Review Article - Interventional Cardiology (2010) Volume 2, Issue 1
Benefits of coronary revascularization in stable patients in the short and long term after acute myocardial infarction
- Corresponding Author:
- Antonio Abbate
Assistant Professor of Medicine, Division of Cardiology/VCU Pauley Heart Center
Virginia Commonwealth University, 1200 East Broad Street – West Hospital
10th Floor, East Wing, Room 1041, PO Box 980281, Richmond, VA 23298-0281, USA
Tel: +1 804 828 0513
Fax: +1 804 828 6765
E-mail: aabbate@mcvh-vcu.edu
Abstract
Keywords
acute myocardial infarction, infarct-related artery, myocardial viability, open artery hypothesis, percutaneous coronary intervention, silent myocardial ischemia
Percutaneous coronary intervention (PCI) has a well-established role in the current management of patients presenting with acute myocardial infarction (AMI). Guidelines published by the American College of Cardiology and the American Heart Association (ACC/AHA) outline the evidence supporting an immediate reperfusion strategy using PCI in patients with ST elevation myocardial infarction (STEMI) within 12 h of presentation, and an early invasive strategy in highrisk patients with non-ST elevation myocardial infarction [1,2]. The indications for revascularization beyond the 12 h window in STEMI are less clear. Evidence from the randomized Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock (SHOCK) trial supports the use of PCI in patients with AMI complicated by cardiogenic shock beyond the first 12 h [3,4]. The benefit of coronary revascularization in stable patients with AMI, on the other hand, has been the subject of considerable debate. Current ACC/AHA guidelines recommend against routine angioplasty for patients with total infarct-related artery (IRA) occlusion in patients presenting with STEMI more than 12 h from symptom onset [1]. In this article, we review the current evidence on the potential short- and long-term benefits of PCI in stable patients presenting with AMI.
The late open artery hypothesis
In 1989, Eugene Braunwald reviewed data from several observational studies showing that patency of the IRA was associated with significantly lower mortality than in patients with persistent IRA occlusion, and proposed that the reperfusion paradigm should be expanded to include late reperfusion (the open artery hypothesis) [5,6]. The open artery hypothesis postulates that in the extended paradigm of AMI, there is both time-dependent myocardial salvage (early) and a time-independent process, whereby late coronary reperfusion appears to interrupt the process of adverse cardiac remodeling, dilation and impairment of systolic function. The influx of inflammatory cells through restoration of blood flow, even when it occurs late, is thought to play a significant role in forming a firm myocardial scar and limiting infarct expansion and remodeling [6,7]. Favorable myocardial stiffening is also thought to occur more readily in the setting of late reperfusion through intramyocardial hemorrhage, cellular edema and contraction band necrosis [8]. Nakagawa et al. studied the effects of late reperfusion in rodents, comparing restoration of flow in the IRA with permanent occlusion [9]. They found increased granulation tissue cellular proliferation with late reperfusion and a reduction in the incidence of granulation cell apoptosis in the subacute phase, contributing to thicker infarct scar formation. In addition, they described a reduction in cardiomyocyte degenerative changes with late reperfusion as compared with permanent occlusion. Abbate et al. described an association between persistent IRA occlusion and increased apoptosis in postmortem human subjects dying after AMI [10]. Furthermore, persistent IRA occlusion has been associated with an increase in markers of ischemia and of an increased apoptotic rate in the peri-infarct region [11]. The progressive loss of cells through apoptosis is thought to contribute to the unfavorable ventricular remodeling, left ventricular dilatation and progressive left ventricular systolic dysfunction [10,11]; thus, late reperfusion may achieve a nontraditional myocardium salvage that presents not as an infarctsparing effect, but rather as a preservation of cell number and density through inhibition of apoptosis and other forms of programmed cell death [9–11]
Support from observational & retrospective clinical data
Numerous studies have addressed the prognostic significance of a patent IRA in predicting mortality in survivors of AMI. White et al. reviewed data from a cohort of patients who had been enrolled in one of two thrombolytic trials, who then proceeded to coronary angiogram at a mean of 28 ± 11 days [12]. Patency of the IRA was assessed and entered into a regression analysis along with other clinical variables to correlate with mortality at a mean follow-up of 39 ± 13 days. The patency of the IRA along with left ventricular ejection fraction (LVEF) were both strong independent predictors of mortality.
Gaudron et al. described a group of 70 patients with AMI, in whom they performed serial measurements of ventricular volumes, ejection fraction and hemodynamic data [13]. They found that Thombolysis In Myocardial Infarction (TIMI) grade flow in the IRA was an early predictor of subsequent progressive dilation of the left ventricle.
Puma et al. analyzed data obtained in the Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO)-1 trial to determine if there was an association between patency of the IRA on initial coronary angiogram and outcomes at 30 days and 1 year [14]. Patients in the GUSTO-1 trial were those presenting with AMI within 6 h of symptom onset and were randomized to one of four primary thrombolytic strategies [15]. In their analysis, Puma et al. examined data from 11,228 patients in whom index admission angiographic data on IRA patency was available. An open IRA (defined as TIMI-III flow) was independently associated with lower 30-day and 1-year mortality rates, even after adjusting for other clinical variables, including LVEF [14]. While these studies certainly identify IRA patency as an important prognostic variable, these data alone are insufficient to establish a causal relationship between therapeutic recanalization of the IRA and a favorable long-term prognosis.
