing to identify such patients with CYP2C19 polymorphisms is not recommended as a routine strategy based on the most current PCI guidelines [83]. Since these guidelines were published, the RAPID–GENE study established that point-of-care genetic testing following PCI can accurately identify CYP2C19*2 carriers leading to subsequent reductions in PRU levels with prasugrel [99]. These results lay the foundation for larger studies to evaluate the role of pharmacogenomics in guiding antiplatelet selection post-PCI.
Lesion-related predictors
Specific findings on angiography are predictive of ST regardless of the procedural strategy. Longer lesions [7,45,52], smaller pre-PCI vessel diameters [49], bifurcation lesions [46,49], type C lesions [100], saphenous vein graft lesions [53] and the lesions associated with thrombus [46,101] have all been found to be associated with future episodes of ST after PCI.
Thrombus burden, in particular, is among the most high-risk lesion-related characteristics. In a retrospective analysis of 812 patients treated with DES for STEMI, patients with a large thrombus burden (defined as ≥2 vessel diameters) experienced higher mortality (HR: 1.76; p = 0.023) compared with patients with a smaller thrombus burden [101]. ST occurred in 1.1% of patients at 30 days and 3.2% at 2 years with significantly more events occurring among patients with larger thrombus burden (8.2 vs 1.3% at 2 years, respectively; p < 0.001). In this study, thrombectomy was associated with fewer ST events during follow-up (HR: 0.11; p = 0.03).
Procedure-related predictors
Achieving optimal technical results during PCI is perhaps the single most important way to avoid ST. Based on studies evaluating findings on angiography and IVUS after stent deployment, numerous predictors for ST have been identified. Angiographic findings associated with ST include evidence of residual dissection [7,48,50,52,102], smaller luminal dimensions [52,102,103] and persistent slow flow [52,103]. In addition, the use of longer stents [7,45,47,50,55,71,104], multiple stents [38,51,52] and overlapping stents [38,50] increases the potential for subsequent events. All of these risk factors have also been shown to predict early ST in the BMS era based on studies utilizing IVUS [50,52]. Among 53 cases in the POST registry, designed to identify IVUS predictors of acute and subacute ST, 94% exhibited at least one significant abnormality on IVUS (including stent underexpansion, stent malapposition, inflow–outflow disease, dissection or thrombus) with only 32% having such abnormalities appreciated on angiography [105]. A smaller series of 12 patients presenting with acute ST identified most as having severe stent underexpansion with no patient fulfilling criteria for optimal stent implantation [106].
Studies utilizing IVUS to evaluate deployment of early BMS found that over 80% of these stents were poorly expanded despite seemingly adequate results on angiography [107,108]. Soon thereafter, Colombo et al. demonstrated that a strategy utilizing routine IVUS to optimize PCI results with adequately sized balloons (balloon-to-vessel ratio 1.17 ± 0.19) and high-pressure inflations (14.9 ± 3.0 atmospheres) can achieve optimal stent expansion in 96% of patients [6]. Subsequently, the CRUISE study the use of IVUS to be associated with larger stent diameters post- PCI and a 44% relative risk reduction (RRR) in the need for repeat revascularization at 9-month follow-up [109], while the OPTICUS study found no reduction in clinical events or restenosis rates with routine IVUS use [110].
In the DES era, the routine use of IVUS to optimize stent deployment and clinical end points was evaluated in an examination of 884 patients [111]. Rates of definite ST were significantly lower compared with propensity score-matched patients who underwent PCI without the aid of IVUS both at 30 days (0.5 vs 1.4%; p = 0.046) and 1 year (0.7 vs 2.0%; p = 0.014). Patients undergoing IVUS-guided PCI underwent less direct stenting, more postdilatation and had greater cutting balloon and rotational atherectomy use. Although there were no significant differences in the rates of death, Q-wave MI or TVR between groups, IVUS guidance was an independent predictor of freedom from cumulative ST at 12 months (adjusted HR: 0.5 [95% CI: 0.1–0.8]; p = 0.02) and was associated with a trend towards lower TLR. A meta-analysis of 11 studies comparing PCI with and without routine IVUS found IVUS-guided DES implantation to also be associated with a reduced ST (HR: 0.58 [95% CI: 0.44–40.77]; p < 0.001) as well as a lower incidence of death (HR 0.59 [95% CI: 0.48–40.73]; p < 0.001) and major adverse cardiac events (HR: 0.87 [95% CI: 0.78–70.96]; p = 0.008) [112]. More recently, results from the IVUS study of ADAPT–DES, a prospective multicenter registry designed to determine the frequency, timing and correlates of DES thrombosis, found that IVUS guidance was independently associated with a reduced 1-year rate of ST (HR: 0.37 [95% CI: 0.20–0.68]; p = 0.0014) with nearly a 50% reduction in nonperiprocedural MI [113]. These data suggest that information gained with IVUS may lead to important differences in procedural approach, which may in turn reduce thrombotic events.
As stent technology and technique has advanced, the majority of stents are adequately and safely deployed without the need for routine IVUS guidance. Indeed, the use of IVUS varies greatly from one center to another. However, emerging data suggest that IVUS can play an important role in optimizing procedural results in order to reduce future clinical events, ST in particular. If IVUS is utilized, the operator should aim to achieve a lumen inside the stent of at least 60–70% of the media–media cross-sectional area. If IVUS is not used, achieving a small ‘step up’ at the entrance of the stent and a ‘step down’, at the exit of the stent along with liberal postdilatation with a noncompliant balloon is likely to achieve similar results [114].
Advances in stent technology
▪ First-generation DES
As discussed previously, the development of first-generation DES heralded a breakthrough in our ability to treat severe coronary lesions while minimizing the need for repeat revascularization. The panic caused by the European Society of Cardiology Firestorm followed by the dissemination of data on the efficacy and safety of these devices soon resulted in the acknowledgement that, although associated with a greater hazard of ST beyond the first year, firstgeneration DES provide superior clinical outcomes with an overall excellent safety profile that justifies their use among appropriately selected patients.
▪ Second-generation DES
Newer stents have since been designed with novel metal–alloy platforms, biocompatible polymers and improved antiproliferative agents that have resulted in thinner struts, a more open-cell design and greater deliverability. In addition, these devices elicit less inflammation and result in more rapid endothelialization compared with first-generation DES, translating into reduced rates of restenosis and ST [115]. The most commonly used platforms of these so-called ‘second-generation’ DES, along with the data supporting their use, are discussed below.
Everolimus-eluting stents
The Xience V EES (Abbott Vascular, IL, USA; also distributed as Promus™ by Boston Scientific) is composed of a cobalt–chromium (CoCr) alloy platform coated with a thin layer of fluoropolymer that elutes everolimus, a sirolimus analog, over a period of several months [116]. The SPIRIT family of trials established the safety and efficacy of EES with a significant reduction in late lumen loss (LLL) and clinical events (MI and TVR) at 1 year compared with PES [117,118]. A pooled analysis of SPIRIT II and III found a sustained 44% RRR in major adverse cardiac events in favor of EES at 3-year follow-up and comparable rates of def/prob ST between EES and PES (n = 10, 1.2% vs n = 7, 1.9%; p = 0.43) [119]. The larger SPIRIT IV trial randomized 3687 patients with greater clinical complexity 2:1 to EES versus PES and found a 38% RRR in target-lesion failure (TLF) favoring EES (4.2 vs 6.8%, p = 0.001) and a 75% RRR in protocol-defined ST (n = 4, 0.17% vs n = 10, 0.85%; p = 0.004) at 1-year follow-up [120]. The incidence of ST in the EES group (0.17% protocol, 0.29% ARC def/prob) was among the lowest reported with any DES at the time [120], with a persistent 64% RRR in ST favoring EES at 2-year follow-up [121]. Published alongside SPIRIT IV, the COMPARE trial randomized 1800 nonselected patients to EES versus PES and demonstrated a 31% RRR in composite death, MI or TVR at 1-year (6 vs 9%, p = 0.02) with a significantly lower rate of ST (n = 6, <1% vs n = 23, 3%; p = 0.002) favoring EES, similar to SPIRIT IV [122]. At 2-year follow-up, the rates of ST continued to be significantly lower in the EES group with an impressive 79% RRR compared with PES (n = 8, 0.9% vs n = 35, 3.9%; p = 0.02) [123].
Further modifications have resulted in the next iteration of EES that are commonly used today. The Promus Element™ EES (Boston Scientific) utilizes a modified platinum–chromium alloy scaffold, but the same drug and polymer as Xience V EES. The incorporation of platinum into the platform results in a stent with better deliverability, radiopacity and fracture resistance [124]. This enhanced design was evaluated in the PLATINUM trial, which randomized 1530 low-risk patients to PCI with a Promus Element platinum–chromium EES versus Xience V CoCr EES, and showed comparably low rates of TLF and ST (0.4 vs 0.4%), supporting noninferiority [124]. The Xience Prime EES (Abbott Vascular) utilizes the same CoCr alloy scaffold, drug and polymer as the Xience V EES, but is designed with a slightly different link configuration, resulting in a longer and taller cell that promotes enhanced flexibility and deliverability. In the SPIRIT PRIME trial, the Xience Prime EES was shown to have comparable rates of TLF to historical events rates with Xience V from the SPIRIT family of trials and included a subset of long lesions during which no ST events occurred [125].
