Review Article - Interventional Cardiology (2011) Volume 3, Issue 2
Biomarkers for diagnosis of the vulnerable atherosclerotic plaque
- Corresponding Author:
- Christos D Liapis
Department of Vascular Surgery
1 Rimini Street , Athens University Medical School
‘Attikon’ University Hospital, Athens, Greece
E-mail: liapis@med.uoa.gr
Abstract
Keywords
atherosclerosis, biomarkers, vulnerable plaque
Atherosclerotic cardiovascular disease is a major cause of morbidity and mortality world-wide, accounting for more than 19 million deaths per year [1]. Atherosclerotic patients present with a significant overlapping of vascular disorders implicating peripheral arterial disease (PAD), coronary heart disease and carotid arterial disease and cerebral disease. Atherosclerosis is a systemic disease affecting large and medium-sized arteries with lipid and fibrous accumulation within the intimal layer.
The genesis and progression of atherosclerotic plaques are accompanied by the release of a series of proteomic mediators of inflammation and significant chemotactic activity. These mediators carry the potential to be utilized as biomarkers, defined as measurable proteins, peptides, genes or metabolic products that represent biologic processes in an organism at a given time [2]. Biomarkers are indicators of disease states and encompass a spectrum of molecules with certain ‘ideal’ characteristics as proposed by Thomas et al. [3]. Even though atherosclerotic plaque imaging can be a much more precise predictor of destabilization, its employment – in the majority of the modalities used to acquire images – can be costly and is not as practical as the measurement of a serum biomarker. In addition, depending on the plaque anatomic location, the appropriate imaging required ranges from the simple, quick and cost effective use of ultrasound to the expensive magnetic resonance technology.
As demonstrated by many authors, the majority of plaque ruptures are clinically silent [4–6]. However, besides serving as surrogate markers of drug efficacy or as markers for patient stratification, the role of biomarkers in detecting the vulnerable (unstable, thrombogenic) plaques is a field of great interest due to its potential to also aid in the prevention of cardiovascular events.
Physiology of atherosclerotic plaque formation
The starting point for atheroma formation is endothelial dysfunction or activation [7,8]. In addition, others have suggested that the primary factor that initiates plaque formation is the response to lipoprotein retention, which in turn reduces the endothelial threshold to sheer stress [9]. At present, the most important contributors of endothelial dysfunction are hemodynamic disturbances, hypercholesterolemia and inflammation. Etiologic factors also include cigarette toxins, homocysteine and a wide spectrum of infectious agents. Chronic endothelial injury eventually results in endothelial dysfunction and increased permeability and induces LDL oxidation and accumulation in the subendothelial space of the intima [10] as well as the expression of adhesion molecules (e.g., vascular cell adhesion molecule [VCAM]-1, ICAM-1, and P selectin) and chemokines (e.g., monocyte chemoattractant peptide [MCP]-1) that participate in platelet aggregation, lymphocyte and monocyte adhesion and infiltration, thus initiating the inflammatory process [11–16]. As monocytes are attracted to the endothelium and migrate to the subendothelial space, they mature into macrophages and uptake oxidized LDL transforming into ‘foam’ cells that eventually form the lipid core of the atherosclerotic plaque after apoptosis occurs [17]. This inflammatory mediator cascade promotes a phenotype change of vascular smooth muscle cells (VSMCs) from the ‘contractile’ phenotype state to the active ‘synthetic’ state. VSMCs in the synthetic state can migrate and proliferate from the media to the intima, where they produce excessive amounts of extracellular matrix (e.g., collagen, elastin and proteoglycans) that transforms the lesion into a fibrous plaque [18]. The typical atherosclerotic plaque comprises of the lipid core and the fibrous cap, and is the most commonly classified histologically by the American Heart Association-recommended Stary classification [19].
The vulnerable atherosclerotic plaque
Vulnerable atherosclerotic plaques (high-risk or unstable plaques) are associated with an increased risk of disruption, distal embolization and vascular events. They are histological lesions with a large lipid core, a thin fibrous cap, and may contain ulceration, intraluminal thrombosis and intraplaque hemorrhage, as well as intense infiltration of macrophages and other inflammatory cells. Inflammation plays a key role in the pathogenesis of atherosclerosis, and the immune system and oxidative stress seem to be involved in the initiation, propagation and activation of such lesions in the arterial wall [19–22]. Unstable plaques are rich in inflammatory cells that destroy the fibrous cap and are responsible for endothelial denudation and therefore thrombogenicity of the plaque contents. Rupture depends on the balance between inflammatory cell activity and the VSMC-driven repair process under the influence of the hemodynamic stress exerted on it [23]. When rupture takes place, the fibrous cap appears to be eroded at the shoulder of the lesion (where the fibrous cap meets the intima of the normal segment of the vessel wall) [24].
Activated macrophages, T cells and mast cells produce a variety of molecules – inflammatory cytokines, proteases, coagulation factors, radicals and vasoactive molecules – that are expressed in the plaques and may modulate extracellular matrix remodeling, cell proliferation, cell death (apoptosis) and ultimately destabilize these lesions [19–22,25]. These molecules include VEGF, VCAM-1, ICAM-1, MCP-1, cathepsins, P selectin, endothelin-1, platelet-activating factor, NF-kB, tumor necrosis factors, interleukins and leukotactin-1. T cells in the region of the fibrous cap produce IFN-g, a potent inhibitor of collagen synthesis, inducing apoptosis of VSMCs [25]. Furthermore, elevated expression/activity of several matrix metalloproteinases (MMPs; including -1, -3 and -9), the main physiological regulators of the extracellular matrix, seems to play an important role in plaque activation [66].
