Review Article - Interventional Cardiology (2015) Volume 7, Issue 6
Baroreflex activation therapy for the treatment of heart failure
- Corresponding Author:
- Edoardo Gronda
Cardiovascular Research Department
IRCCS MultiMedica, Sesto San Giovanni (Milan), Italy
Tel: +39 022 420 9460
Fax: +39 022 420 9051
E-mail: edoardo.gronda@multimedica.it
Submitted: 1 July 2015; Accepted: 4 September 2015; Published online: 12 November 2015
Abstract
Baroreflex activation therapy (BAT) results in centrally mediated reduction of sympathetic outflow and increased parasympathetic activity to the heart via a physiological reflex pathway. In a pilot study in 11 NYHA Class III heart failure (HF) patients, BAT produced a persistent significant reduction of sympathetic activity over 21 months follow-up and a dramatic decrease in the number and length of hospitalizations. In a multinational, prospective, randomized, parallel-controlled, clinical trial in 146 NYHA Class III HF patients, BAT produced a significant N-terminal pro-brain natriuretic peptide reduction (p < 0.02). This was associated with a trend toward fewer in-hospital days for HF. BAT might become a powerful tool to repair autonomic alterations due to HF at their origin and thus, profoundly affect the prognosis of advanced HF patients.
Baroreflex activation therapy (BAT) results in centrally mediated reduction of sympathetic outflow and increased parasympathetic activity to the heart via a physiological reflex pathway. In a pilot study in 11 NYHA Class III heart failure (HF) patients, BAT produced a persistent significant reduction of sympathetic activity over 21 months follow-up and a dramatic decrease in the number and length of hospitalizations. In a multinational, prospective, randomized, parallel-controlled, clinical trial in 146 NYHA Class III HF patients, BAT produced a significant N-terminal pro-brain natriuretic peptide reduction (p < 0.02). This was associated with a trend toward fewer in-hospital days for HF. BAT might become a powerful tool to repair autonomic alterations due to HF at their origin and thus, profoundly affect the prognosis of advanced HF patients.
Keywords
autonomic nervous system, baroreflex, heart failure, nonpharmacologic therapy
Background
Despite the impressive progress achieved in the last decades in its management and outcome, heart failure (HF) remains a major cause of death in the western countries.
The reasons are related not only to the general population aging process, but also to the limit of contemporary HF state-of-the-art therapy. Very recent survival analyses suggest indeed that optimized medical treatment, including β-blockers and cardiac resynchronization plus implantable defibrillator, provides effective benefit in a time frame that usually does not exceed the 15 years span [1].
Many factors contributed both in a positive and negative way to the current status of HF management. On one side, some fundamental aspects of HF pathophysiology were misunderstood for some 20 years leading to an inappropriate approach to the syndrome. Additionally, these pathophysiological mis-understanding reflected in erroneous clinical standards of therapy and consequent developments that stuck in the medical community for many years.
The inotropes saga in HF treatment provides a clear example. While there is no doubt that decreased contractility is a central component of HF pathophysiology, attempts to increase contractility with high doses of inotropic agents shown to increase myocardial work have not proven to be safe.
Similarly, the attempt to translate into clinical benefit the vasodilating action of α-lytic agents, aiming to counteract the peripheral arteriolar vasoconstriction, did not provide convincing benefit in the long-term outcome of HF patients. These unsatisfactory results led to oversight the advantage of the combination of a fixed dose of isosorbide dinitrate and hydralazine in African–Americans that later proved to be more effective of converting enzyme inhibitors (ACE I) in this ethnic group [2].
The critical missing point here was that sympathetic responses to cardiac damage reflect intrinsic individual characteristics of the autonomic functional activity. Such responses are not the consequence of the cardiac negative remodeling but rather its principal cause and, being so, they require an immediate effective modulatory intervention to prevent unfavorable cardiac remodeling that, ultimately, will produce cardiac electrical instability and pump failure [3].
Only 25 years later, indeed, a more complete understanding of the role of neurohormones in HF syndrome development, drove the introduction of ACE I and later, of β-blockers. Administration of β-blockers was unfavorably delayed by the longstanding conceptual bias that their negative inotropic action could represent a predominant threaten to HF patient outcome [4]. At the opposite, the association of β-blocker and ACE I provided stunning results by halting the negative ventricular remodeling and then promoting and consolidating the reversion of this remodeling process both at the structural and molecular level, thus reaching the goal of restoring a more efficient heart phenotype
On the other hand, innovative research, specifically over the last two decades, added to HF armamentarium new devices that implemented significantly quality of life (QoL) and its expectancy. Translated in practical terms the fundamental misunderstanding about its mechanisms was that HF was primarily conceived as a disease of the cardiac myocyte with the consequent therapeutic synthesis that the solution was the inotropic support to the heart. In the meanwhile, adrenergic antagonism was perceived as detrimental and disregarded as the bad witch! Not only that. In the same while, the use of implantable cardioverter defibrillator (ICD) skyrocketed and this intervention was seen as THE therapy of HF while it was the surrender to it as rather than treating the disease and its evolution into sudden death we felt happy by having a device that do rescue the patient in the many instances in which our therapy failed [5].
Hopeless research was carried out thinking that pharmacologic modulation of single ion channels would do the job, specifically on the arrhythmic death, and we had to withstand to the consecutive failures of sodium, calcium and potassium channel blockers. Eventually, when no more ion channels were left to block and α-adrenergic receptors blockade also failed, the medical community accepted to reverse its thinking and to start using β-adrenergic blockers with the new understanding of therapy titration. Now a days, after some 10 years of training cardiologist and internists, the use of β-blockers is really a standard of care in HF. Such a dramatic delay in this fundamental understanding affected the entire line of research on HF and the comprehension of the function of its primary actor, namely the autonomic nervous system (ANS). Thus, we recently apprised the contribution of ANS balance recovery in the efficacy of cardiac resynchronization therapy (CRT) and the bioindustry is now taking the ball to bring the game into the arena of direct neural modulation of afferent and efferent cardiac nerves to eventually reach into the core of the problem, rather than to merely protect the end organ from the excessive release of catecholamine [6].