Zotarolimus-eluting stents
The Endeavor® zotarolimus-eluting stents (E-ZES; Medtronic, MN, USA) are also composed of a CoCr alloy platform but coated with a thinner polymer that releases an alternative sirolimus derivative, zotarolimus, more rapidly than other DES, with 95% released within 2 weeks. Angioscopic and OCT images revealed greater endothelial coverage of E-ZES struts compared with SES and PES [126], supporting the concept of E-ZES exhibiting intermediate behavior between BMS and DES. The ENDEAVOR family of trials established the safety of E-ZES, but noted higher rates of LLL and TLR compared with SES (9.8 vs 3.5%; p = 0.04) [127] and PES (15.3 vs 10.4%; p = 0.284) [128] despite comparable clinical outcomes. Extended follow-up from ENDEAVOR III and IV suggested E-ZES to be safe based on a reduction in mortality and MI compared with SES [129] and PES [128], and significantly less very late ST compared with PES (0.1 vs 1.6%; p = 0.004) [128]. SORT-OUT III subsequently randomized 2332 unselected patients to E-ZES versus SES and found composite cardiac death, MI or TVR to be twice as high in the E-ZES group at 18 months (10 vs 5%; p = 0.0002) with higher mortality in the E-ZES group (4 vs 3%; p = 0.035) [130]. Among this mixed ACS/stable CAD population with more complex lesions than the prior ENDEAVOR trials, ST was not significantly different at 18-month follow-up.
These disappointing results led to design modifications of the E-ZES, resulting in the Resolute ZES (R-ZES) with a unique blend of three polymers allowing for more delayed drug release [131]. Following the reassuring results in initial feasibility studies [132], the RESOLUTE–US observational study evaluated 1402 patients treated with R-ZES and found a 12-month rate of TLF of just 3.7%, much lower than the historical 6.5% rate of E-ZES controls, and an extremely low 0.1% incidence of ST [133]. When pooled with data from other trials evaluating R-ZES, extremely low rates of def/prob ST (1-year 0.78% and 2-year 0.96%) have been reported [134].
To date, only two trials have directly compared different second-generation DES in a randomized fashion. The Resolute All-Comers trial randomized 2292 patients to EES versus R-ZES and found similar rates of 13-month TLF (8.3 vs 8.2%, respectively; p = NS) with significantly fewer episodes of ST favoring EES (definite: 1.2 vs 0.3%; p = 0.01 and def/prob: 1.6 vs 0.7%; p = 0.05) [135]. The TWENTE trial randomized 1391 patients to EES versus R-ZES and found no difference in TVF at 1 year (8.1 vs 8.2%, respectively; p = NS) and similarly low rates of ST in both groups (definite ST: 0 vs 0.58%; p = 0.12 and def/prob: 1.2 vs 0.9%; p = 0.59) [136]. The exclusion criteria of both trials were very limited, with over half with ACS and over three-quaters with at least an ‘off-label’ feature for stenting. ST rates among unselected patients treated with ZES and EES are higher than those in RCTs, but remain considerably low compared with rates previously reported for first-generation DES. An analysis of the ESTROFA-2 registry, including 4768 patients treated with either ZES (2549) or EES (2219), found a cumulative incidence of def/ prob ST of 1.3% at 1 year and 1.7% at 2 years for ZES, and 1.4% at 1 year and 1.7% at 2 years for EES (p = 0.8) [137]. The increment of definite ST between the first and second year was 0.2 and 0.25%, respectively.
▪ Impact of second-generation DES
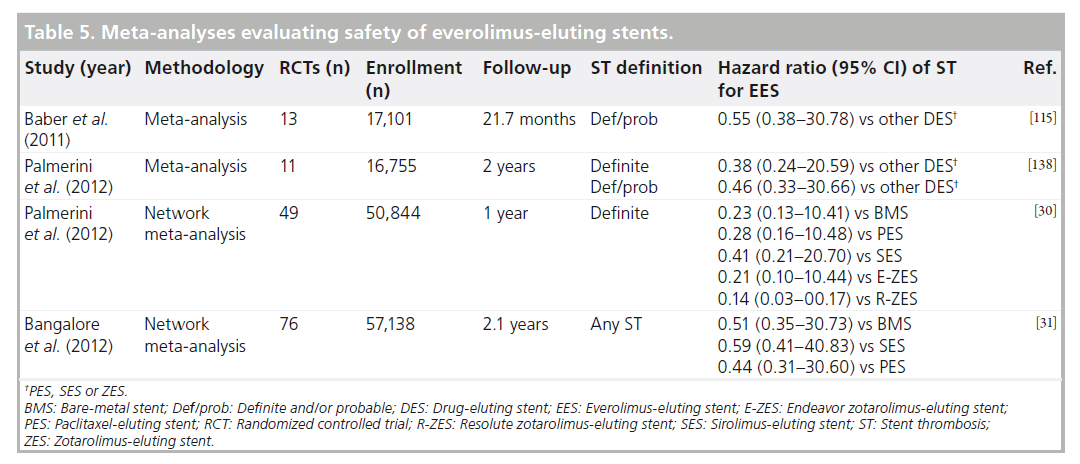
A consistent theme among all trials evaluating EES is the low rate of ST. Multiple meta-analyses have now been published that suggest EES to possess a superior profile in terms of both efficacy and safety (Table 5). Reductions in ST ranging from 45 [115] to 54% [138] compared with other DES have been suggested on the basis of these studies, an impressive finding when considering the greater complexity of patients in contemporary trials. Utilizing methods capable of comparing multiple stents both directly and indirectly by way of a common reference stent, two independently conducted network metaanalyses have recently added to these findings [30,31]. In what may be regarded as a paradigm shift, Palmerini et al. found EES to not only be associated with the lowest rates of definite ST at 1-year of all DES, but also lower rates of definite ST compared to BMS both at 1-year and 2-year follow-up [30]. The reduction in ST with EES compared to BMS was apparent after only 30 days of follow-up and continued to be present between 31 days and 1 year. In a similar analysis of 76 RCTs and 117,762 patient-years of follow-up, Bangalore et al. reported EES to have an 86% probability of having the lowest rate of ST of all stents [31]. Multiple EES components may be contributing to the reduction in thrombotic events including thinner stent struts, polymer characteristics and drug effect. In particular, the fluoropolymers used in EES have been demonstrated to have greater biocompatibility and less thrombogenicity as evidenced by a reduction in platelet adhesion in vitro [139].
Registries comparing outcomes of patients treated with second-generation DES versus first-generation DES in the modern era also support the superior safety and efficacy of newer devices. An analysis of SCAAR demonstrated the risk of definite ST to be 61% lower among second-generation DES recipients compared with BMS and 43% lower compared with first-generation DES [9]. Patients treated with a second-generation DES also had lower mortality compared with both BMS patients (45% RRR) and first-generation DES patients (23% RRR) [9]. An analysis of the Bern–Rotterdam registry found the 4-year incidence of definite ST to be significantly lower with EES (1.4 per 100 person-years) versus SES (2.9 per 100 person-years; HR: 0.41 [95% CI: 0.27–20.62]) and versus PES (4.4 per 100 person-years; HR: 0.33 [95% CI: 0.23–20.48]) [140]. Focusing beyond the first year, very late ST with EES was 67–76% compared with first-generation DES, with an annual incidence of only 0.2% (n = 12) in the EES group versus 0.47% (n = 49) in the SES group and 0.8% (n = 53) in the PES group [140].
▪ Stents with bioabsorbable polymers
A large body of evidence now supports superior efficacy and safety of second-generation DES compared with first-generation DES. However, one limitation continues to be the nonerodible polymer coating that persists for the life of the patient long after its role as a drug depot has been served. These polymers contribute to the delayed vascular healing and chronic inf lammation linked with ST in pathologic studies [34,35]. In an effort to address this potential nidus for ST and restenosis in the long term, newer stents have replaced durable polymer coatings with bioabsorbable polymers (BP), allowing for controlled drug-release followed by subsequent degradation of the polymer coating and, thus, rendering the stent surface similar to that of a BMS. The potential clinical advantage of BP DES over durable polymer DES lies in the potential to reduce the incidence of late adverse events related to impaired vessel healing.
One of the first of these novel devices consists of a microporous stainless steel stent coated with a mixture of rapamycin, BP and resin that completely resorbs in vivo after a 6–9-week period and was evaluated in the ISAR stent project. After ISAR–TEST 3 demonstrated noninferiority of this BP stent compared with a Cypher SES [141], ISAR–TEST 4 randomized 2603 patients to receive a BP stent versus a durable polymer stent platform with an equal number of patients in the durable polymer group receiving either a first-generation Cypher SES or second-generation Xience EES [142]. The BP stent was found to be noninferior with respect to MI, death or TLR at 12-month follow-up with low rates of def/prob ST observed in both groups (1.0 vs 1.5%; p = 0.29). A 3-year follow-up has since reported no significant difference with respect to the primary end point (20.1 vs 20.9%, respectively; p = 0.59) or def/prob ST (1.2 vs 1.7%, respectively; p = 0.32) [143]. The type of durable polymer stent (EES vs SES) was not found to be associated with lower rates of def/prob ST (1.4 vs 1.9%, respectively; p = 0.51).
A large amount of data also exist for the Bio- Matrix™ family of stents (Biosensors International Ltd, Singapore). The polymer consists of a 50:50 matrix of polylactic acid and the antiproliferative agent, Biolimus A9, a semisyn-thetic sirolimus analog with tenfold greater lipo-philicity, which is coated to the abluminal of a flexible stainless-steel stent. This BES polymer coating was previously shown to fully convert to lactic acid by 6 months, followed by further breakdown to water and carbon dioxide in the next few months. The LEADERS trial randomized 1707 patients to PCI with either a BES (n = 857) or SES (n = 850) and found similar rates of death, MI and clinical revascularization at 9-month follow-up [144]. The rate of definite ST at 1-year follow-up was 2.0% in both groups. However, extended follow-up has since demonstrated a 74% RRR in the incidence of very late ST between years 1 and 5 in favor of BES (0.7 vs 2.5%; p = 0.003) [145].