Paucity of randomized trials in stable patients following AMI
While the association between long-term patency of the IRA and improved survival appears quite robust in the aforementioned analyses, it was unclear whether routine mechanical reperfusion would yield the same advantage in prospective experimental trials. Clinical studies examining the efficacy of revascularization of the IRA in stable patients following AMI have demonstrated variable results, with some demonstrating a benefit with PCI and others showing no benefit. Early studies lacked power as they enrolled very small numbers of patients (typically less than 100), had relatively short-term follow-up, and generally failed to show statistically significant benefits in terms of clinical outcomes [16,17]. Methodology amongst the trials performed to date has also been variable, in terms of time from symptom onset to revascularization, inclusion of patients with total occlusion of the IRA versus subtotal occlusion, and the presence or absence of inducible ischemia.
The ‘not-so-late late-comers’
A substantial number of patients present between 12 and 48 h after symptom onset. The Beyond 12 h Reperfusion Alternative Evaluation (BRAVE-2) study specif ically addressed this clinical question by enrolling patients with STEMI between 12 and 48 h [18]. Patients were randomized to an immediate invasive strategy or a conventional conservative strategy, with a primary end point of final left ventricular infarct size. A total of 365 patients were enrolled in the study, with single photon emission computed tomography imaging at a median of 7.1 days showing a statistically significant reduction in the final infarct size in those assigned to the invasive strategy. While the initially published clinical outcomes measured at 30 and 90 days did not show any difference between the two study arms, a recently published update on outcomes at 4 years showed that mortality was significantly lower in the invasive group (11.1%) than in the conservatively managed group (18.9%) reflecting an absolute risk difference of 7.8% and a number needed to treat of only 13 [19]. These findings contribute several important pieces of information to the understanding of this subject. First, while it is clear that reperfusion should occur as early as possible after an AMI, patients continue to benefit from revascularization of the IRA beyond 12 h and up to 48 h. This is longer than the time interval previously thought to be necessary to achieve sufficient myocardial salvage to be clinically relevant. Indeed, this study proved that limitation of the final infarct size can be achieved when PCI is performed as late as 48 h from the index event, possibly through reperfusion of hibernating but still viable myocardium in the peri-infarct region. Second, it seems that longer lengths of follow-up may be necessary to document clinically relevant advantages to performing late revascularization.
Stable patients with silent ischemia
An estimated 50,000 patients per year will have evidence of myocardial ischemia without anginal symptoms following AMI [20]. Silent ischemia in such patients is known to represent an adverse prognostic indicator [21,22]. The Asymptomatic Cardiac Ischemia Pilot (ACIP) study found that clinically stable patients with demonstrable silent ischemia and coronary disease amenable to PCI had more favorable outcomes with routine revascularization [22]. Furthermore, in patients who were not randomized to primary revascularization, those who subsequently underwent PCI on the basis of silent ischemia performed better than those who were revascularized on the basis of symptomatic angina, supporting the concept that silent ischemia may be a more reliable marker of those prone to adverse outcomes than those with recurrent chest pain.
The recently published Swiss Interventional Study on Silent Ischemia Type II (SWISSI-II) study randomized patients with recent AMI (within the past 3 months) with evidence of silent ischemia to routine PCI versus intensive anti-ischemic therapy, and followed these patients for a mean of 10.2 years [23]. For the primary end point of combined major adverse cardiac events (MACE; defined as cardiac death, nonfatal recurrent MI, and/or symptom- driven revascularization with either PCI or coronary artery bypass grafting), PCI was associated with an absolute risk reduction of 6.3% per year compared with optimal medical therapy with a number needed to treat of 16. The SWISSI-II study represents the longest follow-up of patients in a randomized trial of patients receiving late revascularization following AMI, and showed that the benefit of this therapy is maintained for many years after the index event. Secondary end points for which a statistically significant benefit was found included cardiac death (PCI group 0.3%, controls 2.1%; p = 0.01), nonfatal recurrent MI (PCI group 1.2%, controls 4.7%; p = 0.002), symptom-driven revascularization (PCI group 3.1%, controls 6.3%; p = 0.008) and recurrent angina without revascularization (PCI group 1.1%, controls 3.4%; p = 0.09).
A post hoc analysis of the data from the SWISSI-II study was performed to determine which factors may be associated with the incidence of sudden cardiac death (SCD) [24]. Of the 201 patients randomized in SWISSI-II, there were 12 SCD events, one in the PCI arm of the study and 11 in the medically managed arm. Multivariate regression analysis showed that a decline in LVEF was the only independent variable that predicted SCD. The factors that seemed to be most closely associated with a decline in LVEF were treatment allocation (PCI vs medical therapy), the presence of residual MI and recurrent MI [24]. This study highlights the fact that patients with silent ischemia are at substantial risk for SCD and that this risk becomes more apparent as time goes on. In addition, it suggests that SCD is less likely to occur in patients who are revascularized soon after MI if silent ischemia is detected, and that the mechanism for this reduction may be through preservation of LVEF over the long term.
