Device Evaluations - Interventional Cardiology (2012) Volume 4, Issue 1
Biolimus-eluting stent with biodegradable polymer: one step forward in the fight against stent thrombosis vulnerability?
- Corresponding Author:
- Timothy Watson
Department of Cardiology
Cardiac Catheterization Laboratory B2–127
Academic Medical Center
University of Amsterdam Meibergdreef 9, 1105 AZ, Amsterdam, The Netherlands
Tel: +31 (20) 5668672
Fax: +31 (20) 6962609
E-mail: j.j.wykrzykowska@amc.uva.nl
Abstract
Keywords
biodegradable polymer, Biolimus A9™, biolimus-eluting stent, BioMatrix Flex™, polylactic acid
The coronary stent was developed to overcome the shortcomings of coronary angioplasty: acute vessel closure due to coronary dissections and restenosis due to late constrictive recoil [1]. The first coronary stent was bare-metal and selfexpanding. Although these bare-metal stents (BMS) offered an alternative for emergency coronary artery bypass graft surgery as a bailout after complicated coronary balloon angioplasty, they had the major limitation of subacute thrombotic occlusion, observed in more than 10% of cases [2,3]. Anticoagulation regimens were developed and despite the initial concerns about increased bleeding rates [4], both the BENESTENT and the STRESS trials, which compared stent use with balloon angioplasty, showed stent use was a safe technique [5,6].
Besides the risk of subacute thrombosis, another problem concerning stent use arose: instent neointimal hyperplasia [7,8]. This growth of scar tissue within the stent is a result of proliferation and migration of vascular smooth muscle cells. In 1999, the Cypher® stent, which was coated with sirolimus, a macrolide antibiotic that also inhibits the cytokine- and growthfactor- mediated proliferation of lymphocytes and smooth muscle cells, was developed: the drug-eluting stent (DES) was born [9].
Body of review
▪▪ Overview of the market & historical perspective
Follow-up data of the initial first-in-man (FIM) studies on DES indeed showed reduced neointimal proliferation [10,11]. Subsequently, the RAVEL and the SIRIUS trials both demonstrated significantly lower rates of target lesion revascularization (TLR) and major adverse cardiovascular events (MACE) after treatment with the Cypher sirolimus-eluting stent (SES) compared with BMS [12–15]. Other major trials in different clinical settings [16–26] and registries [27,28] confirmed these results.
Almost simultaneously, the paclitaxeleluting stent (PES) was developed. Paclitaxel inhibits smooth muscle cell proliferation through stabilization of microtubules, inhibiting cell division. PES also showed a significantly lower rate of late loss, angiographic restenosis and repeat revascularizations compared with BMS in subsequent trials in different clinical settings up to 4 years followup [29–37]. In addition, 2-year follow-up of an unrestricted single-center registry that used the PES as the default stent for all percutaneous coronary intervention in 576 consecutive real-world patients, showed the same results in suppressing neointimal growth and reduction of restenosis as in the SES registries [38]. The first PES to be approved was the TAXUS® Express® stent. A few years later, this was followed by the TAXUS Liberté™ stent. When comparing Taxus PES with Cypher SES, the pivotal SIRTAX study showed a significant reduction in TLR, whereas no differences in death or myocardial infarction (MI) were noted [39,40]. These results were confirmed in a meta-analysis of 16 randomized trials [41].
Results from the largest meta-analysis on DES to date, which includes more than 18,000 patients from 38 DES trials, indicated TLR reduction of 70% with the use of SES and 58% with PES [42]. Overall, it is justified to conclude that DES had several benefits in terms of reduced rates of restenosis compared with BMS. However, with the introduction of DES, there were also some concerns being raised regarding their safety.
First of all, there were concerns that the use of DES would increase mortality. These concerns were based on four early publications: a meta-analysis including 17 randomized trials [42,43], the single center BASKET-LATE trial, which compared SES with BMS in unselected patients [44], a pooled analysis from the first Cypher SES trials, RAVEL, SIRIUS, E-SIRIUS and C-SIRIUS [45] and the 3-year results of the SCAAR registry [46]. However, several subsequent patient-based meta-analyses [42,47,48] and large registries [49], demonstrated the safety of DES with respect to deaths and MI rates.
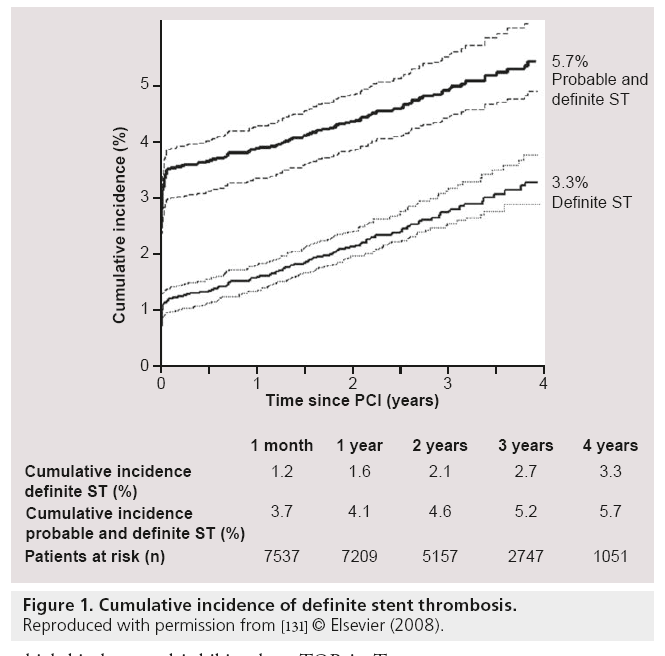
Besides mortality there was a second major concern about the use of DES: stent thrombosis (ST) [50,51]. Several postmarketing surveillance studies and trials of multivessel percutaneous coronary intervention reported a risk for ST within 1 year after DES implantation, ranging from 0.2 to 0.5% [52–54]. Although these rates are similar to BMS, the concerns remained and subsequent meta-analyses were performed to further investigate this phenomenon. These analyses demonstrated similar rates of overall ST between DES and BMS [42,47,48,55–57]. Despite the similar rates of early (<30 days) and late ST (>30 days), there was a significantly higher rate of very late ST (>1 year) seen in DES patients, compared with BMS [42,47,48,55–57]. Furthermore, three large registries showed that the risk of very late ST persists at an annual rate, ranging from 0.36 to 0.6% per year up to 5 years follow-up after DES implantation (Figure 1) [58–61].
Figure 1: Cumulative incidence of definite stent thrombosis.
Reproduced with permission from [131] © Elsevier (2008).
These higher rates of very late ST in first generation DES could be explained by several reasons:
▪▪ Delayed and/or incomplete endothelialization of stent struts in DES;
▪ Inflammatory response to the stent polymer;
▪ Stent malapposition.
Delayed/incomplete endothelialization
Drugs used in DES are antiproliferative to prevent neointimal hyperplasia. The same drugs, on the other hand, could also delay and/or impair endothelialization, resulting in thin and locally incomplete coverage of stent struts. As a consequence, blood is exposed to thrombogenic struts, potentially precipitating ST [50,62–65]. An optical coherence tomography (OCT) study showed that 9 months after SES placement, 12.3% of the struts were still uncovered [66].
Inflammatory response
To ensure controlled release according to pharmacokinetic principles, a polymer coating, which contains the drug to be eluted, is all but mandatory. In the first generation DES, these polymers remain on the stent surface after drug elution is completed. These permanent polymers can cause delayed healing, impaired stent strut endothelialization and a hypersensitivity reaction, which can culminate in ST [62,67–70]. Histopathological findings indicated that ST is precipitated by induced localized vascular inflammation, hypereosinophilia, thrombogenic reactions and apoptosis of smooth muscle cells [69,70].
Stent malapposition
Incomplete stent apposition (ISA) was recognized to be highly prevalent in patients with very late ST after DES implantation [71]. An OCT study showed that ISA is associated with delayed coverage of the stent struts [72], which could explain why ISA is highly prevalent in patients with very late ST.
Consequently, there was a need to develop new DES, using polymers, which are more biocompatible. One of these second-generation DES was the Endeavor® zotarolimus-eluting stent (ZES). The ZES is coated with a phosphorylcholine (PC) polymer. A total of 90% of the phospholipids in the outer membrane of red blood cells contain a PC headgroup. As the PC polymer of the ZES resembles the chemical structure of these PC headgroups, the polymer was thought to be more biocompatible and decreased inflammation of the struts. In animal models, this second-generation DES had displayed higher re-endothelialization percentages compared with first-generation DES [68]. OCT studies in humans demonstrated significantly better strut coverage in ZES than in SES or PES [66,73].
Another second-generation DES was the everolimus-eluting stent (EES), marketed by Abbott Vascular as the Xience V® stent and by Boston Scientific as the Promus™ stent. Its 6–8 μm thick polymer is composed of the biocompatible acrylic and fluorinated polymers, which release about 80% of the drug within 30 days, with almost all of the drug released at 4 months. Randomized controlled trials (RCT) that compared EES and PES, demonstrated superior efficacy and safety with EES and, importantly, a significant reduction in ST rates [74–77]. When comparing EES with SES, four major RCTs showed no benefit of the EES over SES regarding death, MI, TLR or overall definite ST [78–81].
Thus, second-generation DES offered some benefits over first-generation DES. However, challenges remain to further improve the DES technology. Although restenosis rates of the second-generation stents are low, restitution of healthy endothelial lining is still a target of ongoing research. Furthermore, despite the fact that ST after treatment with second-generation DES became a rare phenomenon, with a total risk for very late (probable/definite) ST of 0.5% between 1 and 3 years follow-up [82], it is still an iatrogenic complication, with a potentially high mortality rate. Biocompatible polymers could be a part of the solution to overcome this latter issue.
Introduction to the device
As mentioned above, in DES a polymer was mandatory to carry and gradually release the drug. After the development of more biocompatible polymers, the next logical step was to design a polymer that would degrade after it had released all of the drug. Such a design could potentially prevent the polymer-induced inflammatory and/or prothrombogenic reactions. This concept of a biodegradable polymer led to the development of the Biolimus A9™-eluting stents of the BioMatrix™ famlily (Biosensors International, Morges, Switzerland).
The platform used in the BioMatrix family of stents, is a balloon expandable intracoronary 316L stainless steel stent with Quadrature Link™ design. The stent struts have an average thickness of 120 μm. The struts are coated abluminally with a 50:50 matrix of polylactic acid (PLA) and Biolimus A9™.