The Nobori BES (Terumo, NJ, USA) also utilizes a blend of Biolimus A9 and polylactic acid, and has been tested against various other DES platforms. After demonstrating noninferior angiographic outcomes compared with first-generation Taxus and Cypher stents in a smaller series of patients [146,147], the Noburi BES has been compared with more current second-generation stents in a randomized fashion. In COMPARE II, 2707 ‘all-comers’ patients were randomized 2:1 to the Nobori BES versus Xience/Promus EES and were found to have comparable rates of composite cardiac death, MI or TVR at 1-year (5.2 vs 4.8%, respectively; p = NS) with very low rates of ST observed in both groups (definite ST: 0.7 vs 0.4%, respectively and def/prob ST: 0.8 vs 1.0%, respectively) [148]. Another large RCT reported less optimistic results based on the Noburi BES, failing to meet its prespecified end point of noninferiority compared with first-generation Cypher SES. The SORT OUT V trial randomized 1229 patients (1532 treated lesions) to BES and 1239 patients (1555 treated lesions) to SES and found a similar incidence of 9-month composite cardiac death, MI, definite ST and TVR (4.1 vs 3.1%, respectively) [149]. The observation that definite ST occurred more often among BES recipients (n = 9, 0.7% vs n = 2, 0.2%; p = 0.034) is a cause for concern given that a reduction in ST is a major objective of this technology but long-term follow-up will be necessary to determine whether an advantage towards fewer very late ST events favoring BES becomes apparent, as seen in the LEADERS trial.
In a pooled analysis of three of these RCTs comparing stents with BP with stents with durable polymers (ISAR–TEST 3, ISAR–TEST 4 and LEADERS), the risk of definite ST was significantly reduced among patients randomized to a BP DES (HR: 0.56 [95% CI: 0.35–30.90]) driven by a lower risk of very late ST (HR: 0.22 [95% CI: 0.08–00.61]) and also with a lower incidence of MI among such patients (HR: 0.59 [95% CI: 0.73–0.95]) [150].
▪ Stents with bioabsorbable scaffolds
Beyond stents with BP coatings, the concept of a fully bioabsorbable coronary stent has been a long-term goal of the interventional community that has recently been realized. By providing short-term lumen support during vessel healing prior to being completely resorbed over time, these stents can allow for a return of normal vascular function, avoidance of permanent side branch occlusion, and a reduction in restenosis and ST.
Several companies are developing and testing different bioabsorbable stent designs. Among them, the ABSORB™ Bioresorbable Vascular Scaffold (BVS; Abbott Vascular) has received considerable attention for reporting clinical and imaging outcomes similar to DES. Made out of poly-l-lactide and coated with everolimus, the Absorb BVS releases 80% of the drug by 30 days (similar to Xience) followed by gradual bulk erosion of the polymer over the next 2 years. The design is notable for having thicker stent struts, less tolerance for overexpansion compared with metal stents, but a variable rate of reabsorption. In the ABSORB trial, this novel stent was prospectively evaluated in 30 low-risk patients and was found to have a low 3.3% event rate (only one non- Q-wave MI) at 12-months with no episodes of ST during this period or at 5-year follow-up [151]. Imaging with IVUS and OCT detected an 11–12% reduction in stent area at 6 months raising concerns about stent recoil and prompting design modifications to improve radial strength. However, at 2-year angiographic follow-up, greater luminal area enlargement due to a decrease in plaque size was demonstrated with no change in LLL compared with 6-months [152]. In addition, approximately one-third of stent struts were no longer discernable and return of vasomotion in response to vasoactive agents was demonstrated. The design enhancements of the ABSORB BVS continue to be evaluated in a similarly low-risk cohort of patients and, thus far, has been associated with lower degrees of LLL compared with the previous cohort [153,154]. Further studies are underway to further evaluate the clinical safety and efficacy of these new devices, including the ABSORB II, which will compare BVS with Xience Prime EES.
Looking forward, although the potential advantages of fully bioabsorbable stents are clear, what remains unclear is whether the potential enhanced safety justifies the use of devices that are currently higher profile, less deliverable and untested in lesions of greater complexity. Issues related to the optimal duration of the scaffolding and drug elution also remain to be determined.
Advances in pharmacotherapy
▪ Role of DAPT established
The feasibility of coronary stenting was accompanied by immediate recognition of the thrombotic properties of this new technology, prompting pharmacologic strategies to reduce platelet aggregation and subsequent thrombosis. Combinations of ASA and dipyridamole were first used followed by the addition of anticoagulants to reduce the unacceptable high rates of abrupt vessel closure [4,5]. Such regimens were plagued by high rates of bleeding and vascular complications leading to prolonged hospitalizations and prompting multiple trials to determine the optimal regimen to balance the risks of ST and bleeding. The combination of ASA and ticlopidine was shown to dramatically reduce ischemic events and hemorrhagic complications compared with conventional anticoagulant therapy in ISAR [155] and FANTASTIC [156]. Subsequently, the STARS trial evaluated three different anti-thrombotic regimens and found a significant reduction in 30-day ST with ASA and ticlopidine (0.5%) compared with ASA alone (3.6%) or ASA and warfarin (2.7%) [157]. ASA and ticlopidine was further shown to reduce vascular complications post-PCI in high-risk and unselected patients [156,158]. Soon thereafter, clopidogrel emerged as an alternative thienopyridine, offering advantages of greater potency, more rapid onset of action, and fewer and less serious adverse events compared with ticlopidine. Results from PCI–CURE and CREDO supported the benefit of prolonged treatment with clopidogrel and ASA based on a significant reduction in ischemic events without an increase in major bleeding [159,160], establishing this DAPT regimen as the cornerstone of post-PCI therapy.
▪ Novel P2Y12 antagonists
Efforts to further reduce the incidence of ST have focused on achieving a more pronounced and consistent degree of platelet inhibition. Two P2Y12 receptor antagonists in particular, prasugrel and ticagrelor, have succeeded in this regard by demonstrating improved outcomes in the ACS population compared with clopidogrel.
In TRITON–TIMI 38, treatment of moderate-to- high risk ACS patients with prasugrel was associated with a 19% RRR (12.1 vs 9.9%; p < 0.001) in the incidence of cardiovascular death, MI or stroke compared with clopidogrel, albeit at the cost of more major bleeding (2.4 vs 1.8%; p = 0.03) [59]. Among patients who underwent PCI, the incidence of def/prob ST was significantly reduced with prasugrel (2.4 vs 1.1%; p < 0.001), a difference that persisted among those implanted with both DES (0.8 vs 2.3%) and BMS (1.3 vs 2.4%), and that was apparent both in the early period (71% RRR from 1.44 to 0.42%) and the late period (54% RRR from 0.91 to 0.42%) [161]. A striking reduction in ST was observed in subgroups of patients with diabetes (1.6% absolute risk reduction [ARR]), more than 20 mm of stent (1.5% ARR), bifurcation stents (3.2% ARR) and those with an MI prior to presentation (2.6% ARR).
In the PLATO trial, a similar population of 18,624 ACS patients undergoing planned PCI was randomized to clopidogrel versus ticagrelor [60]. Treatment with ticagrelor was associated with a 16% RRR (11.7 vs 9.8%; p < 0.001) in the composite of vascular death, MI or stroke, again at the cost of more major noncoronary artery bypass graft-related bleeding (4.5 vs 3.8%; p = 0.03) [60]. The incidence of def/prob ST was significantly reduced among patients treated with ticagrelor (2.2 vs 2.9%; p = 0.02).
An adjusted indirect meta-analysis between ticagrelor and prasugrel utilizing patient data from TRITON, PLATO and DISPERSE-2 reported a 39% RRR in ST among patients receiving one of these more potent P2Y12 inhibitors compared with clopidogrel [162]. The data supporting the use of these more potent P2Y12 inhibitors is limited to patients presenting with ACS and has not yet been demonstrated to provide a similar benefit in patients undergoing PCI for stable CAD.
▪ Novel oral anticoagulants
Although inhibition of the coagulation cascade with heparin products or direct thrombin inhibitors is a mainstay of therapy in the periprocedural period, few patients receive pharmacotherapy targeting the generation of thrombin in an outpatient setting after undergoing PCI. The emergence of novel oral inhibitors of the coagulation cascade for the reduction in embolic events in patients with atrial fibrillation and for the management of venous thromboembolism has renewed interest in the potential role of such therapy in patients with CAD who are being treated for ACS. Among these, the factor- Xa inhibitor rivaroxaban, was studied in the ATLAS ACS 2 TIMI 51 trial, which randomized 15,526 patients with recent ACS to receive twice-daily doses of either 2.5 or 5 mg of rivaroxaban or placebo in addition to standard therapy including low-dose ASA and a thienopyridine for a mean of 13 months [163]. Patients randomized to rivaroxaban experienced a 16% reduction in composite cardiovascular death, MI or stroke (8.9 vs 10.7%; p = 0.008) with similar efficacy seen in both doses. Patients who received rivaroxaban also experienced a 31% reduction in ST. Not unexpectedly, patients randomized to rivaroxaban experienced higher rates of noncoronary artery bypass graft major bleeding (2.1 vs 0.6%; p < 0.001) and intracranial hemorrhage (0.6 vs 0.2%; p = 0.009), but fewer fatal bleeding events were observed in patients taking the lower twice daily 2.5-mg dose compared with the 5-mg dose (0.1 vs 0.4%; p = 0.04). Although not yet FDA approved for the management of ACS, the addition of rivaroxaban as a potential adjunct to the current mainstay of DAPT will present new challenges for clinicians seeking to achieve a balance between reducing subsequent ischemic events and minimizing the potential for major bleeds.
