Review Article - Journal of Biochemistry Research (2018) Volume 1, Issue 1
Biochemical study of combined and separated encapsulated rivaroxaban and ubiquinone liposomal nanoparticles and some herbals on the myocardial infracted rat model
- Corresponding Author:
- AbouZaid OAR
Department of Biochemistry, Faculty of Veterinary Medicine, Moshtohor, Benha University, Egypt
E-mail: omayma_ragab55@yahoo.com
Abstract
Background: Anthracyclines such as doxorubicin are highly effective chemotherapy agents used to treat many common malignancies. However, their use is limited by cardiotoxicity. Rivaroxaban, a direct Factor Xa inhibitor, is now approved in United States and elsewhere for the prevention of venous thrombo-embolism. Coenzyme Q is mitochondrial respiratory chain element. Ginger has staring potential for treating cardiovascular disorders (atherosclerosis and hypertension). Murraya koenigii is known as ‘curry patta’ has anti-oxidant, anti-tumor, anti-inflammatory effects. Aim of study: In order to evaluate the protective effect of rivaroxban, ubiquinone separated and combined in the form of liposomes, combined curry and ginger on the myocardial infarcted rat model induced by doxorubicin. In vivo study was carried out, free doxorubicin; different liposomal preparations were assessed by different measurements. Materials and methods: The tested groups received respectively, group (1) Control. (2) Group B:received doxorubicin. (3) Group Q: liposomal ubiquinone and doxorubicin.(4) Group R: liposomal rivaroxban and doxorubicin. (5) Group QR: liposomal rivaroxban and ubiquinone encapusulated on the same liposome and doxorubicin. (6) Group D: blend of ginger, curry and doxorubicin. Particles size, zeta potential and TEM were measured for liposomal preparations. Several parameters have been measured to assess the cardiotoxicity (Albumin, lactate dehydrogenase enzyme (LDH), Aspartate Aminotransferase (AST), Anticardiolipin IgG, Creatine kinase-MB (CK MB), C-reactive protein (CRP), Tumor necrosis factor (TNF α)) and histopathological studies. Results: Rats with free doxorubicin caused significantly elevated levels of all parameters measured compared to control group, contol group vs. dox. treated group was as follow at p<0.05 (albumin 4.435 ± 0.085 vs. 2.910 ± 0.10, anticardiolipin 9.4 ± 0.256 vs.19.31 ± 0.4391, AST 64.60 ± 1.79 vs. 244.8 ± 9.86, CK 14.04 ± 1.794 vs. 73.25 ± 1.240, CRP4.904 ± 0.45 vs. 23.96 ± 0.95, LDH 212 ± 10.1 vs. 754 ± 16.4, TNF α 34.62 ± 1.05 vs.126.6 ± 6.25). In addition there is a significant variation of other groups compared to group B. Conclusionc There is a marked cardiotoxicity of doxorubicin could be decreased by the administration of antithrombic rivaroxban, respiratory chain element ubiquinone, herbals like ginger and curry.
Keywords
Cadiotoxicity, Liposomes, Doxorubicin, Rivaroxban, Ubiquinone, Curry and ginger
Abbreviations
(LDH) Lactate Dehydrogenase Enzyme, (AST) Aspartate Aminotransferase, (CK MB) Creatine Kinase- MB, (CRP) C-reactive Protein, (TNF α) Tumor Necrosis Factor, (dox. gp) Doxorubicin Group, (VKAs) Vitamin K Antagonists, (EDTA) Ethylene Diamine Tetraacetic Acid, (CVD) Cardio Vascular Diseases, (CTAB) Cetyl Trimethylammonium Bromide, (TEOS) Tetraethyl Orthosilicate, (MLV) Multilamellar Vesicles
Introduction
Myocardial infarction is a major cause of death and disability worldwide may be the first manifestation of coronary artery disease (CAD) or it may occur, repeatedly, in patients with established disease. From the epidemiological point of view, the incidence of MI in a population can be used as a proxy for the prevalence of CAD in that population [1]. To overcome the limitations of VKAs, the development of new oral anticoagulants has aimed to find effective treatment options that are more tolerable and more convenient. Among these new classes of anticoagulants are the oral direct thrombin inhibitors (DTIs) and the direct factor Xa inhibitors. Rivaroxaban is approved by (FDA), (EMA), and Health Canada for the reduction of the risk for stroke and systemic embolism in patients with nonvalvular atrial fibrillation [2]. x8European Medicines Agency, Committee for Medicinal Products for Human Use. Summary of Opinion. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000944/smops/Positive/human_smop_000441.jsp&mid=WC0b01ac058001d127. (Accessed November 28, 2012).
See all ReferencesCoenzyme Q10 (CoQ10) is an endogenous provitamin that is also known as ubiquinone acts as a coenzyme in the energyproducing metabolic pathways; therefore, performs a fundamental function in cellular ATP synthesis process. By considering its role in respiratory chain, CoQ10 functions as an antioxidant, scavenging free radicals and inhibiting lipid peroxidation. Consequently, CoQ10 efficiency in CVD such as cardiomyopathy, hypertension, angina pectoris, and atherosclerosis has been discovered over the past decade [3]. In recent years, Coenzyme Q10 (CoQ10) has gained considerable attention as a dietary supplement capable of influencing cellular bioenergetics and counteracting some of the damage caused by free radicals. CoQ10 is a vitamin like, fat-soluble substance existing in all cells. Venous thromboembolism, which includes deep vein thrombosis and pulmonary embolism, is the third most common cause of vascular death after myocardial infarction and stroke. The mainstay of treatment is anticoagulation [4] and in patients without active cancer, guidelines suggest the use of direct oral anticoagulant agents such as rivaroxaban over vitamin K antagonists such as warfarin [5].
