Review Article - Interventional Cardiology (2010) Volume 2, Issue 2
Benchside testing of drug-eluting stent surface and geometry
- Corresponding Author:
- Clemens von Birgelen
Department of Cardiology
Haaksbergerstraat 55
7513ER Enschede, The Netherlands
Tel: +31 534 872 151
Fax: +31 534 872 152
E-mail: c.vonbirgelen@mst.nl
Abstract
Keywords
benchside testing, biodegradable polymer, coating irregularity, drug-eluting stent, durable polymer, kissing balloon, micro-computed tomography, postdilatation, postdilation, scanning electron microscopy, stent geometry, stent surface, stent thrombosis, vessel phantoms
Drug-eluting stents (DES) are effective in reducing restenosis and morbidity in patients undergoing percutaneous coronary interventions [1–4]. However, there are still ongoing discussions with regards to late and very late DES thrombosis, prolonged use of dual antiplatelet therapy following DES implantation, and potential differences between DES types in their suppression of neointimal proliferation and, hence, in prevention of restenosis and reintervention [5–8]. In addition, there may be differences between DES types with regard to their suitability for treatment of certain critical lesions (e.g., left main bifurcations) or use in certain clinical scenarios (e.g., ST-elevati on myocardial infarctions).
The physical properties of DES coatings and stent platforms and the interaction of DES with the local vascular anatomy and streaming blood may, first, contribute to some DES-related problems, and second, may partly explain differences in the clinical performance of different DES types. In conditions of delayed stent endothelialization, such as those observed with DES [7,9], morphology and structure of the stent surface will be of particular importance as the stent surface is in prolonged contact with blood. Since some types of DES occasionally exhibit spots of incomplete re-endothelialization [10,11], coarse irregularities of the DES coating at such spots could play a role in promoting stent thrombosis – in particular once the period of dual platelet therapy is finished.
Benchside testing of contemporary DES types may help to clarify some aspects of their clinical performance. In novel DES, which are supposed to add advantages above the contemporary DES [12], it may be prudent to carefully examine such stents with more challenging percutaneous coronary intervention scenarios in mind. With growing acceptance and increasing use, DES are more often implanted for so-called off-label indications, which have been estimated to constitute up to 60% of all percutaneous coronary intervention procedures with DES [12,13]. For this reason, it makes sense to test the boundaries of coatings and stent platforms of various DES during rigorous benchside testing, which implies the assessment of DES following gentle deployment as well as after more aggressive maneuvers.
In this article, we shed light on currently used techniques for thorough benchside assessment of the contemporary commercially available DES surface and geometry and the results obtained from such examinations, and we discuss the potential implications of such findings.
Surface of DES
▪ Principal function & types of DES coatings
Since antiproliferative drugs do not adhere to smooth metallic stent surfaces, there is a need for technical approaches that load the antiproliferative drug on the metallic stent platform. The application of certain polymer coatings on the metallic stent platform (on entire stent or just abluminal surface) allows binding of the drug with favorable and easily modifiable release kinetics. Therefore, in most DES the homogeneous smooth metallic surface of the bare-metal stent platform is partially or totally covered by a coating. Most current DES have a coating that is composed of a mixture of an antiproliferative drug and a polymer. This coating layer has a somewhat variable texture while its surface (owing to delayed endothelialization) is exposed to blood for a relatively long period of time.
▪ Types of polymers used in current DES coatings
Polymers can be classified as natural versus synthetic polymers. Synthetic polymers are preferred for use in coronary DES owing to their proven efficacy. In addition, polymers used for coronary application are classified into durable polymers (also termed nonerodible, permanent or biostable) versus biodegradable polymers. In the latter, the polymer is slowly hydrolyzed into monomers that can be further metabolized in vivo. Durable polymers with complete coverage of the metallic stent platform were the first successful vehicles for drug-loading and release. As a consequence, at present, the four US FDA-approved DES utilize durable polymer coatings [14].
▪ Which requirements do DES coatings have to meet?
Besides the ability to contain a drug and control its release, DES coatings should be biocompatible. The term biocompatibility is defined as the property of acting with an appropriate host response. While for certain implants in the human body the requirements may be less problematic, vascular implants have to meet more challenging requirements. DES should not elicit an exaggerated inflammatory response in adjacent tissue. In addition, a relatively low thrombogenicity of the DES surface is required owing to its prolonged contact with blood [15,16]. As cardiac motion leads to repetitive cyclic movement of the coronary arteries with various types of stress inside the vascular wall, intracoronary endoprostheses require particularly favorable long-term mechanical properties (e.g., fatigue life, wear resistance and kink resistance) [17]. In addition, the physical integrity of an evenly distributed DES coating may be important as coating irregularities or defects may disturb drug elution kinetics and may affect the neointimal response.
▪ The other side of the coin: possible coating-related problems
Current DES meet the aforementioned demands upon polymeric DES coatings to a large extent, which explains the great clinical success of DES. However, there are some unfavorable aspects that may be partly related to the polymer component of DES, including, DES thrombosis, hypersensitivity reaction to DES and embolization of fragments of the DES coating [18,19]. FDA reports analyzed by Nebeker et al. suggested that DES can provoke hypersensitivity reactions that were mainly observed in sirolimus-eluting stents coated with a poly[ethylene-co-vinyl acetate]/ poly[n-butyl methacrylate] (PEVA/PBMA) copolymer [20]. An exaggerated inflammatory response to DES – leading to DES thrombosis, myocardial infarction and sometimes death – has been interpreted as a manifestation of Kounis syndrome, particularly if associated with allergic reactions [21]. In addition, in aspirates from very-late DES thrombosis, eosinophilic infiltrates were found and inflammation scores were high [22].
DES coating irregularities
▪ DES coating irregularities & potential clinical implications
Owing to the presence of a polymer/drug coating, the surface of DES may be less smooth and could exhibit some irregularities, roughness or defects that are generally absent on the surface of bare-metal stents manufactured with modern technologies. Theoretically, DES surface irregularities (or defects) may be related to unfavorable clinical outcomes through various mechanisms; first, decreased thickness or absence of the DES coating may locally decrease the antirestenotic potential of the DES; second, displacement of coating with or without embolization of fragments (of a relevant size) may lead to (micro)vascular obstruction and periprocedural myocardial necrosis; and third, an increased roughness of the DES surface can increase its thrombogenicity and may promote stent thrombosis [23]. Nevertheless, we cannot exclude the possibility that some mild coating irregularities of DES could occasionally have favorable side effects (e.g., mild roughness of a surface may promote endothelialization) [24].
▪ Imaging methods for assessment of DES coating irregularities
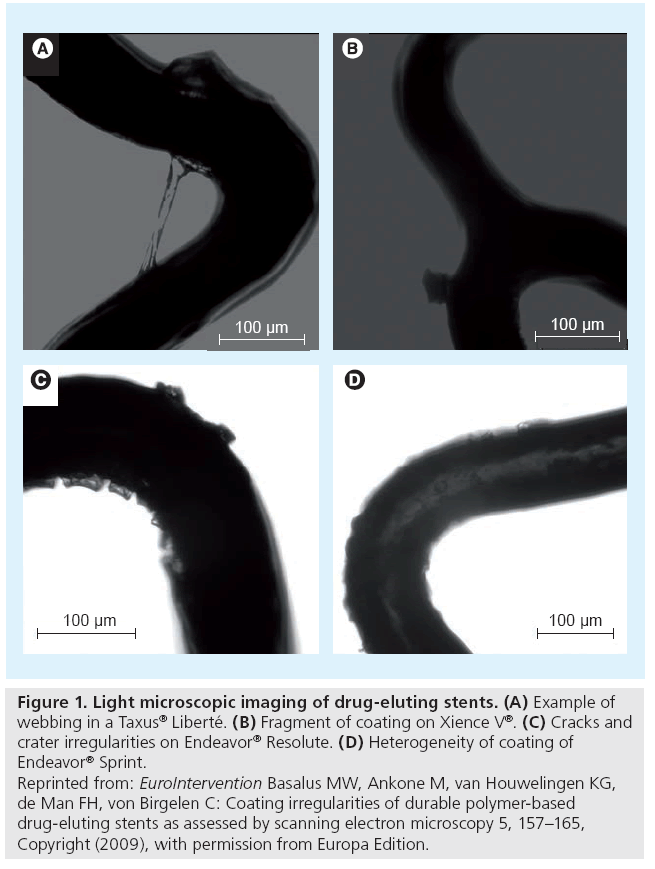
Coating irregularities can be examined by light microscopy (Figure 1). However, the 2D nature of light microscopic examination and light reflections limit the visualization of some DES coating irregularities and make the technique less suitable for quantification purposes. The limitations of light microscopy can be avoided by the use of scanning electron microscopy (SEM). Infrequently, other techniques for even higher magnification have been applied. Transmission electron microscopy, for example, can be used to study drug–polymer miscibility [25]; and further examinations on the nanoscale level could provide interesting information on porosity and mild roughness of the coating [26].