Biomarkers & vulnerable atherosclerotic plaques
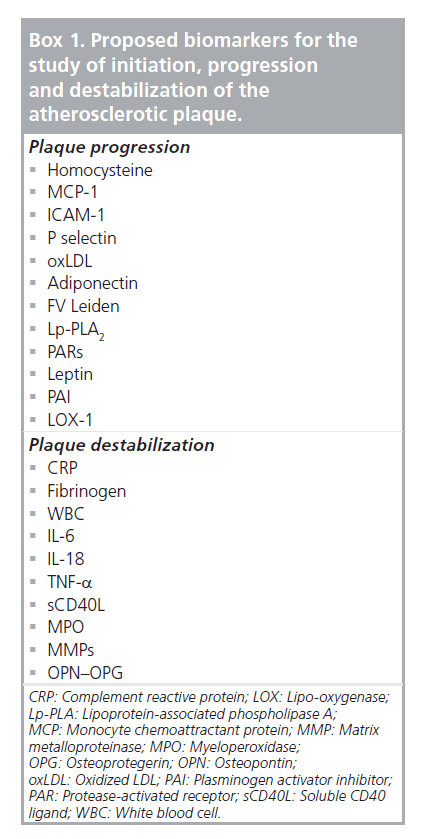
Various biomarkers have been studied as candidates for monitoring the progression of atherosclerotic disease and the majority of them are implicated in different stages of the pathophysiological mechanism of plaque formation and evolution. As a consequence, the diagnostic/prognostic weight of each one of them leans either towards progression or to the direction of plaque destabilization (Box 1) [26]. In addition, some of the changes in circulating marker levels may be a consequence of silent plaque rupture and/or subsequent healing. Detection of vulnerable or rupture-prone lesions is of paramount importance so that necessary clinical steps can be taken to prevent the deleterious clinical sequelae associated with symptomatic plaque rupture.
Box 1. Proposed biomarkers for the study of initiation, progression and destabilization of the atherosclerotic plaque.
Specificity and sensitivity vary for each one of these biological markers and they are also different for each vascular bed. In order to detect a vulnerable atherosclerotic plaque that is more likely to cause cardiovascular events, one should focus on studying biomarkers more associated with destabilization rather than disease progression.
▪ Complement reactive protein
Complement reactive protein (CRP) is an acute phase protein, primarily synthesized by hepatocytes, and induced by IL-6 with synergistic enhancement of IL-1 or TNF [27]. A rise in CRP levels is common in both infectious and noninfectious disorders, including myocardial infarction [28]. Up until now, it is the only inflammatory marker used in clinical practice and it should be emphasized that its predictive value can be estimated only through high-precision assays. It is within these lower ranges that the hs-CRP levels seem to have predictive abilities for cardiovascular events. A hs-CRP level of >10 mg/l, for example, should be discarded and repeated in 2 weeks to allow acute inflammations to subside before retesting.
Studies have consistently reported that elevated CRP serum levels definitely have a prognostic value for cardiovascular events and mortality [29]. CRP has been proposed to induce a prothrombotic state via induction of tissue factor expression in human monocytes [30]. It can activate or inhibit the complement system, driving the inflammation in atherosclerotic lesions [31]. CRP has also been demonstrated to decrease the expression and bioactivity of endothelial nitric oxide synthase [32] with a subsequent effect on vasodilatation. CRP downregulates both basal and VEGF-stimulated angiogenesis, whereas it promotes endothelial apoptosis in a nitrous oxide-dependent fashion [33]. CRP has also been found to synergistically enhance angiotensin II-induced proinflammatory effects, involving cellular migration and proliferation as well as lesion collagen and elastin content [34]. Finally, it induces the release of MCP-1 and endothelin-1 upregulating adhesion molecules and chemoattractant chemokines in endothelial cells and VSMCs [35].
Commercially available high-sensitivity assays for CRP are cost effective and reproducible [36]. Unlike many other inflammatory mediators, CRP is not subject to diurnal fluctuation or biological variance, and CRP concentration appears to be proportional to disease severity [37]. Unfortunately, a major limitation is that CRP is elevated in systemic inflammation [37], which may limit its use as a prognostic marker in postoperative patients.
▪ Fibrinogen
Fibrinogen is a glycoprotein that circulates at a high concentration in blood and initially mediates platelet aggregation. Later in clot formation it is converted to fibrin, which in turn is organized in a matrix defining the clot shape providing strength, flexibility and stability.
More than 40 years ago, fibrinogen was demonstrated to be elevated among patients with acute thrombosis. Hyperfibrinogenemia produces a dense and tight network of fibers which demonstrates reduced fibrinolysis [38]. It increases plasma viscosity and induces VSMC proliferation. The Gothenburg Heart Study was the first prospective trial to demonstrate an association between fibrinogen levels and subsequent cardiovascular disease risk. In another study, higher levels of fibrinogen predicted subsequent acute coronary syndromes (ACS) while lower levels, despite elevated cholesterol levels, were associated with lower risks of ACS [39]. Elevated fibrinogen levels in patients with PAD are associated with increased risk of fatal cardiovascular complications [40].
However, it remains unclear whether elevated fibrinogen levels are a cause or consequence of atherosclerosis. In the Copenhagen City Heart Study the relative risk of developing a stroke was almost double in patients with higher fibrinogen levels. Nevertheless they were not associated with echolucent unstable – and therefore vulnerable – carotid plaques [41].