Role of myocardial viability
The presence of myocardial viability on lowdose dobutamine echocardiography performed several days after an AMI has been demonstrated to predict a tendency towards preservation of left ventricular (LV) volumes and, hence, less LV dilation over time [25]. LV dilation in turn has been shown to be a predictor of significantly worse long-term outcomes in survivors of MI, so it stands to reason that any therapy that might preserve myocardial viability could potentially help to prevent deleterious remodeling over time and, hence, produce a better clinical outcome [12].
Preliminary data from the Viability in Acute Myocardial Infarction (VIAMI) trial have been presented. In the VIAMI trial, patients presenting with STEMI who do not receive primary or rescue PCI and who remain stable over the first 48 h following symptom onset underwent low-dose dobutamine echocardiography to determine the presence or absence of myocardial viability in the infarct area [26]. Viability in this study is defined as an improvement in wall motion abnormalities in the infarct zone of at least two segments. Those with confirmed myocardial viability were then randomized to either an invasive strategy, including routine coronary angiography with intent to perform PCI with stent placement and treatment with abciximab, or a conservative management strategy where patients are medically managed with an ischemia-guided approach to performing angiography. Patients without significant viability in the infarct area were included in a prospective registry. Interim results from the first 216 patients enrolled in this study at 6 months follow-up showed freedom from the combined end point of death; reinfarction or unstable angina was 93.5% in the invasive group and 84.5% in the conservatively managed group (p = 0.04) [27]. Interestingly however, the event rate was lowest in the registry group without viability and without revascularization (5.3% for ischemic events defined as unstable angina or recurrent MI, 14.5% in the conservatively managed patients with viability). This study suggests a potential role for viability testing in stable patients following STEMI, with a clear benefit of revascularization in those with demonstrable viability.
Stable patients with total occlusion of the infarct artery
The Occluded Artery Trial (OAT) investigators recently reported their findings in the largest single randomized trial of this type [28]. They enrolled stable patients with persistent total occlusion of the IRA 3 to 28 days following AMI to a strategy of either routine PCI or optimal medical therapy. Mean duration of followup for this study was 2.9 years. Patients were excluded from the trial if they had evidence of severe ischemia on stress testing. There was no difference in outcomes for the combined primary end point of death, MI or New York Heart Association class IV heart failure. Data published on an online supplement indicate that symptomatic angina pectoris was less frequent in the PCI arm, and maintained a statistically significant benefit up to 24 months [101].
Event rates & benefits from late revascularizaton
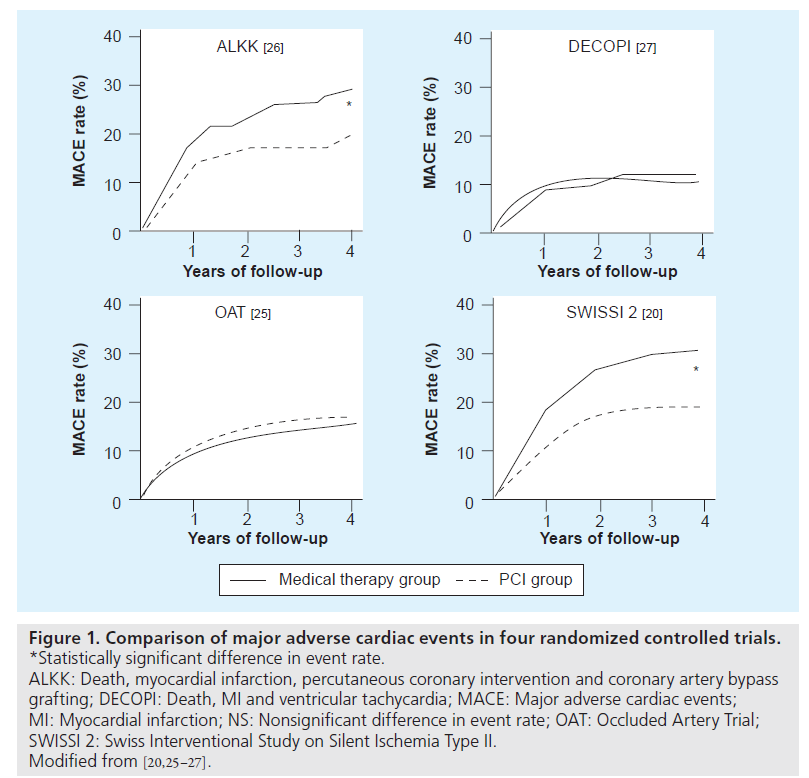
While the patients in the OAT study were described as stable patients with at least one high-risk feature (defined by either LVEF less than 50% or proximal occlusions of the IRA), the 4-year rate of MACE was low at approximately 15%, with a 4-year mortality of approximately 10%. Similarly, the DECOPI study also had a 4-year mortality in the conservative group of less than 10% and showed no benefits of revascularization, despite a benefit in terms of LVEF [29]. It is interesting to note that both the SWISSI-II and the ALKK, on the other hand, found a statistically significant benefit with late coronary revascularization. Subtle differences in enrollment criteria and consequent variances in inherent baseline risk of future adverse events could conceivably account for the variable benefit from revascularization observed in these studies with the benefits of late revascularization being more evident in those cohorts with higher event rates (Figure 1). For example, while the OAT excluded patients with moderate-to-severe ischemia, the SWISSI-II specifically enrolled patients with inducible ischemia. It is indeed possible that PCI performed after 48 h from symptom onset in a population with low event rates may have diminished returns with longer time to PCI with regards to survival advantage. In contrast to this, however, it is worth highlighting that although the SWISSI-II had a mean time to PCI of 23 days, it still showed a long-term survival advantage, indicating that higher risk patients, such as those with silent ischemia, stand to benefit from revascularization even when performed with substantial delay from the index event [23].