Figure 1: Light microscopic imaging of drug-eluting stents. (A) Example of webbing in a Taxus® Liberté. (B) Fragment of coating on Xience V®. (C) Cracks and crater irregularities on Endeavor® Resolute. (D) Heterogeneity of coating of Endeavor® Sprint. Reprinted from: EuroIntervention Basalus MW, Ankone M, van Houwelingen KG, de Man FH, von Birgelen C: Coating irregularities of durable polymer-based drug-eluting stents as assessed by scanning electron microscopy 5, 157–165, Copyright (2009), with permission from Europa Edition.
▪ SEM
The 3D character of SEM and the capacity to obtain highly magnified (up to 100,000-fold) images with a high resolution [27] enables the identification, classification and quantification of DES coating irregularities [28]. In the early pioneering studies, SEM was used for qualitative examination of several early generation DES [29,30]. We recently reported SEM findings of novel coating irregularities in a biolimuseluting biodegradable coating-based DES [31]. In addition, a thorough quantitative analysis of four durable polymer-based DES has been published, including details on incidence and dimensions of various coating irregularities [28].
▪ SEM-based classification of durable polymer-based coating irregularities
Scanning electron microscopy examination of DES revealed a heterogeneous collection of coating abnormalities with varying degrees of severity that ranged from mild to advanced [28,30,32]. In order to categorize them, a SEM-based classification of coating irregularities of durable polymerbased DES has been suggested [28]. Meanwhile, coating irregularities of biodegradable polymerbased, and additional durable polymer-based, DES have been defined with SEM [31].
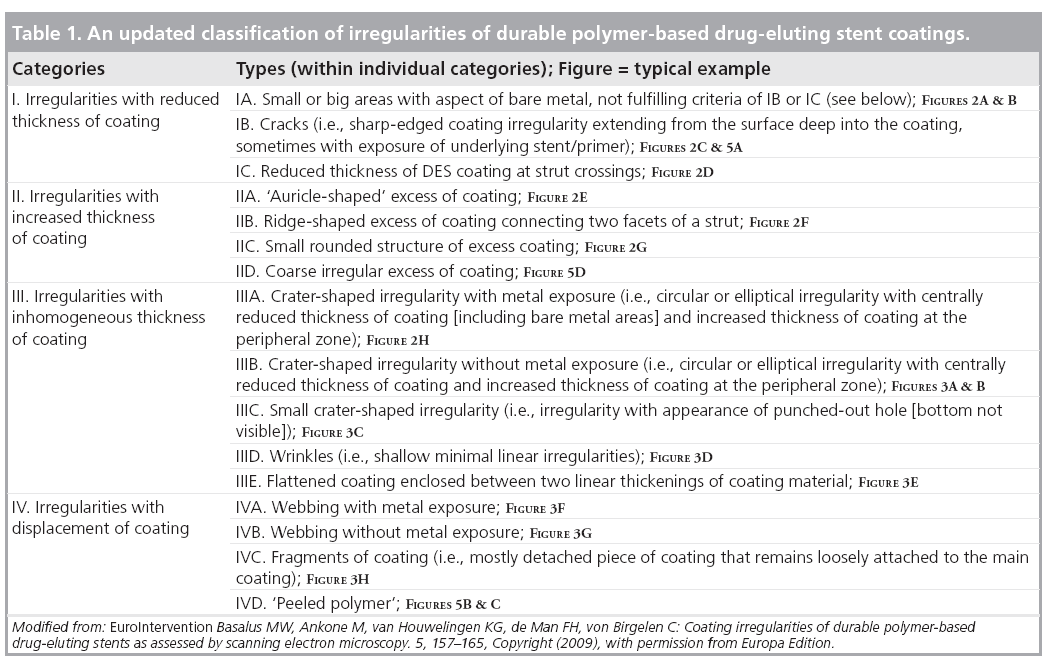
Table 1 presents an update on the SEM-based classification of DES coating irregularities as used by our group. This classification is relatively simple and based on both thickness and displacement of DES coating; it can be used for systematic comparison of contemporary DES with novel/future DES, and may be of some clinical relevance. According to this classification, the coating irregularities are classified into four main categories:
▪ Reduced coating thickness
▪ Increased coating thickness
▪ Inhomogeneous thickness of coating
▪ Displaced coating
Each of these categories was further subdivided into various types of irregularities.
Category I: reduced thickness of coating
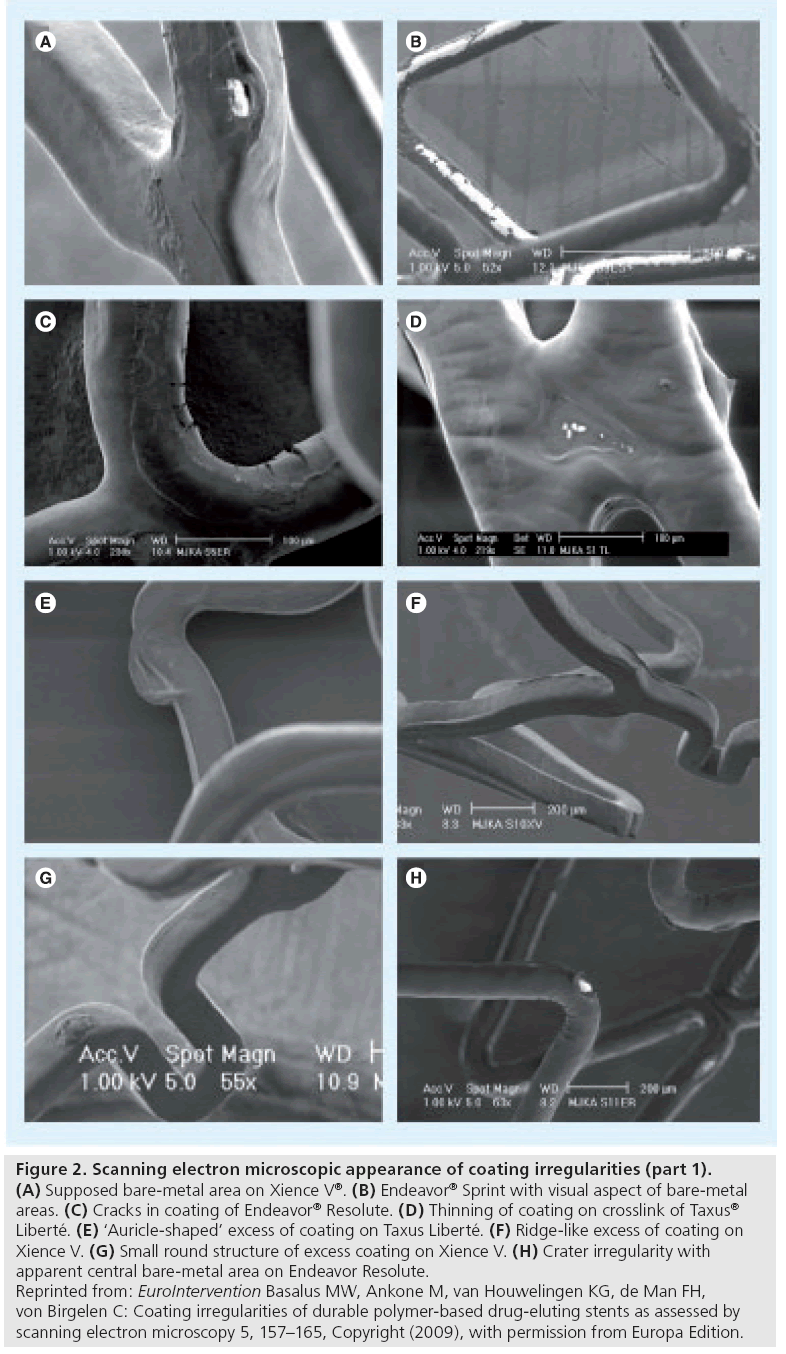
Such irregularities are characterized by a (local) reduction in thickness of the DES coating. Subclassification of irregularities into types was based on their characteristic shape (e.g., cracks; Figure 2C & H) or location (e.g., thinning of coating at strut crossings; Figure 2D). The most extreme form of irregularities from this category was present at sites with a visual aspect of bare metal (Figure 2A,2B). The great variability in size of the areas with bare metal aspects implied further division into small and large areas. A reduced thickness of coating could be related to a certain (local) reduction in antirestenotic potential, while a substantial increase in the roughness of the surface may be associated with some increase in thrombogenicity.
Figure 2: Scanning electron microscopic appearance of coating irregularities (part 1). (A) Supposed bare-metal area on Xience V®. (B) Endeavor® Sprint with visual aspect of bare-metal areas. (C) Cracks in coating of Endeavor® Resolute. (D) Thinning of coating on crosslink of Taxus® Liberté. (E) ‘Auricle-shaped’ excess of coating on Taxus Liberté. (F) Ridge-like excess of coating on Xience V. (G) Small round structure of excess coating on Xience V. (H) Crater irregularity with apparent central bare-metal area on Endeavor Resolute. Reprinted from: EuroIntervention Basalus MW, Ankone M, van Houwelingen KG, de Man FH, von Birgelen C: Coating irregularities of durable polymer-based drug-eluting stents as assessed by scanning electron microscopy 5, 157–165, Copyright (2009), with permission from Europa Edition.