▪ White blood cell count
The white blood cell count in peripheral blood is usually increased in inflammatory and infectious conditions and could also be affected in plaque inflammation. Higher leukocyte count is associated with a greater cardiovascular risk. In a meta-analysis of seven prospective studies comparing the top with the bottom third of the value distribution, the relative risk of coronary disease was 1.4 (95% CI: 1.3–1.5) [42], rendering leukocytes a valuable marker.
▪ IL‑6, IL‑18 & TNF‑a
Cytokines are key regulatory glycoproteins allied to inflammatory/immunological processes which modulate all aspects of vascular inflammation. Many cytokines have been implicated in atheroma formation and complication. The Edinburgh artery [43,44], InCHIANTI [45] and MESA [46] studies have all individually established the role of IL-6 as an independent predictor of PAD in community screening, irrespective of ethnicity. IL-6 enhances cell adhesion molecule expression and enhances the production of acute phase reactants such as CRP and TNF-a by the hepatocytes. While exogenous administration of IL‑18 in mice enhances atherosclerotic lesions [47], there is a converse reduction in atherosclerosis inhibiting IL-18 [48]. IL-18 has a bearing on the progression and stability of human atherosclerotic plaques [49]. TNF-a is involved in atherosclerotic progression from the initial stages of intimal thickening to the subsequent vessel occlusion. It stimulates selectin and adhesion molecule expression and MMP -1–9, -11 and -13 production in the endothelium, VSMCs and macrophages [50]. Locally, within the atheroma, it increases expression of tissue factor, a potent thrombogenic protein [51].
A limitation of IL-6 is the presence of diurnal fluctuations and large biological variance [51]. Furthermore, cytokines are present only in picogram per milliliter quantities, and the production of a standardized, sensitive and specific immunoassay is both difficult and expensive.
▪ Circulating soluble CD40 ligand
Circulating soluble CD40 ligand (sCD40L), largely derived from activated platelets, activates an inflammatory reaction in vascular endothelial cells by the secretion of cytokines and chemokines. Membrane-bound CD40L and sCD40L forms interact with the CD40 receptor molecule, leading to the release of matrix MMPs and subsequent destabilization of the plaque [52]. Elevated plasma concentrations of sCD40L at baseline predict a subsequent increased risk of future cardiovascular events in apparently healthy women and in angina-stable patients.
▪ Vascular calcification markers
Atherosclerotic plaque calcification enhances plaque stability and decreases the likelihood of clinical events [53]. A growing number of stimulatory and inhibitory molecules suggest that vascular calcification is an actively regulated process. Among these molecules osteopontin (OPN), an acidic phosphoprotein, and osteoprotegerin (OPG), a member of the TNF-a receptor superfamily, have recently been demonstrated to inhibit mineral deposition as well as osteoclastogenesis and they are constitutively expressed by a wide range of cell types in the vasculature [53–56].
These bone-matrix proteins, which attenuate vascular calcification, have emerged as novel markers of atherosclerotic plaque composition and cardiovascular disease prognosis. Data derived from clinical studies support the notion that increased serum levels of the aforementioned markers are positively associated with acute cardiovascular events, coronary disease severity and poor longterm cardiovascular outcomes [57–61]. Although the large prospective study by Nybo et al. observed no association of baseline OPG with ischemic stroke [62], recent studies have demonstrated a strong relationship of serum OPN and OPG levels with low carotid plaque echogenicity, while enhanced immunodetection of OPN and OPG in human carotid plaques indicate their contribution to plaque instability [63]. At present, only one study concerning pharmaceutical interventions with intensive lipid-lowering therapy with statins has demonstrated the attenuation of serum OPN and OPG levels and enhanced carotid plaque echogenicity, and thereafter stability, in patients with carotid stenosis [64].
▪ Matrix metalloproteinases
Matrix metalloproteinases are an ever-expanding family of zinc-dependent endopeptidases with proteolytic activity toward one or more components of the extracellular matrix [65]. Growing evidence supports the strong relationship of MMPs with plaque instability and consequent cardiovascular events [66,67]. Histopathological studies and experimental models have revealed the overproduction of MMPs in the rupture-prone regions of atherosclerotic plaques [68,69]. This excessive proteolytic activity facilitates extracellular matrix cleavage and fibrous cap degradation with eventual acute plaque rupture. Concerning clinical studies, increased serum levels of MMPs or MMP genotypes (e.g., MMP-3 polymorphism) have been observed to be closely associated with atherosclerotic manifestations, such as coronary artery disease or ischemic stroke. Moreover, biomarker studies have shown greater levels of MMPs (e.g., MMP-9) in ACS, such as unstable angina and myocardial infarction compared with stable coronary artery disease.
Matrix metalloproteinases are predominantly found at several stages of atherosclerotic plaque development and their activity is tightly regulated at three levels: control of gene transcription by a wide spectrum of factors (e.g., inflammatory cytokines); secretion as latent enzymes and activation by proteases such as other MMPs; and inhibition by tissue inhibitors of MMPs (TIMPs).
Both clinical and experimental studies provide a wealth of information concerning the crucial role of MMPs and TIMPs in intimal thickening and plaque destabilization. Atherosclerotic plaque formation constitutes a net matrix deposition, in part mediated by VSMC migration and proliferation. Until now the evidence for MMP-2 activation and MMP-9 upregulation during neointima formation is abundant. At the initial stages of atherogenesis, MMPs have also been hypothesized to mediate subintimal inflammatory cell infiltration [70]. Therefore, degenerative proteases seem to additionally promote lipid-necrotic core formation in the atherosclerotic plaque [66,71,72].