Anticoagulation therapy is administered for 3 months or longer, depending on the balance between the risk of recurrent venous thromboembolism and the risk of bleeding [6]. In patients without reversible risk factors, the risk of recurrent venous thrombo-embolism is as much as 10% in the first year if anticoagulation therapy is stopped [7]. Patients with venous thrombo-embolism are at increased risk for arterial thrombotic events, including myocardial infarction, stroke, and vascular death. Although extended anticoagulation therapy is effective for the prevention of recurrent venous thromboembolism [8]. Anthracyclines such as doxorubicin and daunomycin are anticancer chemotherapeutic drugs that are widely used in clinical cancer treatment. Unfortunately, these types of drugs are associated with dosedependent acute or chronic cardiotoxicity, which is characterized by hypotension, tachycardia, arrhythmia, transient depression of left ventricular function, and even refractory late-onset cardiomyopathy [9]. Ginger is the rhizome of Zingiber officinale, a perennial plant in the family Zingiberaceae. It is consumed as delicacy, medicine, or spice. There are a variety of uses suggested for ginger as folk medicines in Chinese history, such as stomachic, antiemetic, anti-diarrhea, expectorant, antiasthmatic, haemostatic and cardio tonic for the treatment of several gastrointestinal and respiratory diseases. The most famous traditional medicinal practice of Z. officinaleis to promote the blood circulation for removing blood stasis and the mechanism is related to anti-platelet aggregation activity [10]. Murraya koenigii, popularly known as curry leaves in many Indian languages. It is basically known for its’ aroma and medicinal property. It has an antibacterial, antifungal, antioxidant, anti-inflammatory activities [11].
The present study aimed to investigate the effect of the use of liposomal rivaroxban and ubiquinone and highly beneficial curry plus ginger on myocardial infracted rat model.
Materials and Methods
▪ Materials
Doxorubicin hydrochloride provided from (Eimic company, cairo, egypt), ubiquinone provided from (Mebaco company, cairo, Egypt), rivaroxban provided from (Eldebeki company, cairo, egypt), ginger and curry were provided from farms of EAEA. Phosphatidyl choline provided from (Alandaloscompany, cairo, Egypt), cholesterol provided from (Oxford company, mumbai, egypt). CTAB, TEOS (SiO2>28.0%) and sodium hydroxide (>96%) were purchased from Sigma-Aldrich
▪ Animals
I. Source: The male albino rats were provided by the animal house unit of The National Organization for Drug Control and Research (NODCAR), Giza, Egypt.
II. Diet: The standard diet was a mixture composed of (72.2% carbohydrate, 3.4% fats, 19.8% proteins, 3.6% cellulose, 0.5% vitamins and minerals and 0.5% salts), obtained from Kahira Company for Poultries, Cairo, Egypt.
III. Adaptation: The animals were housed in the animal house unit of NODCAR, and allowed free access to standard diet and water under standardized conditions (12.00 h light: 12.00 h dark, 25 ± 2°C) and away from stressful stimuli. The investigation was complied with the Guide for Care and Use of Laboratory Animals published by the US national institutes of health (NIH Publication NO. 85-23, revised 1996) [12].
▪ Experimental design
This study performed on 60 adult male albino rats with different time duration for each group. The total time period for this research was 24 days, starts from the second of January 2017 till the 25th of the January 2017.
Their weights ranged from 190-220 gm at the time of the experimentation, classified randomly into five groups as follows:
1. Control group (group A): Ten albino rats received saline for 10 days then slaughtered to collect samples.
2. Group B (free doxorubicin administrated group): Ten albino rats received a cumulative doses of doxorubicin (15 mg/kg) [13] given as a dose of (5 mg/kg) every other two days, rats slaughtered and plasma collected after 48 hours from the last given dose. The duration of treatment was 9 days from the first day of treatment.
3. Group Q (doxorubicin free and liposomal ubiquinone administrated group): Ten albino rats received liposomal ubiquinone [14] (10 mg/ kg) from the first day and received a cumulative doses of doxorubicin (15 mg/kg) given as a dose of (5 mg/kg) every other two days, rats slaughtered and plasma collected after 48 hours from the last given dose. The duration of treatment was 9 days from the first day of treatment.
4. Group R (doxorubicin free and liposomal rivaroxban administrated group): Ten albino rats received daily doses of liposomal rivaroxban [15] (1 mg/kg) encapusulated on nanoparticles from the first day and received a cumulative doses of doxorubicin (15 mg/kg) given as a dose of (5 mg/kg) every other two days, rats slaughtered and plasma collected after 48 hours from the last given dose. The duration of treatment was 9 days from the first day of treatment 5. Group QR (doxorubicin free and combined liposomal ubiquinone and rivaroxban administrated group): Ten albino rats received daily doses of rivaroxban (1 mg/kg) and ubiquinone (10 mg/ kg) encapusulated on the same liposome from the first day and received a cumulative doses of doxorubicin (15 mg/kg) given as a dose of (5 mg/kg) every other two days, rats slaughtered and plasma collected after 48 hours from the last dose.s
6. Group D (ginger and curry treated group): Ten albino rats fed on food contains blend of ginger and curry [16] (10% of diet) for 15 days before administration of doxorubicin, then they had received a cumulative doses of doxorubicin (15 mg/kg) given as a dose of (5 mg/kg) every other two day and continued in feeding of herbals, rats slaughtered and plasma collected after 48 hours from the last dose. The duration of treatment was 24 days from the first day of treatment.
▪ Collection of blood samples
Blood sample collected and part of it used as EDTA plasma, and part used for collection of serum for the rest of the parameters, serum was divided into Epindorf tubes, stored at -20°C until analysis.
Heart homogenate preparation. Heart homogenate is prepared according to the method described by 21. Briefly, the homogenate was prepared with ice in the ratio 4 g tissue for 16 mL of phosphate buffer (pH = 7.5) containing 1 mM/L Na2EDTA, 10 mL of 500 mM/L butylated hydroxy toluene (BHT) in acetonitrile was added to the prevent formation of new peroxides during the assay. The homogenates were centrifuged at 2,000 rpm for 4 min at 4°C and the supernatant was frozen at -70°C until analysis [17].
▪ Preparation of ML vs. using thin lipid film method
Stock solution containing phosphatidylcholine, cholesterol and drug either ubiquinone or rivaroxban separately or together in 10:4:1 molar ratios were prepared in chloroform, substances were added in the previous mentioned ratios to chloroform till completely dissolved by vortexing and in water bath sonicator. Appropriate volume of these solutions were transferred to a 250 ml round bottom flask and attached to the rotary vacuum evaporator. The flask was kept immersed in a thermostat water bath, with the temperature set at 42° and rotated at about 150 rpm. Process was allowed to continue till all the liquid had completely evaporated from the solution and a dry lipid film had deposited on the wall of the flask. Flask was rotated under nitrogen gas for another 15 min and. Temperature raised to 45°C and rotated at about 180 rpm, aqueous phase composed of PBS buffer was added to the flask and the flask was rotated with for 30 min or until all the lipid had been removed from the wall of the flask. The suspension was allowed to stand for an optimized period of 2 h at room temperature in order to complete the hydration [18].