Category II: increased thickness of coating
These irregularities were subdivided according to their shape. An increase in the amount of DES coating could theoretically result in a locally increased antiproliferative effect. To date, only relatively small irregularities of this category have been observed; accordingly, their clinical impact may be limited.
Category III: inhomogeneous thickness of coating
This is defined by a characteristic local heterogeneity in distribution of coating, showing areas of increased thickness next to areas with decreased thickness of coating. This may result in a less smooth texture of the DES surface, which could have consequences such as previously discussed.
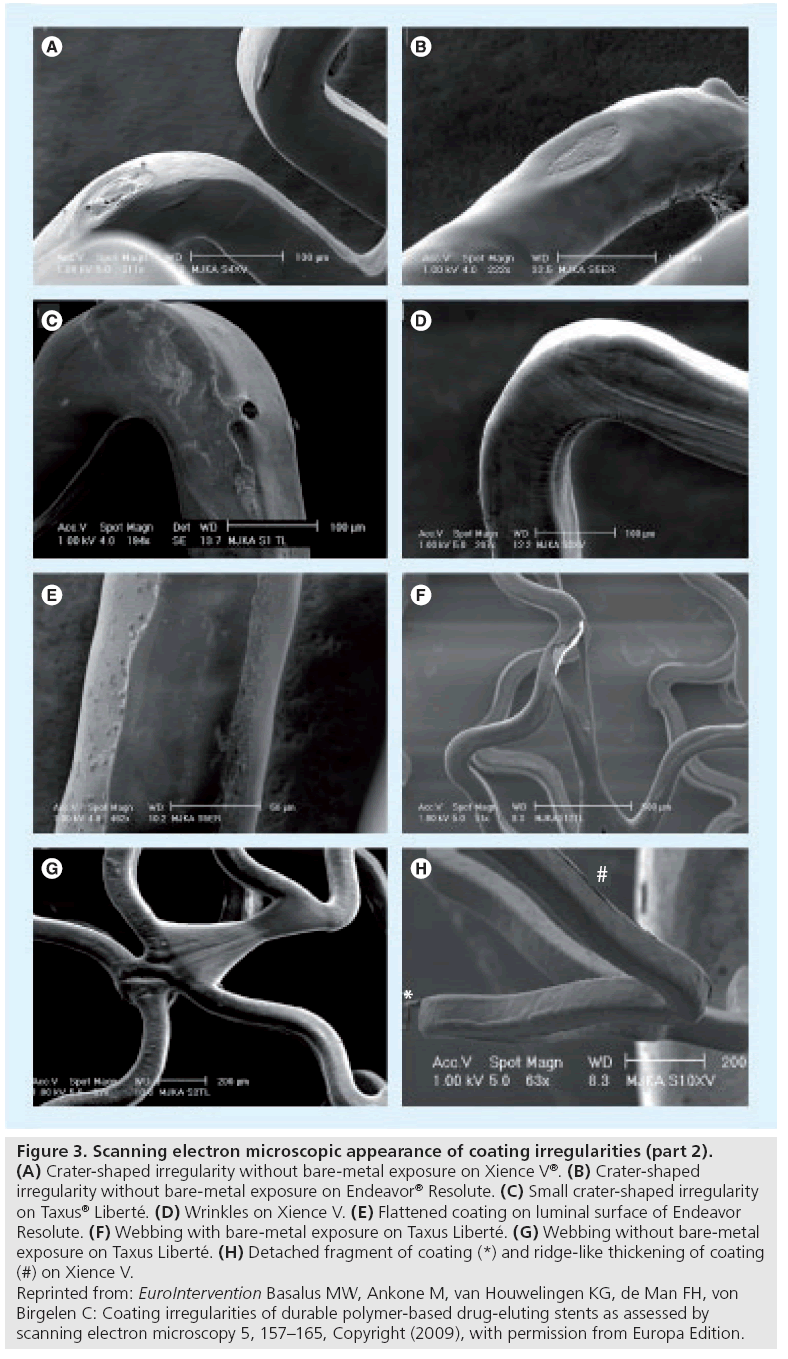
Category IV: displacement of coating
The main characteristic of these irregularities is the displacement of coating, which means that coating material is partly separated from the stent platform. Webbing (Figure 3F & G) is characterized by coating material that is connected between adjacent stent struts. Fragments are pieces of DES coating (Figure 3H) with partial or total loss of contact with the stent platform. The main concern about irregularities of this category is the possibility of (micro)vascular obstruction as a result of embolization of coating fragments and/or jailing of side branches behind webbings.
Figure 3: Scanning electron microscopic appearance of coating irregularities (part 2). (A) Crater-shaped irregularity without bare-metal exposure on Xience V®. (B) Crater-shaped irregularity without bare-metal exposure on Endeavor® Resolute. (C) Small crater-shaped irregularity on Taxus® Liberté. (D) Wrinkles on Xience V. (E) Flattened coating on luminal surface of Endeavor Resolute. (F) Webbing with bare-metal exposure on Taxus Liberté. (G) Webbing without bare-metal exposure on Taxus Liberté. (H) Detached fragment of coating (*) and ridge-like thickening of coating (#) on Xience V. Reprinted from: EuroIntervention Basalus MW, Ankone M, van Houwelingen KG, de Man FH, von Birgelen C: Coating irregularities of durable polymer-based drug-eluting stents as assessed by scanning electron microscopy 5, 157–165, Copyright (2009), with permission from Europa Edition.
▪ Coating irregularities after gentle deployment of durable polymer-based DES
Scanning electron microscope-based analysis of several currently available durable polymer-based DES has been performed, including a thorough examination of the incidence and dimensions of DES coating irregularities. We recently reported quantitative SEM analyses of four durable polymer- based DES following gentle deployment at 14 atm (Figure 4):
▪ Paclitaxel-eluting stents with styrene-B-isobutylene- B-styrene (SIBS) coating (Taxus® Liberté; Boston Scientific Corp., MA, USA);
▪ Zotarolimus-eluting stents with phosphorylcholine coating (Endeavor® Sprint; Medtronic Cardiovascular, CA, USA);
▪ Zotarolimus-eluting stents with Biolinx®- coating (Endeavor® Resolute; Medtronic Cardiovascular, CA, USA);
▪ Everolimus-eluting stents with a fluoropolymer (XIENCE V®; Abbott Vascular, CA, USA) [28].
Coating irregularities were found on all DES types, but the incidence and types of coating irregularities differed considerably.
Figure 4: Sample preparation and examination. (A) Stent deployment (care was taken to avoid dust contamination). (B) Scanning electron microscopic (SEM) examination of drug-eluting stent (DES); DES sample on the examination stage of SEM (insert). (C) Quantitative examination of coating irregularities.
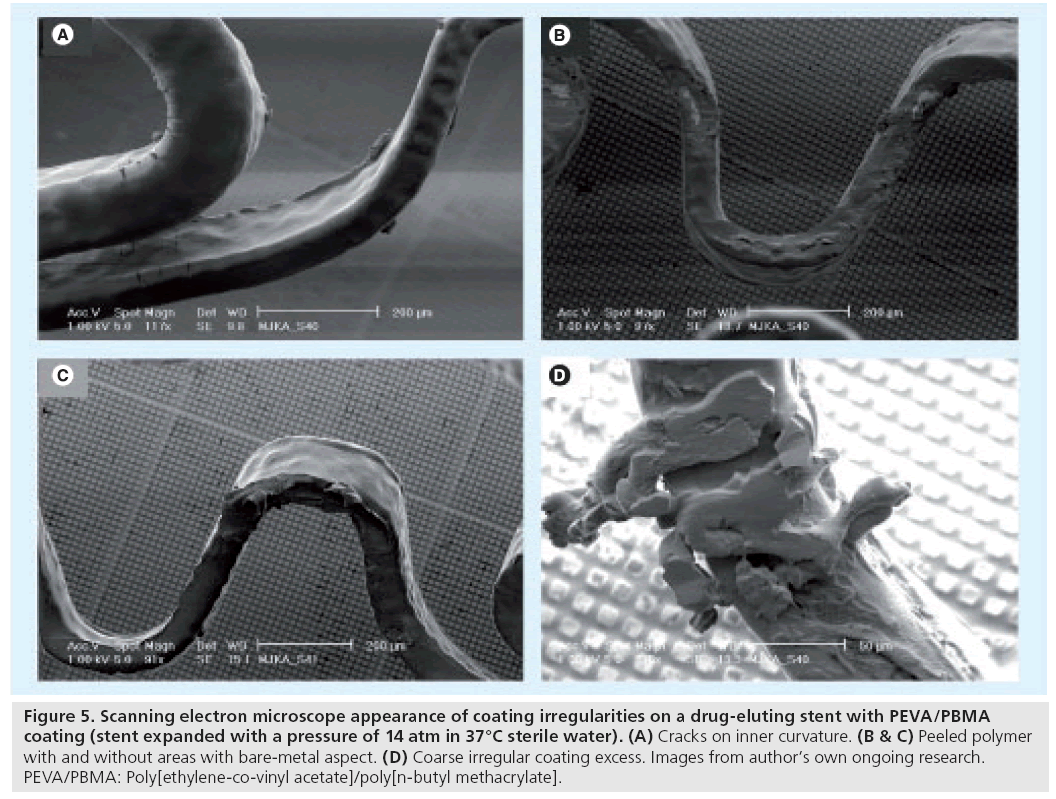
On these four types of durable polymer-based DES, on average a total of seven irregularities were observed within a SEM image at 60-fold magnification. All DES types had areas with a bare-metal aspect, but incidence, shape and size differed largely. In addition, each DES showed some characteristic irregularity that was observed exclusively or mainly on that DES type. Endeavour Sprint showed the largest bare-metal areas. Cracks were found in the coating of Endeavor Sprint and Endeavor Resolute, while wrinkles (Figure 3D) were only seen in Taxus Liberté and Xience V. Taxus Liberté showed no cracks but was the only DES that showed webbing with large bare-metal exposure (Figure 3F). Notably, some irregularities were located at characteristic stent locations exposed to increased mechanical stress. Some DES types even showed a characteristic pattern (distribution) of irregularities [28]. For example, on Endeavor Resolute there is a distinguishing combination of cracks on inner curvatures of stent loops, together with crater lesions on outer curvatures (Figure 2H). Meanwhile, we also obtained preliminary SEM data regarding irregularities on sirolimus-eluting stents with PEVA/PBMA copolymer-based coating (CYPHER SELECT Plus®; Cordis, NJ, USA), which showed cracks together with other irregularities (e.g., ‘peeled polymer’; Figure 5B & C, and coarse irregular excess of coating; Figure 5D). These SEM data from various DES suggest that there is some improvement in the morphology of DES coatings between early and newer generation DES. Differences in coating irregularities between different DES types may contribute to variations in vascular inflammation and fibrin deposits after implantation of various DES as observed in animal studies [33]. Animal studies also demonstrated a disparity in rate and completeness of endothelialization between various DES and, consequently, differences in the period of direct contact between blood and some coating irregularities (being shorter in newer DES) [34].