In one study, MMP-9 plasma concentrations predicted stroke and cardiovascular death in patients with ≥50% carotid stenosis, though not independently [67]. These findings were further confirmed by two nonprospective studies comparing symptomatic and asymptomatic carotid arterial disease patients [73,74]. The positive predictive value of MMP-9 was significantly enhanced when combined with other members of the MMP family (MMP-7 and MMP-8 and their tissue inhibitor TIMP-1) [74] or with plaque echolucency [75]. Histological analysis of specimens obtained from patients with unstable angina has demonstrated a remarkable increase in intracellular MMP-9 levels than in stable angina. Similarly, plaques extracted from symptomatic patients within 1 month before undergoing carotid endarterectomy contain fourfold higher concentrations of MMP-9. It has been demonstrated that inhibition of TGF-b signaling alters plaque stability by altering extracellular matrix components by means of attenuating collagen deposition and increasing MMP activity due to reductions in TIMPs [76–78]. Other members of the MMP family, including excessive MMP-7, MMP-8 and MMP-12, have been observed in carotid plaques with morphological characteristics of vulnerability [75,79–81].
Finally, it has been demonstrated that cyclooxygenase/prostaglandin E (PGE) synthase- 1 (COX-2/mPGES-1) are overexpressed in symptomatic plaques in association with PGE2- dependent MMP biosynthesis and plaque rupture. This seems to be mainly achieved through PGE2 EP4 macrophage receptor interaction inducing MMP production [82–85].
▪ Myeloperoxidase
Myeloperoxidase (MPO) is a hemoprotein produced by polymorphonuclear neutrophils and macrophages and catalyzes the conversion of chloride and hydrogen peroxide to hypochlorite [86]. It is released into the extracellular fluid and general circulation during inflammatory conditions. MPO and its products are involved in the oxidation of lipids contained within LDL particles, and is thought to promote the formation of foam cells in atherosclerotic plaques [86]. Inflammatory cells producing MPO are found more frequently and in higher concentrations in the culprit lesions of patients with ACS than in patients with stable disease [87,88]. Together with MMPs, MPO degrades the collagen layer of atheroma leading to erosion or rupture of plaques and its fatal consequences [87–89]. Thus, MPO has been proposed as a marker of plaque instability even if it is not specific to cardiac diseases, as activation of neutrophils and macrophages can occur in infectious, inflammatory or infiltrative disease processes. Recently, several assays for MPO have been approved for clinical use.
Biomarkers in coronary heart disease
Despite the development of many markers associated with myocardial ischemia and injury, cardiac troponin is still the preferred marker in this category owing to its myocardial tissue specificity and related sensitivity, as well as its established usefulness for therapeutic decisionmaking [90–92]. However, troponin is only elevated in acute coronary syndromes and is used to assess myocardial ischemia.
The hypothesis that MPO is involved in the inflammatory process that precedes the onset of symptomatic coronary artery disease by many years was supported by findings in more than 3000 patients of the European Prospective Investigation into Cancer and Nutrition–Norfolk population study [93]. Elevated levels of MPO independently predicted future risk of coronary artery disease in apparently healthy individuals (odds ratio for the highest quartile of MPO: 1.36; 95% CI: 1.07– 1.73). The potential usefulness of MPO for risk stratification was demonstrated in an analysis of 1090 patients with ACS from the CAPTURE trial [94]. At a cutoff of 350 μg/l, MPO demonstrated an adjusted hazard ratio for the 6‑month incidence of death and acute myocardial infarction of 2.25 (95% CI: 1.32–3.82). The effects were particularly impressive in patients with undetectable cardiac troponin with an adjusted hazard ratio of 7.48 (95% CI: 1.98–28.29). The predictive ability of MPO was independent of the levels of cardiac troponin, CRP and sCD40L, suggesting that MPO levels reflect a different aspect of ACS.
Two additional studies of patients with ACS also demonstrated that the prognostic information from MPO was independent from that of N-terminal pro-brain natriuretic peptide [95,96]. In another cohort of 604 patients presenting with symptoms suggestive for ACS, increasing concentrations of MPO were predictive for major cardiovascular events [97]. MPO levels at baseline independently predicted the risk of acute myocardial infarction and other major adverse coronary events at 30 days, even if patients initially had undetectable cardiac troponin levels, suggesting that MPO might be helpful in the early risk stratification of patients with ACS.
Biomarkers in carotid & cerebral arterial disease
Several studies indicate a higher carotid artery stenting (CAS)-associated risk of macro- and microembolization compared with carotid endarterectomy (CEA) [98–100]. Currently, there are no studies indicating biological markers as an independent risk factor related to a higher embolic potential for CAS when compared with CEA. Biological markers have been associated with increased perioperative risk of embolization, with both CEA and CAS. In a recent study, pre-CEA levels of high senditivity CRP (hsCRP) and fibrinogen have been proposed as independent determinants of new periprocedural cerebrovascular ischemic events [101]. When comparing the group with new diffusion-weighted imaging lesions in particular, the former had significantly higher levels of fibrinogen and hs‑CRP [101]. Correspondingly, pre- CAS-elevated CRP (>5 mg/l) [102] and IL-6 [103] have been demonstrated as powerful predictors of stroke, while elevated preprocedural white blood cell count independently predicted more frequent microembolic signals in transcranial Doppler [104].