Figure 1: Comparison of major adverse cardiac events in four randomized controlled trials. *Statistically significant difference in event rate. ALKK: Death, myocardial infarction, percutaneous coronary intervention and coronary artery bypass grafting; DECOPI: Death, MI and ventricular tachycardia; MACE: Major adverse cardiac events; MI: Myocardial infarction; NS: Nonsignificant difference in event rate; OAT: Occluded Artery Trial; SWISSI 2: Swiss Interventional Study on Silent Ischemia Type II. Modified from [20,25–27].
The importance of total versus subtotal occlusion of the IRA
The majority of trials that enrolled only patients with total occlusion of the IRA following AMI with randomization to PCI or medical therapy showed no significant improvement in survival with PCI [28–32]. On the other hand, several studies including both patients with total and subtotal occlusions of the IRA did show a long-term survival advantage with PCI [18,23,33]. While methodology and patient characteristics between these studies differ in ways other than severity of occlusion of the IRA, it may be reasonable to expect that those with a totally occluded IRA on initial diagnostic angiogram are less likely to see a survival benefit from late revascularization than those with subtotal occlusions, unless there are other high-risk features such as inducible ischemia or significant viability. It is likely that patients with subtotal occlusions are more likely than those with total occlusions to have ongoing ischemia, which is known to induce further cell death, such that late reperfusion beneficially interrupts this process and reduces the amount of adverse remodeling occurring in this setting [10,11,34].
Meta-analysis of late PCI demonstrates an overall survival advantage
Abbate et al. performed a meta-analysis and systematic review of randomized trials comparing late PCI (defined as greater than 12 h from symptom onset) with medical therapy in hemodynamically stable patients, with a primary end point of survival [35]. The analysis pooled results from ten trials with a total of 3560 patients. This analysis included data from the OAT, the SWISSI-II and several of the small studies mentioned above, and as such, may be more representative as a whole with regard to the full spectrum of clinical presentations of stable late comers with AMI seen in real-world practice. PCI was associated with an overall improvement in survival, with an odds ratio of 0.49 (95% CI: 0.26–0.94). In addition, for the studies that provided data on baseline and follow-up LVEF, a greater improvement in cardiac function was observed over time in the patients assigned to PCI than medically managed patients, supporting the concept that late revascularization may be primarily beneficial by means of a reduction in adverse cardiac remodeling over the longer term.
Preservation of left ventricular systolic function over time with PCI
Several randomized trials of late PCI in patients with total occlusion of the IRA after AMI have reported data on changes in LVEF over time [16,29–31,36]. All have shown trends towards greater improvements in LVEF over time with PCI as compared with medical management alone, but results have been inconclusive owing to low statistical power. Abbate et al. showed in their meta-analysis that late PCI of the infarctrelated artery lead to a significantly greater improvement in LVEF. Appleton et al. confirmed these results selecting only those studies enrolling patients with totally occluded infarct arteries to gather pooled data on changes in cardiac function and remodeling over time [37]. PCI was associated with a statistically significant benefit with regard to long-term preservation of systolic function (LVEF difference of +3.1%, with 95% CI: +1.0–+5.2; p = 0.0004). While only three studies reported comparable data on changes in LV dimensions over time, PCI was also associated with a statistically significant beneficial reduction in LV end-systolic and end-diastolic volume. A reduced LVEF following AMI is known to be a strong predictor of long-term mortality, and is therefore an important therapeutic goal for all patients surviving an AMI [38,39].