Figure 5: Scanning electron microscope appearance of coating irregularities on a drug-eluting stent with PEVA/PBMA coating (stent expanded with a pressure of 14 atm in 37°C sterile water). (A) Cracks on inner curvature. (B & C) Peeled polymer with and without areas with bare-metal aspect. (D) Coarse irregular coating excess. Images from author’s own ongoing research. PEVA/PBMA: Poly[ethylene-co-vinyl acetate]/poly[n-butyl methacrylate].
▪ Coating irregularities after postdilatation of durable polymer-based DES
During many percutaneous coronary intervention procedures, DES are postdilated to avoid stent malapposition. This step to optimize the result of DES implantation may be most valuable in lesions with substantial calcification, long stented segments, acute coronary syndromes and diabetic patients. In some cases, an even more aggressive (partial) postdilatation of the DES with an oversized high-pressure balloon is required. This approach is mainly used in DES implanted across major bifurcations with inherent significant vessel tapering, for example, left main bifurcations. Owing to the extreme stress posed on the DES coating, benchside examination of DES following aggressive postdilatation is reasonable. Our preliminary SEM analyses of coatings on durable polymer-based DES after aggressive partial postdilatation suggest that additional defects may occur.
Specific techniques and maneuvers used during bifurcation stenting (e.g., crush technique and kissing balloon inflation) pose an additional challenge to DES coatings, which may lead to an increased damage of the coatings in response to increased mechanical stress. This issue has been addressed previously with SEM in a semiquantitative fashion [35]; further quantitative analyses are warranted.
▪ Coating irregularities of biodegradable polymer-based DES
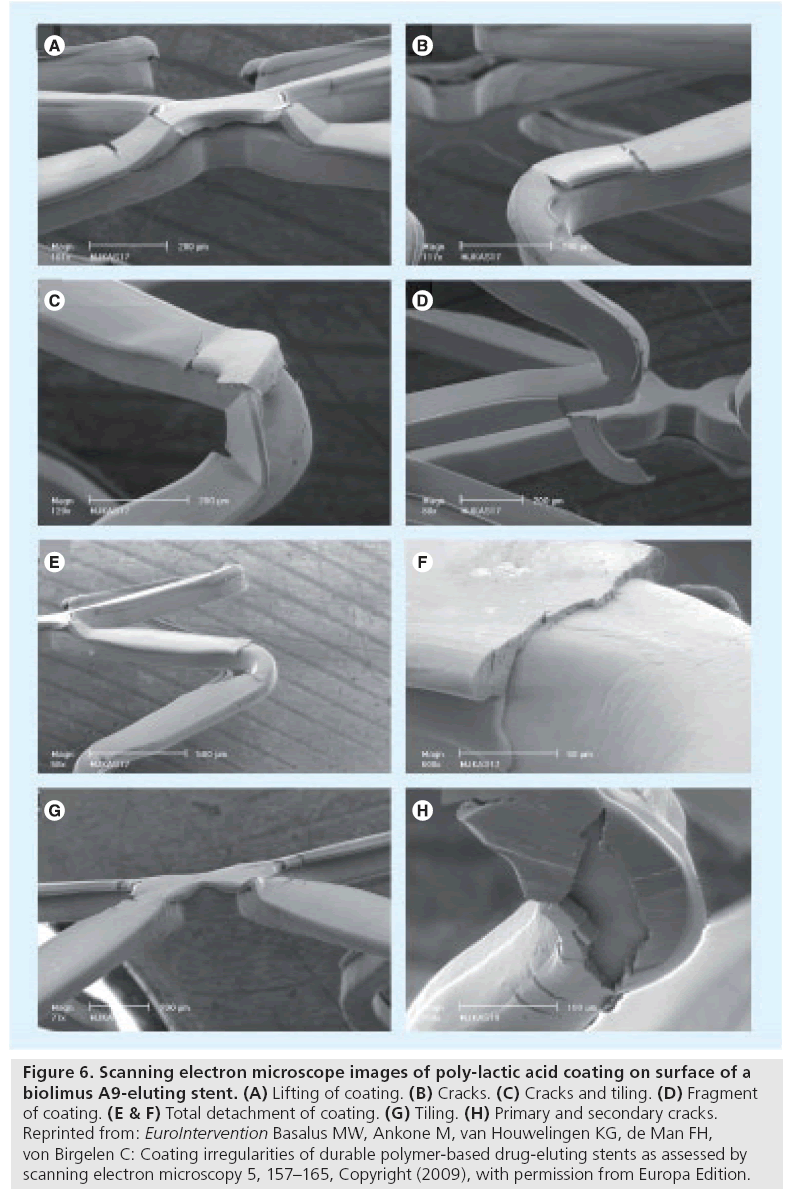
A recent SEM benchside examination of a biolimus A9-eluting stent with poly-lactic acid coating (BioMatrix®; Biosensors Europe SA, Switzerland) demonstrated various (partly novel) irregularities [31], which are defined as follows: first, primary cracks are sharp-edged coating irregularities extending from the coating surface deep into the coating (sometimes with exposure of the underlying stent or the primer layer underneath the coating) (Figure 6B & C); second, tiling is local outward displacement of the coating with formation of tile-like structures, resembling the ridge of a roof (Figure 6G); third, lifting of coating is an upwards displacement of the central portion of the coating on a crosslink that is still connected to the adjacent coating (Figure 6A); fourth, fragments of coating are detached pieces of coating that may or may not keep a minimum contact to the rest of the coating (Figure 6D); and fifth, total detachment of coating is total loss of a portion of the coating with exposure of the underlying stent or primer layer (Figure 6E & F).
Figure 6: Scanning electron microscope images of poly-lactic acid coating on surface of a biolimus A9-eluting stent. (A) Lifting of coating. (B) Cracks. (C) Cracks and tiling. (D) Fragment of coating. (E & F) Total detachment of coating. (G) Tiling. (H) Primary and secondary cracks. Reprinted from: EuroIntervention Basalus MW, Ankone M, van Houwelingen KG, de Man FH, von Birgelen C: Coating irregularities of durable polymer-based drug-eluting stents as assessed by scanning electron microscopy 5, 157–165, Copyright (2009), with permission from Europa Edition.
Aggressive oversized DES postdilatation with a high-pressure balloon resulted in an increase in the number and size of cracks with the occurrence of so-called secondary cracks, which are sharpedged irregularities, extending perpendicularly to the primary cracks (Figure 6H). The size of the fragments observed (Figure 6D) was large enough to potentially obstruct or occlude minor sections of the myocardial microvasculature due to embolization. In the absence of other explanations, this mechanism could be a plausible explanation for the higher incidence of myocardial infarction shortly after bifurcation stenting with this biodegradable polymer-based DES versus the durable polymer-based Cypher® stent (in a subanalysis of the Limus Eluted from A Durable versus Erodable Stent coating [LEADERS] trial) [36].
▪ Why does a particular DES show a certain characteristic coating irregularity?