Biolimus A9, is a macrocylic triene lactone sirolimus derivate with pharmacological properties specifically tailored for stent application and localized drug delivery. It has similar immunosuppressant and anti-inflammatory properties as sirolimus. Due to its high lipophilicity (tentimes higher than sirolimus), it strongly binds to the vessel wall and is rapidly absorbed by tissue, minimizing the systemic exposure of the drug. Similarly to sirolimus, Biolimus A9 likely forms a complex with intracellular FKBP-12, which binds to and inhibits the mTOR in T lymphocytes and smooth muscle cells. With the inhibition of mTOR, a signaling pathway is blocked, resulting in blockage of a protein kinase, which in turn is necessary for ribosomal biosynthesis and translation of the mRNA required for the G1 to S phase transition in the cell cycle. Biolimus A9 has been shown to inhibit T-cell and smooth muscle growth [83].
The polymer used in the biolimus-eluting stents (BES) of the BioMatrix family is a PLA. PLAs were primarily used as materials for sutures, prosthetic implants and in vivo drug delivery systems. In vivo, with the degradation of PLA by hydrolysis of polymer chains, nontoxic lactic acid is released, which is further converted into water and carbon dioxide via the Krebs cycle. By minimizing the polymer weight (possible because of the high drug-carrying capacity) the inflammation and tissue reaction to the polymer is minimized.
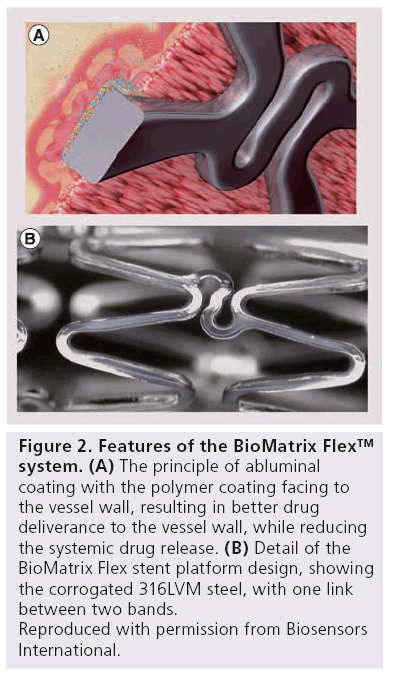
The PLA/Biolimus A9 coating is applied only to the abluminal stent surface, resulting in more targeted drug delivery to the vessel wall, while reducing the systemic drug release. In these sections, PLA degrades uniformly by bulky erosions, resulting in a simultaneous release of drug and polymer. After 6–9 months, both polymer degradation, as well as drug release, are complete (Figure 2).
Figure 2: Features of the BioMatrix Flex™ system.
(A) The principle of abluminal coating with the polymer coating facing to the vessel wall, resulting in better drug deliverance to the vessel wall, while reducing the systemic drug release.
(B) Detail of the BioMatrix Flex stent platform design, showing the corrogated 316LVM steel, with one link between two bands.
Reproduced with permission from Biosensors International.
Clinical studies & postmarketing findings
The STEALTH I trial was a FIM, RCT designed to investigate the safety and efficacy of the BioMatrix stent [84]. This trial compared the BioMatrix stent with the bare-metal S-stent in 120 patients undergoing coronary angioplasty, who were randomly assigned in a 2:1 proportion to the BioMatrix-stent group and the control S-stent group. The primary end point was in-lesion late loss at 6 months. Secondary end points were event-free survival (defined as absence of MACE) and reduction in binary restenosis at 6-month follow-up. After 6 months, there was a significant difference found in in-lesion late loss, with a late loss of 0.14 mm in the BioMatrix group compared with 0.40 mm in the S-stent group (p < 0.004). There was also a difference seen in the in-stent late loss (0.26 vs 0.74 mm; p < 0.001). Binary restenosis rates did not differ significantly between groups (3.9 vs 7.7%; p = 0.40). The event-free survival was also similar for both groups at 6 months. This FIM trial demonstrated that the use of BES was safe and effectively reduced neointimal proliferation and restenosis when used to treat de novo coronary lesions.
The STEALTH PK study investigated the pharmacokinetics of Biolimus A9 [85]. Twenty-seven patients treated with a BioMatrix stent were enrolled in this study. A total of 7 days after implantation, 44% of the patients had Biolimus A9 concentrations below the LLOQ of 10 pg/ml, as assessed with the validated and highly sensitive LC–MS/MS assay. After 28 days, this percentage was 51.8%. At 3 and 6 months, all patients had Biolimus A9 concentrations below the LLOQ. The mean Biolimus A9 concentration between placement and 28 days follow-up was 151 ± 114 pg/ml (mean ± SD). The amount of Biolimus A9 on the stent and the area under the time–concentration curve over the observation period (r = -0.15; p < 0.44; n = 27) or maximum concentration (r = 0.25; p < 0.21; n = 27) were not correlated. No early or late bursts of Biolimus A9 release from the stents were detected. These results showed that the systemic exposure of the drug was relatively low, when compared with other DES, which may be explained by the abluminal coating of the stent and the high lipophilicity of Biolimus A9.
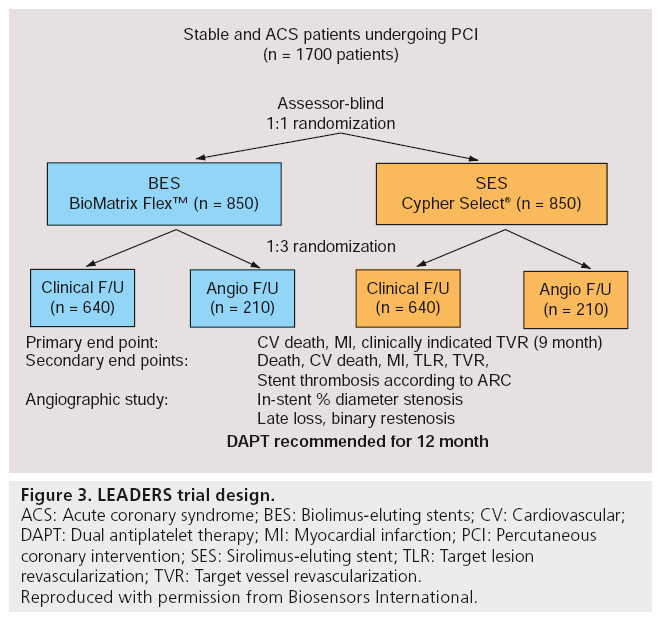
The LEADERS study was a randomized, multicenter, assessor-blind, noninferiority trial performed in ten European centers, which enrolled an all-comers, ‘real world’ patient population [86]. In total, 1707 patients aged 18 years or older with chronic stable coronary artery disease or acute coronary syndromes (ACS) were randomized to treatment with either biodegradable polymer biolimus-eluting (BioMatrix Flex™; n = 857) or permanent polymer sirolimus-eluting (Cypher Select™; n = 850) stents. The primary end point of this trial was MACE rate, defined as the composite of cardiac death, MI or clinically indicated target-vessel revascularization (TVR) within 9 months. In addition, 427 patients were randomly allocated to angiographic follow-up, with in-stent percentage diameter stenosis as the principal outcome measure at 9 months (Figure 3). For the primary end point, the BES turned out to be noninferior to the SES (9 vs 11%; risk ratio [RR]: 0.88; 95% CI: 0.64–1.19; pnoninferiority = 0.003; psuperiority = 0.39), with similar frequencies of cardiac death, MI or TVR between both groups. BES was also noninferior to SES in in-stent percentage diameter stenosis (20.9 vs 23.3%; difference -2.2%; 95% CI: -6.0–1.6; pnoninferiority = 0.001; psuperiority = 0.26). At 12 months follow-up, BES remained noninferior compared with SES for the primary end point (10.6 vs 12% respectively; hazard ratio [HR]: 0.88; 95% CI: 0.66–1.17; psuperiority = 0.37) [87]. These results suggested that the BioMatrix Flex BES was a safe and effective alternative to the reference system of first-generation DES, the Cypher Select SES.
Figure 3: LEADERS trial design.
ACS: Acute coronary syndrome; BES: Biolimus-eluting stents; CV: Cardiovascular;
DAPT: Dual antiplatelet therapy; MI: Myocardial infarction; PCI: Percutaneous
coronary intervention; SES: Sirolimus-eluting stent; TLR: Target lesion revascularization; TVR: Target vessel revascularization.
Reproduced with permission from Biosensors International.
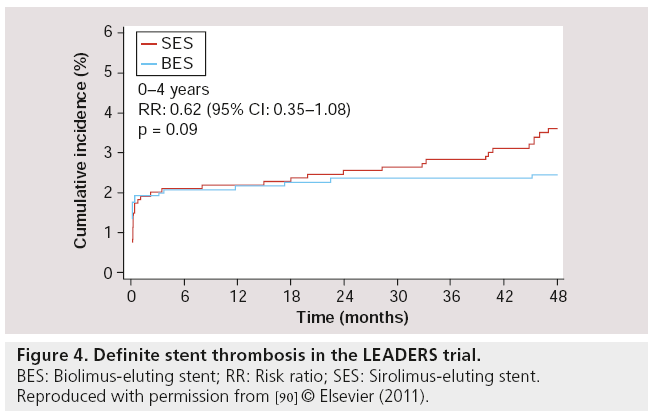
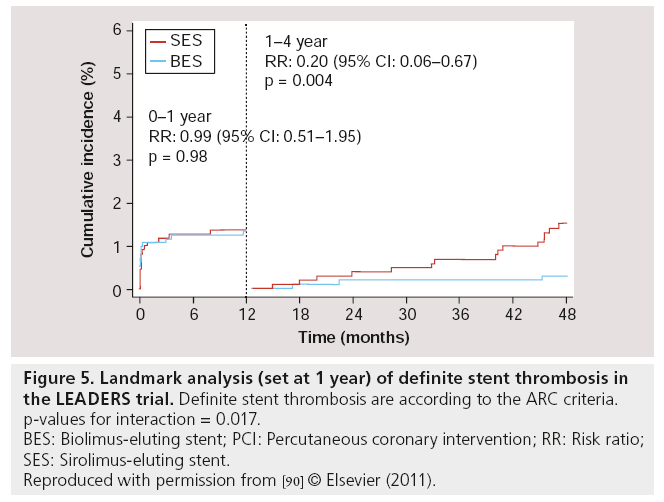
Follow-up results at 2 years [88], 3 years [89] and 4 years [90] of LEADERS were consistent with the 9-month follow-up results. For the primary end point (MACE – defined as the composite of cardiac death, MI or clinically-indicated TVR), BES remained noninferior compared with SES (MACE rates at 4 years: 19 vs 23% respectively; RR: 0.81; 95% CI: 0.66–1.00; p = 0.05), while the Kaplan–Meier event curves showed an increasing divergence with a difference in events rate of 1.4, 2.4, 3.3 and 4% at 1, 2, 3 and 4 years respectively, in favor of BES (with psuperiority = 0.37, 0.18, 0.09 and 0.05 at 1, 2, 3 and 4 years, respectively). At 4-year follow-up, the rates of cardiac death (6 vs 7%; RR: 0.88; 95% CI: 0.60–1.29; p = 0.51), MI (8 vs 9%; RR: 0.96; 95% CI: 0.69– 1.33; p = 0.80) and clinically-indicated TVR (9.4 vs 11%; RR: 0.84; 95% CI: 0.62–1.13; p = 0.25) were numerically lower in BES compared with SES, although no statistically significant difference was found. Most importantly however, the rates of definite ST were 2.4% for BES and 4.0% for SES (HR: 0.62; 95% CI: 0.35–1.08; p = 0.09) at 4-year follow-up. In the BES group, definite ST was increased by 0.4% from 1–4 years, while in the SES group, it was increased by 2.0% in the same period (Figure 4). Furthermore, a landmark analysis (set at 1 year) indicated a significant difference between BES and SES in the development of definite ST from 1–4 years, in favor of BES (Figure 5) [91]. Thus, BES appeared to reduce rates of very late ST.