On the basis of these historical remainders it might then be appreciated why the main reason underlying the limits of the current medical therapy is probably related to the its ability to induce only a partial reversion of the clinical condition and of the complex structural negative remodeling with some variable degree of cardiac dysfunction remaining in place and running on the HF pathophysiologic process.
The unfavorable outcome, prominently associated with the presence of left ventricular dysfunction even in absence of relevant HF symptoms, is the matter of fact in real world HF population [7].
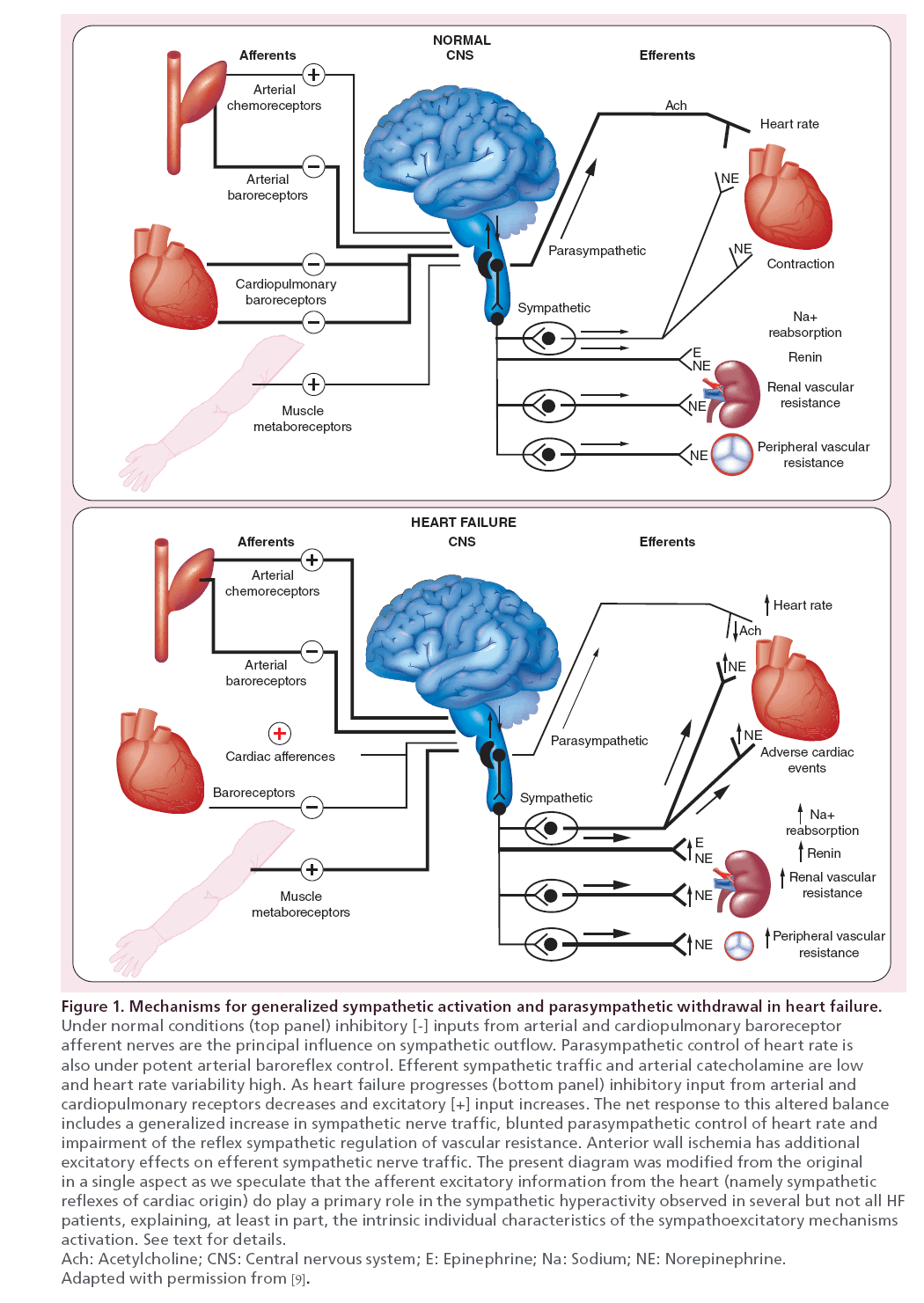
HF pathophysiology evolution is tightly linked to the reflexive enhancement of the sympathetic drive as the sympathetic nervous system hyperactivation in HF adversely impacts the disease progression and the patient survival [8]. Such hyperactivity is primarily driven by the afferent signals from chemo- and mechano- receptors located in the cardiac chambers and sustained by the progressive baroreceptorial unloading due to cardiac output drop. This latter, indeed, results in a central release of the sympathetic activity from the baroreceptorial restrain. Thus, in the entire autonomic functional remodeling resulting also from the decreased outflow of the counterbalancing action of the vagal nerve [9] the cardiac pump dysfunction becomes then the leading drive of the syndrome. This set up has then major consequences on the whole vascular bed including both arterial and venous vessels. The progressive unbalance between the two limbs of the ANSs, driven by the aggravation of HF hemodynamic derangement, is mostly expressed by the increasing plasma norepinephrine spillover that closely mirrors sympathetic tone overactivation and is tightly related to HF patient prognosis [10].
Beside cardiac pump dysfunction, other cardiovascular insults such as myocardial ischemia or injuries directed toward the whole vascular beds contribute to the pathophysiological process of the sympathetic drive reflexive enhancement and to the decreased outflow of the counterbalancing vagal nerve action [11].
It is indeed now recognized that in HF syndrome, the time course and magnitude of sympathetic activation are not generalized but they target specific organ independently from ventricular systolic function. Moreover, diminution of cardiac reflex vagal modulation is primarily attributed to the dysfunction of two sets of sympathoinhibitory reflexes engaged by stimulation of mechanoreceptor afferents: arterial baroreceptors, which are less stretched as systolic and pulse pressures diminish; and cardiopulmonary receptors, which may be damaged by prior myocardial infarction, or maybe less stimulated as myocardial inotropy diminishes and ventricular chambers dilate [11]. Thus, cardiac sympathoexcitatory reflex due to increased cardiopulmonary filling pressure and to individual variation in nonbaroreflex-mediated sympathoexcitatory mechanisms, including coexisting sleep apnea, myocardial ischemia, obesity and reflexes from exercising muscle become the predominant autonomic force to the heart and the entire cardiovascular system [11,12].