A comparative study of risk-adjusted patients with elevated biological markers, randomized to CEA or CAS, would identify whether biological markers have a role in technique selection. In the absence of available data, this issue remains unresolved and is open for further investigation. The Stent Protected Angioplasty versus Carotid Endarterectomy 2 (SPACE-2) study has included substudies on biomarkers that will be available within the next 5 years [105].
Biomarkers in PAD
There is a strong clinical need for more specific biomarkers for PAD. A blood test for PAD would increase recognition of the disease and thereby improve clinical care. It is likely that a biomarker panel with high sensitivity and high specificity for PAD will be composed of biomarkers that circulate systemically but reflect the activity of local pathophysiologic processes [106–116]. Patients with PAD are at increased risk of cardiovascular morbidity and mortality. Identifying high-risk patients, especially those undergoing noncardiac vascular surgery, and aggressively managing their risk factors are the priority and the ultimate goal of a biomarker. There are multiple biomarkers that appear to stratify patients with PAD at risk of cardiovascular morbidity and mortality, but none of them are currently being used in clinical practice. With the number of biomarkers continuing to increase, other integrated strategies need to be adopted, such as the use of panels of biomarkers to improve identification of susceptible patients.
A study of a cohort of 540 high-risk individuals revealed that b2 microglobulin (b2M), cystatin C, hs-CRP and glucose were associated with PAD independently of the traditional risk factors of age, diabetes mellitus, hyperlipidemia, hypertension and tobacco use. Among the plasma markers tested, b2M and cystatin C had the highest correlation with ankle brachial index, higher than any of the conventional risk factors of age, smoking status and diabetes status. A biomarker panel score derived from b2M, cystatin C, hs-CRP and glucose had an increased association with PAD status [117], independently of the traditional risk factors. However, no association was found with the destabilization of the atherosclerotic plaque.
Conclusion
Current literature on biological markers and atherosclerotic disease is increasingly expanding, yet the subject is a complicated entity, fraught with measurement variability – as a result of either technique or effects of the disease state – as well as with complex and intertwined pathophysiology. Atherosclerosis is a systematic disease, and inflammatory markers are generated from all vascular beds including coronary circulation, cerebrovascular arterial tree and peripheral arteries. Biomarkers are substances involved in various stages of the pathophysiology of atherosclerotic plaque formation, progression and destabilization. Therefore, their diagnostic and prognostic value varies accordingly.
In order to approach the diagnosis of vulnerable plaque, a series of biological markers indicating imminent destabilization needs to be addressed. Such markers are CRP, fibrinogen, white blood cell count, cytokines, MMPs, MPO, sCD40L and vascular calcification markers (OPN and OPG). There are readily available commercial kits for determining most of these markers at costs that should be taken into account when planning clinical or research utilization of these techniques. Finally, the special characteristics of each vascular bed should also be included in the evaluation algorithm of biomarker detection.
Future perspective
Patients with disease affecting one vascular bed are at increased risk of overall cardiovascular morbidity and mortality. There are multiple biomarkers that appear to stratify patients at risk of cardiovascular morbidity and mortality, but none are currently being used in clinical practice. In addition, they are not without limitations.
For any single biomarker (such as CRP), a certain percentage of subjects with abnormal levels will not have the disease (false positives), whereas those with disease may have normal levels (false negatives). One approach to addressing this problem is to use a panel, in which each of the biomarkers contributes independent diagnostic information. Biomarker panels and index scores are beginning to be used in medicine to refine diagnosis and to aid in prognostication. For example, such index scores incorporating novel biomarkers have been utilized to predict clinical outcomes in hepatocellular and breast malignancies [118,119]. Recently, Wang et al. combined multiple biomarkers from the Framingham study to predict cardiovascular outcomes and death and they found only moderate addition of predicting value on conventional biomarkers [120]. However, this study assessed biomarkers as risk factors for disease progression and incorporated all measurable biomarkers, not only those implicated in transforming the plaque into a vulnerable one. Similarly designed studies are required, focusing on the vulnerable plaque biomarker group and incorporating novel ones in search of a panel with significant predictive value.
Safe recommendations cannot be included in daily practice, as randomized controlled trials and meta-analyses are still lacking. Modern risk scores and charts for identifying patients at high risk of cardiovascular events should include biomarkers as one of their major cumulative components. Even though not all plaque ruptures are symptomatic, randomized controlled trials incorporating vascular bed-specific biomarker panel assessment are needed in order to lead us to a future of being able to detect patients with atherosclerotic plaques that are vulnerable and thus at high risk of undergoing cardiovascular events. In such a setting, decision-making about the ‘how’ and ‘when’ of interventions and medical treatment will become easier and more efficient.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Introduction
▪ A serum biomarker should be easy and cost effective to measure.
Physiology of atherosclerotic plaque formation
▪ Endothelial dysfunction plays a key role in the pathophysiology of atherosclerosis.
▪ Various circulating proteomic mediators are involved in disease progression and can be used as biomarkers.
The vulnerable atherosclerotic plaque
▪ A ruptured plaque that will give rise to cardiovascular events is defined as a vulnerable one, but not all ruptures lead to symptomatology.
▪ The balance between vascular smooth muscle cell repair activity and inflammation determine the plaque rupture and destabilization.
Biomarkers & vulnerable atherosclerotic plaques
▪ Not all biomarkers are associated with plaque transformation into a vulnerable one, but they have all been used as a measure of disease progression.
Biomarkers in different vascular beds
▪ Biomarker value in detecting plaque vulnerability can be different in each vascular bed (coronary, peripheral and carotid/cerebral arterial vasculature).