The aforementioned SEM studies demonstrated an evident association of the examined DES with characteristic coating irregularities such as: peeled polymer and coarse irregular excess of coating on Cypher; webbing of Taxus Liberté; large bare-metal areas on Endeavor Sprint; wrinkles on Xience V; distinctive cracks in the coating of Endeavor Resolute; and fragments of the coating in BioMatrix stents.
In certain DES coatings, the presence of cracks (and in some coatings even fragments) at high shear-stress locations suggests that these particular coatings are less elastic than other DES coatings that show wrinkles at corresponding spots. Adhesions of the coating layer on adjacent stent struts can only lead to the development of strands – so-called webbing – in the presence of a particularly high elasticity of the polymer. Crater lesions are generally located at spots, where stent struts may have come into contact with each other (in the crimped state); this suggests that some adhesion of coating material may be involved in the formation of such irregularities, if the coating is not elastic enough to form webbing. However, without exact knowledge of all technical details of the complex process of DES production, it is very difficult to attribute the irregularities to a particular cause. Large bare-metal areas on the luminal DES surface, as observed after deployment of Endeavor Sprint, suggest a potential role of the stent-balloon interaction in the formation of such irregularities; however, an uneven distribution of the coating (thinner layer on luminal side of stent struts) and the proportion of drug to polymer (9:1) may contribute to this phenomenon.
Thus, the typical shape and size of certain coating irregularities may essentially result from multiple factors: the stent design and material (i.e. the stent platform); the type and thickness of the polymer; the proportion of drug to polymer; and potential technical shortcomings in applying the coating onto the stent platform.
Spatial configuration & geometry of DES
▪ Stent geometry & size of stent cells in DES
The term stent geometry comprises not only the geometry of individual stent struts (i.e., strut thickness and cross-sectional shape) [37,38] and the angular burden [39], but also the spatial relation of stent struts to each other, which defines the size of the stent cells.
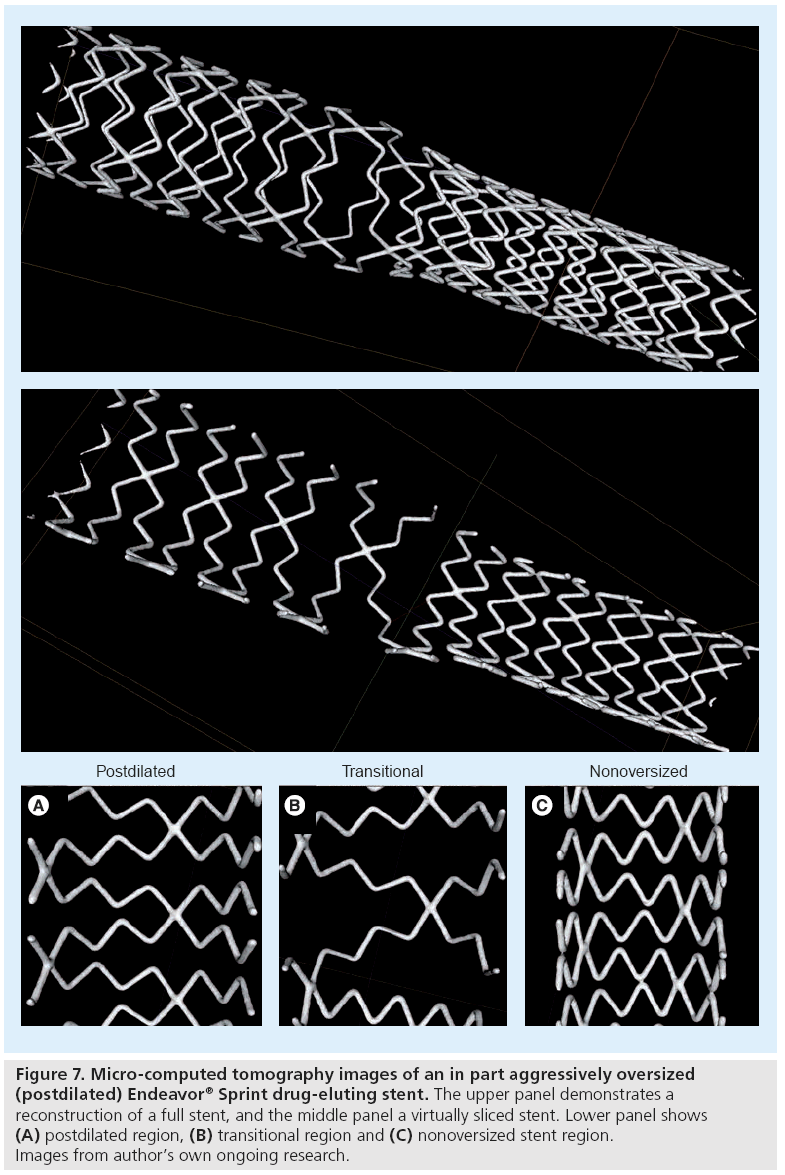
The size of stent cells provides valuable information about scaffolding properties, distribution of the antiproliferative drug and the risk of jailing side branches. The cell size of DES varies widely from gentle stent deployment to aggressive (partial) postdilatation or complex bifurcation techniques. While the cell size is highly predictable in the case of gentle stent deployment during benchside testing, (partial) stent postdilatation or complex bifurcation techniques may lead to a substantial variation in cell size, as suggested by preliminary data from ongoing research (Figure 7).
Figure 7: Micro-computed tomography images of an in part aggressively oversized (postdilated) Endeavor® Sprint drug-eluting stent. The upper panel demonstrates a reconstruction of a full stent, and the middle panel a virtually sliced stent. Lower panel shows (A) postdilated region, (B) transitional region and (C) nonoversized stent region. Images from author’s own ongoing research.
▪ Factors determining final stent geometry
The final DES geometry results from the interaction of different factors. Even the material of the stent platform is important. Most stent platforms are currently made from 316L stainless steel or cobalt–chromium [40]. The latter permits manufacturing of stents with thinner stent struts [37]. Other novel alloys have recently been introduced [41].
The design of the stent platform (e.g., more open versus more closed cell design) is of particular importance and varies between current DES. Stents with more open cell designs are more flexible and can adapt more easily (with or without postdilataton) to different anatomies. While in bifurcation stenting the possibility to easily achieve a large cell size is advantageous for side branch access, it may be disadvantageous with regard to plaque scaffolding and, perhaps, drug distribution. Conversely, a more closed cell design limits the ability of a DES to adapt to the local anatomical situation and carries an increased risk of DES malapposition [42–44].
Anatomical factors such as plaque composition (i.e., calcifications may limit stent expansion), tortuousity of the lesion segment (i.e., larger cells in the outer curvature) and local arterial compliance have an additional effect on stent geometry as they determine the ideal implantation pressure and the need for postdilatation. Finally, the inflation of a balloon in a side branch changes the local stent geometry significantly.
▪ Clinical implications of different stent geometries
Drug-eluting stents with a more open-cell design allow an easier access to side branches [45], while DES with a more closed cell design are less capable of adapting to the local anatomy. This explains the increased risk of malapposition of such DES as recently demonstrated by optical coherence tomography [43], which has a higher capacity to detect malappositon [46].
Conversely, the more closed cell design DES may display a more uniform distribution of stent struts, as demonstrated by intravascular ultrasound [47], which is a valuable technique for stent examination in vivo [48,49]. Stent geometry – in particular the distribution of the struts – may have implications for drug delivery and the uniformity of drug distribution [50,51]. Clinical research with intravascular ultrasound and optical coherence tomography demonstrated an association between nonuniform strut distribution and increased neointimal hyperplasia [47,52].
Curved or calcified lesion segments may also show a less homogeneous distribution of stent struts and increased incidence of stent malapposition with potential implications for the formation of restenosis [53,54].
Drug-eluting stents differ in type, shape and number of links (i.e., interconnections) between subsequent stent rings, which in the in vivo setting may affect shear stress of the stented vessel wall and thus the risk of in-stent restenosis [55].
Following DES postdilatation and/or complex bifurcation techniques, examination of the stent geometry provides insight into the direction and extent of strut displacement. The frequency and extent of strut protrusion could promote the formation of instent restenosis and/or thrombus. In two-stent strategies, stent geometry will be even more complex. During bifurcation maneuvers, the geometry of stent(s) determines ostial scaffolding of the side branch, which is important for the prevention of restenosis.
Assessment of DES geometry
▪ Methods for examination of stent geometry
Finite element analysis is a computer-based calculation method that is frequently used to predict the geometry of stents following deployment and/or postdilatation [56]. Pioneering examinations of the geometry of real coronary stents have been performed by taking highly magnified photographs of stents [57], while the interior of the stents was examined with a pediatric endoscope [58,59]. These techniques provided very interesting information on stent geometry and stent deformation in the context of bifurcation stenting [57–59]. Nevertheless, it is quite obvious that light-based techniques that provide 2D images have a limited capacity with regard to the representation of the spatial (3D) geometry of stents. Therefore, micro-computed tomography (micro-CT) – a high resolution imaging modality that permits nondestructive assessment and 3D reconstruction of spatial objects – has been used to examine stent geometry [60].