Figure 4: Definite stent thrombosis in the LEADERS trial.
BES: Biolimus-eluting stent; RR: Risk ratio; SES: Sirolimus-eluting stent.
Reproduced with permission from [90] © Elsevier (2011).
Figure 5: Landmark analysis (set at 1 year) of definite stent thrombosis in the LEADERS trial. Definite stent thrombosis are according to the ARC criteria.
p-values for interaction = 0.017.
BES: Biolimus-eluting stent; PCI: Percutaneous coronary intervention; RR: Risk ratio;
SES: Sirolimus-eluting stent.
Reproduced with permission from [90] © Elsevier (2011).
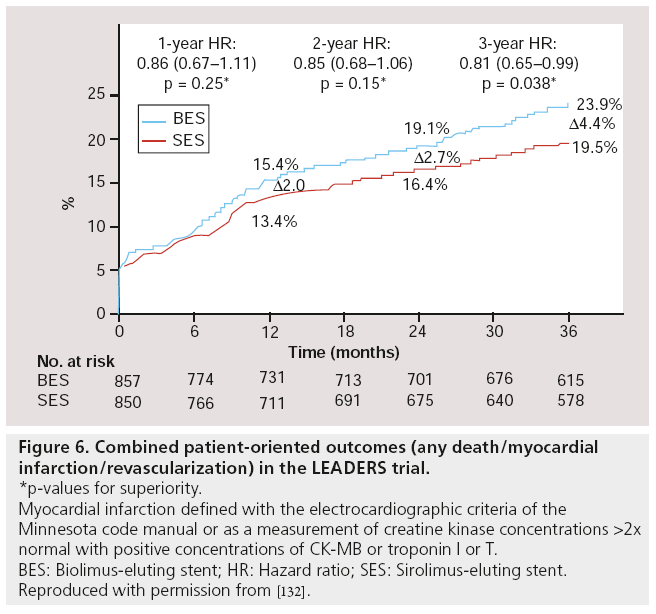
A post hoc analysis using a patient-oriented composite end point of any death, MI or revascularization, rather than the device/lesion- oriented primary end point used in LEADERS (composite of cardiac death, MI or clinically indicated TVR), BES performed significantly better than SES (16.6 vs 23.9%; HR: 0.81; 95% CI: 0.65–0.99; p = 0.038, Figure 6) [91].
Figure 6: Combined patient-oriented outcomes (any death/myocardial infarction/revascularization) in the LEADERS trial.
*p-values for superiority.
Myocardial infarction defined with the electrocardiographic criteria of the Minnesota code manual or as a measurement of creatine kinase concentrations >2x normal with positive concentrations of CK-MB or troponin I or T.
BES: Biolimus-eluting stent; HR: Hazard ratio; SES: Sirolimus-eluting stent.
Reproduced with permission from [132].
In LEADERS, stratified analyses of the primary end point were prespecified according to the presence or absence of the following characteristics: diabetes, ACS or de novo lesions. Additionally, post hoc analyses were performed stratified according to acute ST-segment elevation MI (STEMI), multivessel disease, smallvessel disease, long lesions, bifurcation lesions (BL) and patients who were at higher risk (according to the SYNTAX score [SX]).
In diabetic patients, the RR for the primary end point (MACE) at 4-year follow-up in BES versus SES was 1.00 (95% CI: 0.70–1.44; p = 0.88), whereas in nondiabetic patients the RR was 0.70 (95% CI: 0.54–0.91; p = 0.007), with a nonsignificant p-value for interaction [90].
In patients who were treated for STEMI in the LEADERS trial, a significant benefit was found with respect to the primary end point of MACE at 4 years follow-up in the BES group compared with the SES group. RR in the STEMI group was 0.45 (95% CI: 0.24–0.83; p = 0.009) and the RR in patients without STEMI was 0.88 (95% CI: 0.70–1.10; p = 0.26), with p = 0.043 for interaction [90].
Since neointimal hyperplasia is usually independent of vessel size [92,93], small vessels are more prone to restenose [94,95] (a late lumen loss of 0.7 mm is obviously of greater hemodynamical importance in a small vessel with 2 mm diameter then in a larger vessel with 3.5 mm diameter). In a LEADERS substudy, small vessels were defined as vessels with a reference diameter of ≤2.75 mm. Of the 1707 patients included in the LEADERS trial, there were 429 patients in the BES group and 434 patients in the SES group who were treated for small vessels only. In this small-vessel subgroup, there were no significant differences seen between the BES and SES in overall MACE rate (12.1 vs 11.8%; p = 0.89) or TLR rates (9.6 vs 7.4%; p = 0.26). Overall, MACE and TLR rates were significantly different between the small-vessel group and the large-vessel group. This substudy showed BES was noninferior in the treatment of small vessels compared with SES in this ‘all-comer’ population [96].
It is widely accepted that, when compared with BMS, DES reduce restenosis rates and the need for repeat revascularizations [42]. However, although superior to BMS, the first-generation DES still have higher repeat revascularization rates in long lesions compared with ‘short’ lesions [97,98]. Of the 1707 patients included in the LEADERS trial, 305 patients had long lesions, defined as a lesion of >20 mm in length (153 BES vs 151 SES patients). In this subgroup, the MACE rate was similar between BES and SES patients (17 vs 14.6%; p = 0.62). There was also no significant difference found in late loss (0.32 ± 0.69 vs 0.24 ± 0.57; p = 0.59). On the other hand, there was a significant difference seen in binary in-segment restenosis, with a restenosis rate of 23.2% in the BES group compared with 13.1% in the SES group (p = 0.042). Furthermore, there was a trend towards higher overall TLR rate with BES (12.4 vs 6.0%; HR: 2.06; p = 0.07) and clinically driven TLR (10.5 vs 5.3%; HR: 1.94; p = 0.13). In conclusion, BES and SES appeared similar with respect to MACE in long lesions in an ‘all-comers’ patient population. However, long lesions had a higher rate of binary in-segment restenosis following BES when compared with SES treatment, at 1-year follow-up.
Another subgroup analysis was performed in patients with BLs. In the BMS era, BLs were associated with lower procedural success and poorer clinical outcome [99]. The introduction of DES has significantly decreased in-stent restenosis and consequently lowered the repeat revascularization rates [100,101]. However, safety concerns have emerged, especially with respect to ST [102]. Of the 1707 patients included in LEADERS, 497 patients underwent treatment of at least one BL. At 12 months, there was no difference in the primary end point MACE (BES 12.8 vs SES 16.3%; p = 0.31). There were no differences in definite ST after 12 months (BES 1.9 vs SES 2.5%; p = 0.77). Cardiac death (BES 2.7 vs SES 2.9%; p = 1.00) and MI (BES 8.9 vs SES 5.4%; p = 0.17) rates were not statistically significantly different, but there was a significant difference in clinically indicated revascularization (BES 4.3 vs SES 11.3%; p = 0.004) in this subgroup. Of the total of 534 treated BLs in LEADERS, 232 were ‘true’ BLs (i.e., lesions involving both main and side branch [SB]; medina score [103] 1.1.1, 1.0.1, 0.1.1), with 131 patients treated with BES and 102 patients with SES. The use of BES was associated with significantly lower rates of percutaneous revascularization (TLR and TVR) within this true bifurcation group (p < 0.05). In LEADERS, the treatment strategy for BLs was not prespecified in the protocol but left to the discretion of the operator. The most often used strategy was the single-stent approach, being used in over 80% of cases, with a low crossover rate to a two-stent strategy of 5.3% and comparable MACE rates of 14.0 and 16.7% for one- and two-stent strategies, respectively [104]. The clinically indicated revascularization rate of 4.3% in the BES group was lower if compared with the 5.3% of TLR rate, which was found in two large meta-analyses, comparing clinical outcomes in 1641 patients with BLs between one- or two-stent technique in the DES era [105,106].