Conclusion
ST is a rare, but devastating, complication associated with high morbidity and mortality. Patients typically present with acute MI and do not often survive long enough to present for clinical evaluation. Risk factors for ST include those relating to patient characteristics (comorbid clinical conditions, stable versus unstable presentation, antiplatelet therapy compliance and/or resistance), lesion characteristics (length, thrombus burden, bifurcation) and the procedural details (including stent characteristics). Recognition of these risk factors can aid the operator in reducing the incidence of ST with careful patient and stent selection and meticulous technique. Pathologic studies and coronary imaging modalities have advanced our understanding of the mechanisms contributing to ST during different time intervals. These insights have led to advancements in stent design and pharmacotherapy resulting in a reduction in event rates. Despite these gains, ST continues to represent a threat, particularly among patients of greater complexity. Ongoing studies may help determine the optimal duration of DAPT following stent placement and the role of novel stents with bioabsorbable materials.
Executive summary
Definitions for stent thrombosis
▪ Academic Research Consortium definitions have standardized the way in which stent thrombosis (ST) is diagnosed and reported, allowing for more meaningful comparisons across clinical trials. These definitions characterize events based on degrees of certainty (definite, probable and possible), as well as timing (early: 0–30 days, late: 30 days to 1 year and very late: beyond 1 year).
Incidence & timing
▪ The timing and incidence of ST varies depending on the type of stent placed and the complexity of the patient population studied, with higher rates typically observed in registries of nonselected patient compared with rates reported from randomized controlled trials.
▪ Early and late ST occurs at similar rates with both bare-metal stents and first-generation drug-eluting stents (DES).
▪ First-generation DES are associated with a risk of very late ST, ranging from 0.2% in patients with noncomplex coronary artery disease to as high as 0.6% in higher risk patients.
▪ Second-generation DES have less long-term follow-up data available, but have been shown to have lower rates of ST across all time periods.
Mechanisms
▪ The underlying mechanisms of ST vary depending on the timing of the event and the type of stent placed.
▪ Early ST is often related to suboptimal procedural results or medical noncompliance, and is similar between recipients of both bare-metal stents and DES.
▪ Late and very late ST is often related to the inflammatory response to one or more components of DES that can manifest as hypersensitivity, delayed arterial healing and late acquired malapposition. Very late ST may also result from unique mechanisms such as neoatherosclerosis.
Predictors
▪ Multiple risk factors for ST have been identified based on retrospective and prospective registries and are often classified as being related to the patient, the lesion or the procedure.
▪ Acute coronary syndrome increases the risk for subsequent ST similarly for both bare-metal stents and first-generation DES. Emerging data suggest that this risk may be reduced with newer DES.
▪ Early discontinuation of dual antiplatelet therapy is the most consistent and powerful predictor of ST.
▪ Studies comparing shorter versus longer durations of dual antiplatelet therapy have not demonstrated a significant clinical benefit from longer prescriptions of dual antiplatelet therapy. Until the results of ongoing randomized controlled trials are able to provide further insight into the optimal duration, individual assessment of each patient’s unique risks for both thrombosis and bleeding should be used to guide clinical decision-making.
▪ Clopidogrel hyporesponsiveness is associated with higher rates of ST post-percutaneous coronary intervention. Platelet function testing and genetic testing can identify patients at risk for ischemic events, but a routine testing strategy has not been demonstrated to improve clinical outcomes in randomized controlled trials.
Advances in stent technology
▪ Second-generation DES have incorporated design enhancements including thinner struts, more biocompatible polymers and enhanced deliverability that have contributed to their enhanced efficacy and safety compared with first-generation DES.
▪ Among the DES that are commonly used today, multiple analyses suggest everolimus-eluting stents to possess a superior profile in terms of efficacy and reduction in ST; however, head-to-head comparisons with Resolute zotarolimus-eluting stents demonstrate comparable clinical results.
▪ Novel stents with bioabsorbable polymers and scaffolds provide theoretical benefit in further reducing ischemic events during long-term follow-up, but more data are necessary before they replace the current generation of DES.
Advances in pharmacotherapy
▪ Both prasugrel and ticagrelor provide more rapid and intense platelet inhibition with less variability than clopidogrel and have been shown to significantly reduce subsequent ischemic events, including ST, in the acute coronary syndrome population.
▪ Rivaroxaban, a factor-Xa inhibitor, was shown to reduce ischemic events, cardiac death and ST in the acute coronary syndrome population. Novel oral anticoagulants may play an important role in the future as an adjunct to antiplatelet therapy.
▪ Among patients deemed to be at higher risk for ST based on other clinical characteristics, the benefit of intensifying P2Y12 inhibition must be carefully weighed against the risks of major bleeding.
Conclusion
▪ Advances in both stent technology and pharmacotherapy have reduced the incidence of ST, but the ongoing risk of events mandates a thoughtful assessment of risk factors for both thrombosis and bleeding in order individualize decision-making for each patient.
Future perspective
▪ Early studies of stents with bioabsorbable polymers have demonstrated promise in their ability to reduce the rates of very late ST. Long-term follow-up is necessary to fully assess the safety of these devices.
▪ Stents with fully bioabsorbable scaffolds represent an attractive concept to reduce the long-term burden percutaneous coronary intervention. Whether the current limitations of these devices can be overcome to realize these benefits remains to be determined.
Future perspective
Although ST continues to be a serious complication associated with PCI, advancements in stent technology and pharmacotherapy have progressively reduced the incidence to the lowest rates observed. The next generation of stents with BP coatings may potentially reduce ST even further by eliminating one of the remaining contributors to late events and have shown promise in early clinical follow-up. Beyond that, stents that fully degrade into nontoxic byproducts months-to-years after treating coronary lesions are currently being developed and tested, but are several years away from clinical practice. The reductions in ST observed with both prasugrel and ticagrelor highlight the benefits of more potent antiplatelet therapy among select populations. The addition of alternative antithrombotic agents, such as factor-Xa inhibitors, have the potential to further reduce ST but also afford greater risks of bleeding. Moving forward, clinicians will increasingly face challenges of balancing the risks of ischemic events and the risks of bleeding, both of which lead to adverse outcomes, as novel antithrombotic therapy is introduced. Expert operator techniques will always be needed to ensure good outcomes and to prevent complications with PCI, but ongoing improvements in stent design and pharmaco-therapy promise to mitigate the effects of complex anatomy and other high-risk clinical features on the incidence of ST, allowing for the expanded use of stents in patients who can potentially benefit from coronary revascularization.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- Cutlip DE, Windecker S, Mehran R et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation 115(17), 2344–2351 (2007).
- Cutlip DE, Nakazawa G, Krucoff MW et al. Autopsy validation study of the academic research consortium stent thrombosis definition. JACC Cardiovasc. Interv. 4(5), 554–559 (2011).
- van Werkum JW, Heestermans AA, Zomer AC et al. Predictors of coronary stent thrombosis: the Dutch Stent Thrombosis Registry. J. Am. Coll. Cardiol. 53(16), 1399–1409 (2009).
- Serruys PW, Strauss BH, Beatt KJ et al. Angiographic follow-up after placement of a self-expanding coronary–artery stent. N. Engl. J. Med. 324(1), 13–17 (1991).
- Schatz R, Baim D, Leon M et al. Clinical experience with the Palmaz–Schatz coronary stent. Initial results of a multicenter study. Circulation 83(1), 148–161 (1991).
- Colombo A, Hall P, Nakamura S et al. Intracoronary stenting without anticoagulation accomplished with intravascular ultrasound guidance. Circulation 91(6), 1676–1688 (1995).
- Cutlip DE, Baim DS, Ho KKL et al. Stent thrombosis in the modern era: a pooled analysis of multicenter coronary stent clinical trials. Circulation 103(15), 1967–1971 (2001).
- Lagerqvist B, Carlsson J, Frobert O et al. Stent thrombosis in Sweden: a report from the Swedish Coronary Angiography and Angioplasty Registry. Circulation Cardiovasc. Interv. 2(5), 401–408 (2009).
- Sarno G, Lagerqvist B, Frobert O et al. Lower risk of stent thrombosis and restenosis with unrestricted use of ‘new-generation’ drugeluting stents: a report from the nationwide Swedish Coronary Angiography and Angioplasty Registry (SCAAR). Eur. Heart J. 33(5), 606–613 (2012).
- Doyle B, Rihal CS, O’sullivan CJ et al. Outcomes of stent thrombosis and restenosis during extended follow-up of patients treated with bare-metal coronary stents. Circulation 116(21), 2391–2398 (2007).
- Finn AV, Otsuka F. Neoatherosclerosis: a culprit in very late stent thrombosis. Circulation Cardiovasc. Interv. 5(1), 6–9 (2012).
- Serruys PW, Unger F, Sousa JE et al. Comparison of coronary-artery bypass surgery and stenting for the treatment of multivessel disease. N. Engl. J. Med. 344(15), 1117–1124 (2001).
- Wessely R. New drug-eluting stent concepts. Nat. Rev. Cardiol. 7(4), 194–203 (2010).
- Moses JW, Leon MB, Popma JJ et al. Sirolimuseluting stents versus standard stents in patients with stenosis in a native coronary artery. N. Engl. J. Med. 349(14), 1315–1323 (2003).
- Stone GW, Ellis SG, Cox DA et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N. Engl. J. Med. 350(3), 221–231 (2004).
- Maisel WH. Unanswered questions – drug-eluting stents and the risk of late thrombosis. N. Engl. J. Med. 356(10), 981–984 (2007).
- Muni NI, Gross TP. Problems with drugeluting coronary stents – the FDA perspective. N. Engl. J. Med. 351(16), 1593–1595 (2004).