▪ Micro-computed tomography examination of DES
The use of micro-CT for the assessment of stents was established by Ormiston et al., who used it for the investigation of bifurcation stenting [61], and has since been adapted by other groups [62,63]. In fact, this technique is also very suitable for the accurate measurement of small distances between adjacent stent struts. A recent micro-CT study demonstrated that after crush stenting, the ostial stenosis of sidebranches was minimized by use of the minicrush technique, two-step kissing postdilation, and stents with a larger cell size [61]. Our group recently used micro-CT to gain insight into the geometry of several stent platforms of current DES. Figure 7 shows an example of an Endeavor Sprint that is based on the cobalt–chromium modular Driver stent platform [28,64]. In addition, we quantitatively examined the effect of aggressive partial postdilatation of DES on stent geometry. Such postdilatation is sometimes required to achieve good stent apposition in coronary segments with significant tapering, for example, when DES are implanted across the left main bifurcation.
▪ Relevance of stent geometry for interpretation of coating irregularities as seen by SEM
The presence of characteristic coating irregularities on certain typical DES locations suggests that there is a relation between irregularities and stent geometry. For example, the incidence and severity of cracks of the coating on stent loops could be related to the angle of that particular stent loop. In addition, no thorough examination of the incidence and extent of DES coating irregularities following aggressive postdilatation have been performed so far. Therefore, it may be valuable to examine both DES coating morphology and geometry in the same samples. This may allow interventional cardiologists to match the degree of stent overstretch and deformation versus potential changes in coating morphology as seen with SEM.
Limitations
▪ Limitations of current DES benchside testing
Benchside studies do not exactly mimic conditions in vivo. DES with a somewhat less favorable appearance may be clinically highly efficacious and safe; for example, a higher hydrophilicity of certain DES coatings may compensate for a somewhat higher incidence of certain irregularities on these coatings [65]. Hence, clinical data are most important in order to form a prudent opinion on DES. Nevertheless, meticulous benchside studies (including quantitative assessment) are important because they add valuable information to the overall picture of DES and may sometimes help clinicians to understand clinical data. Results of benchside testing of delicate DES implants are clearly related to the method of handling samples. However, for SEM examination for example, this can be minimized by avoiding gold sputtering and using a lowvoltage electron beam as high-voltage electron beams may produce some artefacts.
Current benchside testing of DES is performed without the use of vascular phantoms or by using silicon or perspex phantoms. Unquestionably, such phantoms do not resemble diseased coronary vessels in a perfect way. During stent delivery in clinical practice, potential shear between the abluminal DES surface and the vessel wall may lead to additional defects, depending on characteristics of target lesion and vessel (e.g., vessel tortuosity, calcification and lesion location) [66,67]. Not only during stent delivery, but also after stent delivery, shear flow and mechanical strain may worsen some coating irregularities, for example, dislodge loosely attached polymer fragments. Moreover, stent fracture, which is estimated to occur in 1–8% of patients [68], is an example where impairment of the stent geometry can affect the integrity of DES polymer coating. In a recent study, stent fracture was reported more frequently in the closed-cell designed Cypher stent compared with the more open-cell designed Taxus® Express stent [69]. Fatigue of the stent platform and its material can be tested at the benchside. Other factors associated with stent fracture, such as the lack of vascular wall support (e.g., in coronary aneurysms or sites of substantial stent malapposition), depend greatly on the local vascular anatomic situation [70]. The assessment of these complex issues would require sophisticated phantoms with properties that are very similar to atherosclerotic and aneurysmatic vessels. Avoidance of the use of phantoms eliminates the need for sample harvesting (i.e., for SEM studies) that could otherwise cause additional artificial defects; nevertheless, this approach may intensify a few coating irregularities (e.g., in biodegradable coatings). Moreover, DES expansion in water followed by drying could theoretically affect more hydrophilic coatings. This possible effect may be minimized by gradual passive drying without temperature changes. Critical point drying may be an alternative method of drying samples; however, such an approach may be associated with other limitations related to the high concentration of alcohol used. The use of environmental SEM could theoretically avoid this problem to some extent, but this imaging technique is less suitable for quantitative studies.
Optimization of in vitro DES research may be achieved by the cooperation of clinicians and scientists, who should bring together good knowledge on biophysical characteristics of (diseased) coronary vessel walls and various biomaterials. Polymer experts may help not only to interpret the findings of benchside research but also to develop vascular phantoms in order to closely simulate diseased coronary vessels. Current meticulous protocols for SEM assessment are relatively time consuming. Automation of scanning with SEM and perhaps even the use of computer-based semiautomated pattern recognition algorithms for detection (and scoring) of coating irregularities may help to save time and to further standardize DES assessment.
Future perspective
Scanning electron microscope observations suggest that some coating irregularities may result from adhesion between coating material on adjacent stent struts; therefore, refinement of the process of loading DES coatings on the metallic stent platform and/or subsequent handling of the freshly coated DES may help to avoid this. This process of refinement has been initiated but will require further effort, as the ongoing development of novel DES is very dynamic and involves the use of novel drugs, stent designs and alloys. Examples of some recent attempts to improve the process of applying the coating on stents may be the use of electrostatic dry powder deposition technology [71] or sol-gel technology, which involves a chemical synthetic technique with transition of a liquid coating precursor into a (solid) coating [72].
In the expanding market of DES, many more new DES concepts (or modified existing stent concepts) may soon be introduced [73]. The significance of the changes in a DES system for second-generation DES will directly influence the amount of additional nonclinical and/or clinical testing needed to support the safety and efficacy of a modified DES [74]. Therefore, sharpening of the controls by regulatory bodies may be useful or even necessary. In this light, extended benchside testing of DES coatings and stent platforms may be considered as one piece of the mosaic of evidence that is used for decisionmaking. In parallel with the efforts in the field of durable polymer-based DES one may expect further innovations in biodegradable DES coatings. An example of recently applied novel biodegradable DES coatings may be salicylate-based polymers [75]. A significant reduction in the required amount of polymer can be achieved by the use of reservoir-based DES concepts [76].
Recently developed polymer-free DES (e.g., stents with drugs loaded on a nanoporous hydroxyapatite surface coating) may provide a significant antirestenotic effect without some potential side effects of polymer-based DES that have occasionally been attributed to the polymer layer [77]. Preclinical studies suggest safety and efficacy of such concepts; however, this still needs to be confirmed in large clinical trials [78,79]. Another concept to create polymerfree DES may be a drug-filled hollow stent that serves as a drug reservoir and releases the drug through small holes on the abluminal side [80]. In addition, there are ongoing efforts to improve fully bioabsorbable stent technologies in order to create a future DES [81,82]. Nevertheless, there are still some open questions with regard to the radial strength, stent recoil, drug release kinetics, and last but not least, the sequence of DES resorption, which may be important to avoid potential embolization of fragments.
In certain challenging lesion types, clinicians may consider data from benchside testing as valuable complementary information that can sometimes help to make a rational choice for DES. In addition, insights obtained from benchside testing can improve stent implantation techniques. Considering the various concepts of ongoing DES innovation and development (only some are briefly mentioned in this article), there is an increased need for careful benchside studies of DES, including thorough quantitative examinations. Such research can indeed provide valuable information that may complement preclinical and clinical studies.
It may be questionable whether the full picture of DES benchside findings is easily accessible for the interventional community in the absence of independent benchside research. The same accounts for high-resolution images of DES, taken at high magnification levels. Clinicians have to consider that SEM images displayed in brochures are generally not taken with the aim to detect the coating irregularities that independent research has consistently demonstrated. Accordingly, benchside studies of DES by independent research parties may be valuable and should be encouraged. Ideally, benchside research of DES should be performed by a multidisciplinary team of scientists, embedded in a high-level research institute with various advanced technical facilities and the expertise to guarantee a prompt reaction to the shifting demands as can be expected in response to novel DES concepts.
Financial & competing interests disclosure
The research department of Thoraxcentrum Enschede has received in the past unrestricted research grants and/or has participated in clinical studies funded by: Abbott Vascular, Biosensors International, Biotronik, Boston Scientific, Cordis Corporation and Medtronic. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Drug-eluting stent polymer coatings: function, requirements & types
▪ Act as a reservoir for antiproliferative drugs and control the drug-release kinetics.
▪ Have to be biocompatible, hemocompatible and of high mechanical durability.
▪ Polymer coatings used in drug-eluting stents (DES) can be durable or biodegradable.
Surface morphology of contemporary DES
▪ Various irregularities have been described on the surface of contemporary DES.
▪ Shape, size and incidence of irregularities varies between different DES types.
▪ Durable polymer irregularities may show decreased, increased, inhomogenous or displaced polymer coating.
▪ Postdilatation and/or bifurcation maneuvers could affect DES coating.
▪ Some DES irregularities might be related to risk of stent thrombosis or restenosis.
Geometry of DES
▪ Various factors determine final stent geometry (e.g., stent material, design and local vascular anatomy).
▪ Stent geometry has implications on plaque scaffolding, drug distribution and side branch access.
▪ Micro-computed tomography is a valuable method to examine stent geometry.