▪ Size reduction of ML vs. system
Vibronics -250W probe type ultrasonicator was used for size reduction of MLV dispersion 19 Sample (30 ml), placed in a 50 ml falcon tube and the probe was dipped into it. Total ultrasonication period was 3 min including intermittant stoppage of 30 s.
Encapsulation Efficiency Determination
Encapsulation efficiency is an expression of the amount of drug incorporated into the liposome and is normally defined as the percentage of drug bound to liposomes relative the total amount of drug. Determination of this parameter generally requires separation of free drug from the liposomal formulation. Analysis of drug in both the free and encapsulated drug fractions allow calculation of encapsulation efficiency [19,20]. The encapsulation efficiency of ubiquinone and rivaroxban were expressed as the percent of drug encapsulated and calculated using the following formula: Percent encapsulated of ubiquinone=[- Total ubiquinone]−[Free ubiquinone]/[Total ubiquinone] × 100%. Percent encapsulated of rivaroxban=[Total rivaroxban]−[Free rivaroxban]/[ Total rivaroxban] × 100%
▪ Nanoparticles characterization
Transmission electron microscopy (TEM)
TEM is a procedure that is used to characterize the morphology of NPs. TEM is a microscopy technique whereby a beam of electrons is transmitted through an ultra thin specimen. An image is formed from the electrons transmitted through the specimen, magnified and focused by an objective lens and appears on an imaging screen. TEM operates on the same basic principles as the light microscope but uses electrons instead of light [21].
Dynamic Light Scattering (DLS)
DLS is a non-invasive technique for measuring the size of particles and molecules in suspension. The technique of DLS measures the speed of particles which undergoing Brownian motion (Brownian motion is the random movement of particles due to collisions caused by bombardment of the solvent molecules that surround them).
The speed of the Brownian motion is influenced by particle size, sample viscosity and temperature.
Velocity of the Brownian motion is defined by the translational diffusion coefficient (D), and the translational diffusion coefficient can be converted into a particle size using the Stokes- Einstein equation.
dh=(kT)/(3ny D)
Where: dh=hydrodynamic diameter, k=Boltzmann’sconstant, T=absolute temperature, y=viscosity coefficient, D = diffusion coefficient.
The hydrodynamic diameter will depend not only on the size of the particle “core”, but also on any surface structure, as well as the type and concentration of any ions in the medium.
Zeta potential measurements
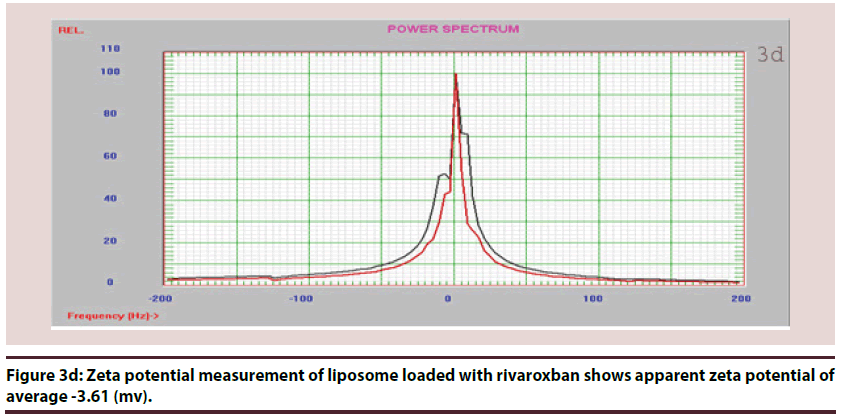
Zeta potential has often been used for characterizing colloidal drug delivery systems. It is a measure of the surface electrical charge of the particles. The magnitude of zeta potential gives an indication of the potential stability of a colloidal system. If all the particles have relatively large negative or positive zeta potentials, they will repel each other and create dispersion stability. If the particles have low zeta potential values, there is no force to prevent the particles from agglomerating and there is dispersion instability [22].
Measurement of plasma cardiac function enzymes
Cardiac dysfunction was assessed by measuring the elevation in serum levels of LDH5, AST 23, CKMB5 using commercially available kits. The results were expressed in u/l [23].
Measurement of plasma cardiac function proteins
Cardiac dysfunction was assessed by measuring the alteration in plasma levels of albumin, and elevation in the concentration of anticardiolipion and albumin, CRP, TNFα using commercially available kits.
Histopathological studies
Specimens excised after dissection, the average size of each section block is 0.6 cm3. Tissue blocks are immediately fixed at 10% buffered formaldehyde solution. Sections submitted in formalin include a minimum of four sections of the ventricles (interventricular septum, anterior wall, lateral wall, and posterior wall); a section of any valve lesions; a section of the mitral and aortic valves; representative sections of each of the coronary arteries and any additional area showing gross pathologic changes. After fixation, these blocks are embedded in paraffin and cut into serial 4 μm thick tissue sections (average size of 80 mm2) for histological staining. Myocardial tissue sections are stained with hematoxylineosin according to standard protocols. Coronary vessel sections are stained with hematoxylin eosin. Documented histological feature of each section has been done: inflammation, interstitial fibrosis, fibrofatty replacement, hypertrophy, atrophy, cellular edema, necrosis [24].
Statistical analysis
Data are expressed as mean ± SEM. The level of statistical significance was taken at P<0.05, using one way analysis of variance (ANOVA), followed by Tukey–Kramer multiple comparison test to judge the difference between various groups. All analysis and graphics were performed using Instant and graph pad Prism computer program.