The overall concept that underlies the ANS involvement in the genesis of HF syndrome is addressed in Figure 1 (modified from [9]).
Rationale & applications of baroreflex activation therapy in heart failure management
Signaling from baroreceptors interacts with global afferent information coming from the heart and the periphery that is integrated in the medulla with the nucleus of the solitary tract serving as a central relay station. This integrated system becomes inefficient when the impairment of baroreflex activity in HF allows excess sympathetic activation to go unrestrained [9,11,12].
Thus, sympathetic activation in the setting of impaired systolic function reflects the net balance and interaction between appropriate reflex compensatory responses to impaired systolic function and excitatory stimuli that elicit adrenergic responses in excess of homeostatic requirements.
In this complex setting, the baroreceptors may represent the therapeutic gate to rebalance cardiovascular and autonomic function deranged by HF.
It is of note that baroreceptors regulatory action on cardiovascular function is primitively disengaged by low cardiac output, but a number of researches concordantly address the fact that arterial baroreflex control of sympathetic nerve activity is still effective in HF. Floras provided key observations on this matter [11]. They include:
• Preserved muscle sympathetic nerve activity (MSNA) pulse synchronicity and the fact that MSNA variations reflect pulsus alternans [13];
• Immediate reflex augmentation of MSNA burst amplitude and duration in response to the long post-extrasystolic pause with a subsequent suppression of MSNA proportional to the diastolic pressure overshoot [14];
• MSNA drop when diastolic pressure rises modestly upon left or biventricular pacing [15];
• A similar inhibition of MSNA by aortic and ventricular mechanoreceptor stimulation in subjects with normal and impaired ventricular systolic function [16];
• A similar reflex increases in total body norepinephrine spillover in patients and control subjects with nitroprusside infusion to achieve comparable baroreceptor unloading [17]; and,
• A similar gain, in subjects with and without HF, of the cross-spectral transfer function between BP (stimulus) and MSNA (response) across all frequency bands [15].
Other information, originated from an advanced HF study population, documents the inverse relationship between baroreflex sensitivity (BRS) and event risk in patients with depressed left ventricular systolic function, whether or not are treated with β-blocker therapy [18].
It is possible to speculate that a depressed BRS in HF patients may be related to a less than optimal response to β-blocker therapy or that they may belong to specific genotypes, yet to be defined, associated with less survival benefits and less left ventricular ejection fraction (LVEF) improvement [19].
A closer data analysis provides information that suggests intriguing speculations by just looking at the study survival curves. It can be noted that 5 years survival in patients with depressed baroreflex response, despite β-blocker therapy, equals survival of those patients not receiving β-blocker therapy, but presenting a preserved baroreflex response. It can be speculated the preserved baroreflex response, that is, a preserved cardiac vagal control, provides benefit in HF patient survival that might be comparable to the one provided by β-blocker drugs. The mirroring consideration, though, is that β-blockers therapy can add moderate benefit in HF patients whose risk profile is maintained low by his/her high vagal activity.
All these evidences concur to support baroreflex activation therapy (BAT) as a potential tool to rebalance the sympathetic nerve activity in the intricate HF milieu where the device therapy seems the only solution that can restart appropriate response for specific therapeutic aims, without creating undesired systemic side effects. Moreover, direct baroreceptor stimulation takes a very specific approach, in comparison, for instance, to vagal nerve stimulation, by targeting an afferent pathway that corrects, via integrative responses of brainstem centers, both the autonomic and the consequent hemodynamic dysfunctions including the decreased arterial and venous compliance and the augmented peripheral resistance.
BAT: from the proof-of-concept data to the clinical prospective
On the basis of such background recently a mechanistic pilot study has been performed in 11 advanced HF, NYHA f. Cl. III patients [20], with reduced LVEF (HFrEF) of 31% (± 7.3) associating severe functional limitation with moderately impaired renal function.
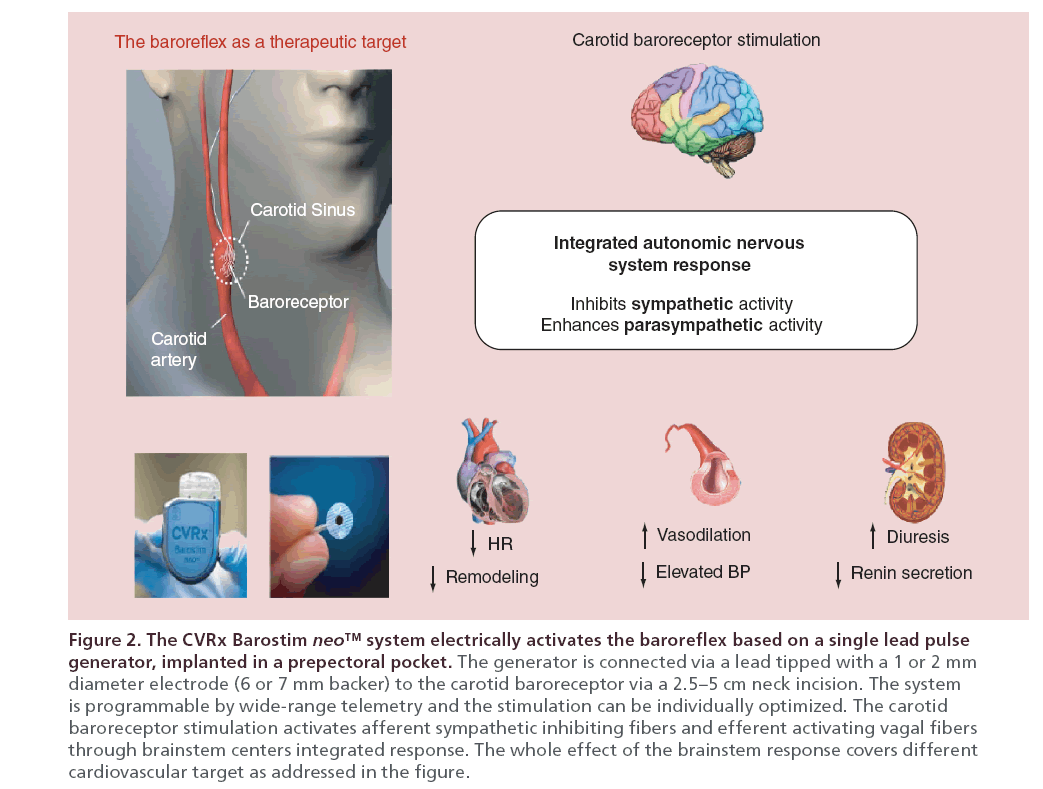
In the study population, despite optimized medical therapy, an elevated sympathetic activity (MSNA) was detected by peroneal nerve microneurography. After autonomic modulation by BAT using the chronic electrical stimulation of the carotid baroreceptors (Barostim, CVRx, Inc. MN, USA, Figure 2. For details on device characteristic and implantation technique see foot-page note 1 at the end of this section), a rapid and sustained 30% reduction in MSNA was observed with persistence, throughout 6 months follow-up. At study baseline recorded bursts/min were 47.4 ± 6.2, and they dropped to 13.6 ± 1.6 and 15.0 ± 2.6 burst/min after 3 and 6 months (p < 0.001). This impressive change occurred in optimally treated HF patients and was coupled with a recovery of the BRS (arbitrary units from 0.11 ± 0.13 to 1.26 ± 0.16, p < 0.001).