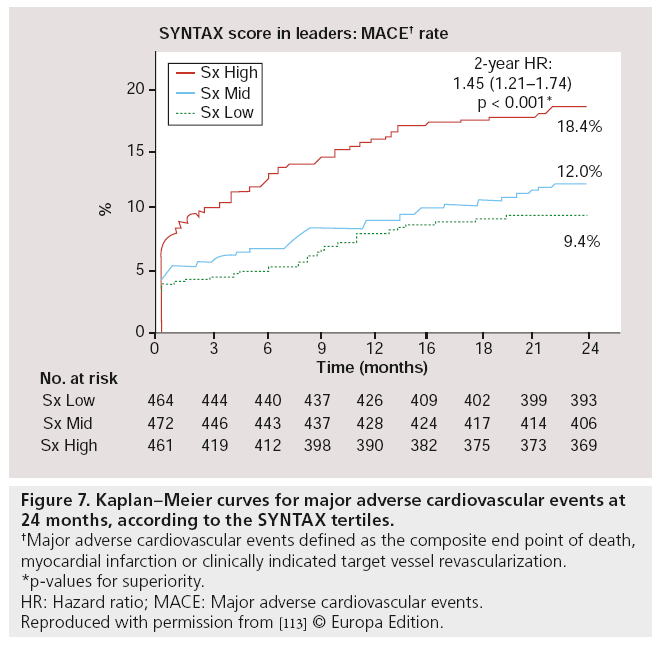
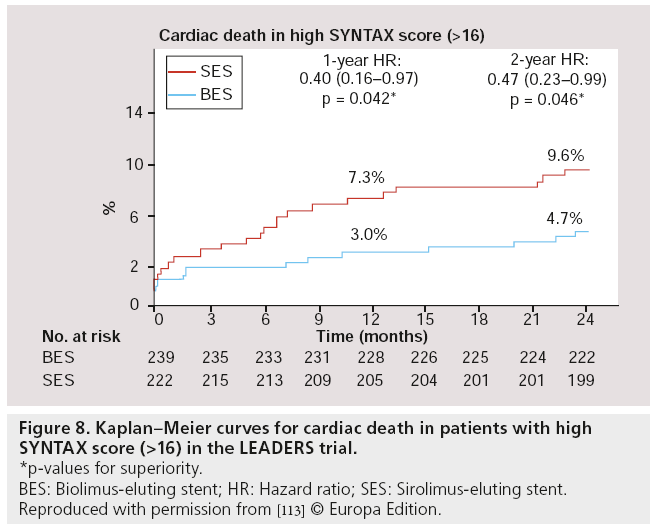
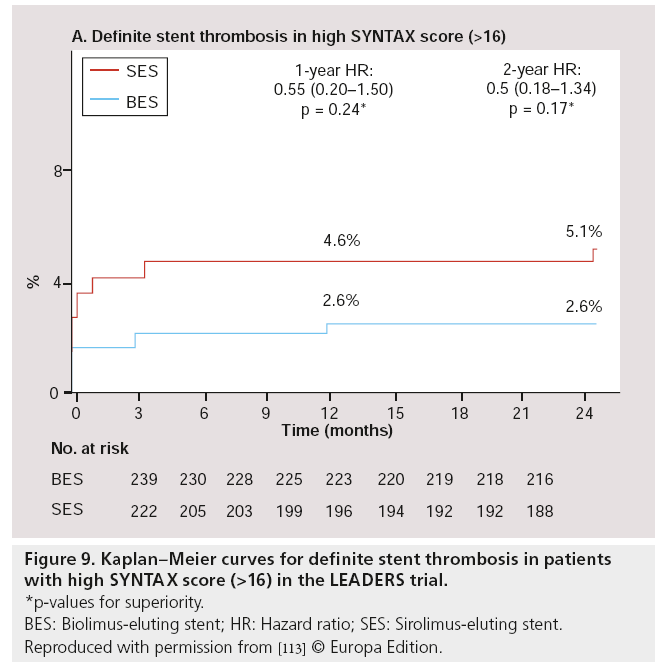
Besides post hoc subgroup analyses on high-risk lesions, another major substudy was performed, focussed on high-risk patients as defined by the SX. The SX is a comprehensive angiographic scoring system derived from the coronary anatomy and lesion characteristics [107,108] initially designed to quantify coronary lesion complexity, which could be used as a decision-making tool for treatment allocation. Additional analyses have subsequently demonstrated its ability to predict MACE, following percutaneous revascularization in patients with multivessel coronary artery disease at follow-up ranging from 1 to 5 years [109–111]. In the LEADERS substudy, SX was collected prospectively in 1397 ‘all-comer’ patients [112]. Patients were divided according to their SX into tertiles defined as: SX low ≤ 8 (n = 467), SX mid 9–16 (n = 472) and SX high >16 (n = 461). Overall, patients stratified to the highest SX, showed increased rates of MACE (Figure 7), cardiac death, TVR and TLR, whereas no differences were found in MI rates among the different SYNTAX groups, after 2-year followup. If BES was compared with SES within the SX high group, overall MACE (again defined as the composite of cardiac death, MI and clinically- indicated TVR) rate tended to be lower in the BES treated group (15.3 vs 21.8%; HR: 0.68; 95% CI: 0.44–1.04; p = 0.08) after 2-year follow-up. Cardiac death was significantly lower in this SX high group if patients were treated with BES compared with SES (4.7 vs 9.5%; HR: 0.48; 95% CI: 0.23–0.99; p = 0.046; Figure 8). Moreover, both definite (2.6 vs 5.1%; p = 0.17) as well as probable ST (2.6 vs 4.8%; p = 0.23) were nonsignificantly lower in the SX high group treated with BES when compared with the SES treated SX high group, at 2-year follow-up (Figure 9) [113].
Figure 7: Kaplan–Meier curves for major adverse cardiovascular events at 24 months, according to the SY NTAX tertiles.
†Major adverse cardiovascular events defined as the composite end point of death, myocardial infarction or clinically indicated target vessel revascularization.
*p-values for superiority.
HR: Hazard ratio; MACE: Major adverse cardiovascular events.
Reproduced with permission from [113] © Europa Edition.
Figure 8: Kaplan–Meier curves for cardiac death in patients with high SY NTAX score (>16) in the LEADERS trial.
*p-values for superiority.
BES: Biolimus-eluting stent; HR: Hazard ratio; SES: Sirolimus-eluting stent.
Reproduced with permission from [113] © Europa Edition.
Figure 9: Kaplan–Meier curves for definite stent thrombosis in patients with high SY NTAX score (>16) in the LEADERS trial.
*p-values for superiority.
BES: Biolimus-eluting stent; HR: Hazard ratio; SES: Sirolimus-eluting stent.
Reproduced with permission from [113] © Europa Edition.
Although these subgroup analyses may have been underpowered and post hoc analyses have to be interpreted with caution, they show a trend towards superiority of biolimus-eluting biodegradable-polymer stents in particularly challenging lesion subsets and patients, except in the long-lesion subgroup.
Another substudy of LEADERS evaluated 56 patients at 9-month follow-up with OCT. Twenty patients (29 lesions, with 4592 struts) included in this substudy were treated with BES and 26 (35 lesions, with 6476 struts) with SES. At 9 months, a total of 83 struts were uncovered in the BES group and 407 in the SES group (weighted difference -1.4%; 95% CI: -3.7–0.0; p = 0.04). There remained a difference after adjustment for preprocedure lesion length, reference vessel diameter, number of implanted study stents and presence of overlapping stents. Three lesions in BES and 15 in SES had >5% uncovered struts (difference -33.1%; 95% CI: -61.7 to -10.3; p < 0.01). In conclusion, it seems that strut coverage appears to be more complete in BES after 9 months [114]. However, at 24 months follow-up, strut coverage was improved in SES, resulting in similar stent coverage in BES and SES [115].
The higher rate of very late ST of BES compared with SES in the LEADERS trial at 4 years follow-up (as shown with a landmark analysis set at 1 year), could not be explained by the lower rate of uncovered struts in BES, as shown in the OCT study at 9 months, because at 24 months there was a ‘catch-up’ of strut coverage observed in Cypher Select SES.
Alternative biolimus-eluting devices
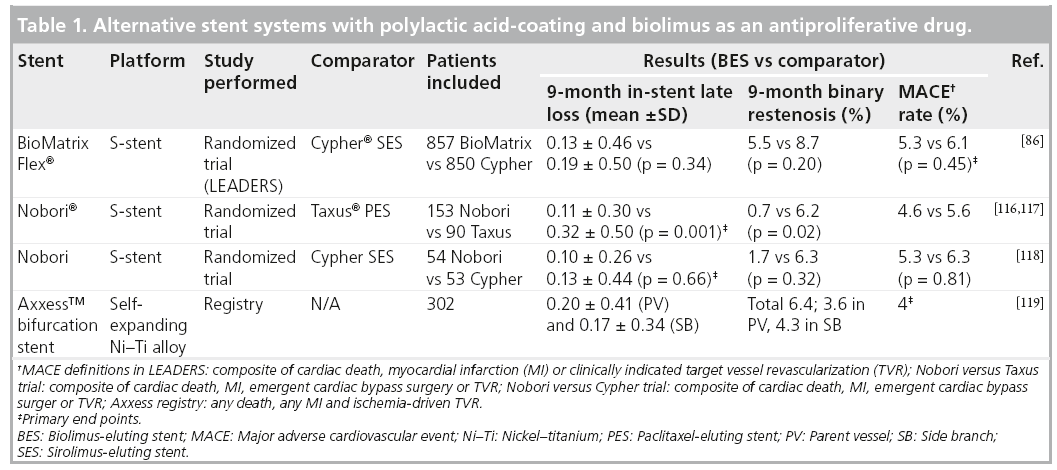
Besides the stents of the BioMatrix family, there exist stents which are coated with PLA and use Biolimus A9 as an antiproliferative drug (Table 1). The first of these stents is the Nobori® stent (Terumo Corporation, Tokyo, Japan), with the S-stent™ platform (as in the BioMatrix stent). The Nobori stent proved to be safe when compared with the Taxus PES in 243 randomized patients (153 Nobori and 90 Taxus) [116,117]. Nobori showed to have significantly lower instent late loss at 9 months compared with PES (0.11 ± 0.30 vs 0.32 ± 0.50 mm, respectively), both the primary hypothesis of noninferiority (p < 0.001) and the secondary hypothesis of superiority (p = 0.001) reached significance [117]. There was also a significantly lower binary restenosis rate in the Nobori group (0.7 vs 6.2%; p = 0.02) and neointimal volume obstruction as found with intravascular ultrasound (1.8 vs 5.5%; p = 0.01) [117]. Although not significant, MACE rate (4.6 vs 5.6%) and ST rate (0 vs 4.4%) were numerically lower in the Nobori group. In addition, 107 patients were randomized between the Nobori stent (n = 54) and the Cypher SES (n = 53). The primary end point – in-stent late loss at 9 months – was equal between both stents (Nobori 0.10 ± 0.26 mm vs Cypher 0.13 ± 0.44 mm; p = 0.66). There was a significantly lower in-stent diameter stenosis found in the Nobori group (13 ± 10% vs 20 ± 12%; p = 0.002), without significant difference in binary restenosis (Nobori 1.7% vs Cypher 6.3%; p = 0.32) [118]. At 12 months follow-up, MACE rate was low in both groups (Nobori 1.9% vs Cypher 4.1%). In conclusion, the Nobori stent was shown to be safe and effective (with respect to angiographic end points).
Another alternative system, which uses PLA polymer coating in combination with biolimus drug is the CE-approved Axxess™ (Biosensors Int., Morges, Switzerland). It is a dedicated bifurcation stent and its platform, which is a nickel–titanium alloy, allows the stent to selfexpand [119]. The stent has a conical-shaped design, with a distal wide end, which has to be positioned at the level of the carina and then seated within the ostia of both the parent vessel as well as the SB, providing access to these vessels for additional stent placement. A registry, which enrolled 302 patients where 299 patients were treated with the Axxess stent, showed a 9-month MACE rate of 7.7% [120]. Intravascular ultrasound and angiographical follow-up at 9 months showed subacute and late ST of 0.7 and 0.3%, respectively. Binary restenosis rate was 6.4%, with 3.6% in the main branch and 4.3% in the SB. The SB restenosis rate was particularly low compared with bifurcation treatment with other nondedicated DES. Late loss was 0.20 ± 0.41 mm (parent vessel) and 0.17 ± 0.34 mm (SB), whereas in the Axxess stent segment, percentage neointimal volume obstruction was 4.3 ± 5.2%. These results suggested that the use of this device is safe, but randomized trials are necessary to confirm this.