- Nordmann AJ, Briel M, Bucher HC. Mortality in randomized controlled trials comparing drug-eluting vs. bare metal stents in coronary artery disease: a meta-analysis. Eur. Heart J. 27(23), 2784–2814 (2006).
- Camenzind E, Steg PG, Wijns W. Stent thrombosis late after implantation of firstgeneration drug-eluting stents: a cause for concern. Circulation 115(11), 1440–1455; discussion 1455 (2007).
- Pfisterer M, Brunner-La Rocca HP, Buser PT et al. Late clinical events after clopidogrel discontinuation may limit the benefit of drugeluting stents: an observational study of drugeluting versus bare-metal stents. J. Am. Coll. Cardiol. 48(12), 2584–2591 (2006).
- Mauri L, Hsieh WH, Massaro JM, Ho KK, D’agostino R, Cutlip DE. Stent thrombosis in randomized clinical trials of drug-eluting stents. N. Engl. J. Med. 356(10), 1020–1029 (2007).
- Stone GW, Moses JW, Ellis SG et al. Safety and efficacy of sirolimus- and paclitaxeleluting coronary stents. N. Engl. J. Med. 356(10), 998–1008 (2007).
- Stettler C, Wandel S, Allemann S et al. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network metaanalysis. Lancet 370(9591), 937–948 (2007).
- Kastrati A, Mehilli J, Pache J et al. Analysis of 14 trials comparing sirolimus-eluting stents with bare-metal stents. N. Engl. J. Med. 356(10), 1030–1039 (2007).
- Stone GW, Ellis SG, Colombo A et al. Long-term safety and efficacy of paclitaxeleluting stents final 5-year analysis from the TAXUS Clinical Trial Program. JACC Cardiovasc. Interv. 4(5), 530–542 (2011).
- Caixeta A, Leon MB, Lansky AJ et al. 5-year clinical outcomes after sirolimus-eluting stent implantation insights from a patient-level pooled analysis of 4 randomized trials comparing sirolimus-eluting stents with baremetal stents. J. Am. Coll. Cardiol. 54(10), 894–902 (2009).
- Daemen J, Wenaweser P, Tsuchida K et al. Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large two-institutional cohort study. Lancet 369(9562), 667–678 (2007).
- Wenaweser P, Daemen J, Zwahlen M et al. Incidence and correlates of drug-eluting stent thrombosis in routine clinical practice. 4-year results from a large 2-institutional cohort study. J. Am. Coll. Cardiol. 52(14), 1134–1140 (2008).
- Raber L, Wohlwend L, Wigger M et al. Five-year clinical and angiographic outcomes of a randomized comparison of sirolimuseluting and paclitaxel-eluting stents: results of the Sirolimus-Eluting Versus Paclitaxel- Eluting Stents for Coronary Revascularization LATE trial. Circulation 123(24), 2819–2828, 2816 p following 2828 (2011).
- Palmerini T, Biondi-Zoccai G, Della Riva D et al. Stent thrombosis with drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. Lancet 379(9824), 1393–1402 (2012).
- Bangalore S, Kumar S, Fusaro M et al. Shortand long-term outcomes with drug-eluting and bare-metal coronary stents/clinical perspective. Circulation 125(23), 2873–2891 (2012).
- Virmani R, Guagliumi G, Farb A et al. Localized hypersensitivity and late coronary thrombosis secondary to a sirolimus-eluting stent: should we be cautious? Circulation 109(6), 701–705 (2004).
- Nebeker JR, Virmani R, Bennett CL et al. Hypersensitivity cases associated with drugeluting coronary stents: a review of available cases from the Research on Adverse Drug Events and Reports (RADAR) Project. J. Am. Coll. Cardiol. 47(1), 175–181 (2006).
- Joner M, Finn AV, Farb A et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J. Am. Coll. Cardiol. 48(1), 193–202 (2006).
- Finn AV, Joner M, Nakazawa G et al. Pathological correlates of late drug-eluting stent thrombosis. Circulation 115(18), 2435–2441 (2007).
- Mintz GS. What to do about late incomplete stent apposition? Circulation 115(18), 2379–2381 (2007).
- Hassan AK, Bergheanu SC, Stijnen T et al. Late stent malapposition risk is higher after drug-eluting stent compared with bare-metal stent implantation and associates with late stent thrombosis. Eur. Heart J. 31(10), 1172–1180 (2010).
- Cook S, Wenaweser P, Togni M et al. Incomplete stent apposition and very late stent thrombosis after drug-eluting stent implantation. Circulation 115(18), 2426–2434 (2007).
- Lee CW, Kang S-J, Park D-W et al. Intravascular ultrasound findings in patients with very late stent thrombosis after either drug-eluting or bare-metal stent implantation. J. Am. Coll. Cardiol. 55(18), 1936–1942 (2010).
- Inoue K. Pathological perspective of drugeluting stent thrombosis. Thrombosis 2012, 219389 (2012).
- Cook S, Ladich E, Nakazawa G et al. Correlation of intravascular ultrasound findings with histopathological analysis of thrombus aspirates in patients with very late drug-eluting stent thrombosis. Circulation 120(5), 391–399 (2009).
- Alfonso F, Dutary J, Paulo M et al. Combined use of optical coherence tomography and intravascular ultrasound imaging in patients undergoing coronary interventions for stent thrombosis. Heart 98(16), 1213–1220 (2012).
- Guagliumi G, Sirbu V, Musumeci G et al. Examination of the in vivo mechanisms of late drug-eluting stent thrombosis: findings from optical coherence tomography and intravascular ultrasound imaging. JACC Cardiovasc. Interv. 5(1), 12–20 (2012).
- Kume T, Akasaka T, Kawamoto T et al. Assessment of coronary arterial thrombus by optical coherence tomography. Am. J. Cardiol. 97(12), 1713–1717 (2006).
- Park DW, Park SW, Park KH et al. Frequency of and risk factors for stent thrombosis after drug-eluting stent implantation during longterm follow-up. Am. J. Cardiol. 98(3), 352–356 (2006).
- Iakovou I, Schmidt T, Bonizzoni E et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA 293(17), 2126–2130 (2005).
- de la Torre-Hernandez JM, Alfonso F, Hernandez F et al. Drug-eluting stent thrombosis: results from the multicenter Spanish registry ESTROFA (Estudio ESpanol sobre TROmbosis de stents FArmacoactivos). J. Am. Coll. Cardiol. 51(10), 986–990 (2008).
- Aoki J, Lansky AJ, Mehran R et al. Early stent thrombosis in patients with acute coronary syndromes treated with drug-eluting and bare metal stents. Circulation 119(5), 687–698 (2009).
- Kuchulakanti PK, Chu WW, Torguson R et al. Correlates and long-term outcomes of angiographically proven stent thrombosis with sirolimus- and paclitaxel-eluting stents. Circulation 113(8), 1108–1113 (2006).
- Schuhlen H, Kastrati A, Dirschinger J et al. Intracoronary stenting and risk for major adverse cardiac events during the first month. Circulation 98(2), 104–111 (1998).
- D’Ascenzo F, Bollati M, Clementi F et al. Incidence and predictors of coronary stent thrombosis: evidence from an international collaborative meta-analysis including 30 studies, 221,066 patients, and 4276 thromboses. Int. J. Cardiol. doi:10.1016/j.ijcard.2012.01.080 (2012) (Epub ahead of print).
- Moussa I, Di Mario C, Reimers B, Akiyama T, Tobis J, Colombo A. Subacute stent thrombosis in the era of intravascular ultrasound-guided coronary stenting without anticoagulation: frequency, predictors and clinical outcome. J. Am. Coll. Cardiol. 29(1), 6–12 (1997).
- Waksman R, Torguson R, Kaneshige K. TCT-621 Drug-Eluting Stent Event Registry of Thrombosis (DESERT): the International Drug-Eluting Stent Thrombosis Registry, angiographic assessment. J. Am. Coll. Cardiol. 60(Suppl. 17), B180–B181 (2011).
- McKee SA, Applegate RJ, Hoyle JR, Sacrinty MT, Kutcher MA, Sane DC. Cocaine use is associated with an increased risk of stent thrombosis after percutaneous coronary intervention. Am. Heart J. 154(1), 159–164 (2007).
- Kimura T, Morimoto T, Nakagawa Y et al. Very late stent thrombosis and late target lesion revascularization after sirolimus-eluting stent implantation: five-year outcome of the j-Cypher Registry. Circulation 125(4), 584–591 (2012).
- Tolleson TR, Newby LK, Harrington RA et al. Frequency of stent thrombosis after acute coronary syndromes (from the SYMPHONY and 2nd SYMPHONY trials). Am. J. Cardiol. 92(3), 330–333 (2003).
- Dangas GD, Caixeta A, Mehran R et al. Frequency and predictors of stent thrombosis after percutaneous coronary intervention in acute myocardial infarction/clinical perspective. Circulation 123(16), 1745–1756 (2011).
- Nakazawa G, Finn AV, Joner M et al. Delayed arterial healing and increased late stent thrombosis at culprit sites after drug-eluting stent placement for acute myocardial infarction patients: an autopsy study. Circulation 118(11), 1138–1145 (2008).
- Wiviott SD, Braunwald E, McCabe CH et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 357(20), 2001–2015 (2007).
- Wallentin L, Becker RC, Budaj A et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 361(11), 1045–1057 (2009).
- Stone GW, Witzenbichler B, Guagliumi G et al. Bivalirudin during primary PCI in acute myocardial infarction. N. Engl. J. Med. 358(21), 2218–2230 (2008).
- Dangas GD, Claessen BE, Mehran R et al. Clinical outcomes following stent thrombosis occurring in-hospital versus out-of-hospital: results from the HORIZONS–AMI (Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction) trial. J. Am. Coll. Cardiol. 59(20), 1752–1759 (2012).