The study demonstrated the BAT safety and the associated MSNA reduction, and it was strengthened by an impressive improvement in HF clinical status documented by consistent changes in NYHA Class, QoL score and 6–min hall walk (6 MHW) distance, cardiac structure and function, assessed by 3D echo-cardiography.
It is important also to observe that heart rate (HR) did not show significant changes throughout the study follow-up. The reasons for the lack of this response might be different but appeared related to the fact that all but one of the patient study group received β-blocker therapy, three presented permanent atrial fibrillation an one had an atrial stimulating pace maker. All of these are possible causes for the absence of detectable effect on HR. It has also to be noted that in the chronic vagal nerve stimulation Phase II study, where the CardioFit (BioControl Medical) implantable system was tested, the acute change in HR during stimulation was modest and, in the average, the HR did not change during the 24-h ambulatory ECG. The finding would suggest that HR is not the prominent mediator of the possible favorable effects of vagus nerve action. It seems more probable that vagal nerve activation may have positive direct effects at myocardial level independent of its HR change. This suggestion is concordant with findings in conscious dogs where the vagally mediated protection from ventricular fibrillation was, in approximately half of the cases, not dependent from HR changes [21].
On note, other recent animal studies support the hypothesis that vagal activation operates through mechanisms other than HR decrease to ameliorate cardiac performance [22].
HR-independent effects may include antiadrenergic effects at ventricular level due to sympathetic–parasympathetic interaction, antiapoptotic effects, increase in nitric oxide, reduction in the ischemia/reperfusion damage as well as the so-called vagal anti-inflammatory reflex [23–25].
All the positive effects documented in animal models are yet to be proven in human beings, but the BAT overall clinical benefit in HF patients was, somewhat unexpectedly, addressed by the accrued HF-related hospital days in the 12 months following BAT activation as compared with the 12 months prior to implant (125 vs 18 days spent in hospital) [20]. The contemporary after BAT kidney function was stabilized with a trend toward improvement, while mean HR, blood pressure and medical therapy remained unchanged
Data on kidney are highlighted consistently by ATRAMI study results. In this trial BRS impairment and BUN were both independent outcome predictors, meaning that impaired or worsened renal function correlates with BRS. The link also exists in chronic kidney disease as BRS impairment significantly correlates with cardiac mortality [26].
Baroreflex activation therapy: from the clinical prospective to the confirmatory data of the clinical controlled study
The promising, but preliminary data found extensive confirmation in the results of the recently published multicenter, randomized, controlled trial [27]. In this controlled study 146 patients at 45 centers were randomized. Of these, 70 patients served as control group and managed with optimal medical therapy while 76 were assigned to BAT. One patient in the control group died prior to his/her activation date, and 5 dropped out from the study, 140 completed the trial: 71 in the treated arm and 69 in the control arm.
The two groups were similar with respect to baseline characteristics, except for a significantly worse QoL score and a significantly higher rate of diuretic use in the treatment group.
The majority of BAT patients (93%) had pre-existing cardiac devices for heart rhythm management. Of the 69 patients assigned to the control group who reached their activation date, 15 did not complete 6 months of follow-up – four patients died, five with drew consent, three were lost to follow-up and three missed the visit. Of the 71 patients implanted with the BAT system reaching their activation date, 7 did not complete the 6 months of follow-up – five died and two withdrew consent.
Of note, during the 6 months prior to enrollment, there was an apparent difference between groups in the annualized rate of HF hospitalization and the average number of days hospitalized for HF, especially in USA (p = 0.08 and p = 0.05, respectively). Globally, there was a significant reduction in the rate of HF hospitalization from pre- to postenrollment in the treatment group (0.63 ± 1.5 to 0.14 ± 0.5 hospitalization/ patient/year, p = 0.01), with no change seen in the control group (0.36 ± 1.1 to 0.31 ± 0.97 hospitalizations/ patient/year, p = 0.85).
However, the between-group difference in the post-randomization rate of HF hospitalization did not reach statistical significance (p = 0.35). The effect of BAT on the average number of days hospitalized for HF followed a similar pattern of significance and nonsignificance. However, the between-group difference in postrandomization days spent in-hospital for HF demonstrated a trend favoring the treatment group (p = 0.08).
The overall data of the controlled trial confirmed the BAT benefit on sparing hospital resources observed in the preliminary mechanistic study, but it was able to add more meaningful information on BAT efficacy. Importantly NT-pro-BNP, reported as median (interquartile range), was reduced in the treatment group while it increased in the control group, with a significant between-group difference (-69.0 pg/ml [-504 pg/ml, 198 pg/ml] vs 129.0 pg/ml [-67 pg/ml, 619 pg/ml], p = 0.02). This drop is a specific indicator of BAT positive effect on patient biology and hemodynamic balance and it was associated to highly significant improvement of others study end points such as NYHA f. Cl. (decrease in 77% of patients; p < 0.002), 6MHW distance (59.6 ± 14.1 m; p = 0.004) and Minnesota QoL Leaving with HF score (-17.4 ± 2.8; p < 0.001), thus mirroring the positive impact of BAT on patient daily life [24].