How the technology fits into the field of medical devices
The BioMatrix Flex DES is indicated for improving coronary luminal diameter and reducing stent restenosis for the treatment of de novo lesions in native coronary arteries with a reference diameter ranging from 2.25–4.00 mm. Stents with length 33 and 36 mm are only available for artery diameters ranging between 2.5 and 3.5 mm. The BioMatrix Flex DES with stent lengths up to 28 mm is also indicated for use in patients with ACS (including ACS-STEMI, ACS-NSTEMI and unstable angina) and diabetes mellitus. The BioMatrix Flex DES is available in the European Union, South America, Africa and Asia Pacific (excluding China and Japan).
Conclusion
The results of the LEADERS trial showed that the BioMatrix Flex BES is noninferior to the Cypher Select SES regarding to the composite primary end point of cardiac death, MI or clinically-indicated TVR. LEADERS was an ‘all-comers’ trial with an off-label use of 80%, therefore different subgroups of interest could be identified and analyzed, with a follow-up period of up to 4 years. In some of these subgroups, BES was shown to be superior to SES with respect to the primary and/or one of the secondary end points. Overall, the superiority of BES over SES in the primary composite end point in LEADERS approached statistical significance at 4 years follow-up, while a landmark analysis on very late ST actually showed clear superiority of BES over DES in the overall group.
Although these subgroup and post hoc analyses should be interpreted with caution and ST was only superior in BES if a landmark analysis was performed, these results suggest that the theoretical advantage of the BioMatrix Flex stent design with a biodegradable polymer and a highly lipophilic drug, could be translated also into an advantage in real-world clinical practice. Better strut coverage at 9 months in BES, as shown with an OCT study [114], could only partly explain lower rates of very late ST, because strut coverage was equal between BES and SES at 24 months follow-up, and therefore no conclusions could be drawn regarding the mechanism of reduction of the very late ST rates with BES. Whether complete polymer degradation at 6–9 months indeed decreases inflammatory and prothrombogenic reactions, and is therefore protective for very late ST, could not be concluded with the current evidence. The clinical results at 4 years follow-up of the LEADERS trial, however, seem to indicate a clinical advantage of this technology.
Currently, the clinical program of the BioMatrix family involves more than 14,000 patients, including a randomized trial comparing BES with BMS in STEMI patients (COMFORTABLE AMI) and the planned Global LEADERS (>10,000 patients) trial.
Future perspective
Although biodegradable polymer metallic devices appear to offer advantages over first-generation DES, there may be other solutions to prevent ST. The reasons for ST are multifactorial. Besides patient characteristics and environment, there are the aforementioned device related factors, such as delayed and/or incomplete endothelialization, inf lammatory response to the stent polymer and stent malapposition.
▪▪ Delayed and/or incomplete endothelialization
To promote endothelialization after stent placement, the Genous™ endothelial progenitor cell (EPC)-capturing stent was developed. This stent is coated with CD34+ antibodies specifically targeting the circulating EPC population. These bone-marrow-derived EPCs are associated with arterial repair and it was hypothesized that both ST and restenosis rates would decrease by improving endothialization of stent struts, resulting in fast coverage with healthy endothelium. The first registry studies showed promising results regarding ST rates (12 months definite ST rates of 0.5 and 0.6%), where TLR rates at 12 months were moderately high (5.7 and 10.9%) [121,122]. A large RCT, randomizing Genous against DES (Taxus PES, Cypher SES, Endeavor ZES or Xience V EES, at the discretion of the operator), was stopped early (after inclusion of 50% of patients), as recommended by the data and safety monitoring board, due to an excessive TLR rate in the Genous arm. A new EPC-capturing stent was developed with an abluminal coating of a sirolimus-eluting biodegradable polymer: the Combo™ dualtherapy stent. In the REMEDEE trial, where the Combo stent was randomized against Taxus PES, the Combo stent was noninferior to Taxus regarding the primary angiographic end point of late lumen loss at 9 months (0.39 ± 0.45 mm in the Combo stent and 0.44 ± 0.56 mm in the Taxus) [123]. Whether the combination of the EPC-capturing technique with an abluminal sirolimus eluting, biodegradable polymer coating could decrease restenosis and ST rates, when compared with the currently widely used second-generation DES, should be further investigated.
▪▪ Inflammatory response to the stent polymer
Currently, a stent system, which elutes biolimus from a polymer-free drug-coated stent, the BioFreedom™ (BFD), is under investigation. A pilot study of 182 patients (60 patients treated with BFD standard dose, 62 with BFD low dose and 60 with Taxus Liberté) shows that the BFD standard dose is noninferior to the Taxus Liberté concerning the in-stent late lumen loss at 1 year (0.17 vs 0.35 mm; pnoninferior = 0.001; psuperior = 0.11), whereas no differences were found between groups in MACE rates (defined as the composite of all death, Q wave MI and non-Q wave MI, emergency coronary artery bypass graft surgery and TLR) [124].
Although currently not commercially available, an everolimus-eluting device, with a fully biodegradable scaffolding, could potentially offer a definitive solution for the long-term risk of very late ST, as the scaffold is completely metabolized to CO2 and water after 9 months and the normal vessel architecture is restored at 3 years (i.e., the struts are completely integrated into the vessel wall) [125]. The FIM ABSORB trial, including 30 patients, showed a composite end point of cardiac death, MI or ischemiadriven TLR of only 3.4% after 3 years follow-up [126–128]. Further studies are necessary to fully assess the performance of the fully degradable device, especially in complex lesions. Its deployment may require lesion preparation and certainly mandates very careful device sizing, making it at this time challenging to use for most high volume operators.
▪▪ Stent malapposition
Lastly, a new stent system, the Stentys® selfdeploying stent, could offer a solution to the problem of stent malapposition. In the setting of STEMI, the presence of thrombus and epicardial vasoconstriction could result in undersizing of the stent, potentially resulting in ST [102]. The Stentys stent is a nickel–titanium alloy, self-expanding stent, which further expands even days after placement [129]. In a FIM study, Stentys were shown to be safe and feasible, with a procedural success rate of 95.5% and a MACE rate at 30 days of 5.1% [130]. In a study with STEMI patients only, Stentys were shown to have a 6 months in-stent late lumen loss of 0.71 ± 0.71 [129]. In the same study, Stentys were shown to expand to the same extent as the vessel vasodilates (18 and 19%, respectively) and potentially offer a solution of stent undersizing and stent malapposition, especially in the setting of STEMI.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. No writing assistance was utilized in the production of this manuscript.
Executive summary
Device description: BioMatrix™ family
▪ Platform: a balloon-expandable, corrugated 316 LVM steel-ring stent, with Quadrature Link™ design.
▪ Polymer: polylactic acid, which is fully absorbed after 6–9 months. Polylactic acid acts as a carrier for the drug and biodegrades along with the drug elution.
▪ Drug: Biolimus A9™, is a semisynthetic rapamycin derivative with enhanced pharmacokinetic properties, which strongly blocks a signaling pathway by inhibiting mTOR, with blockage of protein kinase as result, inhibiting T-cell and smooth muscle cell growth.
▪ Abluminal coating: The PLA/Biolimus A9-coating of the stent is abluminal, resulting in more targeted tissue release and less systemic exposure.
Safety
▪ The LEADERS trial with biodegradable polymer showed that BioMatrix Flex was noninferior regarding major adverse cardiovascular events (cardiac death, myocardial infarction and clinically-indicated target lesion revascularizations) at 9 months follow-up (pnoninferiority = 0.003).
▪ Biolimus-eluting stents (BES) remained noninferior at 4 years follow-up.
Clinical efficacy
▪ LEADERS 4 years follow-up: BioMatrix Flex approached superiority regarding the primary composite end point (19 vs 23%; risk ratio (RR): 0.81; 95% CI: 0.66–1.00; psuperiority = 0.05).
▪ A landmark analysis, set at 1 year, showed significantly less stent thrombosis in BES (probable/definite stent thrombosis between 1 and 4 years; RR: 0.20; 95% CI: 0.06–0.067; p = 0.004).
▪ Patients with ST-segment elevation myocardial infarction treated with BES in LEADERS were shown to have a lower rate of the composite end point, compared with sirolimus-eluting stent (RR: 0.45; 95% CI: 0.24–0.83; p = 0.009).
▪ Patients with a high SYNTAX score treated with BES within the LEADERS trial showed significantly lower cardiac death rates at 2 years follow-up compared with SES (4.7 vs 9.6%; hazard ratio: 0.48; 95% CI: 0.23–0.99; p = 0.046).
▪ In a LEADERS bifurcation substudy, patients in the BES group had significantly lower clinically-indicated revascularization rates at 1-year follow-up compared with the sirolimus-eluting stent group (4.3 vs 11.3%; p = 0.004).
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- Sigwart U, Puel J, Mirkovitch V, Joffre F, Kappenberger L. Intravascular stents to prevent occlusion and restenosis after transluminal angioplasty. N. Engl. J. Med. 316(12), 701–706 (1987).
- Serruys PW, Strauss BH, Beatt KJ et al. Angiographic follow-up after placement of a self-expanding coronary-artery stent. N. Engl. J. Med. 324(1), 13–17 (1991).
- Nath FC, Muller DW, Ellis SG et al. Thrombosis of a flexible coil coronary stent: frequency, predictors and clinical outcome. J. Am. Coll. Cardiol. 21(3), 622–627 (1993).
- van Domburg RT, Foley DP, de Jaegere PP et al. Long term outcome after coronary stent implantation: a 10-year single center experience of 1000 patients. Heart 82(Suppl. 2), II27–II34 (1999).
- Serruys PW, de Jaegere PP, Kiemeneij F et al. A comparison of balloon-expandable stent implantation with balloon angioplasty in patients with coronary artery disease. Benestent Study Group. N. Engl. J. Med. 331(8), 489–495 (1994).
- Fischman DL, Leon MB, Baim DS et al. A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. N. Engl. J. Med. 331(8), 496–501 (1994).
- Gordon PC, Gibson CM, Cohen DJ, Carrozza JP, Kuntz RE, Baim DS. Mechanisms of restenosis and redilation within coronary stents: quantitative angiographic assessment. J. Am. Coll. Cardiol. 21(5), 1166–1174 (1993).
- Hoffmann R, Mintz GS, Dussaillant GR et al. Patterns and mechanisms of in-stent restenosis. A serial intravascular ultrasound study. Circulation 94(6), 1247–1254 (1996).
- Sousa JE, Costa MA, Abizaid A et al. Lack of neointimal proliferation after implantation of sirolimus-coated stents in human coronary arteries: a quantitative coronary angiography and three-dimensional intravascular ultrasound study. Circulation 103(2), 192–195 (2001).