Conclusion
▪ Biomarkers involved in destabilization pathophysiology are complement reactive protein, fibrinogen, white blood cell count, interleukins, soluble CD40 ligand, myeloperoxidase, metalloproteinases and vascular calcification markers (osteopontin, osteoprotegerin).
▪ More randomized controlled trials are needed to reach safe recommendations regarding the use of biomarkers to identify the rupture-prone atherosclerotic plaque.
Future perspective
▪ Serum biomarker determination and assessment in a panel may be valuable in identifying patients at high risk of cardiovascular events.
References
Papers of special note have been highlighted as:
▪ of interest
- Naghavi M, Libby P, Falk E et al.: From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: part I. Circulation 108, 1664–1672 (2003).
- Becker RC: Emerging paradigms, platforms, and unifying themes in biomarker science. J. Am. Coll. Cardiol. 50(18), 1777–1780 (2007).
- Thomas JC, Vohra RS, Beer S et al.: Biomarkers in peripheral arterial disease. Trends Cardiovasc. Med. 19, 147–151 (2009).
- Yazdani SK, Vorpahl M, Ladich E, Virmani R: Pathology and vulnerability of atherosclerotic plaque: identification, treatment options, and individual patient differences for prevention of stroke. Curr. Treat. Options Cardiovasc. Med. 12(3), 297–314 (2010).
- Thim T, Hagensen MK, Bentzon JF, Falk E: From vulnerable plaque to atherothrombosis. J. Intern. Med. 263(5), 506–516 (2008).
- Shalhoub J, Owen DR, Gauthier T, Monaco C, Leen EL, Davies AH: The use of contrast enhanced ultrasound in carotid arterial disease. Eur. J. Vasc. Endovasc. Surg. 39(4), 381–387 (2010).
- Liapis CD: Role of risk factors. In: Rutherford’s Vascular Surgery (7th Edition). Cronenwett JL, Johnston KW (Eds).
- Elsevier Publishing, PA, USA, 8211 (2010) 8 Holtzman JL: Atherosclerosis and oxidant stress, a new perspective. Springer Science and Business Media, LLC, PA, USA (2008).
- Williams KJ, Tabas I: The response-toretention hypothesis of early atherogenesis. Arterioscler. Thromb. Vasc. Biol. 15, 551–561 (1995).
- Flavahan NA: Atherosclerosis or lipoprotein induced endothelial dysfunction: potential mechanisms underlying reduction in EDRF/ nitric oxide activity. Circulation 85, 1927–1938 (1992).
- Cybulsky MI, Gimbrone MAJ: Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science 251(4995), 788–7891 (1991).
- Berliner JA, Navab M, Fogelman AM et al.: Atherosclerosis: basic mechanisms. Oxidation, inflammation, and genetics. Circulation 91(9), 2488–2496 (1995).
- Tsao PS, Buitrago R, Chan JR et al.: Fluid flow inhibits endothelial adhesiveness: nitric oxide and transcriptional regulation of VCAM-1. Circulation 94(7), 1682–1689 (1996).
- Tsao PS, Wang B, Buitrago R et al.: Nitric oxide regulates monocyte chemotactic protein-1. Circulation 96(3), 934–940 (1997).
- Ross R: Cellular and molecular studies of atherosclerosis. Atherosclerosis 131, S3–S4 (1997).
- Berliner JA, Territo MC, Sevanian A et al.: Minimally modified low density lipoprotein stimulates monocyte endothelial interactions. J. Clin. Invest. 85, 1260–1266 (1990).
- Ross R: Cell biology of atherosclerosis. Annu. Rev. Physiol. 57, 791–804 (1995).
- Doran AC, Meller N, McNamara CA: Role of smooth muscle cells in the initiation and early progression of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 28(5), 812–819 (2008).
- Stary HC: Natural history and histological classification of atherosclerotic lesions: an update. Arterioscler. Thromb. Vasc. Biol. 20(5), 1177–1178 (2000).
- Golledge J, Greenhalgh RM, Davies AH: The symptomatic carotid plaque. Stroke 31(3), 774–781 (2000).
- Hansson GK: Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 352(16), 1685–1695 (2005).
- Daskalopoulou SS, Daskalopoulos ME, Perrea D et al.: Carotid artery atherosclerosis: what is the evidence for drug action? Curr. Pharm. Des. 13(11), 1141–1159 (2007).
- Falk E: Why do plaques rupture? Circulation 86(6 Suppl.), III30–III42 (1992).
- Davies JR, Rudd JF, Fryer TD et al.: Targeting the vulnerable plaque: the evolving role of nuclear imaging. J. Nucl. Cardiol. 12, 234–246 (2005).
- Rosner D, Stoneman V, Littlewood T et al.: Interferon-g induces Fas trafficking and sensitization to apoptosis in vascular smooth muscle cells via a PI3K- and Akt-dependent mechanism. Am. J. Pathol. 168, 2054–2063 (2006).
- Defraigne JO: Pathophysiology of atherosclerosis. In: Vascular Surgery, European Manual of Medicine Series. Liapis CD, Balzer K, Fernandes e Fernandes J et al. (Eds).
- Springer-Verlag, Berlin, Heidelberg, Germany, 23–25 (2007) 27 Volanakis JE: Human C-reactive protein: expression, structure, and function. Mol. Immunol. 38(2–3), 189–197 (2001).
- Pietila K, Harmoinen A, Hermens W et al.: Serum C-reactive protein and infarct size in myocardial infarct patients with a closed versus an open infarct-related coronary artery after thrombolytic therapy. Eur. Heart J. 14(7), 915–919 (1993).