Results
▪ Preparations results
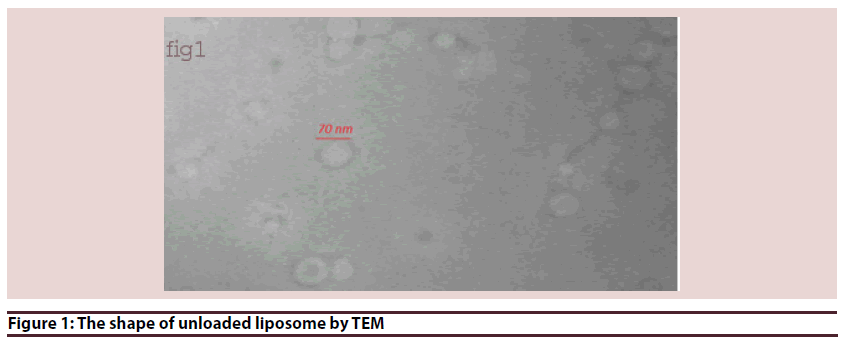
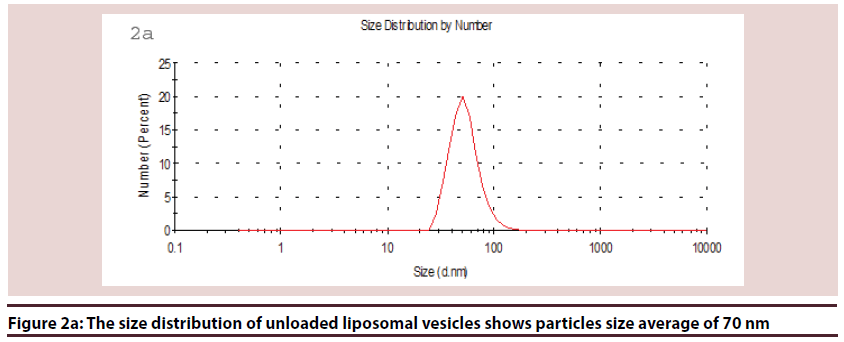
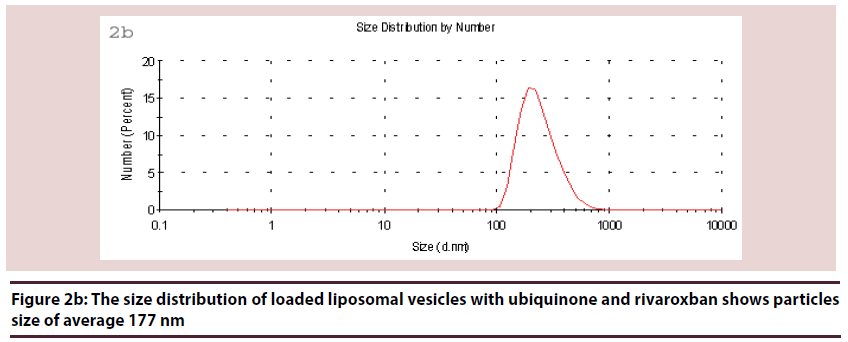
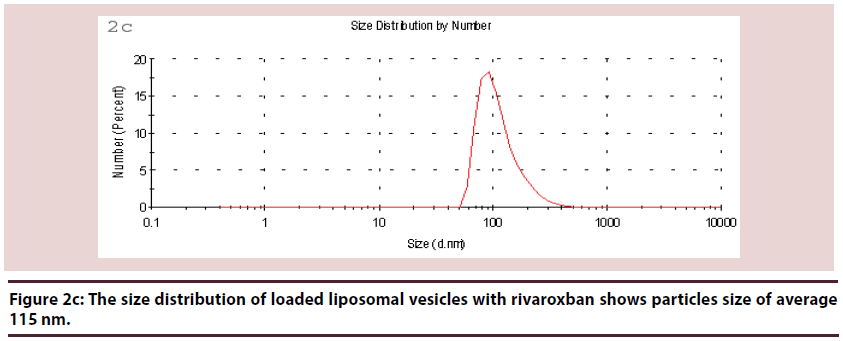
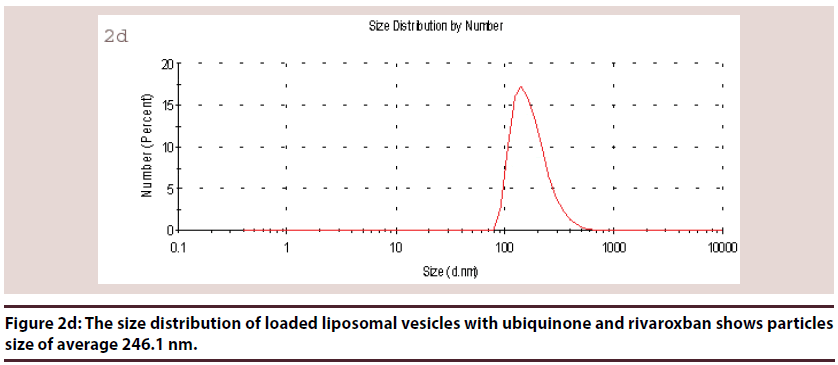
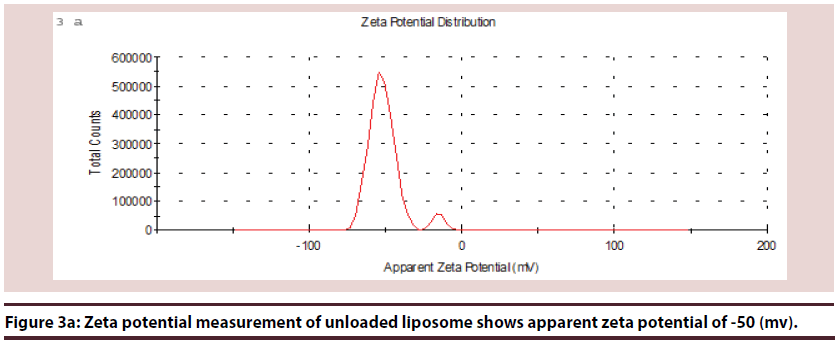
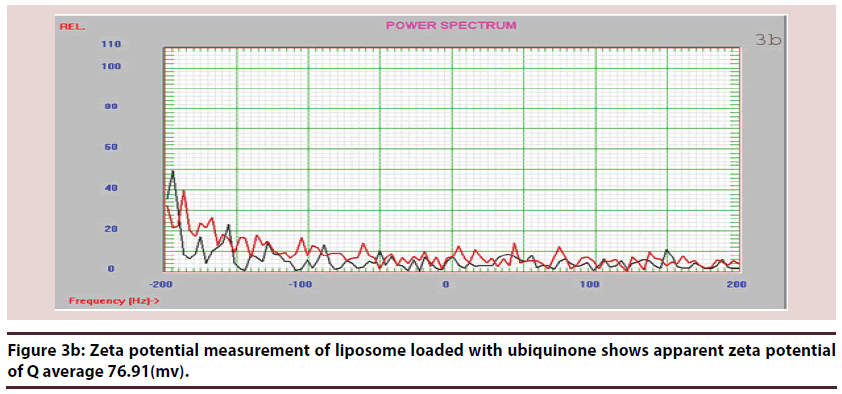
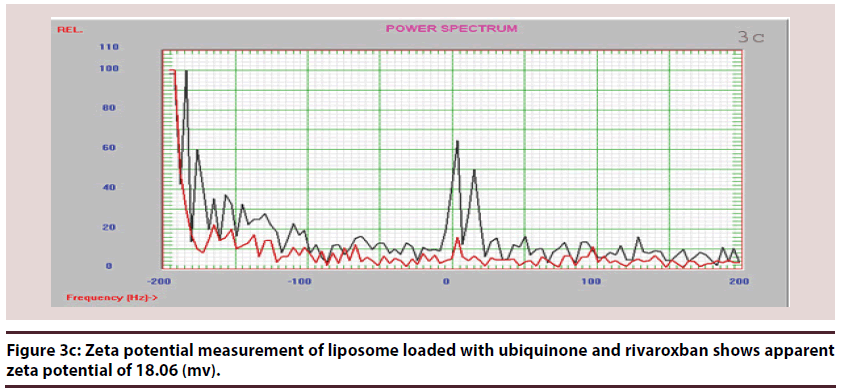
Liposomes (Figures 1-6 and Table 1)
| Group A | Control group |
| Group B | Doxorubicin alone group |
| Group D | Ginger and curry group |
| Group Q | Liposomal ubiquinone |
| Group QR | Liposomal rivaroxban& Ubiquinone |