Challenges of late revascularization
Late revascularization of the IRA may be considered a peculiar type of PCI, as success rates are sensibly lower than those of elective PCI in stable coronary disease, yet peri-procedural complications occur less frequently. The total occlusion typical of an IRA being treated late after symptom onset is often associated with a significant thrombotic burden, yet with a thrombus that is at least partially organized and thus mechanically more resistant to guidewire recanalization given its relatively advanced age. In addition, the risk of ongoing microvascular damage implies that, despite apparent successful dilatation and expansion of the culprit lesion, epicardial TIMI-3 flow and TIMI-3 perfusion grades cannot be achieved in all cases. This leads to success rates (defined as residual diameter stenosis <20%, epicardial TIMI-3 grade flow) not exceeding 85–90% [28]. Despite this, peri-procedural complications occur only in a minority of patients with adverse coronary lesion features (e.g., unprotected left main disease, true bifurcation lesion and severe calcification) or those with extensive thrombus burden. The OAT trial provides interesting data on the risk of procedure-related myocardial infarction, which occurred in six (0.6%) out of 1082 patients undergoing revascularization versus one (0.1%) out of 1084 undergoing medical therapy (p = 0.069), figures that are much lower than those typical of current unselected elective or urgent patients [28,40].
Whereas clinical decision making should be best individualized, with a revascularization strategy based on patient features, coronary anatomy, evidence of ischemia/viability and surgical risk, it is clear that angiographic features predictive of peri-procedural complications play a major role in decision making, as a short occlusion with minimal thrombus burden of the proximal left anterior descending warrants revascularization much more than a long, calcific occlusion in a remote posterolateral branch. From a procedural standpoint, timing in itself should not play a major role in decision-making. Actually, early intervention may be associated with more remodeling benefits, despite a somewhat greater likelihood of distal embolization in case friable thrombus is present. Indeed, when thrombus is angiographically evident, aggressive antithrombotic therapy (e.g., aspirin, thienopyridines and glycoprotein IIb/IIIa inhibitors) and mechanical thrombectomy prior to stent implantation can minimize the risk of distal embolization, microvascular plugging and recurrent myocardial infarction. The remaining interventional strategy is best decided on a case-by-case basis, as other patientand lesion-specific features impact more than the presence of an IRA on the choice between bare-metal versus drug-eluting stents, use of debulking or imaging (e.g., intravascular ultrasound or optical coherence tomography), appropriateness for routine angiographic follow- up, or short versus long duration of dual antiplatelet therapy.
Proposed strategy for managing stable patients following AMI
The 2007 update to the ACC/AHA guidelines for STEMI state that PCI of a totally occluded infarct artery is not recommended more than 24 h after the onset of symptoms in patients with STEMI, or in those who are asymptomatic with one to two vessel disease if they are hemodynamically and electrically stable and do not have evidence of severe ischemia [3]. This updated recommendation appears to be largely based on data from the OAT trial [28]. Furthermore, the guidelines give a Class IIb recommendation, stating that PCI may be considered as part of an invasive strategy in patients with a hemodynamically significant stenosis of a patent infarct artery greater than 24 h after STEMI.
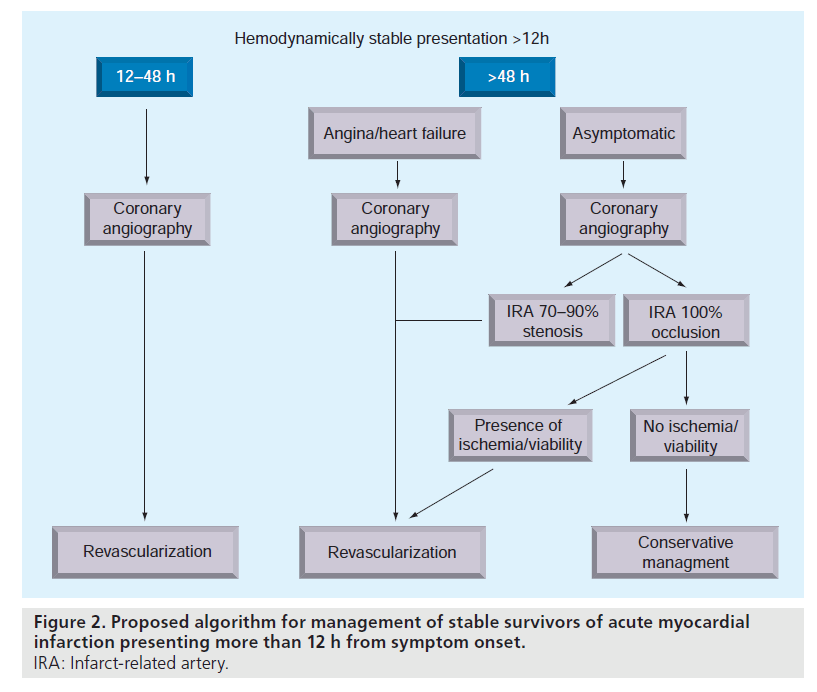
We contend that the available evidence supports a strategy based on a comprehensive analysis of multiple patient variables, with the final decision to proceed with PCI or pursue noninvasive optimal medical management based on the aggregate of these factors on a case-by-case basis. Figure 2 demonstrates our proposed management algorithm for real-world practice based on our interpretation of the current literature. While we believe this is a reasonable approach, it is worth noting that this strategy differs substantially from the current guidelines and no single randomized trial has proven this strategy to be more effective than any other.