- Sousa JE, Costa MA, Abizaid AC et al. Sustained suppression of neointimal proliferation by sirolimus-eluting stents: one-year angiographic and intravascular ultrasound follow-up. Circulation 104(17), 2007–2011 (2001).
- Rensing BJ, Vos J, Smits PC et al. Coronary restenosis elimination with a sirolimus eluting stent: first European human experience with 6-month angiographic and intravascular ultrasonic follow-up. Eur. Heart J. 22(22), 2125–2130 (2001).
- Morice MC, Serruys PW, Sousa JE et al. A randomized comparison of a sirolimuseluting stent with a standard stent for coronary revascularization. N. Engl. J. Med. 346(23), 1773–1780 (2002).
- Morice MC, Serruys PW, Barragan P et al. Long-term clinical outcomes with sirolimuseluting coronary stents: five-year results of the RAVEL trial. J. Am. Coll. Cardiol. 50(14), 1299–1304 (2007).
- Weisz G, Leon MB, Holmes DR Jr. et al. Two-year outcomes after sirolimus-eluting stent implantation: results from the sirolimuseluting stent in de novo native coronary lesions (SIRIUS) trial. J. Am. Coll. Cardiol. 47(7), 1350–1355 (2006).
- Weisz G, Leon MB, Holmes DR Jr. et al. Five-year follow-up after sirolimus-eluting stent implantation results of the SIRIUS (sirolimus-eluting stent in de novo native coronary lesions) trial. J. Am. Coll. Cardiol. 53(17), 1488–1497 (2009).
- Schofer J, Schluter M, Gershlick AH et al. Sirolimus-eluting stents for treatment of patients with long atherosclerotic lesions in small coronary arteries: double-blind, randomized controlled trial (E-SIRIUS). Lancet 362(9390), 1093–1099 (2003).
- Jimenez-Quevedo P, Sabate M, Angiolillo DJ et al. Long-term clinical benefit of sirolimuseluting stent implantation in diabetic patients with de novo coronary stenoses: long-term results of the DIABETES trial. Eur. Heart J. 28(16), 1946–1952 (2007).
- Baumgart D, Klauss V, Baer F et al. One-year results of the SCORPIUS study: a German multicenter investigation on the effectiveness of sirolimus-eluting stents in diabetic patients. J. Am. Coll. Cardiol. 50(17), 1627–1634 (2007).
- Atary JZ, van der Hoeven BL, Liem SS et al. Three-year outcome of sirolimus-eluting versus bare-metal stents for the treatment of ST-segment elevation myocardial infarction (from the MISSION! Intervention Study). Am. J. Cardiol. 106(1), 4–12 (2010).
- Violini R, Musto C, De FF et al. Maintenance of long-term clinical benefit with sirolimuseluting stents in patients with ST-segment elevation myocardial infarction 3-year results of the SESAMI (sirolimus-eluting stent versus bare-metal stent in acute myocardial infarction) trial. J. Am. Coll. Cardiol. 55(8), 810–814 (2010).
- Spaulding C, Teiger E, Commeau P et al. Four-year follow-up of TYPHOON (trial to assess the use of the CYPHer sirolimus-eluting coronary stent in acute myocardial infarction treated with balloon angioplasty). JACC Cardiovasc. Interv. 4(1), 14–23 (2011).
- Pache J, Dibra A, Mehilli J, Dirschinger J, Schomig A, Kastrati A. Drug-eluting stents compared with thin-strut bare stents for the reduction of restenosis: a prospective, randomized trial. Eur. Heart J. 26(13), 1262–1268 (2005).
- Suttorp MJ, Laarman GJ, Rahel BM et al. Primary stenting of totally occluded native coronary arteries II (PRISON II), a randomized comparison of bare-metal stent implantation with sirolimus-eluting stent implantation for the treatment of total coronary occlusions. Circulation 114(9), 921–928 (2006).
- Menozzi A, Solinas E, Ortolani P et al. Twenty-four months clinical outcomes of sirolimus-eluting stents for the treatment of small coronary arteries: the long-term SES-SMART clinical study. Eur. Heart J. 30(17), 2095–2101 (2009).
- Kelbaek H, Klovgaard L, Helqvist S et al. Longterm outcome in patients treated with sirolimus-eluting stents in complex coronary artery lesions: 3-year results of the SCANDSTENT (stenting coronary arteries in nonstress/benestent disease) trial. J. Am. Coll. Cardiol. 51(21), 2011–2016 (2008).
- Vermeersch P, Agostoni P, Verheye S et al. Increased late mortality after sirolimuseluting stents versus bare-metal stents in diseased saphenous vein grafts: results from the randomized DELAYED RRISC trial. J. Am. Coll. Cardiol. 50(3), 261–267 (2007).
- Serruys PW, Onuma Y, Garg S et al. 5-year clinical outcomes of the ARTS II (arterial revascularization therapies study II) of the sirolimus-eluting stent in the treatment of patients with multivessel de novo coronary artery lesions. J. Am. Coll. Cardiol. 55(11), 1093–1101 (2010).
- Lemos PA, Serruys PW, van Domburg RT et al. Unrestricted utilization of sirolimuseluting stents compared with conventional bare stent implantation in the ‘real world’: the rapamycin-eluting stent evaluated at Rotterdam Cardiology Hospital (RESEARCH) registry. Circulation 109(2), 190–195 (2004).
- Grube E, Silber S, Hauptmann KE et al. TAXUS I: six- and twelve-month results from a randomized, double-blind trial on a slow-release paclitaxel-eluting stent for de novo coronary lesions. Circulation 107(1), 38–42 (2003).
- Silber S, Colombo A, Banning AP et al. Final 5-year results of the TAXUS II trial: a randomized study to assess the effectiveness of slow- and moderate-release polymer-based paclitaxel-eluting stents for de novo coronary artery lesions. Circulation 120(15), 1498–1504 (2009).
- Ellis SG, Stone GW, Cox DA et al. Long-term safety and efficacy with paclitaxel-eluting stents: 5-year final results of the TAXUS IV clinical trial (TAXUS IV-SR: Treatment of de novo Coronary Disease Using a Single Paclitaxel-Eluting Stent). JACC Cardiovasc. Interv. 2(12), 1248–1259 (2009).
- Stone GW, Ellis SG, Cannon L et al. Comparison of a polymer-based paclitaxeleluting stent with a bare-metal stent in patients with complex coronary artery disease: a randomized controlled trial. JAMA 294(10), 1215–1223 (2005).
- Grube E, Dawkins K, Guagliumi G et al. TAXUS VI final 5-year results: a multicentre, randomised trial comparing polymer-based moderate-release paclitaxel-eluting stent with a bare-metal stent for treatment of long, complex coronary artery lesions. EuroIntervention 4(5), 572–577 (2009).
- Stone GW, Parise H, Witzenbichler B et al. Selection criteria for drug-eluting versus bare-metal stents and the impact of routine angiographic follow-up: 2-year insights from the HORIZONS-AMI (harmonizing outcomes with revascularization and stents in acute myocardial infarction) trial. J. Am. Coll. Cardiol. 56(19), 1597–1604 (2010).
- Di LE, Sauro R, Varricchio A et al. Benefits of drug-eluting stents as compared to bare-metal stent in ST-segment elevation myocardial infarction: four year results of the paclitaxel or sirolimus-eluting stent vs bare-metal stent in primary angioplasty (PASEO) randomized trial. Am. Heart J. 158(4), e43–e50 (2009).
- Vink MA, Dirksen MT, Suttorp MJ et al. 5-year follow-up after primary percutaneous coronary intervention with a paclitaxel-eluting stent versus a bare-metal stent in acute ST-segment elevation myocardial infarction: a follow-up study of the PASSION (paclitaxeleluting versus conventional stent in myocardial infarction with ST-segment elevation) trial. JACC Cardiovasc. Interv. 4(1), 24–29 (2011).
- Erglis A, Narbute I, Kumsars I et al. A randomized comparison of paclitaxel-eluting stents versus bare-metal stents for treatment of unprotected left main coronary artery stenosis. J. Am. Coll. Cardiol. 50(6), 491–497 (2007).
- Ong AT, Serruys PW, Aoki J et al. The unrestricted use of paclitaxel- versus sirolimuseluting stents for coronary artery disease in an unselected population: one-year results of the Taxus-stent evaluated at Rotterdam Cardiology Hospital (T-SEARCH) registry. J. Am. Coll. Cardiol. 45(7), 1135–1141 (2005).
- Windecker S, Remondino A, Eberli FR et al. Sirolimus-eluting and paclitaxel-eluting stents for coronary revascularization. N. Engl. J. Med. 353(7), 653–662 (2005).
- Raber L, Wohlwend L, Wigger M et al. Five-year clinical and angiographic outcomes of a randomized comparison of sirolimus-eluting and paclitaxel-eluting stents: results of the sirolimus-eluting versus paclitaxel-eluting stents for coronary revascularization LATE trial. Circulation 123(24), 2819–2828 (2011).
- Schomig A, Dibra A, Windecker S et al. A meta-analysis of 16 randomized trials of sirolimus-eluting stents versus paclitaxel-eluting stents in patients with coronary artery disease. J. Am. Coll. Cardiol. 50(14), 1373–1380 (2007).
- Stettler C, Wandel S, Allemann S et al. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet 370(9591), 937–948 (2007).
- Nordmann AJ, Briel M, Bucher HC. Mortality in randomized controlled trials comparing drug-eluting vs. bare-metal stents in coronary artery disease: a meta-analysis. Eur. Heart J. 27(23), 2784–2814 (2006).
- Pfisterer M, Brunner-La Rocca HP, Buser PT et al. Late clinical events after clopidogrel discontinuation may limit the benefit of drug-eluting stents: an observational study of drug-eluting versus bare-metal stents. J. Am. Coll. Cardiol. 48(12), 2584–2591 (2006).
- Camenzind E, Steg PG, Wijns W. Stent thrombosis late after implantation of first-generation drug-eluting stents: a cause for concern. Circulation 115(11), 1440–1455 (2007).
- Lagerqvist B, James SK, Stenestrand U, Lindback J, Nilsson T, Wallentin L. Long-term outcomes with drug-eluting stents versus bare-metal stents in Sweden. N. Engl. J. Med. 356(10), 1009–1019 (2007).
- Stone GW, Moses JW, Ellis SG et al. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N. Engl. J. Med. 356(10), 998–1008 (2007).
- Kastrati A, Mehilli J, Pache J et al. Analysis of 14 trials comparing sirolimus-eluting stents with bare-metal stents. N. Engl. J. Med. 356(10), 1030–1039 (2007).
- Douglas PS, Brennan JM, Anstrom KJ et al. Clinical effectiveness of coronary stents in elderly persons: results from 262,700 Medicare patients in the American College of Cardiology-National Cardiovascular Data Registry. J. Am. Coll. Cardiol. 53(18), 1629–1641 (2009).