- Raber L, Windecker S. Primary percutaneous coronary intervention and risk of stent thrombosis: a look beyond the HORIZON. Circulation 123(16), 1709–1712 (2011).
- Spaulding C, Henry P, Teiger E et al. Sirolimus-eluting versus uncoated stents in acute myocardial infarction. N. Engl. J. Med. 355(11), 1093–1104 (2006).
- Laarman GJ, Suttorp MJ, Dirksen MT et al. Paclitaxel-eluting versus uncoated stents in primary percutaneous coronary intervention. N. Engl. J. Med. 355(11), 1105–1113 (2006).
- Brar SS, Leon MB, Stone GW et al. Use of drug-eluting stents in acute myocardial infarction: a systematic review and meta-analysis. J. Am. Coll. Cardiol. 53(18), 1677–1689 (2009).
- Sabate M, Cequier A, Iñiguez A, Serra A, Hernandez-Antolin R, Mainar V. The EXAMINATION trial: a randomized comparison between everolimus-eluting stent and bare metal stent in patients with STsegment elevation myocardial infarction. Presented at: European Society of Cardiology Congress. Paris, France, 27–31 August 2011.
- Raber L, Kelbaek H, Ostojic M et al. Effect of biolimus-eluting stents with biodegradable polymer vs bare-metal stents on cardiovascular events among patients with acute myocardial infarction: the COMFORTABLE AMI randomized trial. JAMA 308(8), 777–787 (2012).
- Jeremias A, Sylvia B, Bridges J et al. Stent thrombosis after successful sirolimuseluting stent implantation. Circulation 109(16), 1930–1932 (2004).
- Spertus JA, Kettelkamp R, Vance C et al. Prevalence, predictors, and outcomes of premature discontinuation of thienopyridine therapy after drug-eluting stent placement: results from the PREMIER registry. Circulation 113(24), 2803–2809 (2006).
- Airoldi F, Colombo A, Morici N et al. Incidence and predictors of drug-eluting stent thrombosis during and after discontinuation of thienopyridine treatment. Circulation 116(7), 745–754 (2007).
- Schulz S, Schuster T, Mehilli J et al. Stent thrombosis after drug-eluting stent implantation: incidence, timing, and relation to discontinuation of clopidogrel therapy over a 4-year period. Eur. Heart J. 30(22), 2714–2721 (2009).
- Lemesle G, Torguson R, Bonello L et al. Relation between clopidogrel discontinuation and early cardiovascular events after percutaneous coronary intervention with drug-eluting stents. EuroIntervention 6(9), 1053–1059 (2011).
- Eisenstein EL, Anstrom KJ, Kong DF et al. Clopidogrel use and long-term clinical outcomes after drug-eluting stent implantation. JAMA 297(2), 159–168 (2007).
- Park SJ, Park DW, Kim YH et al. Duration of dual antiplatelet therapy after implantation of drug-eluting stents. N. Engl. J. Med. 362(15), 1374–1382 (2010).
- Valgimigli M, Kastrati A. PRODIGY: Randomized comparison of six versus twentyfour months clopidogrel therapy after balancing anti-intimal hyperplasia stent potency in all comer patients undergoing percutaneous coronary intervention. Results of the PRODIGY trial. Presented at: European Society of Cardiology Congress. Paris, France, 27–31 August 2011.
- Gwon H. The randomized comparison of six-month versus 12-month duration of dual antiplatelet therapy after implantation of drug eluting stent: from comparison of Everolimus versus Sirolimus-Eluting Stents for Coronary Revascularization (EXCELLENT) trial. Presented at: American College of Cardiology. San Francisco, CA, USA, 29 June–1 July 2011.
- Kim BK, Hong MK, Shin DH et al. A new strategy for discontinuation of dual antiplatelet therapy: the RESET Trial (REal Safety and Efficacy of 3-month dual antiplatelet Therapy following Endeavor zotarolimus-eluting stent implantation). J. Am. Coll. Cardiol. 60(15), 1340–1348 (2012).
- Valgimigli M. Prolonging Dual Antiplatelet Treatment after Grading Stent-induced Intimal Hyperplasia Study (PRODIGY). Presented at: European Society of Cardiology Congress. Paris, France, 30 August 2011.
- 80 Mauri L, Kereiakes DJ, Normand SL et al. Rationale and design of the dual antiplatelet therapy study, a prospective, multicenter, randomized, double-blind trial to assess the effectiveness and safety of 12 versus 30 months of dual antiplatelet therapy in subjects undergoing percutaneous coronary intervention with either drug-eluting stent or bare metal stent placement for the treatment of coronary artery lesions. Am. Heart J. 160(6), 1035–1041, 1041.e1 (2010).
- Byrne RA, Schulz S, Mehilli J et al. Rationale and design of a randomized, double-blind, placebo-controlled trial of 6 versus 12 months clopidogrel therapy after implantation of a drug-eluting stent: the Intracoronary Stenting and Antithrombotic Regimen: Safety And EFficacy of 6 Months Dual Antiplatelet Therapy After Drug- Eluting Stenting (ISAR–SAFE) study. Am. Heart J. 157(4), 620–624.e2 (2009).
- Feres F, Costa RA, Bhatt DL et al. Optimized duration of clopidogrel therapy following treatment with the endeavor zotarolimuseluting stent in real-world clinical practice (OPTIMIZE) trial: rationale and design of a large-scale, randomized, multicenter study. Am. Heart J. 164(6), 810–816.e3 (2012).
- Levine GN, Bates ER, Blankenship JC et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J. Am. Coll. Cardiol. 58(24), e44–e122 (2011).
- Mehta SR, Bassand JP, Chrolavicius S et al. Dose comparisons of clopidogrel and aspirin in acute coronary syndromes. N. Engl. J. Med. 363(10), 930–942 (2010).
- Mehta SR, Tanguay JF, Eikelboom JW et al. Double-dose versus standard-dose clopidogrel and high-dose versus low-dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENT–OASIS 7): a randomised factorial trial. Lancet 376(9748), 1233–1243 (2010).
- Bonello L, Tantry US, Marcucci R et al. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J. Am. Coll. Cardiol. 56(12), 919–933 (2010).
- Gurbel PA, Becker RC, Mann KG, Steinhubl SR, Michelson AD. Platelet function monitoring in patients with coronary artery disease. J. Am. Coll. Cardiol. 50(19), 1822–1834 (2007).
- Aradi D, Komocsi A, Vorobcsuk A et al. Prognostic significance of high on-clopidogrel platelet reactivity after percutaneous coronary intervention: systematic review and metaanalysis. Am. Heart J. 160(3), 543–551 (2010).
- Price MJ, Endemann S, Gollapudi RR et al. Prognostic significance of post-clopidogrel platelet reactivity assessed by a point-of-care assay on thrombotic events after drug-eluting stent implantation. Eur. Heart J. 29(8), 992–1000 (2008).
- Marcucci R, Gori AM, Paniccia R et al. Cardiovascular death and nonfatal myocardial infarction in acute coronary syndrome patients receiving coronary stenting are predicted by residual platelet reactivity to ADP detected by a point-of-care assay: a 12-month follow-up. Circulation 119(2), 237–242 (2009).
- Brar SS, Ten Berg J, Marcucci R et al. Impact of platelet reactivity on clinical outcomes after percutaneous coronary intervention. A collaborative meta-analysis of individual participant data. J. Am. Coll. Cardiol. 58(19), 1945–1954 (2011).
- Price MJ, Berger PB, Teirstein PS et al. Standard- vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial. JAMA 305(11), 1097–1105 (2011).
- Trenk D, Stone GW, Gawaz M et al. A randomized trial of prasugrel versus clopidogrel in patients with high platelet reactivity on clopidogrel after elective percutaneous coronary intervention with implantation of drug-eluting stents: results of the TRIGGER–PCI (Testing Platelet Reactivity In Patients Undergoing Elective Stent Placement on Clopidogrel to Guide Alternative Therapy With Prasugrel) study. J. Am. Coll. Cardiol. 59(24), 2159–2164 (2012).
- Collet JP, Cuisset T, Range G et al. Bedside monitoring to adjust antiplatelet therapy for coronary stenting. N. Engl. J. Med. 367(22), 2100–2109 (2012).
- Gurbel PA, Erlinge D, Ohman EM et al. Platelet function during extended prasugrel and clopidogrel therapy for patients with acs treated without revascularization: the TRILOGY ACS platelet function substudy. JAMA 308(17), 1785–1794 (2012).
- Stone GW. ADAPT–DES one year: a large-scale, multicenter, prospective, observational study of the impact of clopidogrel and aspirin hyporesponsiveness on patient outcomes. Presented at: Transcatheter Therapeutics. Miami, FL, USA, 22–26 October 2012.
- Shuldiner AR, O’connell JR, Bliden KP et al. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA 302(8), 849–857 (2009).
- Mega JL, Simon T, Collet JP et al. Reducedfunction CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta-analysis. JAMA 304(16), 1821–1830 (2010).
- Roberts JD, Wells GA, Le May MR et al. Point-of-care genetic testing for personalisation of antiplatelet treatment (RAPID GENE): a prospective, randomised, proof-of-concept trial. Lancet 379(9827), 1705–1711 (2012).
- Orford JL, Lennon R, Melby S et al. Frequency and correlates of coronary stent thrombosis in the modern era: analysis of a single center registry. J. Am. Coll. Cardiol. 40(9), 1567–1572 (2002).
- Sianos G, Papafaklis MI, Daemen J et al. Angiographic stent thrombosis after routine use of drug-eluting stents in ST-segment elevation myocardial infarction: the importance of thrombus burden. J. Am. Coll. Cardiol. 50(7), 573–583 (2007).