The figure illustrates that we believe timing of intervention remains an important consideration, and that most stable survivors of AMI presenting between 12–48 h should undergo coronary angiogram with intent to proceed with PCI. For those presenting after 48 h, we suggest that those with recurrent symptomatic angina or objective evidence of ischemia should also undergo coronary revascularization. Asymptomatic patients who undergo coronary angiography after 48 h and who have 100% occlusion of the IRA should be managed conservatively in the first instance, especially if there is no evidence of large areas of viable myocardium or inducible ischemia, whereas asymptomatic patients with subtotal (70–99%) occlusions may derive benefit from late revascularization.
Conclusions
Early reperfusion (within the first 12 h) following onset of symptoms in AMI remains the primary therapeutic goal. Many survivors of AMI, however, continue to present late, and remain at substantial risk for both short- and long-term adverse outcomes. A strategy for the management of complex patients needs to take into account multiple variables to account for the highly variable range of presentations encountered in clinical practice. The ‘perfect’ trial to answer the complex question of whether to perform revascularization in stable survivors of AMI may be impossible to perform, and a thorough understanding of existing trial data is imperative if the clinician is to offer their patient the best therapeutic option for their specific situation.
Future perspective
The identification of patients who stand to benefit the most from late PCI following aMI remains a challenge, and there is an urgent need to develop and refine practical methods that can be used in routine clinical practice. The rapid evolution and improvements in noninvasive imaging modalities may enable the development of novel methods to assist in patient selection for PCI. Larger randomized trials in subgroups such as those presenting between 12 and 24 h and in those with myocardial viability or ischemia may help to confirm and substantiate the benefits seen in smaller trials. Finally, refinements in techniques used to achieve percutaneous coronary revascularization are urgently needed to reduce long-term complications, such as recurrent MI or need for repeat PCI. Improvements in adjunctive pharmacotherapy with newer antiplatelet agents and anticoagulants may also help to make PCI safer and with more durable responses.
Executive summary
▪ The late open artery hypothesis proposes that coronary revascularization may yield clinically relevant benefit even when performed more than 12 h from symptom onset in a time-independent fashion. Proposed mechanisms for this include limitation of infarct expansion and remodeling through the influx of inflammatory cells and reduction in cellular degeneration through apoptosis.
▪ Early support for this theory came from retrospective observation studies that found patency of the infarct-related artery (IRA) following thrombolytic therapy to be a favorable prognostic sign.
▪ The BRAVE-2 study demonstrated that patients treated with an immediate invasive strategy within 12–48 h of acute myocardial infarction had reduced mortality at 4 years.
▪ Patients with evidence of silent ischemia showed significant benefit from late percutaneous coronary intervention (PCI) with a reduction in major adverse clinical events over a 10-year follow-up period in the Swiss Interventional Study on Silent Ischemia Type II study.
▪ Myocardial viability may also be an important factor in determining who may benefit from late PCI based on early results from the Viability in Acute Myocardial Infarction trial.
▪ Stable asymptomatic patients presenting more than 3 days from symptom onset who have total occlusion of the IRA and no evidence of severe silent ischemia showed no difference in overall mortality or major adverse clinical events in the Open Artery Trial.
▪ Studies with higher event rates in the control arm have shown greater benefit from late PCI, suggesting that it is higher risk patients that have the most to gain from late revascularization.
▪ Trials that enrolled patients with subtotal occlusion tended to show more benefit from PCI than those that only enrolled patients with total IRA occlusion, suggesting those with subtotal occlusions are more likely to have underlying viability and/or ischemia.
▪ A meta-analysis of randomized trials comparing late PCI to medical therapy demonstrated improved survival and greater improvement in cardiac function over time.
▪ Late revascularization presents special challenges to the interventional cardiologist, requiring careful analysis of patient and angiographic variables to determine the most appropriate strategy.
▪ Given the complexity and variety of possible factors in the spectrum of stable patients presenting late with acute myocardial infarction, we propose a patient-tailored approach and a management strategy based on the existing evidence.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
- Antman EM, Hand M, Armstrong PWet al.: 2007 focused update of the ACC/AHA 2004 guidelines for the managementof patients with ST-elevation myocardialinfarction: a report of the American Collegeof Cardiology/American Heart AssociationTask Force on Practice Guidelines.J. Am. Coll. Cardiol. 51(2), 210–247(2008).
- Anderson JL, Adams CD, Antman EM et al.:ACC/AHA 2007 guidelines for themanagement of patients with unstableangina/non-ST-elevation myocardialinfarction: a report of the American College of Cardiology/American HeartAssociation Task Force on PracticeGuidelines (Writing Committee to Revisethe 2002 Guidelines for the Management ofPatients With Unstable Angina/Non-STElevation Myocardial Infarction) developedin collaboration with the American Collegeof Emergency Physicians, the Society forCardiovascular Angiography andInterventions, and the Society of ThoracicSurgeons endorsed by the AmericanAssociation of Cardiovascular andPulmonary Rehabilitation and the Societyfor Academic Emergency Medicine.J. Am. Coll. Cardiol. 50(7), E1–E157 (2007).
- King SB 3rd, Smith SC Jr, Hirshfeld JW Jret al.: 2007 focused update of the ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention:a report of the American College of Cardiology/American Heart AssociationTask Force on practice guidelines.J. Am. Coll. Cardiol. 51(2), 172–209(2008).