- Buffon A, Liuzzo G, Biasucci LM et al.: Preprocedural serum levels of C-reactive protein predict early complications and late restenosis after coronary angioplasty. J. Am. Coll. Cardiol. 34, 1512–1521 (1999).
- Paffen E, Vos HL, Bertina RM: C-reactive protein does not directly induce tissue factor in human monocytes. Arterioscler. Thromb. Vasc. Biol. 24(5), 975–981 (2004).
- Giannakis E, Male DA, Ormsby RJ et al.: Multiple ligand binding sites on domain seven of human complement factor H. Int. Immunopharmacol. 1(3), 433–443 (2001).
- Venugopal SK, Devaraj S, Yuhanna I et al.: Demonstration that C-reactive protein decreases eNOS expression and bioactivity in human aortic endothelial cells. Circulation 106(12), 1439–1441 (2002).
- Verma S, Kuliszewski MA, Li SH et al.: C-reactive protein attenuates endothelial progenitor cell survival, differentiation, and function: further evidence of a mechanistic link between C-reactive protein and cardiovascular disease. Circulation 109, 2058–2067 (2004).
- Wang CH, Li SH, Weisel RD et al.: C-reactive protein upregulates angiotensin type 1 receptors in vascular smooth muscle. Circulation 107, 1783–1790 (2003).
- Pasceri V, Chang J, Willerson JT et al.: Modulation of C-reactive protein-mediated monocyte chemoattractant protein-1 induction in human endothelial cells by anti-atherosclerosis drugs. Circulation 103(21), 2531–2534 (2001).
- Ridker PM, Cannon CP, Morrow D et al.: C-reactive protein levels and outcomes after statin therapy. N. Engl. J. Med. 352, 20–28 (2005).
- Zaninotto M, Mion MM, Novello E et al.: New biochemical markers: from bench to bedside. Clin. Chim. Acta 381, 14–20 (2007).
- Collet JP, Park D, Lesty C et al.: Influence of fibrin network conformation and fibrin fiber diameter on fibrinolysis speed: dynamic and structural approaches by confocal microscopy. Arterioscler. Thromb. Vasc. Biol. 20, 1354–1361 (2000).
- Thompson SG, Keinast J, Pyke SDM et al.: For the European Concerted Action on Thrombosis and Disabilities Angina Pectoris Study Group. Hemostatic factors and the risk of myocardial infarction or sudden death in patients with angina pectoris. N. Engl. J. Med. 332(10), 635–641 (1995).
- Doweik L, Maca T, Schillinger M et al.: Fibrinogen predicts mortality in high risk patients with peripheral artery disease. Eur. J. Vasc. Endovasc. Surg. 26, 381–386 (2003).
- Kofoed SC, Wittrup HH, Sillesen H et al.: Fibrinogen predicts ischaemic stroke and advanced atherosclerosis, but not echolucent, rupture-prone carotid plaques. Eur. Heart J. 24(6), 567–576 (2003).
- Danesh J, Collins R, Appleby P et al.: Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA 279, 1477–1482 (1998).
- Tzoulaki I, Murray GD, Lee AJ et al.: C-reactive protein, interleukin-6, and soluble adhesion molecules as predictors of progressive peripheral atherosclerosis in the general population: Edinburgh Artery Study. Circulation 112(7), 976–983 (2005).
- Tzoulaki I, Murray GD, Price JF et al.: Hemostatic factors, inflammatory markers, and progressive peripheral atherosclerosis: the Edinburgh Artery Study. Am. J. Epidemiol. 163, 334–341 (2006).
- McDermott MM, Guralnik JM, Corsi A et al.: Patterns of inflammation associated with peripheral arterial disease: the In CHIANTI study. Am. Heart J. 150, 276–281 (2005).
- Allison MA, Criqui MH, McClelland RL et al.: The effect of novel cardiovascular risk factors on the ethnic-specific odds for peripheral arterial disease in the Multi-Ethnic Study of Atherosclerosis (MESA). J. Am. Coll. Cardiol. 48, 1190–1197 (2006).
- Mallat Z, Corbaz A, Scoazec A et al.: Interleukin-18/interleukin-18 binding protein signaling modulates atherosclerotic lesion development and stability. Circ. Res. 89(7), E41–E45 (2001).
- Elhage R, Jawien J, Rudling M et al.: Reduced atherosclerosis in interleukin-18 deficient apolipoprotein E-knockout mice. Cardiovasc. Res. 59, 234–240 (2003).
- Mallat Z, Corbaz A, Scoazec A et al.: Expression of interleukin-18 in human atherosclerotic plaques and relation to plaque instability. Circulation 104(14), 1598–1603 (2001).
- Young JL, Libby P, Schonbeck U: Cytokines in the pathogenesis of atherosclerosis. Thromb. Haemost. 88(4), 554–567 (2002).
- Taubman MB, Fallon JT, Schecter AD et al.: Tissue factor in the pathogenesis of atherosclerosis. Thromb. Haemost. 78, 200–204 (1997).
- André P, Nannizzi-Alaimo L, Prasad SK et al.: Platelet derived CD40L. The switch hitting player of cardiovascular disease. Circulation 106(8), 896–899 (2002).
- Abedin M, Tintut Y, Demer LL: Vascular calcification: mechanisms and clinical ramifications. Arterioscler. Thromb. Vasc. Biol. 24(7), 1161–1170 (2004).
- Johnson RC, Leopold JA, Loscalzo J: Vascular calcification: pathobiological mechanisms and clinical implications. Circ. Res. 99(10), 1044–1059 (2006).