It has also to be stressed that the magnitude of improvement of 6MHW in BAT studies is well beyond the possible expected change in a placebo group [28].
Moreover in BAT controlled study, the QoL score improvement was superior to the one achieved in the MIRACLE ICD trial [29],
In this study the QoL improved in the control group by 11 points, with 95% CI: 6–16 points. Thus, the average effect observed in the present study (-17.4 points) exceeds the 95th percentile of the control group distribution of the MIRACLE ICD trial and it is actually identical to the effect observed in the active treatment group.
It is worth noting that, prior to the BAT controlled study, the only device therapy that was able to reduce NT pro-BNP level in advanced HF patients was the CRT as addressed in the Cardiac Resynchronization- Heart Failure trial. In this study, the BNP decrease after 3 months from implantation closely resembles the one achieved in the BAT HF study and it is coupled with comparable improvement in LVEF [30]. A further analogy with CRT is also the efficacy of BAT in reducing the adrenergic drive.
A relevant complimentary finding of the study was the upward trend of systolic blood pressure in the treatment group, while, on the opposite, systolic blood pressure trended downward in control patients. This difference in effect of BAT on systolic BP reached statistical significance (8.5 ± 3.8 mmHg, p = 0.03), while pulse pressure also demonstrated a significant increase (9.6 ± 3.2 mmHg, p = 0.004). The increasing in systolic pressure coupled with a nonsignificant trend toward LVEF improvement in the BAT group and a slight reduction in the control group, with a nonsignificant between-group difference of 2.5 ± 1.7% (p = 0.15), suggests a possible gain in cardiac pump performance. These study data match the clinical benefit addressed by the decrease of HF hospitalizations observed in BAT pilot study. They also mirror the benefits achieved in the treated arm of the cardiac resynchronization trial comparison of medical therapy, pacing, and defibrillation in heart failure [COMPANION 31], also performed in advanced HF patients. It is noteworthy to recognize that low systolic pressure is a powerful marker of poor prognosis in HFrEF patients [32] and the systolic pressure increase, achieved without increasing cardiac oxygen consumption, is an appropriate indicator of better heart performance that correlates with more positive patient outcome as obtained in patient with resynchronization therapy [33].
A recently published post hoc analysis on controlled study data also points out to a prominent aspect of BAT benefit in HF patients [34]. Among the 71 patients that underwent BAT activation, 24 have had received already CRT. The study was not powered to evaluate the clinical outcome, but, the effect of BAT in the no- CRT and in CRT patients on the rate of HF hospitalization and on the average number of days hospitalized for HF was explored for future research purpose. A significant reduction in both the rate of HF hospitalization and the average number of days hospitalized for HF from pre- to post-enrollment was present in no-CRT patients. The lack of an evident effect in the CRT-treated patients was concerning but the clinical and instrumental profile of this subgroup described a picture of nonresponders mostly as NYHA class was III and LVEF averaged around 23%. These clinical data are consistent with improvement in NT pro-BNP concentration and are not biased by potential placebo effect between the two groups. On the other hand, the limited number of CRT patients included in the Zile’s analysis does not allow to draw conclusive remarks on BAT inefficacy in patients that already received CRT, but highlights the possibility of a selective targeting of the patient population with the highest likelihood of responding the BAT.
On note the basic difference between CRT and BAT is that CRT decreases the sympathetic activity by providing LV function recovery whereas BAT works directly on symaptho/vagal balance with obvious, and now documented, consequences on cardiac function. Given their different mechanisms of action, CRT and BAT might become, complementary tools to manage selected HF patients.
Another question left open about BAT was its persistency. The long lasting benefit of BAT in resistant hypertensive patients was well assessed in the recent study performed in the long-term follow-up of Rheos pivotal trial [35] and this positive data boosted the need to confirm the long-term persistence of BAT benefit in HF patients. The question was not appropriately addressed in the most recent studies all designed upon a 6 months follow-up.
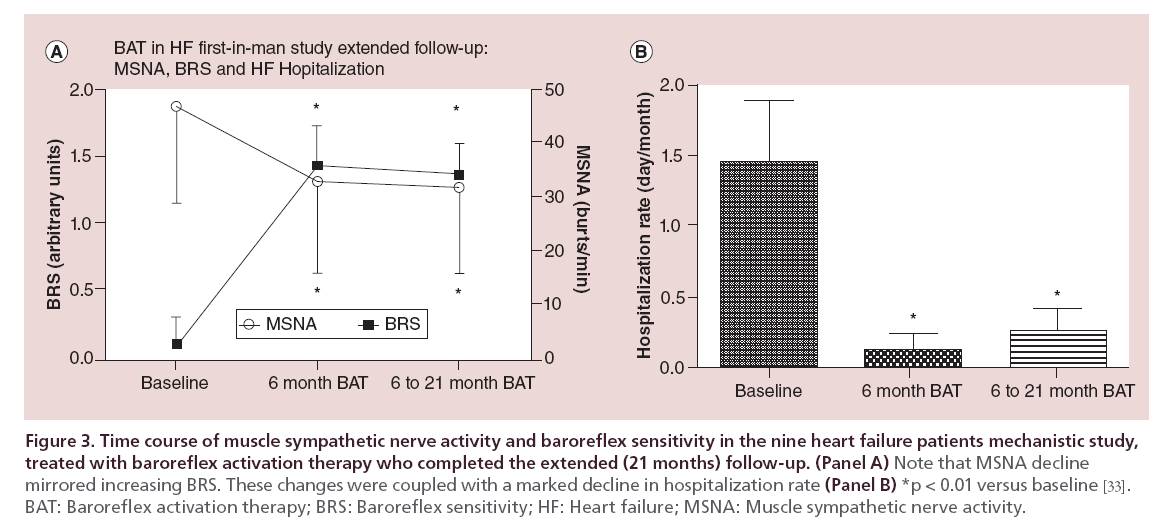
Aiming at this goal, microneurography assessment of MSNA was repeated in the nine survivors of the mechanistic study at 21 months, in the extended follow-up research. The MSNA and the calculated baroreceptor sensitivity closely mirrored the data obtained at 6 months follow-up, confirming the persistent power of BAT in restraining the sympathetic nerve activation and restoring baroreflex sensitivity (Figure 3, panel A). These data were paralleled by a long lasting clinical benefit in terms of HF hospital staying. It is noteworthy to observe, indeed, that the hospitalization rate measured as days/month decreased from 1.44 + 1.3 preimplant to 0.13 + 0.33 in the 6 months postactivation and was 0.27 + 0.44 between 6 and 21 months (p < 0.01, Figure 2, panel B) [36].