| Group R | Liposomal rivaroxban |
Table 1: Abbreviations of different groups
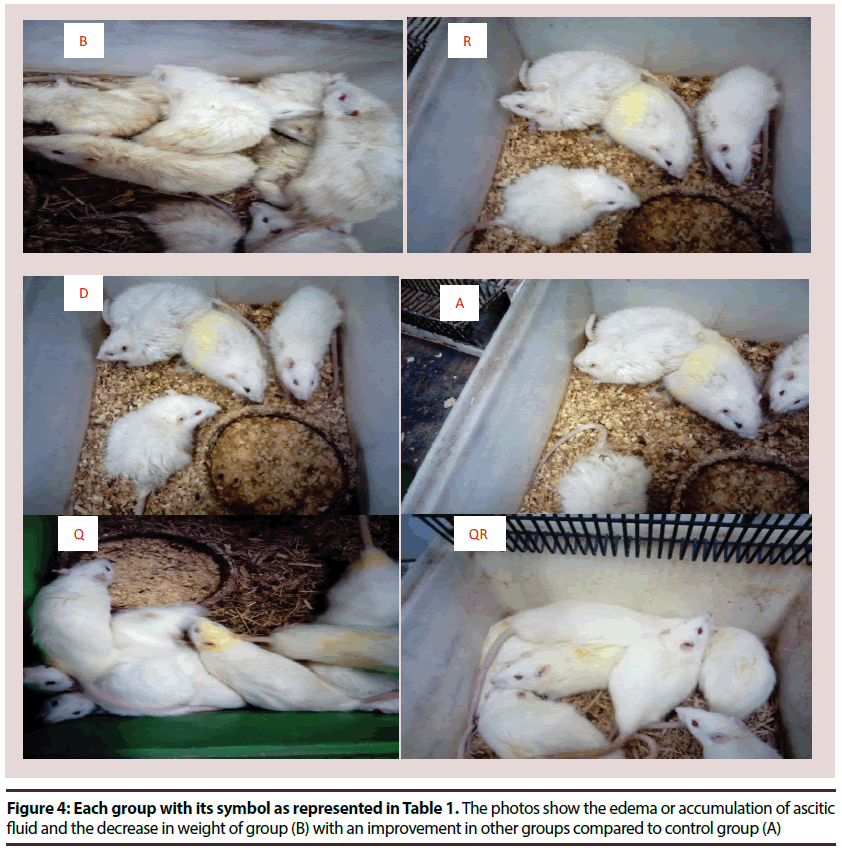
• Photos describe rats’ physical shape as in Figure 4: The photos show the edema or accumulation of ascitic fluid and the decrease in weight of group (B) with an improvement in other groups compared to control group (A)
Parameters evaluate the cardiotoxicity:
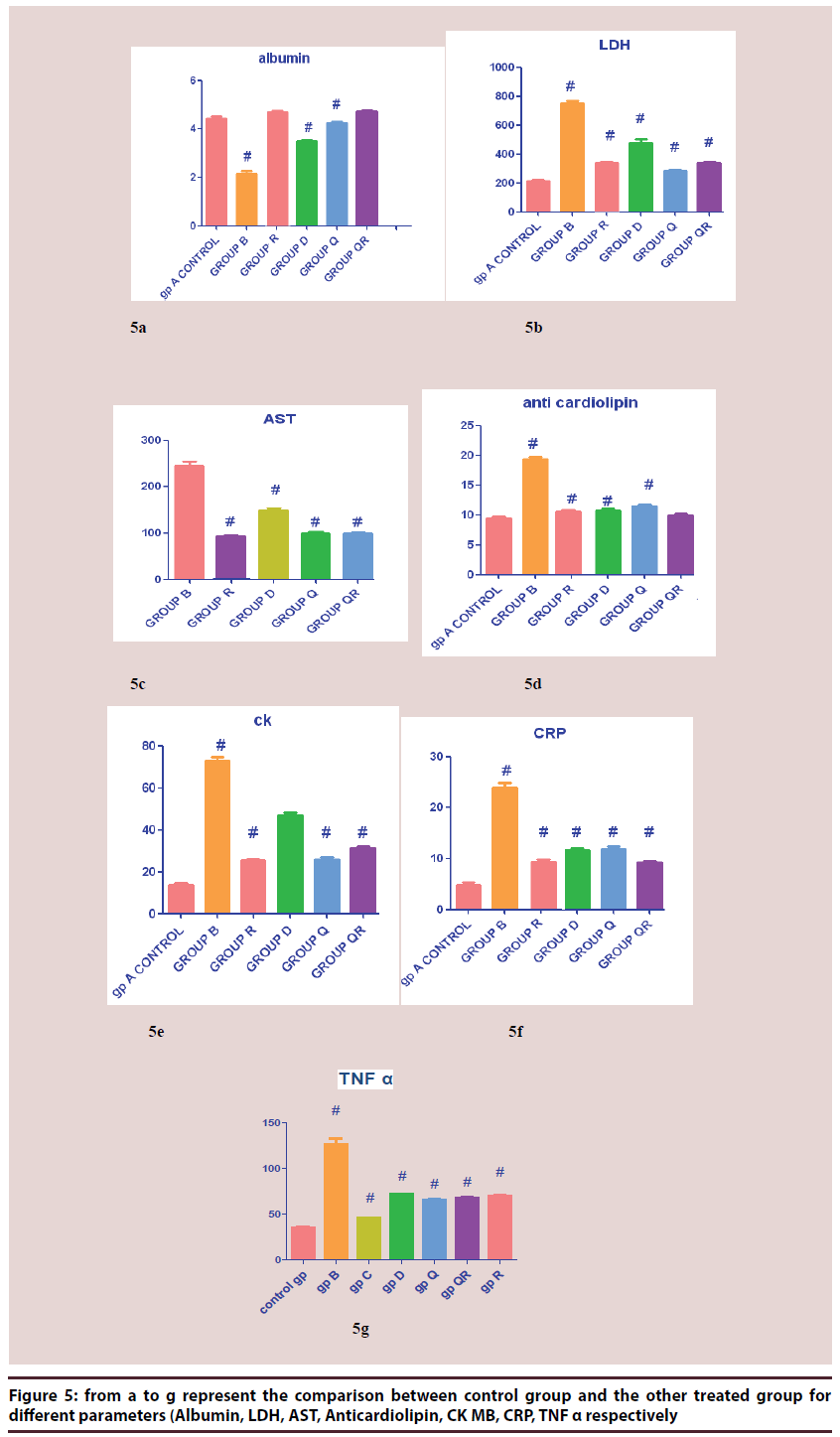
From Figure 5A TO 5G represent the comparison between control group and the other treated group for different parameters (Albumin, LDH, AST, Anticardiolipin, CK MB, CRP, TNF α respectively).