- Hochman JS, Sleeper LA, White HDet al.: One-year survival followingearly revascularization for cardiogenicshock. JAMA 285(2), 190–192(2001).
- Braunwald E: Myocardial reperfusion,limitation of infarct size, reductionof left ventricular dysfunction, andimproved survival. Should the paradigm beexpanded? Circulation 79(2), 441–444(1989).
- Kim CB, Braunwald E: Potential benefits oflate reperfusion of infarcted myocardium.The open-artery hypothesis. Circulation88(5 Pt 1), 2426–2436 (2003).
- Hammerman H, Schoen FJ, Braunwald Eet al.: Drug-induced expansion of infarct:morphologic and functional correlations.Circulation 69(3), 611–617 (1984).
- Pirzada FA, Weiner JM, Hood WB Jr:Experimental myocardial infarction. 14.Accelerated myocardial stiffening related tocoronary reperfusion following ischemia.Chest 74(2), 190–195 (1978).
- Nakagawa M, Takemura G, Kanamori Het al.: Mechanisms by which latecoronary reperfusion mitigatespostinfarction cardiac remodeling. Circ. Res.103(1), 98–106 (2008).
- Abbate A, Bussani R, Biondi-Zoccai GGet al.: Persistent infarct-related arteryocclusion is associated with an increasedmyocardial apoptosis at postmortemexamination in humans late after an acutemyocardial infarction. Circulation 106(9),1051–1054 (2002).
- Abbate A, Bussani R, Biondi-Zoccai GGet al.: Infarct-related artery occlusion, tissuemarkers of ischaemia, and increasedapoptosis in the peri-infarct viablemyocardium. Eur. Heart J. 26(19),2039–2045 (2005).
- White HD, Cross DB, Elliott JM et al.:Long-term prognostic importance ofpatency of the infarct-related coronaryartery after thrombolytic therapy for acutemyocardial infarction. Circulation 89(1),61–67 (1994).
- Gaudron P, Eilles C, Kugler I et al.:Progressive left ventricular dysfunction andremodeling after myocardial infarction.Potential mechanisms and early predictors.Circulation 87(3), 755–763 (1993).
- Puma JA, Sketch MH Jr, Thompson TDet al.: Support for the open-artery hypothesisin survivors of acute myocardial infarction:analysisof 11,228 patients treated withthrombolytic therapy. Am. J. Cardiol. 83(4),482–487 (1999).
- The effects of tissue plasminogen activator,streptokinase, or both on coronary-arterypatency, ventricular function, and survivalafter acute myocardial infarction.The GUSTO Angiographic Investigators.N. Engl. J. Med. 329(22), 1615–1622(1993).
- Dzavik V, Beanlands DS, Davies RF et al.:Effects of late percutaneous transluminalcoronary angioplasty of an occludedinfarct-related coronary artery on leftventricular function in patients with a recent(<6 weeks) Q-wave acute myocardial infarction (Total Occlusion Post-MyocardialInfarction Intervention Study [TOMIIS]– a pilot study). Am. J. Cardiol. 73(12),856–861 (1994).
- Ellis SG, Mooney MR, George BS et al.:Randomized trial of late elective angioplastyversus conservative management for patientswith residual stenoses after thrombolytictreatment of myocardial infarction.Treatment of Post-Thrombolytic Stenoses(TOPS) Study Group. Circulation 86(5),1400–1406 (1992).
- Schoemig A, Mehilli J, Antoniucci D et al.Mechanical reperfusion in patients withacute myocardial infarction presenting morethan twelve hours from symptom onset.JAMA 293, 2865–2872 (2005).
- Ndrepepa G, Kastrati A, Mehilli J et al.:Mechanical reperfusion and long-termmortality in patients with acute myocardialinfarction presenting 12 to 48 hours fromonset of symptoms. JAMA 301(5), 487–488(2009).
- Cohn PF, Fox KM, Daly C: Silentmyocardial ischemia. Circulation 108(10),1263–1277 (2003).
- Conti CR: Silent cardiac ischemia. Curr.Opin. Cardiol. 17(5), 537–542 (2002).
- Davies RF, Goldberg AD, Forman S et al.:Asymptomatic Cardiac Ischemia Pilot(ACIP) study two-year follow-up: outcomesof patients randomized to initial strategies ofmedical therapy versus revascularization.Circulation 95(8), 2037–2043 (1997).
- Erne P, Schoenenberger AW, Burckhard Det al.: Effects of percutaneous coronaryinterventions in silent ischemia aftermyocardial infarction: the SWISSI IIrandomized controlled trial. JAMA 297(18),1985–1991 (2007).
- Schoenenberger AW, Kobza R, Jamshidi Pet al.: Sudden cardiac death in patients withsilent myocardial ischemia after myocardialinfarction (from the Swiss InterventionalStudy on Silent Ischemia Type II [SWISSIII]). Am. J. Cardiol. 104(2), 158–163(2009).