Figure 3: Time course of muscle sympathetic nerve activity and baroreflex sensitivity in the nine heart failure patients mechanistic study, treated with baroreflex activation therapy who completed the extended (21 months) follow-up.
On note the outstanding safety profile of BAT has been concordantly addressed in all the performed studies either in hypertensive or HF patients and particularly no cardiovascular adverse events occurred within the 30 days postimplant observation time window.
Note #1. The system for delivering BAT (Barostim neo system, CVRx, Inc., MN, USA) consists of a generator of pulse and a carotid sinus lead. The lead comprises a 40–cm lead that connects a circular backer 7 mm in diameter with a 2 mm iridium system implantation is generally performed by a vascular surgeon. The pulse generator is implanted in a pacemaker like prepectoral pocket. The sinus region is mapped by temporarily placing the electrode in various locations and applying electrical stimulation to determine the location with greatest sensitivity to BAT. Figure 1 addresses CVRx Barostim neo™ system allocation and a scheme of the overall cardiovascular effect provided by the electrical activation of carotid sinus. Sensitivity is measured by observing hemodynamic changes associated with acute baroreflex activation, namely, reductions in HR and/or BP associated with increased parasympathetic traffic and/or decreased sympathetic traffic, respectively. With the correct position identified, the electrode is directly affixed by applying sutures around the perimeter of the electrode backer, through the backer and adventitia. The therapy is initiated at a moderate level in the absence of side effects such as excessive reductions in HR or BP. At later follow-up visits, therapy levels are increased as the patient is able to tolerate higher doses, with the objective of achieving full titration at around 3 months. On average, pulse amplitude upon activation ranges between 4.5 ± 2.5 mA and steadily increased to reach 6.8 ± 2.4 mA at 3 months, or more in some cases, remaining stable thereafter. The eventfree rate of all system- and procedure-related major adverse neurological and cardiovascular events in the controlled study was 97.2%, lower 95% confidence bound 91.4% [32].
The device currently average battery life is 30 months. New technology next available will extend average battery life up to 5 years.
Conclusion & future perspective
Current evidence does not allow any definitive conclusion to be drawn on BAT efficacy in advanced HF patients. It is worth noting, though, that the data generated in experimental and human studies are consistent in describing a persistent high cardiac sympathetic drive in most cases even in optimally treated HF patients, a condition predisposing to adverse outcome [18]. Data from first human study prove that BAT may successfully diminish the excessive sympathetic tone over the long term.
All the evidences concordantly address that BAT clinical benefit is coupled with an improvement of metrics that currently describe HF severity and that, such improvement portends benefits on top of current optimized medical treatment. In the multicenter controlled study a significant increase of the arterial systolic and pulse pressure was achieved in treated patients together with a 6 MHW distance improvement beyond the limit of the placebo effect. These clinical effects were coupled with a relevant decrease of NT pro-BNP concentration and a LVEF increase.
A significant decrease of hospital resources utilization also occurred among patients in the treated arm. It is, however, somewhat unexpected and still matter of scrutiny that most of the described improvements occurred in NYHA f. Cl. III patient that did not qualify for CRT in comparison to same NYHA f. Cl. patients that already received CRT.
Also, so far, BAT appeared safe both in the implant phase as well when therapy is active. The current major limitation to the widespread use of BAT stands in the need of implanting a second battery operated device as patients currently eligible to this therapy are affected by HFrEF and, thus almost always, already carrying an ICD. Another limitation might be encountered when dealing with a patients with neurological disorders affecting the reflexogenic loop or with untreatable vascular diseases at the carotid level. Thus far no such cases have been described in current trails but this matter deserves specific targeted sub-studies. Overall, larger studies are obviously to be performed before reaching a comprehensive understanding of benefit and limitations of BAT.
On this perspective a next large trial aiming to assess BAT clinical benefit will be based on a multicenter randomized controlled study design with the combined end point of mortality plus hospital admissions, with an open design where ∼600 patients will be randomized to device therapy or control and followed for a minimum of 18 months. The end points committee will adjudicate events in a blinded fashion.
Beyond the hurdle
ANS alterations, resulting in sympathetic prevalence, are the key mechanisms of HF development and unfavorable progression. Such a fundamental concept was clear and documented since the 70s but neglected for some 30 years. Thus, it is no surprise that the same time span needed to reach the moon after the first transatlantic flight was needed for the β-blockers from being absolutely contraindicated to become first line therapy in HF. This misunderstanding in HF pathophysiology delayed the research investments in this area and diverged them with the weak hope that the solution of the problem stood in the inotropic support to the cardiac muscle or in the block of an ion channel. We are now beyond this hurdle, far later than it should have been, but the current research in electrical modulation of the nervous cardiac autonomic input/output is attracting the right attention and investments that it deserves, with the concrete hope that the best has yet to come.
Financial & competing interests disclosure
E Gronda has received research grant support from CVRx. E Gronda and E Vanoli are presently paid consultants or advisors of CVRx, Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Background
• Sympathetic activation often accompanies ischemic heart disease from its first manifestation. When this happens progression of the ischemic disease into left ventricular dysfunction and overt heart failure (HF) is very likely to occur.
• Elevated cardiac sympathetic drive is predominant element that increases morbidity and mortality in HF patients.
• The critical aspect of this pathophysiologic aspect of HF that has been misunderstood for decades is that sympathetic hyperactivity is NOT the mere consequence of a loss of left ventricular performance but it is rather the consequence of an exaggerated afferent excitatory information originating from the heart itself and from the entire cardiorespiratory and muscular apparatus.