Albumin measurement
The results clearly show statistically significant decrease in mean value of albumin concentration in groups B (Dox. alone treat) Mean ± SEM 2.9 g/dl, D (Herb. treat. Gp) Mean ± SEM 3.49 g/dl, the most deteriorated group was group B and the least was group Q (Q10 treat.), Mean ± SEM 4.24 g/dl compared to control group Mean ± SEM 4.44 g/dl, and show statistically non significant decrease in mean value of other groups compared to control group. The data shows the significant decrease of albumin concentration in group B (Dox. alone treat) compared to control group and the improvement of albumin concentration in other groups especially in group R (Riv. treat.) Mean ± SEM 4.745 g/ dl, QR ((Riv. & Q10 treat.), Mean ± SEM 4.736 g/dl, Q (Q10 treat.) Mean ± SEM 4.24 g/dl.
LDH measurement
The results clearly showed statistically significant increase in mean value of all groups, the most deteriorated group was group B (Dox. alone treat) Mean ± SEM 754 U/L compared to control group Mean ± SEM 212U/L. The most significant increase compared to control group was group B and the least was group Q (Q10 treat.) Mean ± SEM 283 U/L. Also the data shows the significant decrease of LDH mean values in all groups compared to group B (Dox. alone treat) and decrease in LDH activity in all groups compared to group B (Dox. alone treat).
AnticardiolipinIg G measurement
The results clearly show statistically significant increase in mean value of all groups compared to control group with Mean ± SEM 9.4 IU/L. In group B (Dox. alone treat) Mean ± SEM 19.31 IU/L, it showed more than duplicate in the mean value of it compared to control group and there is a significant decrease in the mean values of other groups if compared to group B which shows the effects of different treatments on the improvements of the mean values reflected by the anticardiolipin concentration.
AST measurement
The results show statistically significant decrease in mean value of all groups, the most deteriorated group was group B (Dox. alone treat) with Mean ± SEM 244.8 U/L compared to control group Mean ± SEM 64.60. The least significant increase was group R (Riv. treat.) 92.14 U/L compared to control group and the most significant increase compared to control group was group B Mean ± SEM 244.8 U/L. (Dox. alone treat). Also the data shows the significant decrease of AST mean values in all groups compared to group B (Dox. alone treat) and the improvement of AST concentration in all groups compared to group B (Dox. alone treat)
CK MB measurement
The results clearly showed statistically significant increase in mean value of all groups, the most deteriorated group was group B (Dox. alone treat) Mean ± SEM 73.25 (U/L) compared to control group Mean ± SEM 14.04 U/L. The most significant increase compared to control group was group B (Dox. alone treat) 73.25 (U/L). Also the data shows the significant decrease of CK MB mean values in all groups compared to group B and the improvement of CK MB concentration in all groups compared to group B (Dox. alone treat).
CRP measurement
The results show statistically significant decrease in mean value of all groups, the most deteriorated group was group B (Dox. alone treat) Mean ± SEM 23.96 mg/L, compared to control group Mean ± SEM 4.904 mg/L. The least significant increase was in group QR (Q10 & riv. group) Mean ± SEM 9.240 mg/L compared to control group Mean ± SEM 4.904 mg/L, and the most significant increase compared to control group was group B Mean ± SEM 23.96 mg/L. Also the data shows the significant decrease of AST mean values in all groups compared to group B and the improvement of AST concentration in all groups compared to group B (Dox. alone treat).
TNF alpha measurement
The results clearly showed statistically significant increase in mean value of all groups, the most deteriorated group was group B (Dox. alone treat). 126.6 U/L compared to control group 34.62 U/L. The most significant increase compared to control group was group B and the least was group Q. Also the data shows the significant decrease of TNF alpha mean values in all groups compared to group B and the decrease of TNF alpha concentration in all groups compared to group B (Dox. free).
Histopathological studies
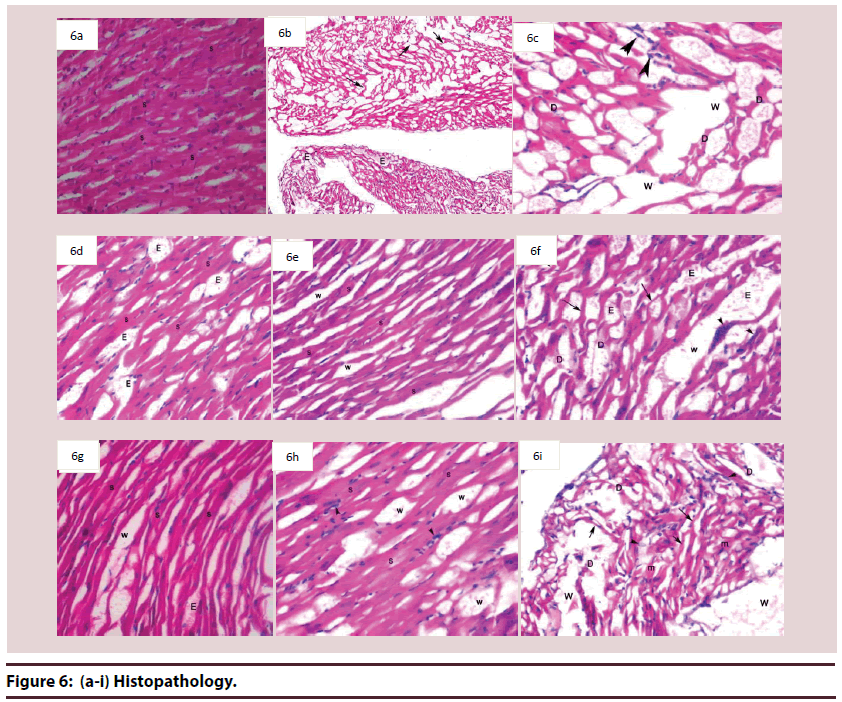
Histological examination of cardiac tissues in the control group (A) Figure 6A showed normal histological architecture, where cardiac myocytes exhibited acidophilic sarcoplasm and centrally located nuclei. Blood capillaries appeared in the intercellular spaces.