- McFadden EP, Stabile E, Regar E et al. Late thrombosis in drug-eluting coronary stents after discontinuation of antiplatelet therapy. Lancet 364(9444), 1519–1521 (2004).
- Kerner A, Gruberg L, Kapeliovich M, Grenadier E. Late stent thrombosis after implantation of a sirolimus-eluting stent. Catheter. Cardiovasc. Interv. 60(4), 505–508 (2003).
- Urban P, Gershlick AH, Guagliumi G et al. Safety of coronary sirolimus-eluting stents in daily clinical practice: one-year follow-up of the e-Cypher registry. Circulation 113(11), 1434–1441 (2006).
- Iakovou I, Schmidt T, Bonizzoni E et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA 293(17), 2126–2130 (2005).
- Park DW, Park SW, Park KH et al. Frequency of and risk factors for stent thrombosis after drug-eluting stent implantation during long-term follow-up. Am. J. Cardiol. 98(3), 352–356 (2006).
- Spaulding C, Daemen J, Boersma E, Cutlip DE, Serruys PW. A pooled analysis of data comparing sirolimus-eluting stents with bare-metal stents. N. Engl. J. Med. 356(10), 989–997 (2007).
- Mauri L, Hsieh WH, Massaro JM, Ho KK, D’Agostino R, Cutlip DE. Stent thrombosis in randomized clinical trials of drug-eluting stents. N. Engl. J. Med. 356(10), 1020–1029 (2007).
- Roukoz H, Bavry AA, Sarkees ML et al. Comprehensive meta-analysis on drug-eluting stents versus bare-metal stents during extended follow-up. Am. J. Med. 122(6), 581–510 (2009).
- Daemen J, Wenaweser P, Tsuchida K et al. Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large two-institutional cohort study. Lancet 369(9562), 667–678 (2007).
- Wenaweser P, Daemen J, Zwahlen M et al. Incidence and correlates of drug-eluting stent thrombosis in routine clinical practice. 4-year results from a large 2-institutional cohort study. J. Am. Coll. Cardiol. 52(14), 1134–1140 (2008).
- Pinto Slottow TL, Steinberg DH, Roy PK et al. Observations and outcomes of definite and probable drug-eluting stent thrombosis seen at a single hospital in a four-year period. Am. J. Cardiol. 102(3), 298–303 (2008).
- James SK, Wallentin L, Lagerqvist B. The SCAAR-scare in perspective. EuroIntervention 5(4), 501–504 (2009).
- Joner M, Finn AV, Farb A et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J. Am. Coll. Cardiol. 48(1), 193–202 (2006).
- Farb A, Burke AP, Kolodgie FD, Virmani R. Pathological mechanisms of fatal late coronary stent thrombosis in humans. Circulation 108(14), 1701–1706 (2003).
- Finn AV, Joner M, Nakazawa G et al. Pathological correlates of late drug-eluting stent thrombosis: strut coverage as a marker of endothelialization. Circulation 115(18), 2435–2441 (2007).
- Ong AT, McFadden EP, Regar E, de Jaegere PP, van Domburg RT, Serruys PW. Late angiographic stent thrombosis (LAST) events with drug-eluting stents. J. Am. Coll. Cardiol. 45(12), 2088–2092 (2005).
- Kim JS, Jang IK, Kim JS et al. Optical coherence tomography evaluation of zotarolimus-eluting stents at 9-month follow-up: comparison with sirolimus-eluting stents. Heart 95(23), 1907–1912 (2009).
- van der Giessen WJ, Lincoff AM, Schwartz RS et al. Marked inflammatory sequelae to implantation of biodegradable and nonbiodegradable polymers in porcine coronary arteries. Circulation 94(7), 1690–1697 (1996).
- Joner M, Nakazawa G, Finn AV et al. Endothelial cell recovery between comparator polymer-based drug-eluting stents. J. Am. Coll. Cardiol. 52(5), 333–342 (2008).
- Virmani R, Guagliumi G, Farb A et al. Localized hypersensitivity and late coronary thrombosis secondary to a sirolimus-eluting stent: should we be cautious? Circulation 109(6), 701–705 (2004).
- Nebeker JR, Virmani R, Bennett CL et al. Hypersensitivity cases associated with drug-eluting coronary stents: a review of available cases from the Research on Adverse Drug Events and Reports (RADAR) project. J. Am. Coll. Cardiol. 47(1), 175–181 (2006).
- Cook S, Wenaweser P, Togni M et al. Incomplete stent apposition and very late stent thrombosis after drug-eluting stent implantation. Circulation 115(18), 2426–2434 (2007).
- Gutierrez-Chico JL, Regar E, Nuesch E et al. Delayed coverage in malapposed and side-branch struts with respect to well-apposed struts in drug-eluting stents: in vivo assessment with optical coherence tomography. Circulation 124(5), 612–623 (2011).
- Guagliumi G, Musumeci G, Sirbu V et al. Optical coherence tomography assessment of in vivo vascular response after implantation of overlapping bare-metal and drug-eluting stents. JACC Cardiovasc. Interv. 3(5), 531–539 (2010).
- Stone GW, Rizvi A, Newman W et al. Everolimus-eluting versus paclitaxel-eluting stents in coronary artery disease. N. Engl. J. Med. 362(18), 1663–1674 (2010).
- Stone GW, Rizvi A, Sudhir K et al. Randomized comparison of everolimus- and paclitaxel-eluting stents 2-year follow-up from the SPIRIT (clinical evaluation of the XIENCE V everolimus eluting coronary stent system) IV trial. J. Am. Coll. Cardiol. 58(1), 19–25 (2011).
- Kedhi E, Joesoef KS, McFadden E et al. Second-generation everolimus-eluting and paclitaxel-eluting stents in real-life practice (COMPARE): a randomised trial. Lancet 375(9710), 201–209 (2010).
- Smits PC, Kedhi E, Royaards KJ et al. 2-year follow-up of a randomized controlled trial of everolimus- and paclitaxel-eluting stents for coronary revascularization in daily practice COMPARE (comparison of the everolimus eluting XIENCE-V stent with the paclitaxel eluting TAXUS LIBERTE stent in all-comers: a randomized open label trial). J. Am. Coll. Cardiol. 58(1), 11–18 (2011).
- Kaiser C, Galatius S, Erne P et al. Drug-eluting versus bare-metal stents in large coronary arteries. N. Engl. J. Med. 363(24), 2310–2319 (2010).
- Jensen LO, Thayssen P, Hansen HS. A prospective, randomized trial of everolimuseluting and sirolimus-eluting stents in patients with coronary artery disease: The SORT OUT IV trial. Presented at: Transcatheter Cardiovascular Therapeutics. San Francisco, CA, USA, 7–11 November 2011.
- Byrne RA, Kastrati A, Tiroch K, et al. Two-year outcomes after everolimus- or sirolimus-eluting stents in patients with coronary artery diseasein the ISAR-TEST 4 trial. Presented at: Transcatheter Cardiovascular Therapeutics. Washington, DC, USA, 21–25 September 2010.
- Park KW, Chae IH, Lim DS et al. Everolimus-eluting versus sirolimus-eluting stents in patients undergoing percutaneous coronary intervention the EXCELLENT (efficacy of xience/promus versus cypher to reduce late loss after stenting) randomized trial. J. Am. Coll. Cardiol. 58(18), 1844–1854 (2011).
- Caixeta A, Lansky AJ, Serruys PW et al. Clinical follow-up 3 years after everolimusand paclitaxel-eluting stents: a pooled analysis from the SPIRIT II (a clinical evaluation of the XIENCE V everolimus eluting coronary stent system in the treatment of patients with de novo native coronary artery lesions) and SPIRIT III (a clinical evaluation of the investigational device XIENCE V everolimus eluting coronary stent system [EECSS] in the treatment of subjects with de novo native coronary artery lesions) randomized trials. JACC Cardiovasc. Interv. 3(12), 1220–1228 (2010).
- Bohler T, Waiser J, Budde K et al. The in vivo effect of rapamycin derivative SDZ RAD on lymphocyte proliferation. Transplant. Proc. 30(5), 2195–2197 (1998).
- Grube E, Hauptmann KE, Buellesfeld L, Lim V, Abizaid A. Six-month results of a randomized study to evaluate safety and efficacy of a Biolimus A9 eluting stent with a biodegradable polymer coating. EuroIntervention 1(1), 53–57 (2005).
- Ostojic MC, Perisic Z, Sagic D et al. The pharmacokinetics of Biolimus A9 after elution from the BioMatrix II stent in patients with coronary artery disease: the stealth PK study. Eur. J. Clin. Pharmacol. 67(4), 389–398 (2011).
- Windecker S, Serruys PW, Wandel S et al. Biolimus-eluting stent with biodegradable polymer versus sirolimus-eluting stent with durable polymer for coronary revascularisation (LEADERS), a randomized noninferiority trial. Lancet 372(9644), 1163–1173 (2008).
- Garg S, Sarno G, Serruys PW et al. The twelve-month outcomes of a biolimus-eluting stent with a biodegradable polymer compared with a sirolimus eluting stent with a durable polymer. EuroIntervention 6(2), 233–239 (2010).
- Klauss V. LEADERS: Two-year follow-up from a prospective randomized trial of Biolimus A9-eluting stents with a bioabsorbable polymer vs sirolimus-eluting stents with a durable polymer. Data presented at: Transcatheter Cardiovascular Therapeutics. San Francisco, CA, USA, 21–26 September 2009.
- Wykrzykowska J, Serruys P, Buszman P et al. The three year follow-up of the randomised ‘all-comers’ trial of a biodegradable polymer biolimus-eluting stent versus permanent polymer sirolimus-eluting stent (LEADERS). EuroIntervention 7(7), 789–795 (2011).
- Stefanini GG, Kalesan B, Serruys PW et al. Long-term clinical outcomes of biodegradable polymer biolimus-eluting stents versus durable polymer sirolimus-eluting stents in patients with coronary artery disease (LEADERS), 4-year follow-up of a randomised noninferiority trial. Lancet 378(9807), 1940–1948 (2011).
- Windecker S. BioMatrix Flex: new generation DES. Presented at: EuroPCR. Paris, France, 18 May 2011.
- Mauri L, Orav EJ, Kuntz RE. Late loss in lumen diameter and binary restenosis for drug-eluting stent comparison. Circulation 111(25), 3435–3442 (2005).
- Meier B, Sousa E, Guagliumi G et al. Sirolimus-eluting coronary stents in small vessels. Am. Heart J. 151(5), 1019–1017 (2006).
- Togni M, Eber S, Widmer J et al. Impact of vessel size on outcome after implantation of sirolimus-eluting and paclitaxel-eluting stents: a subgroup analysis of the SIRTAX trial. J. Am. Coll. Cardiol. 50(12), 1123–1131 (2007).