- Nijland F, Kamp O, Verhorst PM et al.:Myocardial viability: impact on leftventricular dilatation after acute myocardialinfarction. Heart 87(1), 17–22 (2002).
- van Loon RB, Veen G, Kamp O et al.:Early and long-term outcome of electivestenting of the infarct-related artery inpatients with viability in the infarct-area:Rationale and design of the Viability-guidedAngioplasty after acute MyocardialInfarction-trial (The VIAMI-trial). Curr.Control Trials Cardiovasc. Med. 5(1), 11(2004).
- Veen G: VIAMI (Viability-GuidedAngioplasty after Acute MyocardialIschemia). Clin. Cardiol. 29, 550 (2006).
- Hochman JS, Lamas GA, Buller CE et al.:Coronary intervention for persistentocclusion after myocardial infarction.N. Engl. J. Med. 355(23), 2395–2407(2006).
- Steg PG, Thuaire C, Himbert D et al.:DECOPI (Desobstruction Coronaire enPost-Infarctus): a randomized multi-centretrial of occluded artery angioplasty afteracute myocardial infarction. Eur. Heart J.25(24), 2187–2194 (2004).
- Silva JC, Rochitte CE, Junior JS et al.: Latecoronary artery recanalization effects on leftventricular remodelling and contractility bymagnetic resonance imaging. Eur. Heart J.26(1), 36–43 (2005).
- Horie H, Takahashi M, Minai K et al.:Long-term beneficial effect of latereperfusion for acute anterior myocardialinfarction with percutaneous transluminalcoronary angioplasty. Circulation 98(22),2377–2382 (1998).
- Yousef ZR, Redwood SR, Bucknall CAet al.: Late intervention after anteriormyocardial infarction: effects on leftventricular size, function, quality of life,and exercise tolerance: results of the OpenArtery Trial (TOAT Study). J. Am. Coll.Cardiol. 40(5), 869–876 (2002).
- Zeymer U, Uebis R, Vogt A et al.:Randomized comparison of percutaneoustransluminal coronary angioplasty andmedical therapy in stable survivors ofacute myocardial infarction with singlevessel disease: a study of theArbeitsgemeinschaft LeitendeKardiologische Krankenhausarzte.Circulation 108(11), 1324–1328 (2003).
- Abbate A, Biondi-Zoccai GG, Baldi A et al.:The ‘open-artery hypothesis’: new clinicaland pathophysiologic insights. Cardiology100(4), 196–206 (2003).
- Abbate A, Biondi-Zoccai GG, Appleton DLet al.: Survival and cardiac remodelingbenefits in patients undergoing latepercutaneous coronary intervention of theinfarct-related artery: evidence from ameta-analysisof randomized controlled trials.J. Am. Coll. Cardiol. 51(9), 956–964 (2008).
- Dzavik V, Buller CE, Lamas GA et al.:Randomized trial of percutaneous coronaryintervention for subacute infarct-relatedcoronary artery occlusion to achieve long-term patency and improve ventricularfunction: the Total Occlusion Study ofCanada (TOSCA)-2 trial. Circulation114(23), 2449–2457 (2006).
- Appleton DL, Abbate A, Biondi-Zoccai GG:Late percutaneous coronary intervention forthe totally occluded infarct-related artery:a meta-analysisof the effects on cardiacfunction and remodeling. CatheterCardiovasc. Interv. 71(6), 772–781 (2008).
- Mukharji J, Rude RE, Poole WK et al.: Riskfactors for sudden death after acutemyocardial infarction: two-year follow-up.Am. J. Cardiol. 54(1), 31–36 (1984).
- Risk stratification and survival aftermyocardial infarction. N. Engl. J. Med.309(6), 331–336 (1983).
- Biondi-Zoccai GG, Sangiorgi GM,Antoniucci D et al.: Testing prospectivelythe effectiveness and safety of paclitaxelelutingstents in over 1000 very high-riskpatients: design, baseline characteristics,procedural data and in-hospital outcomes ofthe multicenter Taxus in Real-life UsageEvaluation (TRUE) Study. Int. J. Cardiol.117(3), 349–354 (2007).
▪ Demonstrates support for revascularization 12–48 h from onset of acute myocardial infarction.
▪ Both references [23 & 24]; these unique studies demonstrate the benefit of late revascularization over a long period of follow up (>10 years) in patients with asymptomatic (silent) ischemia.
▪ Both references [23 & 24]; these unique studies demonstrate the benefit of late revascularization over a long period of follow up (>10 years) in patients with asymptomatic (silent) ischemia.
▪ Patients who demonstrate myocardial viability benefit from late revascularization.
▪ Stable patients with totally occluded arteries and no evidence of ischemia with acute MI 3–28 days from onset demonstrated no difference in outcomes over a short follow-up period of 3 years.
▪ Meta-analysis of randomized, controlled trials of late revascularization demonstrating an overall survival benefit.
▪ Website
101 Supplement to Hochman JS, Lamas GA, Buller CE et al.: Coronary intervention for persistent occlusion after myocardial infarction. N. Engl. J. Med. 355, 2395–2407 (2006).