• Lack of understanding of the pathophysiologic autonomic process in HF has lead to the denial of the β-blockers use in this setting under the wrong belief that restoration of cardiac performance, by stimulating the residual functional myocardium, would have ameliorated the autonomic balance and, consequently, prognosis. As matter of fact, such an approach improved temporarily quality of life together, however, with an increase in mortality and it was eventually abandoned.
• After decades of this denial, β-blockers are now first line therapy in HF. However, the intrinsic limit of β-adrenergic receptors blockade is that it operates at the cardiac level only, leaving untouched the neural reflex mechanisms that are the primary cause of this elevated sympathetic drive the heart. This might explain the currently observed recurrence of morbidity and mortality at 10–15 years from the initial manifestation despite an optimal neurohumoral therapy.
Rationale & applications of baroreflex activation therapy in HF management
• Baroreflex activation therapy (BAT) provides an effective and physiological intervention to inhibit centrally sympathetic cardiac drive by augmenting afferent vagal input into the system.
• Analysis of the baroreflex control of heart rate (baroreflex sensitivity) has a strong predictive value in HF.
• The theory of BAT efficacy has been fostered by a pilot study in 11 patients in which BAT chronically diminished sympathetic activity and, as clinical correlate, dramatically reduced the need for hospitalization (- 80%).
• Such pilot information has been recently confirmed by a controlled multicenter study involving 140 patients with NYHA functional Class III HF.
• In this study BAT implemented indexes of quality of life and physical performance and reduced hospitalization rate.
• The long lasting benefit of BAT in resistant hypertensive patients was well assessed in the recent study performed in the longterm follow-up of Rheos pivotal trial and this positive data boosted the need to confirm the long-term persistence of BAT benefit in HF patients.
• Aiming at this goal, microneurography assessment of muscle sympathetic nerve activity was repeated in the nine survivors of the mechanistic study at 21 months, in the extended follow-up research. The muscle sympathetic nerve activity and the calculated baroreceptor sensitivity closely mirrored the data obtained at 6 months follow-up, confirming the staying power of BAT in restraining the sympathetic nerve activation. This data were paralleled by a long lasting clinical benefit in term of survival free from HF hospital admission: the hospitalization rate measured as days/month decreased from 1.44+ 1.3 preimplant to 0.13+ 0.33 in the 6 months postactivation and was 0.27 + 0.44 between 6 and 21 months (p < 0.01).
• The pilot study data found a convincing confirmation in a controlled multicenter study of 140 advanced HF patients.
Future perspective
• Thus far BAT might represent an effective tool able to control sympathetic hyperactivity at its origin, providing then an effective therapeutic action to the entire cardiovascular system.
• BAT appeared safe both in the implant Phase as well when therapy is active. The current major limitation to the widespread use of BAT stands in the need of implanting a second battery operated device as patients currently eligible to this therapy are affected by HFrEF and, thus almost always, already carrying an implantable cardioverter defibrillator. Another limitation might be encountered when dealing with a patients with neurological disorders affecting the reflexogenic loop or with untreatable vascular diseases at the carotid level. Thus far no such cases have been described in current trails but this matter deserves specific targeted substudies. Overall, larger studies are obviously to be performed before reaching a comprehensive understanding of benefit and limitations of BAT. On this perspective, a next large trial aiming to assess BAT clinical benefit will be based on a multicenter randomized controlled study design with the combined end point of mortality plus hospital admissions, with an open design where approximately 600 patients will be randomized to device therapy or control and followed for a minimum of 18 months. The end points committee will adjudicate events in a blinded fashion.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- Barsheshet A, Moss AJ, Eldar M et al. Time-dependent benefit of preventive cardiac resynchronization therapy after myocardial infarction. Eur. Heart J. 32(13), 1614–1621 (2010).
- Taylor AL, Zieshe S, Yancy C et al. African American Heart Failure Trial Investigators. Combination of isosrbide dinitrarìate and hydralazine in blacks with heart failure. N. Engl. J. Med. 351, 2049–2057 (2004).
- Massie BM, Swedberg K, Cohn JN. Is neurohormonal activation deleterious to the long-term outcome of patients with congestive heart failure? J. Am. Coll. Cardiol. 12, 547–558 (1988).
- Waagstein F, Hjalmarson A, Varnauskas E, Wallentin I. Effect of chronic beta-adrenergic receptor blockade in congestive cardiomyopathy. Br. Heart J. 37, 1022–1023 (2004).
- Schron EB, Exner DV, Yao Q et al. Quality of life in the antiarrhythmics versus implantable defibrillators trial: impact of therapy and influence of adverse symptoms and defibrillator shocks. Circulation 105, 589–594 (2002).
- Hamdan MH, Zagrodzky JD, Joglar JA et al. Biventricular pacing decreases sympathetic activity compared with right ventricular pacing in patients with depressed ejection fraction. Circulation 102, 1027–1032 (2000).
- Wang TJ, Evans JC, Benjamin EJ, Levy D, LeRoy EC, Vasan RS. Natural history of asymptomatic left ventricular systolic dysfunction in the community. Circulation 108(8), 977–982 (2003).
- Kaye DM, Lefkovits J, Jennings GL, Bergin P, Broughton A, Esler MD. Adverse consequences of high sympathetic nervous activity in the failing human heart. J. Am. Coll. Cardiol. 26, 1257–1263 (1995).
- Floras JS. Clinical aspects of sympathetic activation and parasympathetic withdrawal heart failure. J. Am. Coll. Cardiol. 22, A72–A84 (1993).
- Cohn JN, Levine TB, Olivari MT et al. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart fail. N. Engl. J. Med. 311(13), 819–823 (1984).
- Floras JS. Sympathetic nervous system activation in human heart failure. Clinical implications of an updated model. J. Am. Coll. Cardiol. 54, 375–385 (2009).
- Floras JS. Arterial baroreceptor and cardiopulmonary reflex control of sympathetic outflow in human heart failure. Ann. NY Acad. Sci. 940, 500–513 (2001).