Microscopical examination of cardiac tissues from animals of group B (Dox. alone treat) revealed wide areas display distorted architecture, atrophy muscle, sever edema and marked separation of cardiomyocytes (Figure 6B). In addition to, degenerative changes of cardiomyocytes, marked lipid-laden macrophages were also seen Figure 6C.
Examination by light microscope of cardiac section we observed that sections in tissues of group R (Riv. treat) showed moderate improvement, striated cardiomyocytes with mild edema in some areas Figure 6D. However, other areas display distorted architecture, atrophied cardiomyocytes.
Cardiac tissues from animals of group Q (Q10 treat.) showed marked improvement as evidence by restore of normal striated cardiomyocytes with mild separation between cardiomyocytes in most areas (Figure 6E). However, others areas still display moderate distorted architecture, edema, and mild degenerated cardiomyocytes. Beside atrophied muscle fibers, mild separation between cardiomyocytes, this is beside the occurrence of basophilic granules in cardiomyocytes (Figure 6F).
Examining of cardiac tissues of animals of group QR (Q10 & riv. group) showed moderate improvement compared to group B, as evidenced by striated cardiomyocytes, mild edematous interstitial spaces and mild separation between cardiomyocytes compared with B group (Figure 3G).
Histological examination of cardiac tissues from animals of group D (Herbal group) showed moderate improvement in some areas, striated cardiomyocytes muscle and mild separation between cardiomyocytes (Figure 4H). Meanwhile, other areas showed no signs of improvement, where distorted architecture, degenerated cardiomyocytes and marked separation between cardiac muscles, and lipidladen macrophage were could be detected (Figure 4I).
Discussion
According to the preceding view about the cardiotoxicity induced by doxorubicinin group B, and it was clear the toxicity resulted from the administration of doxorubicin (15 mg/kg) in the light of measuring different parameters which is matched by the work of 5, they stated that doxorubicin is a highly effective anticancer agent but causes cardiotoxicity in many patients. The mechanisms of doxorubicininduced cardiotoxicity remain incompletely understood. But recently one of the mechanisms of doxorubicin induced cardiotoxicity by the expression of death receptors in iPS-derived cardiomyocytes. Many substances could be used to decrease the cardiotoxicity in general or the cardiotoxicity induced by doxorubicin. In our study there was a clear evidence of toxicity induced by doxorubicin which has been chosen as a model of cardiotoxic drugs, the toxicity indicated by increase in AST, CKMB, LDH enzymes concentration and also in increase in inflammatory mediators like CRP, TNFα, also in thrombosis related proteins like anticardiolipin and decrease in albumin plasma concentration. One of the main causes of doxorubicin toxicity induced by initiation of ROS, as Asensio et al., [25]. In this regard, doxorubicin is a potent and exogenous ROS generator that may eventually be involved in delayed cardiomyopathy when used in cancer chemotherapy. Stated that the whole nanoparticles system leads to a special function related to treating, preventing or diagnosing diseases sometimes called smartdrugs or the agnostics [26]. It could be seen that rivaroxban liposome may act as protective against doxorubicin toxicity; rivaroxban is a potent anticoagulant acts as Xa factor inhibitor; it is obvious from parameters measured that the rivaroxban significantly changed the parameters compared to doxorubicin treated group. In addition, the histopathological studies showed improvement compared to group (B). One of the most common mechanisms of cardiotoxicity is thrombosis as was mentioned by (kumar et al.,) [5], DOX can induce procoagulant activity in platelets, an important contributor to thrombus formation. Consistently, DOXtreated platelets and generated MPs induced thrombin generation, a representative marker for procoagulant activity. DOX-induced PS exposure appeared to be from intracellular Ca²⁺ increase and ATP depletion, which resulted in the activation of scramblase and inhibition of flippase. The exact mechanism of cardiotoxicity of doxorubicin is somewhat controversial. There are two main theories (i) iron-related free radicals and formation of doxorubicinol metabolite and (ii) mitochondrial disruption [27], from this point ubiquinone was selected as a basic component in the respiratory chain [28], could compensate the disruption results from doxorubicin. Also, ubiquinone is a powerful antioxidant [29], that has been proved in our work in which the group treated with ubiquinone was significantly improved in comparison with group B. The combination of rivaroxban and ubiquinone did not add much of effect as if they are given alone, may probably result from an interaction happened between the two drugs, although it was effectively, a method to decrease the harmful effect of doxorubicin which appeared in the decrease of the doxorubicin cardiotoxicity. Ginger and curry are models of herbals that have been used in many diseases especially C vs. diseases. The consumption of ginger could decrease many substances like cholesterol, thromboxane-B and PGE which plays a very important role as an anti-inflammatory and anti thrombotic agent, Leaf extract of M. Koenigii has been reported to have strong anti-oxidative, antiinflammatory, anti-platelet and nitric oxide scavenging activities as we have seen in our results that combination of both herbs exerted a beneficial effect on decreasing the toxicity of doxorubicin illustrated with decrease in AST, CRP, anticardiolipin, LDH, with increase in albumin concentration [30].
Conclusion
It can be extracted from the obtained data, the application of liposomal rivaroxban as an antithrombic, with ubiquinone as a powerful antioxidant and also as a pivotal substance in the respiratory chain could reduce the toxicity of doxorubin. In addition, the administration of ginger and curry mixed in diets could also be one of the factors that lessen the harmful effect of doxorubicin.
Conflicts of interest
There is no conflict of interest
Acknowledgement
We would like to express our great appreciation to Prof. DR/ Mohamed Kandeel for his guidance.
We also would like to offer our special thanks to DR/ Mohamed Fathi Elasaal for His great efforts and cooperation.