- Cheneau E, Leborgne L, Mintz GS et al. Predictors of subacute stent thrombosis. Circulation 108(1), 43–47 (2003).
- Choi SY, Witzenbichler B, Maehara A et al. Intravascular ultrasound findings of early stent thrombosis after primary percutaneous intervention in acute myocardial infarction: a Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction (HORIZONS–AMI) substudy. Circulation Cardiovasc. Interv. 4(3), 239–247 (2011).
- Moreno R, Fernandez C, Hernandez R et al. Drug-eluting stent thrombosis: results from a pooled analysis including 10 randomized studies. J. Am. Coll. Cardiol. 45(6), 954–959 (2005).
- Uren NG, Schwarzacher SP, Metz JA et al. Predictors and outcomes of stent thrombosis: an intravascular ultrasound registry. Eur. Heart J. 23(2), 124–132 (2002).
- Alfonso F, Suarez A, Angiolillo DJ et al. Findings of intravascular ultrasound during acute stent thrombosis. Heart 90(12), 1455–1459 (2004).
- Goldberg SL, Colombo A, Nakamura S, Almagor Y, Maiello L, Tobis JM. Benefit of intracoronary ultrasound in the deployment of Palmaz–Schatz stents. J. Am. Coll. Cardiol. 24(4), 996–1003 (1994).
- Nakamura S, Colombo A, Gaglione A et al. Intracoronary ultrasound observations during stent implantation. Circulation 89(5), 2026–2034 (1994).
- Fitzgerald PJ, Oshima A, Hayase M et al. Final results of the Can Routine Ultrasound Influence Stent Expansion (CRUISE) study. Circulation 102(5), 523–530 (2000).
- Mudra H, di Mario C, de Jaegere P et al. Randomized comparison of coronary stent implantation under ultrasound or angiographic guidance to reduce stent restenosis (OPTICUS study). Circulation 104(12), 1343–1349 (2001).
- Roy P, Steinberg DH, Sushinsky SJ et al. The potential clinical utility of intravascular ultrasound guidance in patients undergoing percutaneous coronary intervention with drug-eluting stents. Eur. Heart J. 29(15), 1851–1857 (2008).
- Zhang Y, Farooq V, Garcia-Garcia HM et al. Comparison of intravascular ultrasound versus angiography-guided drug-eluting stent implantation: a meta-analysis of one randomised trial and ten observational studies involving 19,619 patients. EuroIntervention 8(7), 855–865 (2012).
- Witzenbichler BAM, Weisz G, Neumann FJ et al. Use of IVUS reduces stent thrombosis: results from the prospective, multicenter ADAPT–DES study. Presented at: Transcatheter Therapeutics. Miami, FL, USA, 22–26 October 2012.
- Colombo A. Operator techniques to reduce stent thrombosis. Presented at: Transcatheter Therapeutics. San Francisco, CA, USA, 7–11 November 2011.
- Baber U, Mehran R, Sharma SK et al. Impact of the Everolimus-eluting stent on stent thrombosis: a meta-analysis of 13 randomized trials. J. Am. Coll. Cardiol. 58(15), 1569–1577 (2011).
- Serruys PW, Ong AT, Piek JJ et al. A randomized comparison of a durable polymer Everolimus-eluting stent with a bare metal coronary stent: the SPIRIT first trial. EuroIntervention 1(1), 58–65 (2005).
- Serruys PW, Ruygrok P, Neuzner J et al. A randomised comparison of an everolimus-eluting coronary stent with a paclitaxel-eluting coronary stent: the SPIRIT II trial. EuroIntervention 2(3), 286–294 (2006).
- Stone GW, Midei M, Newman W et al. Comparison of an Everolimus-eluting stent and a paclitaxel-eluting stent in patients with coronary artery disease. JAMA 299(16), 1903–1913 (2008).
- Caixeta A, Lansky AJ, Serruys PW et al. Clinical follow-up 3 years after everolimusand paclitaxel-eluting stents: a pooled analysis from the SPIRIT II (A Clinical Evaluation of the XIENCE V Everolimus Eluting Coronary Stent System in the Treatment of Patients With De Novo Native Coronary Artery Lesions) and SPIRIT III (A Clinical Evaluation of the Investigational Device XIENCE V Everolimus Eluting Coronary Stent System [EECSS] in the Treatment of Subjects With De Novo Native Coronary Artery Lesions) randomized trials. JACC Cardiovasc. Interv. 3(12), 1220–1228 (2010).
- Stone GW, Rizvi A, Newman W et al. Everolimus-eluting versus paclitaxel-eluting stents in coronary artery disease. N. Engl. J. Med. 362(18), 1663–1674 (2010).
- Stone GW, Rizvi A, Sudhir K et al. Randomized comparison of everolimus- and paclitaxel-eluting stents. 2-year follow-up from the SPIRIT (Clinical Evaluation of the XIENCE V Everolimus Eluting Coronary Stent System) IV trial. J. Am. Coll. Cardiol. 58(1), 19–25 (2011).
- Kedhi E, Joesoef KS, Mcfadden E et al. Second-generation everolimus-eluting and paclitaxel-eluting stents in real-life practice (COMPARE): a randomised trial. Lancet 375(9710), 201–209 (2010).
- Smits PC, Kedhi E, Royaards KJ et al. 2-year follow-up of a randomized controlled trial of everolimus- and paclitaxel-eluting stents for coronary revascularization in daily practice. COMPARE (comparison of the everolimus eluting XIENCE-V stent with the paclitaxel eluting TAXUS LIBERTE stent in all-comers: a randomized open label trial). J. Am. Coll. Cardiol. 58(1), 11–18 (2011).
- Stone GW, Teirstein PS, Meredith IT et al. A prospective, randomized evaluation of a novel everolimus-eluting coronary stent: the PLATINUM (a prospective, randomized, multicenter trial to assess an everolimuseluting coronary stent system [PROMUS Element] for the treatment of up to two de novo coronary artery lesions) trial. J. Am. Coll. Cardiol. 57(16), 1700–1708 (2011).
- Costa M. SPIRIT PRIME clinical trial. Presented at: Transcatheter Therapeutics. San Francisco, CA, USA, 7–11 November 2011.
- Garg S, Serruys PW. Coronary stents: Current Status. J. Am. Coll. Cardiol. 56(Suppl. 10), S1–S42 (2010).
- Kandzari DE, Leon MB, Popma JJ et al. Comparison of zotarolimus-eluting and sirolimus-eluting stents in patients with native coronary artery disease: a randomized controlled trial. J. Am. Coll. Cardiol. 48(12), 2440–2447 (2006).
- Leon MB, Nikolsky E, Cutlip DE et al. Improved late clinical safety with zotarolimuseluting stents compared with paclitaxel-eluting stents in patients with de novo coronary lesions: 3-year follow-up from the ENDEAVOR IV (Randomized Comparison of Zotarolimusand Paclitaxel-Eluting Stents in Patients With Coronary Artery Disease) trial. JACC Cardiovasc. Interv. 3(10), 1043–1050 (2010).
- Kandzari DE, Mauri L, Popma JJ et al. Late-term clinical outcomes with zotarolimusand sirolimus-eluting stents. 5-year follow-up of the ENDEAVOR III (a randomized controlled trial of the Medtronic Endeavor drug [ABT-578] eluting coronary stent system versus the Cypher sirolimus-eluting coronary stent system in de novo native coronary artery lesions). JACC Cardiovasc. Interv. 4(5), 543–550 (2011).
- Rasmussen K, Maeng M, Kaltoft A et al. Efficacy and safety of zotarolimus-eluting and sirolimus-eluting coronary stents in routine clinical care (SORT OUT III): a randomised controlled superiority trial. Lancet 375(9720), 1090–1099 (2010).
- Udipi K, Melder RJ, Chen M et al. The next generation Endeavor Resolute stent: role of the BioLinx Polymer System. EuroIntervention 3(1), 137–139 (2007).
- Meredith IT, Worthley S, Whitbourn R et al. Clinical and angiographic results with the nextgeneration resolute stent system: a prospective, multicenter, first-in-human trial. JACC Cardiovasc. Interv. 2(10), 977–985 (2009).
- Yeung AC, Leon MB, Jain A et al. Clinical evaluation of the Resolute zotarolimus-eluting coronary stent system in the treatment of de novo lesions in native coronary arteries: the RESOLUTE US clinical trial. J. Am. Coll. Cardiol. 57(17), 1778–1783 (2011).
- Kandzari DE. ENDEAVOR zotarolimuseluting stent program from clinical trials to clinical practice. Presented at: Transcatheter Therapeutics. San Francisco, CA, USA, 7–11 November 2011.
- Serruys PW, Silber S, Garg S et al. Comparison of zotarolimus-eluting and everolimus-eluting coronary stents. N. Engl. J. Med. 363(2), 136–146 (2010).
- Von Birgelen C, Basalus MW, Tandjung K et al. A randomized controlled trial in secondgeneration zotarolimus-eluting Resolute stents versus everolimus-eluting Xience V stents in real-world patients: the TWENTE trial. J. Am. Coll. Cardiol. 59(15), 1350–1361 (2012).
- de la Torre Hernandez JM, Alfonso F, Gimeno F et al. Thrombosis of secondgeneration drug-eluting stents in real practice results from the multicenter Spanish registry ESTROFA-2 (Estudio Espanol Sobre Trombosis de Stents Farmacoactivos de Segunda Generacion-2). JACC Cardiovasc. Interv. 3(9), 911–919 (2010).
- Palmerini T, Kirtane AJ, Serruys PW et al. Stent thrombosis with everolimus-eluting stents: meta-analysis of comparative randomized controlled trials. Circulation Cardiovasc. Interv. 5(3), 357–364 (2012).