- Ando S, Dajani HR, Senn BL, Newton GE, Floras JS. Sympathetic alternans. Evidence for arterial baroreflex control of muscle sympathetic nerve activity in congestive heart failure. Circulation 95, 316–319 (2001).
- Grassi G, Seravalle G, Bertinieri G, Stella ML, Turri C, Mancia G. Sympathetic response to ventricular extrasystolic beats in hypertension and heart failure. Hypertension 39, 886–891 (2002).
- Ando S, Dajani HR, Floras JS. Frequency domain characteristics of muscle sympathetic nerve activity in heart failure and healthy humans. Am. J. Physiol. 273, R205–R212 (1997).
- Bradley TD, Tkacova R, Hall MJ, Ando S, Floras JS. Augmented sympathetic neural response to simulated obstructive sleep apnoea in human heart failure. Clin. Sci. 104, 231–238 (2003).
- Newton GE, Parker JD. Cardiac sympathetic responses to acute vasodilation: normal ventricular function versus congestive heart failure. Circulation 94, 3161–3167 (1996).
- La Rovere MT, Pinna GD, Maestri R, et al. Prognostic implications of baroreflex sensitivity in heart failure patients in the beta-blocking era. J. Am. Coll. Cardiol. 53(2), 193–199 (2008).
- Shin J, Jhonson JA. Beta-blocker pharmaco-genetics in heart failure. Heart Failure Rev. 15, 187–196 (2010).
- Gronda E, Seravalle G, Brambilla G et al. Chronic baroreflex activation effects on sympathetic nerve traffic, baroreflex function, and cardiac haemodynamics in heart failure: a proof-of-concept study. Eur. J. Heart Fail. 16, 977–983 (2014).
- Vanoli E, De Ferrari GM, Stramba-Badiale M, Hull SS Jr, Foreman RD, Schwartz PJ. Vagal stimulation and prevention of sudden death in conscious dogs with a healed myocardial infarction. Circ. Res. 68(5), 1471–1481 (1991).
- Zhang Y, Popovic ZB, Bibevski S et al. Chronic vagus nerve stimulation improves autonomic control and attenuates systemic inflammation and heart failure progression in a canine high rate pacing model. Circ. Heart Fail. 2, 692–699 (2009).
- Hamann JJ, Ruble SB, Stolen C et al. Vagus nerve stimulation improves left ventricular function in a canine model of chronic heart failure Eur. J. Heart Fail. 15(12), 1319–1326 (2013).
- Tracy KJ. The inflammatory reflex. Nature 420, 853–859 (2002).
- Calvillo L, Vanoli E, Andreoli E et al. Vagal stimulation, through its nicotinic action, limits infarct size and the inflammatory response to myocardial ischemia and reperfusion. J. Cardiovasc. Pharmacol. 58, 500–507 (2011).
- Johansson M, Gao SA, Friberg P et al. Baroreflex effectiveness predicts all cause mortality and sudden death in hypertensive patients with chronic renal failure. J. Hypertens. 25, 163–168 (2007).
- Abraham WT, Zile MR, Weaver FA et al. Baroreflex activation therapy for the treatment of heart failure with a reduced ejection fraction. J. Am. Coll. Cardiol. 3(6), 487–496 (2015).
- Olsson L, Swedberg GS, Clark AL, Witte KK, Cleland JGF. Six minute corridor walk test as an outcome measure for the assessment of treatment in randomized, blinded intervention trials of chronic heart failure: a systematic review Eur. Heart J. 26, 778–793 (2005).
- Young JB, Abraham WT, Smith AL et al. Multicenter InSync ICD Randomized Clinical Evaluation (MIRACLE ICD) Trial Investigators. Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: the MIRACLE ICD Trial. JAMA 289(20), 2685–2694 (2003).
- Cleland JG, Daubert JC, Erdmann E et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N. Engl. J. Med. 352(15), 1539–1549 (2005).
- Bristow MR, Saxon LA, Boehmer J et al. Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Investigators. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N. Engl. J. Med. 350(21), 2140–2150 (2004).
- Ather S, Chan W, Chillar A et al. Association of systolic blood pressure with mortality in patients with heart failure with reduced ejection fraction: a complex relationship. Am. Heart J. 161(3), 567–573 (2011).
- Nelson GS, Berger RD, Fetics BJ et al. Left ventricular or biventricular pacing improves cardiac function at diminished energy cost in patients with dilated cardiomyopathy and left bundle-branch block. Circulation 102(25), 3053–3059 (2000).
- Zile MR, Abraham WT, Weaver FA et al. Baroreflex activation therapy for the treatment of heart failure with a reduced ejectionfraction: safety and efficacy in patients with and without cardiac resynchronizationtherapy. Eur. J. Heart Fail. doi:10.1111/ejhf.299 (2015)(Epub ahead of print).
- Bakris GL, Nadim MK, Haller H, Lovett EG, Schafer JE, Bisognano JD. Baroreflex activation therapy provides durable benefit in patients withresistant hypertension: results of long-term follow-up in the Rheos. Pivotal Trial. J. Am. Soc. Hypertens. 6, 152–158 (2012).
- Gronda E, Seravalle G, Quarti Trevano F et al. Long-termchronic baroreflex activation: persistentefficacy in patients with heart failure and reduced ejection fraction. J. Hypertens. 33, 1704–1708 (2015).
•• Provides the integrative model of sympathetic nervous system activation in chronic human heart failure (HF) that has direct implications for both disease progression and survival.
• Addresses in detail all tests that proved baroreflex remains present and can work effectively in HF patients.
• Demonstrated for the first time the lack of HF prognosis relationship between β-blocker therapy and decreased baroreflex sensitivity.
•• First in man study that proved baroreflex activation therapy (BAT) can provide significant muscle sympathetic nerve activity decrease in NYHA functional Class III, HF patients coupling significant clinical improvement.
• First animal study providing evidence that vagal activity can effectively increase the fibrillatory threshold in the scarred myocardium, decreasing arrhythmia susceptibility.
•• BAT pivotal controlled study that provides evidence on BAT clinical effect confirming clinical aspects observed in the first in man study (see [20]).
• Confirms the long lasting positive action of BAT on muscle sympathetic nerve activity and on baroreceptor sensitivity coupled with persisting HF clinical stability.