Finally, we would like to thank our Colleagues and our Professors for their encouragement to finish this work.
Author’s Contribution
Omayma Ahmed Ragab and Osama Hassan Ahour carried out the experiment.
Ibraheem Abulyazed wrote the manuscript
Mamdouh Salah EL-din Zohni had derived the models and analysed the data
All authors provided critical feedback and helped shape the research, analysis and manuscript.
References
- Qanitha A, de Mol BAJM, Burgner DP, et al. Pregnancy-related conditions and premature coronary heart disease in adult offspring. Heart Asia. 9(1), 90-95 (2017).
- Ansell J. New oral anticoagulants should not be used as first-line agents to prevent thromboembolism in patients with atrial fibrillation. Circulation. 125, 165-170 (2012).
- Pourmoghaddas M, Rabbani M, Shahabi J, et al. Combination of atorvastatin/coenzyme Q10 as adjunctive treatment in congestive heart failure: A double-blind randomized placebo-controlled clinical trial. ARYA Atheroscler. 10, 1-5 (2014).
- Quinzii CM, Emmanuele V, Hirano M. Clinical presentations of coenzyme Q10 deficiency syndrome. Mol. Syndromol. 5, 141-146 (2014).
- Kumar L, Yadav YS, Rathnanand M. Simultaneous determination of linezolid and levamisole hydrochloride in a fixed dose combination. Indian. J. Pharm. 51, 613-619 (2017).
- Toth PP. Considerations for long-term anticoagulant therapy in patients with venous thromboembolism in the novel oral anticoagulant era. Vasc. Health Risk Manag. 12, 23-34 (2016).
- Keo HH, Fahrni J, Husmann M, et al. Assessing the risk of recurrent venous thromboembolism &ndash; a practical approach. Vasc. Health Risk Manag. 451 (2015).
- Weitz JI, Lensing AWA, Prins MH, et al. Rivaroxaban or Aspirin for Extended Treatment of Venous Thromboembolism. N. Engl. J. Med. 376, 1211-1222 (2017).
- Zhang D, Contu R, Latronico MVG, et al. MTORC1 regulates cardiac function and myocyte survival through 4E-BP1 inhibition in mice. J. Clin. Invest. 120(8), 2805-2816 (2010).
- Liao YR, Leu YL, Chan YY, et al. Anti-platelet aggregation and vasorelaxing effects of the constituents of the rhizomes of zingiber officinale. Molecules. 17, 8928-8937 (2012).
- Jain V, Momin M, Laddha K. Murraya koenigii. An Updated Review. Int. J. Ayurvedic Herb. Med. 2, 607-627 (2012).
- Gannon WL, Sikes RS. Guidelines of the American Society of Mammalogists for the use of Wild Mammals in Research. J. Mammalogy. 88(3), 809-823 (2007).
- Zhu W, Shou W, Payne RM, et al. A mouse model for juvenile doxorubicin-induced cardiac dysfunction. Pediatr Res. 64(5), 488-494 (2008).
- Garrido-Maraver J, Cordero MD, Oropesa-Ávila M, et al. Coenzyme Q10 Therapy. Mol Syndromol. 5(3-4), 187-197 (2014).
- Parry TJ, Huang Z, Chen C, et al. Arterial antithrombotic activity of rivaroxaban, an orally active factor Xa inhibitor, in a rat electrolytic carotid artery injury model of thrombosis. Blood Coagul Fibrinolysis. 22(8), 720-726 (2011).
- Reddy E, Ramya P. Use of certain herbal preparations in broiler feeds - A review Tirupathi. 6(3), 172-179 (2013).
- Noori S, Azmat M, Mahboob T. Study on antioxidant effects of cinnamon and garlic extract in liver, kidney and heart tissue of rat. Biol Res. 9(1), 17-22 (2012).
- Song J, Xing Y, Chen X, et al. Processing of the Explanted Heart. N Am J Med Sci. 6(12), 613-617 (2014).
- Rathod S, Deshpande SG. Design and evaluation of liposomal formulation of pilocarpine nitrate. Indian J. Pharm. Sci. 72(2), 155-160 (2010).
- Schroeder A, Kost J, Barenholz Y. Ultrasound, liposomes, and drug delivery: principles for using ultrasound to control the release of drugs from liposomes. Chem Phys Lipids. 162(1-2), 1-16 (2009).
- Ong SGM, Ming LC, Lee KS, et al. Influence of the encapsulation efficiency and size of liposome on the oral bioavailability of griseofulvin-loaded liposomes. Pharmaceutics. 8(3), (2016).
- Thomas S, Jovic A, Morana B, et al. Characterization of Thermal Expansion Coefficient of LPCVD Polycrystalline SiC Thin Films Using Two Section V-beam Actuators. Procedia Eng. 168, 1144-1147 (2016).
- Hanaor DAH, Michelazzi M, Leonelli C, et al. The Effects of Carboxylic Acids on the Aqueous Dispersion and Electrophoretic Deposition of ZrO 2. J. Eur. Ceram. Soc. 32(1), 235-244 (2012).
- Huang XJ, Choi YK, Im HS, et al. Aspartate Aminotransferase (AST/GOT) and Alanine Aminotransferase (ALT/GPT) Detection Techniques. Sensors. 6 (7), 756-782 (2006).
- Asensio-López MC, Soler F, Pascual-Figal D, et al. Doxorubicin-induced oxidative stress: The protective effect of nicorandil on HL-1 cardiomyocytes. PLoS One. 12, 1-21 (2017).
- De Jong WH, Borm PJA. Drug delivery and nanoparticles:applications and hazards. Int. J. Nanomedicine. 3(2), 133-149 (2008).
- Thorn C, Oshiro C, Marsh S, et al. Doxorubicin pathways:pharmacodynamics and adverse effects. Pharmacogenet Genomics. 21(7), 440-446 (2012).
- Papa S, Capitanio G, Papa F. The mechanism of coupling between oxido-reduction and proton translocation in respiratory chain enzymes. Biol. Rev. Camb. Philos. Soc. 93(1), 322-349 (2017).
- Calogero AE, Condorelli RA, Russo GI, et al. Conservative Nonhormonal Options for the Treatment of Male Infertility: Antibiotics, Anti-Inflammatory Drugs, and Antioxidants. Biomed. Res. Int. 2017, 4650182 (2017).
- Punuru P, Sujatha D, Kumari BP, et al. Evaluation of aqueous extract of Murraya koenigii in unilateral renal ischemia reperfusion injury in rats. Indian J. Pharmacol. 46(2), 171-175 (2014).