DES coating irregularities & geometry of DES
▪ The presence of characteristic coating irregularities on typical DES locations suggests a relationship with stent geometry (and the forces involved in creating that geometry).
▪ It may be valuable to examine DES coating morphology and geometry together.
Future of DES benchside testing
▪ Novel concepts of drug loading on metallic stents and biodegradable DES are approaching.
▪ Benchside studies of DES should preferably be performed by independent multidisciplinary research teams, embedded in high-level research institutes.
▪ Fast development of new DES concepts requires adaptation and further development of techniques used for benchside testing.
References
Papers of special note have been highlighted as:
▪ of interest
- Grube E, Silber S, Hauptmann KE et al.: TAXUS I: six- and twelve-month results from a randomized, double-blind trial on a slow-release paclitaxel-eluting stent for de novo coronary lesions. Circulation 107, 38–42 (2003).
- Morice MC, Serruys PW, Sousa JE et al.: A randomized comparison of a sirolimuseluting stent with a standard stent for coronary revascularization. N. Engl. J. Med. 346, 1773–1780 (2002).
- Moses JW, Leon MB, Popma JJ et al.: Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N. Engl. J. Med. 349, 1315–1323 (2003).
- Stone GW, Midei M, Newman W et al.: Comparison of an everolimus-eluting stent and a paclitaxel-eluting stent in patients with coronary artery disease: a randomized trial. JAMA 299, 1903–1913 (2008).
- Kastrati A, Mehilli J, Pache J et al.: Analysis of 14 trials comparing sirolimus-eluting stents with bare-metal stents. N. Engl. J. Med. 356, 1030–1039 (2007).
- Stettler C, Wandel S, Allemann S et al.: Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet 370, 937–948 (2007).
- Finn AV, Nakazawa G, Kolodgie FD, Virmani R: Temporal course of neointimal formation after drug-eluting stent placement: is our understanding of restenosis changing? JACC. Cardiovasc. Interv. 2, 300–302 (2009).
- Schulz S, Schuster T, Mehilli J et al.: Stent thrombosis after drug-eluting stent implantation: incidence, timing, and relation to discontinuation of clopidogrel therapy over a 4-year period. Eur. Heart J. 30, 2714–2721 (2009).
- Finn AV, Nakazawa G, Joner M et al.: Vascular responses to drug eluting stents: importance of delayed healing. Arterioscler. Thromb. Vasc. Biol. 27, 1500–1510 (2007).
- Jimenez-Valero S, Moreno R, Sanchez-Recalde A: Very late drug-eluting stent thrombosis related to incomplete stent endothelialization: in vivo demonstration by optical coherence tomography. J. Invasive. Cardiol. 21, 488–490 (2009).
- Joner M, Finn AV, Farb A et al.: Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J. Am. Coll. Cardiol. 48, 193–202 (2006).
- Ellis SG: A generation 2.5 drug-eluting stent? JACC. Cardiovasc. Interv. 2, 986–988 (2009).
- Brodie BR, Stuckey T, Downey W et al.: Outcomes and complications with off-label use of drug-eluting stents: results from the STENT (Strategic Transcatheter Evaluation of New Therapies) group. JACC. Cardiovasc. Interv. 1, 405–414 (2008).
- Byrne RA, Joner M, Kastrati A: Polymer coatings and delayed arterial healing following drug-eluting stent implantation. Minerva Cardioangiol. 57, 567–584 (2009).
- Vroman L: The life of an artificial device in contact with blood: initial events and their effect on its final state. Bull. NY Acad. Med. 64, 352–357 (1988).
- van der Giessen WJ, Lincoff AM, Schwartz RS et al.: Marked inflammatory sequelae to implantation of biodegradable and nonbiodegradable polymers in porcine coronary arteries. Circulation 94, 1690–1697 (1996).
- How TV: Mechanical properties of arteries and arterial grafts. In: Cardiovascular Biomaterials. Springer-Verlag Ltd, London, UK 1–36 (1992).
- Hunter WL: Drug-eluting stents: beyond the hyperbole. Adv. Drug Deliv. Rev. 58, 347–349 (2006).
- Schwartz RS: Pathophysiology of restenosis: interaction of thrombosis, hyperplasia, and/or remodeling. Am. J. Cardiol. 81, E14–E17 (1998).
- Nebeker JR, Virmani R, Bennett CL et al.: Hypersensitivity cases associated with drug-eluting coronary stents: a review of available cases from the Research on Adverse Drug Events and Reports (RADAR) project. J. Am. Coll. Cardiol. 47, 175–181 (2006).
- Kounis NG, Kounis GN, Kouni SN et al.: Allergic reactions following implantation of drug-eluting stents: a manifestation of Kounis syndrome? J. Am. Coll. Cardiol. 48, 592–593 (2006).
- Cook S, Ladich E, Nakazawa G et al.: Correlation of intravascular ultrasound findings with histopathological analysis of thrombus aspirates in patients with very late drug-eluting stent thrombosis. Circulation 120, 391–399 (2009).
- Hecker JF: Roughness and thrombogenicity of the outer surfaces of intravascular catheters. J. Biomed. Mater Res. 19, 381–395 (1985).
- Palmaz JC: Influence of surface topography on endothelialization of intravascular metallic material. J. Vasc. Interv. Radiol. 10, 448–460 (1999).
- Pinchuk L, Wilson GJ, Barry JJ et al.: Medical applications of poly(styrene-blockisobutylene- block-styrene) (‘SIBS’). Biomaterials 29, 448–460 (2008).
- Biela SA, Su Y, Spatz JP, Kemkemer R: Different sensitivity of human endothelial cells, smooth muscle cells and fibroblasts to topography in the nano–micro range. Acta Biomater. 5, 2460–2466 (2009).
- Introduction to Biomedical Engineering. Enderle JD, Brozino JD, Blanchard SM (Eds). Academic Press, Elsevier (2005).
- Basalus MW, Ankone MJ, van Houwelingen GK, de Man FH, von Birgelen C: Coating irregularities of durable polymer-based drug-eluting stents as assessed by scanning electron microscopy. EuroIntervention 5, 157–165 (2009).
- Ormiston JA, Panther M, Pornratanarangsi S: The ‘pitiful’ polymer, scanning electron microscope observations. Presented at: Transcatheter Cardiovascular Therapeutics Symposium. Washington DC, USA, 17 October, 2005.
- Otsuka Y, Chronos NA, Apkarian RP, Robinson KA: Scanning electron microscopic analysis of defects in polymer coatings of three commercially available stents: comparison of BiodivYsio®, Taxus® and Cypher® stents. J. Invasive. Cardiol. 19, 71–76 (2007).
- Basalus MW, van Houwelingen KG, Ankone M, de Man FH, von Birgelen C: Scanning electron microscopic assessment of the biodegradable coating on expanded biolimus-eluting stents. EuroIntervention. 5, 505–510 (2009).
- Ormiston JA, Webster M, Ruygrok P: Polymer integrity after Cypher® and Taxus® stent implantation: scanning electron microscope study. Presented at: DES symposium at Transcatheter Cardiovascular Therapeutics. Washington DC, USA, 1 October, 2004.
- Wilson GJ, Nakazawa G, Schwartz RS et al.: Comparison of inflammatory response after implantation of sirolimus- and paclitaxeleluting stents in porcine coronary arteries. Circulation 120, 141–142 (2009).
- Joner M, Nakazawa G, Finn AV et al.: Endothelial cell recovery between comparator polymer-based drug-eluting stents. J. Am. Coll. Cardiol. 52, 333–342 (2008).
- Guerin P, Pilet P, Finet G: Drug-eluting stents in bifurcations: bench study of strut deformation and bench study of strut deformation and coating coating lesions. Presented at: EuroPCR. Barcelona, Spain, 21 May (2009).
- Garg S: Does stent design impact the outcome in bifurcation treatment? Presented at: EuroPCR. Barcelona, Spain, 20 May (2009).
- Rittersma SZ, de Winter RJ, Koch KT et al.: Impact of strut thickness on late luminal loss after coronary artery stent placement. Am. J. Cardiol. 93, 477–480 (2004).
- Pache J, Kastrati A, Mehilli J et al.: Intracoronary stenting and angiographic results: strut thickness effect on restenosis outcome (ISAR-STEREO-2) trial. J. Am. Coll. Cardiol. 41, 1283–1288 (2003).
- Schulz C, Herrmann RA, Beilharz C, Pasquantonio J, Alt E: Coronary stent symmetry and vascular injury determine experimental restenosis. Heart 83, 462–467 (2000).
- Mani G, Feldman MD, Patel D, Agrawal CM: Coronary stents: a materials perspective. Biomaterials 28, 1689–1710 (2007).
- Parsonage E, Girton T, Knapp D: Biomaterial considerations for drug-eluting stents. Conf. Proc. IEEE Eng Med. Biol. Soc. 1, 2416–2417 (2009).
- Okabe T, Asakura Y, Ishikawa S et al.: Evaluation of scaffolding effects of five different types of stents by intravascular ultrasound analysis. Am. J. Cardiol. 84, 981–986 (1999).