- Ellis SG, Popma JJ, Lasala JM et al. Relationship between angiographic late loss and target lesion revascularization after coronary stent implantation: analysis from the TAXUS-IV trial. J. Am. Coll. Cardiol. 45(8), 1193–1200 (2005).
- Wykrzykowska JJ, Serruys PW, Onuma Y et al. Impact of vessel size on angiographic and clinical outcomes of revascularization with biolimus-eluting stent with biodegradable polymer and sirolimus-eluting stent with durable polymer the LEADERS trial substudy. JACC Cardiovasc. Interv. 2(9), 861–870 (2009).
- Degertekin M, Arampatzis CA, Lemos PA et al. Very long sirolimus-eluting stent implantation for de novo coronary lesions. Am. J. Cardiol. 93(7), 826–829 (2004).
- Kim YH, Park SW, Lee CW et al. Comparison of sirolimus-eluting stent, paclitaxel-eluting stent, and bare-metal stent in the treatment of long coronary lesions. Catheter. Cardiovasc. Interv. 67(2), 181–187 (2006).
- Al Suwaidi J, Yeh W, Cohen HA, Detre KM, Williams DO, Holmes DR Jr. Immediate and one-year outcome in patients with coronary bifurcation lesions in the modern era (NHLBI dynamic registry). Am. J. Cardiol. 87(10), 1139–1144 (2001).
- Ge L, Tsagalou E, Iakovou I et al. In-hospital and nine-month outcome of treatment of coronary bifurcational lesions with sirolimuseluting stent. Am. J. Cardiol. 95(6), 757–760 (2005).
- Thuesen L, Kelbaek H, Klovgaard L et al. Comparison of sirolimus-eluting and bare-metal stents in coronary bifurcation lesions: subgroup analysis of the stenting coronary arteries in nonstress/benestent disease trial (SCANDSTENT). Am. Heart J. 152(6), 1140–1145 (2006).
- van Werkum JW, Heestermans AA, Zomer AC et al. Predictors of coronary stent thrombosis: the Dutch Stent Thrombosis Registry. J. Am. Coll. Cardiol. 53(16), 1399–1409 (2009).
- Medina A, de Lezo JS, Pan M. A new classification of coronary bifurcation lesions. Rev. Esp. Cardiol. 59(2), 183 (2006).
- Garg S, Wykrzykowska J, Serruys PW et al. The outcome of bifurcation lesion stenting using a biolimus-eluting stent with a bio-degradable polymer compared to a sirolimus-eluting stent with a durable polymer. EuroIntervention 6(8), 928–935 (2011).
- Brar SS, Gray WA, Dangas G et al. Bifurcation stenting with drug-eluting stents: a systematic review and meta-analysis of randomised trials. EuroIntervention 5(4), 475–484 (2009).
- Katritsis DG, Siontis GC, Ioannidis JP. Double versus single stenting for coronary bifurcation lesions: a meta-analysis. Circ. Cardiovasc. Interv. 2(5), 409–415 (2009).
- Sianos G, Morel MA, Kappetein AP et al. The SYNTAX Score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention 1(2), 219–227 (2005).
- Serruys PW, Onuma Y, Garg S et al. Assessment of the SYNTAX score in the Syntax study. EuroIntervention 5(1), 50–56 (2009).
- Serruys PW, Morice MC, Kappetein AP et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N. Engl. J. Med. 360(10), 961–972 (2009).
- Serruys PW, Onuma Y, Garg S et al. 5-year clinical outcomes of the ARTS II (arterial revascularization therapies study II) of the sirolimus-eluting stent in the treatment of patients with multivessel de novo coronary artery lesions. J. Am. Coll. Cardiol. 55(11), 1093–1101 (2010).
- Valgimigli M, Serruys PW, Tsuchida K et al. Cyphering the complexity of coronary artery disease using the syntax score to predict clinical outcome in patients with three-vessel lumen obstruction undergoing percutaneous coronary intervention. Am. J. Cardiol. 99(8), 1072–1081 (2007).
- Wykrzykowska JJ, Garg S, Girasis C et al. Value of the SYNTAX score for risk assessment in the all-comers population of the randomized multicenter LEADERS (limus eluted from a durable versus erodable stent coating) trial. J. Am. Coll. Cardiol. 56(4), 272–277 (2010).
- Wykrzykowska JJ, Garg S, Onuma Y et al. Implantation of the biodegradable polymer biolimus-eluting stent in patients with high SYNTAX score is associated with decreased cardiac mortality compared to a permanent polymer sirolimus-eluting stent: two year follow-up results from the ‘all-comers’ LEADERS trial. EuroIntervention 7(5), 605–613 (2011).
- Barlis P, Regar E, Serruys PW et al. An optical coherence tomography study of a biodegradable vs. durable polymer-coated limus-eluting stent: a LEADERS trial sub-study. Eur. Heart J. 31(2), 165–176 (2010).
- Gutierrez-Chico JL, Juni P, Garcia-Garcia HM et al. Long-term tissue coverage of a biodegradable polylactide polymer-coated biolimus-eluting stent: Comparative sequential assessment with optical coherence tomography until complete resorption of the polymer. Am. Heart J. 162(5), 922–931 (2011).
- Chevalier B, Serruys PW, Silber S et al. Randomised comparison of Nobori, biolimus A9-eluting coronary stent with a Taxus(R), paclitaxel-eluting coronary stent in patients with stenosis in native coronary arteries: the Nobori 1 trial. EuroIntervention 2(4), 426–434 (2007).
- Chevalier B, Silber S, Park SJ et al. Randomized comparison of the Nobori Biolimus A9-eluting coronary stent with the Taxus Liberte™ paclitaxel-eluting coronary stent in patients with stenosis in native coronary arteries: the NOBORI 1 trial: Phase II. Circ. Cardiovasc. Interv. 2(3), 188–195 (2009).
- Ostojic M, Sagic D, Beleslin B et al. First clinical comparison of Nobori-biolimus A9-eluting stents with Cypher-sirolimus-eluting stents: Nobori Core nine months angiographic and one year clinical outcomes. EuroInternvention 3(5), 574-579 (2008).
- Verheye S, Trauthen B. Axxess Biolimus A9(R) eluting bifurcation stent system. EuroIntervention 2(4), 506–508 (2007).
- Verheye S, Agostoni P, Dubois CL et al. 9-month clinical, angiographic, and intravascular ultrasound results of a prospective evaluation of the Axxess self-expanding biolimus A9-eluting stent in coronary bifurcation lesions: the DIVERGE (drugeluting stent intervention for treating side branches effectively) study. J. Am. Coll. Cardiol. 53(12), 1031–1039 (2009).
- Klomp M, Beijk MA, Tijssen JG, de Winter RJ. One-year clinical outcome in an unselected patient population treated with the Genous endothelial progenitor cell capturing stent. Catheter. Cardiovasc. Interv. 77(6), 809–817 (2011).
- Silber S, Damman P, Klomp M et al. Clinical results after coronary stenting with the Genous bioengineered R stent: 12-month outcomes of the e-HEALING (healthy endothelial accelerated lining inhibits neointimal growth) worldwide registry. EuroIntervention 6(7), 819–825 (2011).
- Haude M. REMEDEE: a prospective, randomized trial of a combination bioabsorbable polymer-based anti-CD34 antibody sirolimus-eluting stent vs. a durable polymer-based paclitaxel-eluting stent. Presented at: Transcatheter Cardiovascular Therapeutics. San Francisco, CA, USA, 7–11 November 2011.
- Grube E. BioFreedom: a prospective randomized trial of polymer-free Biolimus A9-eluting stents and paclitaxeleluting stents in patients with coronary artery disease. Presented at: Transcatheter Cardiovascular Therapeutics. Washington, DC, USA. 21–25 September 2010.
- Onuma Y, Serruys PW, Perkins LE et al. Intracoronary optical coherence tomography and histology at 1 month and 2, 3 and 4 years after implantation of everolimuseluting bioresorbable vascular scaffolds in a porcine coronary artery model: an attempt to decipher the human optical coherence tomography images in the ABSORB trial. Circulation 122(22), 2288–2300 (2010).
- Ormiston JA, Serruys PW, Regar E et al. A bioabsorbable everolimus-eluting coronary stent system for patients with single de novo coronary artery lesions (ABSORB), a prospective open-label trial. Lancet 371(9616), 899–907 (2008).
- Serruys PW, Ormiston JA, Onuma Y et al. A bioabsorbable everolimus-eluting coronary stent system (ABSORB), 2-year outcomes and results from multiple imaging methods. Lancet 373(9667), 897–910 (2009).
- Onuma Y, Serruys PW, Ormiston JA et al. Three-year results of clinical follow-up after a bioresorbable everolimus-eluting scaffold in patients with de novo coronary artery disease: the ABSORB trial. EuroIntervention 6(4), 447–453 (2010).
- Amoroso G, van Geuns RJ, Spaulding C et al. Assessment of the safety and performance of the STENTYS selfexpanding coronary stent in acute myocardial infarction: results from the APPOSITION I study. EuroIntervention 7(4), 428–436 (2011).
- Verheye S, Grube E, Ramcharitar S et al. First-in-man (FIM) study of the Stentys bifurcation stent: 30 days results. EuroIntervention 4(5), 566–571 (2009).
- Wenaweser P, Daemen J, Zwahlen M et al. Incidence and correlates of drug-eluting stent thrombosis in routine clinical practice. 4-year results from a large 2-institutional cohort study. J. Am. Coll. Cardiol. 52(14), 1134–1140 (2008).
- Morice M. Benefits of biodegradable polymer DES technology in complex patient populations enrolled in an all comers trial. Presented at: EuroPCR. Paris, France, 18–20 May 2011.
▪ Two-center registry shows the increased risk of (first-generation) drug-eluting stents for development of very late stent thrombosis.
▪▪ The originial paper of the LEADERS trial, comparing BioMatrix™ biolimus-eluting stent with Cypher® Select sirolimus-eluting stent. Demonstrates noninferiority of BioMatrix for the primary composite end point at 9-month follow-up.
▪▪ The 4-year follow-up results of the LEADERS study comparing BioMatrix biolimus-eluting stent with Cypher Select sirolimus-eluting stent. Shows a difference in favor of BioMatrix concerning very late stent thrombosis.
▪ LEADERS substudy on small vessels showing noninferiority of the BioMatrix biolimus-eluting stent compared with the Cypher Select sirolimus-eluting stent.
▪ Demonstrates some benefit of the BioMatrix biolimus-eluting stent compared with Cypher Select sirolimus-eluting stent in patient with a high SYNTAX score.
▪ Shows that at 24 months, Cypher Select sirolimus-eluting stent and BioMatrix biolimus-eluting stent have similar strut coverage.