- Bezenek S, Hermiller J, Lansky A et al. Low stent thrombosis risk with the XIENCE V® Everolimus-Eluting Coronary Stent: evidence from randomized and single-arm clinical trials. J. Interv. Cardiol. 24(4), 326–341 (2011).
- Raber L, Magro M, Stefanini GG et al. Very late coronary stent thrombosis of a newer-generation everolimus-eluting stent compared with early-generation drug-eluting stents: a prospective cohort study. Circulation 125(9), 1110–1121 (2012).
- Mehilli J, Byrne RA, Wieczorek A et al. Randomized trial of three rapamycin-eluting stents with different coating strategies for the reduction of coronary restenosis. Eur. Heart J. 29(16), 1975–1982 (2008).
- Byrne RA, Kastrati A, Kufner S et al. Randomized, non-inferiority trial of three limus agent-eluting stents with different polymer coatings: the Intracoronary Stenting and Angiographic Results: Test Efficacy of 3 Limus-Eluting Stents (ISAR–TEST-4) trial. Eur. Heart J. 30(20), 2441–2449 (2009).
- Byrne RA, Kastrati A, Massberg S et al. Biodegradable polymer versus permanent polymer drug-eluting stents and everolimusversus sirolimus-eluting stents in patients with coronary artery disease: 3-year outcomes from a randomized clinical trial. J. Am. Coll. Cardiol. 58(13), 1325–1331 (2011).
- Windecker S, Serruys PW, Wandel S et al. Biolimus-eluting stent with biodegradable polymer versus sirolimus-eluting stent with durable polymer for coronary revascularisation (LEADERS): a randomised non-inferiority trial. Lancet 372(9644), 1163–1173 (2008).
- Serruys P. LEADERS 5-year: a prospective, randomized trial of bioabsorbable polymerbased biolimus-eluting vs. sirolimus-eluting stents. Presented at: Transcatheter Therapeutics. Miami, FL, USA, 22–26 October 2012.
- Chevalier B, Silber S, Park SJ et al. Randomized comparison of the Nobori Biolimus A9-eluting coronary stent with the Taxus Liberte paclitaxel-eluting coronary stent in patients with stenosis in native coronary arteries: the NOBORI 1 trial – Phase 2.Circulation Cardiovasc. Interv. 2(3), 188–195 (2009).
- Kadota K, Muramatsu T, Iwabuchi M et al. Randomized comparison of the Nobori Biolimus A9-eluting stent with the sirolimuseluting stent in patients with stenosis in native coronary arteries. Catheter. Cardiovasc. Interv. 80(5), 789–796 (2012).
- Smits PC, Hofma S, Togni M et al. Abluminal biodegradable polymer biolimuseluting stent versus durable polymer everolimus-eluting stent (COMPARE II): a randomised, controlled, non-inferiority trial. Lancet 381(9867), 651–660 (2013).
- Christiansen EH, Jensen LO, Thayssen P et al. Biolimus-eluting biodegradable polymer-coated stent versus durable polymer-coated sirolimus-eluting stent in unselected patients receiving percutaneous coronary intervention (SORT OUT V): a randomised non-inferiority trial. Lancet 381(9867), 661–669 (2013).
- Stefanini GG, Byrne RA, Serruys PW et al. Biodegradable polymer drug-eluting stents reduce the risk of stent thrombosis at 4 years in patients undergoing percutaneous coronary intervention: a pooled analysis of individual patient data from the ISAR-TEST 3, ISARTEST 4, and LEADERS randomized trials. Eur. Heart J. 33(10), 1214–1222 (2012).
- Ormiston JA, Serruys PW, Regar E et al. A bioabsorbable everolimus-eluting coronary stent system for patients with single de-novo coronary artery lesions (ABSORB): a prospective open-label trial. Lancet 371(9616), 899–907 (2008).
- Serruys PW, Ormiston JA, Onuma Y et al. A bioabsorbable everolimus-eluting coronary stent system (ABSORB): 2-year outcomes and results from multiple imaging methods. Lancet 373(9667), 897–910 (2009).
- Serruys PW, Onuma Y, Ormiston JA et al. Evaluation of the second generation of a bioresorbable everolimus drug-eluting vascular scaffold for treatment of de novo coronary artery stenosis: six-month clinical and imaging outcomes. Circulation 122(22), 2301–2312 (2010).
- Serruys PW, Onuma Y, Dudek D et al. Evaluation of the second generation of a bioresorbable everolimus-eluting vascular scaffold for the treatment of de novo coronary artery stenosis: 12-month clinical and imaging outcomes. J. Am. Coll. Cardiol. 58(15), 1578– 1578 (2011).
- Schomig A, Neumann FJ, Kastrati A et al. A randomized comparison of antiplatelet and anticoagulant therapy after the placement of coronary-artery stents. N. Engl. J. Med. 334(17), 1084–1089 (1996).
- Bertrand ME, Legrand V, Boland J et al. Randomized multicenter comparison of conventional anticoagulation versus antiplatelet therapy in unplanned and elective coronary stenting. The full anticoagulation versus aspirin and ticlopidine (fantastic) study. Circulation 98(16), 1597– 1603 (1998).
- Leon MB, Baim DS, Popma JJ et al. A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. Stent Anticoagulation Restenosis Study Investigators. N. Engl. J. Med. 339(23), 1665– 1671 (1998).
- Urban P, Macaya C, Rupprecht HJ et al. Randomized evaluation of anticoagulation versus antiplatelet therapy after coronary stent implantation in high-risk patients: the multicenter aspirin and ticlopidine trial after intracoronary stenting (MATTIS). Circulation 98(20), 2126–2132 (1998).
- Mehta SR, Yusuf S, Peters RJ et al. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet 358(9281), 527–533 (2001).
- Steinhubl SR, Berger PB, Mann JT 3rd et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA 288(19), 2411–2420 (2002).
- Wiviott SD, Braunwald E, McCabe CH et al. Intensive oral antiplatelet therapy for reduction of ischaemic events including stent thrombosis in patients with acute coronary syndromes treated with percutaneous coronary intervention and stenting in the TRITON-TIMI 38 trial: a subanalysis of a randomised trial. Lancet 371(9621), 1353–1363 (2008).
- Biondi-Zoccai G, Lotrionte M, Agostoni P et al. Adjusted indirect comparison metaanalysis of prasugrel versus ticagrelor for patients with acute coronary syndromes. Int. J. Cardiol. 150(3), 325–331 (2011).
- Mega JL, Braunwald E, Wiviott SD et al. Rivaroxaban in patients with a recent acute coronary syndrome. N. Engl. J. Med. 366(1), 9–19 (2012).
- Spaulding C, Daemen J, Boersma E, Cutlip DE, Serruys PW. A pooled analysis of data comparing sirolimus-eluting stents with baremetal stents. N. Engl. J. Med. 356(10), 989–997 (2007).
- Kimura T, Morimoto T, Nakagawa Y et al. Antiplatelet therapy and stent thrombosis after sirolimus-eluting stent implantation. Circulation 119(7), 987–995 (2009).
- Valgimigli M, Campo G, Percoco G et al. Randomized comparison of 6- versus 24-month clopidogrel therapy after balancing anti-intimal hyperplasia stent potency in all-comer patients undergoing percutaneous coronary intervention design and rationale for the PROlonging Dual-antiplatelet treatment after Grading stent-induced Intimal hyperplasia study (PRODIGY). Am. Heart J. 160(5), 804–811 (2010).
• Large Dutch registry of 437 episodes of definite stent thrombosis (ST) providing insight into the timing and predictors of ST.
•• Large registry of over 94,000 stent implantations demonstrating a significantly lower risk of definite ST among patients treated with ‘newer-generation’ drug-eluting stents (DES) compared with both first-generation DES and bare-metal stents.
• Network meta-analysis comparing clinical performance of first-generation DES and bare-metal stents, demonstrating a similar reduction in mortality and ST for all stent types at 4-year follow-up.
•• Large network meta-analysis including data from 76 randomized controlled trials with 117,762 patient-years of follow-up comparing short- and long-term efficacy and safety outcomes among different types of stents, demonstrating everolimus-eluting stents to have the highest probability of being the safest stent based on a reduction in myocardial infarction and ST.
• Prospective observational cohort study evaluating the incidence, predictors and clinical outcomes of ST after implantation of first-generation DES among ‘real-world’ patients in practice, which identifies premature discontinuation of dual antiplatelet therapy as the strongest predictor of ST.
• Collaborative systematic review on incidence and predictors of ST including data from 30 studies, 221,066 patients and 4276 events identifying multiple predictors of ST from a total of 47 candidate variables.
• Analysis of the HORIZONS–AMI trial evaluating the frequency and predictors of stent thrombosis at 2-year follow-up after primary percutaneous coronary intervention for ST-elevation myocardial infarction that found a similar incidence of events regardless of the type of stent (bare-metal stents vs DES) or anticoagulation strategy (bivalirudin alone vs unfractionated heparin/glycoprotein IIb/IIIa inhibitors), but with important differences in the timing of ST between anticoagulation strategies.
• Pooled analysis of patient data from three large randomized controlled trials (ISARTEST 3, ISAR-TEST 4 and LEADERS) comparing biodegradable polymer DES with durable polymer sirolimus-eluting stent, demonstrating a significant reduction in target-vessel revascularization and ST (driven by a reduction in very late ST) through 4-year follow-up.
• A trial of over 15,000 patients hospitalized with a recent acute coronary syndrome randomized to rivaroxaban (low or very low dose) versus placebo, demonstrating a reduction in composite cardiovascular death, myocardial infarction or stroke, as well as a significant reduction in ST among patients randomized to rivaroxaban.