- Tanigawa J, Barlis P, Dimopoulos K et al.: The influence of strut thickness and cell design on immediate apposition of drugeluting stents assessed by optical coherence tomography. Int. J. Cardiol. 134, 180–188 (2009).
- von Birgelen C, Kutryk MJ, Serruys PW: Three-dimensional intravascular ultrasound analysis of coronary stent deployment and in-stent neointimal volume: current clinical practice and the concepts of TRAPIST, ERASER, and ITALICS. J. Invasive. Cardiol. 10, 17–26 (1998).
- Ako J, Bonneau HN, Honda Y, Fitzgerald PJ: Design criteria for the ideal drug-eluting stent. Am. J. Cardiol. 100, 3M-9M (2007).
- Barlis P: Use of optical coherence tomography in interventional cardiology. Interv. Cardiol. 1, 63–71 (2009).
- Sano K, Mintz GS, Carlier SG et al.: Volumetric intravascular ultrasound assessment of neointimal hyperplasia and nonuniform stent strut distribution in sirolimus-eluting stent restenosis. Am. J. Cardiol. 98, 1559–1562 (2006).
- von Birgelen C, Erbel R: The stent is here to stay: a note on stenting, ultrasound imaging, and the prevention of restenosis. Eur. Heart J. 23, 595–597 (2002).
- von Birgelen C, Mintz GS, Bose D et al.: Impact of moderate lesion calcium on mechanisms of coronary stenting as assessed with three-dimensional intravascular ultrasound in vivo. Am. J. Cardiol. 92, 5–10 (2003).
- Hara H, Nakamura M, Palmaz JC, Schwartz RS: Role of stent design and coatings on restenosis and thrombosis. Adv. Drug Deliv. Rev. 58, 377–386 (2006).
- Hwang CW, Wu D, Edelman ER: Physiological transport forces govern drug distribution for stent-based delivery. Circulation 104, 600–605 (2001).
- Takebayashi H, Mintz GS, Carlier SG et al.: Nonuniform strut distribution correlates with more neointimal hyperplasia after sirolimus-eluting stent implantation. Circulation 110, 3430–3434 (2004).
- Mosseri M, Satler LF, Pichard AD, Waksman R: Impact of vessel calcification on outcomes after coronary stenting. Cardiovasc. Revasc. Med. 6, 147–153 (2005).
- Tanigawa J, Barlis P, Di MC: Heavily calcified coronary lesions preclude strut apposition despite high pressure balloon dilatation and rotational atherectomy: in-vivo demonstration with optical coherence tomography. Circ. J. 72, 157–160 (2008).
- Balossino R, Gervaso F, Migliavacca F, Dubini G: Effects of different stent designs on local hemodynamics in stented arteries. J. Biomech. 41, 1053–1061 (2008).
- Migliavacca F, Petrini L, Colombo M, Auricchio F, Pietrabissa R: Mechanical behavior of coronary stents investigated through the finite element method. J. Biomech. 35, 803–811 (2002).
- Ormiston JA, Webster MW, Ruygrok PN et al.: Stent deformation following simulated side-branch dilatation: a comparison of five stent designs. Catheter. Cardiovasc. Interv. 47, 258–264 (1999).
- Ormiston JA, Currie E, Webster MW et al.: Drug-eluting stents for coronary bifurcations: insights into the crush technique. Catheter. Cardiovasc. Interv. 63, 332–336 (2004).
- Ormiston JA, Webster MW, El JS et al.: Drug-eluting stents for coronary bifurcations: bench testing of provisional side-branch strategies. Catheter. Cardiovasc. Interv. 67, 49–55 (2006).
- Ritman EL: Micro-computed tomographycurrent status and developments. Annu. Rev. Biomed. Eng 6, 185–208 (2004).
- Ormiston JA, Webster MW, Webber B et al.: The ‘crush’ technique for coronary artery bifurcation stenting: insights from microcomputed tomographic imaging of bench deployments. JACC. Cardiovasc. Interv. 1, 351–357 (2008).
- Hikichi Y, Inoue T, Node K: Benefits and limitations of cypher stent-based bifurcation approaches: in vitro evaluation using micro-focus CT scan. J. Interv. Cardiol. 22, 128–134 (2009).
- Mortier P, Van LD, De BM et al.: Comparison of drug-eluting stent cell size using micro-CT: important data for bifurcation stent selection. EuroIntervention. 4, 391–396 (2008).
- Sketch MH Jr, Ball M, Rutherford B et al.: Evaluation of the Medtronic (Driver) cobalt–chromium alloy coronary stent system. Am. J. Cardiol. 95, 8–12 (2005).
- Hezi-Yamit A, Sullivan C, Wong J et al.: Novel high-throughput polymer biocompatibility screening designed for SAR (structure–activity relationship): application for evaluating polymer coatings for cardiovascular drug-eluting stents. Comb. Chem. High Throughput. Screen. 12, 664–676 (2009).
- Wiemer M, Butz T, Schmidt W et al.: Scanning electron microscopic analysis of different drug eluting stents after failed implantation: from nearly undamaged to major damaged polymers. Catheter Cardiovasc. Interv. DOI: 10.1002/ccd.22347 (2010) (Epub ahead of print).
- von Birgelen C, Basalus MW: On the loss of the phosphorylcholine-based DES coating on the abluminal surface of Endeavor® stents. Catheter Cardiovasc Interv. DOI: 10.1002/ ccd.22497 (2010) (Epub ahead of print).
- Canan T, Lee MS: Drug-eluting stent fracture: incidence, contributing factors, and clinical implications. Catheter Cardiovasc. Interv. 75, 237–245 (2010).
- Shaikh F, Maddikunta R, Djelmami-Hani M et al.: Stent fracture, an incidental finding or a significant marker of clinical in-stent restenosis? Catheter Cardiovasc. Interv. 71, 614–618 (2008).
- Doi H, Maehara A, Mintz GS et al.: Classification and potential mechanisms of intravascular ultrasound patterns of stent fracture. Am. J. Cardiol. 103, 818–823 (2009).
- Nukala RK, Boyapally H, Slipper IJ, Mendham AP, Douroumis D: The application of electrostatic dry powder deposition technology to coat drug-eluting stents. Pharm. Res. 27, 72–81 (2009).
- Coradin T, Boissiere M, Livage J: Sol-gel chemistry in medicinal science. Curr. Med. Chem. 13, 99–108 (2006).
- Silber S: Non-us physician–scientist perspectives: device regulation and approvals. fda town hall meeting. Presented at: Transcatheter Cardiovascular Therapeutics. Barcelona, Spain, 23 September, 2009.
- Center for Devices and Radiological Health: Non-clinical tests and recommended labeling for intravascular stents and associated delivery systems. Guidance for Industry and FDA Staff (2005).
- Jabara R, Chronos N, Robinson K: Novel bioabsorbable salicylate-based polymer as a drug-eluting stent coating. Catheter. Cardiovasc. Interv. 72, 186–194 (2008).
- Krucoff MW, Kereiakes DJ, Petersen JL et al.: A novel bioresorbable polymer paclitaxeleluting stent for the treatment of single and multivessel coronary disease: primary results of the COSTAR (Cobalt Chromium Stent With Antiproliferative for Restenosis) II study. J. Am. Coll. Cardiol. 51, 1543–1552 (2008).
- Byrne RA, Mehilli J, Iijima R et al.: A polymer-free dual drug-eluting stent in patients with coronary artery disease: a randomized trial vs. polymer-based drug-eluting stents. Eur. Heart J. 30, 923–931 (2009).
- Costa JR, Jr., Abizaid A, Costa R et al.: 1-year results of the hydroxyapatite polymerfree sirolimus-eluting stent for the treatment of single de novo coronary lesions: the VESTASYNC I trial. JACC. Cardiovasc. Interv. 2, 422–427 (2009).
- Virmani R: Non-polymeric drug-eluting stents – BIOFREEDOM. EuroPCR (2009).
- Leon MB: The endeavor and endeavor resolute zotarolimus-eluting stent: comprehensive update of the clinical trial program (featuring the first presentation of the endeavor iv 3-year results). Presented at: Transcatheter Cardiovascular Therapeutics. San Francisco, CA, USA, 21 September, 2009.
- Erbel R, Di MC, Bartunek J et al.: Temporary scaffolding of coronary arteries with bioabsorbable magnesium stents: a prospective, non-randomised multicentre trial. Lancet 369, 1869–1875 (2007).
- Serruys PW, Ormiston JA, Onuma Y et al.: A bioabsorbable everolimus-eluting coronary stent system (ABSORB): 2-year outcomes and results from multiple imaging methods. Lancet 373, 897–910 (2009).
▪ Thorough quantification of durable polymer-based coating irregularities on various drug-eluting stents (DES), including comprehensive classification.
▪ Qualitative study of coating irregularities on the surface of early generation DES.
▪ Qualitative study of coating irregularities of a biodegradable coating-based DES.
▪ Pioneering paper on early benchside assessment of stent geometry.
▪ Pioneering study that used micro-computed tomography for benchside examination of DES geometry.