- O’Brien ER, Garvin MR, Stewart DK et al.: Osteopontin is synthesized by macrophage, smooth muscle, and endothelial cells in primary and restenotic human coronary atherosclerotic plaques. Arterioscler. Thromb. 14(10), 1648–1656 (1994).
- Schoppet M, Preissner KT, Hofbauer LC: RANK ligand and osteoprotegerin: paracrine regulators of bone metabolism and vascular function. Arterioscler. Thromb. Vasc. Biol. 22(4), 549–553 (2002).
- Kiechl S, Schett G, Wenning G et al.: Osteoprotegerin is a risk factor for progressive atherosclerosis and cardiovascular disease. Circulation 109(18), 2175–2180 (2004).
- Jono S, Ikari Y, Shioi A et al.: Serum osteoprotegerin levels are associated with the presence and severity of coronary artery disease. Circulation 106(10), 1192–1194 (2002).
- Ohmori R, Momiyama Y, Taniguchi H et al.: Plasma osteopontin levels are associated with the presence and extent of coronary artery disease. Atherosclerosis 170(2), 333–337 (2003).
- Kurata M, Okura T, Watanabe S et al.: Osteopontin and carotid atherosclerosis in patients with essential hypertension. Clin. Sci. (Lond), 111(5), 319–324 (2006).
- Golledge J, McCann M, Mangan S et al.: Osteoprotegerin and osteopontin are expressed at high concentrations within symptomatic carotid atherosclerosis. Stroke 35(7), 1636–1641 (2004).
- Nybo M, Johnsen SP, Dethlefsen C et al.: Lack of observed association between high plasma osteoprotegerin concentrations and ischemic stroke risk in a healthy population. Clin. Chem. 54, 1969–1974 (2008).
- Kadoglou NPE, Gerasimidis T, Golemati S et al.: The relationship between serum levels of vascular calcification inhibitors and carotid plaque vulnerability. J. Vasc. Surg. 47(1), 55–62 (2008).
- Kadoglou NP, Gerasimidis T, Moumtzouoglou A et al.: Intensive lipidlowering therapy ameliorates novel inflammatory and calcification markers and GSM score in patients with carotid stenosis. Eur. J. Vasc. Endovasc. Surg. 35(6), 661–668 (2008).
- Kadoglou NP, Liapis CD: Matrix metalloproteinases: contribution to pathogenesis, diagnosis, surveillance and treatment of abdominal aortic aneurysms. Curr. Med. Res. Opin. 20, 419–432 (2004).
- Alvarez B, Ruiz C, Chacon P, Alvarez-Sabin J, Matas M: Serum values of metalloproteinase-2 and metalloproteinase-9 as related to unstable plaque and inflammatory cells in patients with greater than 70% carotid artery stenosis. J. Vasc. Surg. 40, 469–475 (2004).
- Eldrup N, Gronholdt ML, Sillesen H, Nordestgaard BG: Elevated matrix metalloproteinase-9 associated with stroke or cardiovascular death in patients with carotid stenosis. Circulation 114, 1847–1854 (2006).
- Libby P, Ridker PM, Hansson GK et al.: Inflammation in atherosclerosis: from pathophysiology to practice. J. Am. Coll. Cardiol. 54(23), 2129–2138 (2009).
- Halim SA, Newby LK: Prognostic biomarkers in individuals with prevalent coronary heart disease. Dis. Markers 26(5–6), 265–271 (2009).
- Dorweiler B, Torzewski M, Dahm M et al.: Subendothelial infiltration of neutrophil granulocytes and liberation of matrixdestabilizing enzymes in an experimental model of human neo-intima. Thromb. Haemost. 99(2), 373–381 (2008).
- Lijnen HR: Metalloproteinases in development and progression of vascular disease. Pathophysiol. Haemost. Thromb. 33(5–6), 275–281 (2003-2004).
- Galis ZS, Khatri JJ: Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ. Res. 90(3), 251–262 (2002).
- Jefferis BJ, Whincup P, Welsh P et al.: Prospective study of matrix metalloproteinase-9 and risk of myocardial infarction and stroke in older men and women. Atherosclerosis 208, 557–563 (2010).
- Heider P, Pelisek J, Poppert H, Eckstein HH: Evaluation of serum matrix metalloproteinases as biomarkers for detection of neurological symptoms in carotid artery disease. Vasc. Endovasc. Surg. 43, 551–560 (2009).
- Kadoglou NP, Daskalopoulou SS, Perrea D, Liapis CD: Matrix metalloproteinases and diabetic vascular complications. Angiology 56(2), 173–189 (2005).
- Fabunmi RP, Sukhova GK, Sugiyama S et al.: Expression of tissue inhibitor of metalloproteinases-3 in human atheroma and regulation in lesion-associated cells: a potential protective mechanism in plaque stability. Circ. Res. 83(3), 270–278 (1998).
- Mallat Z, Gojova A, Marchiol-Fournigault C et al.: Inhibition of transforming growth factor-b signaling accelerates atherosclerosis and induces an unstable plaque phenotype in mice. Circ. Res. 89(10), 930–934 (2001).
- Lutgens E, Gijbels M, Smook M, et al.: Transforming growth factor-b mediates balance between inflammation and fibrosis during plaque progression. Arterioscler. Thromb. Vasc. Biol. 22(6), 975–982 (2002).
- Jiang X, Zeng HS, Guo Y et al.: The expression of matrix metalloproteinases-9, transforming growth factor-b1 and transforming growth factor-b receptor I in human atherosclerotic plaque and their relationship with plaque stability. Chin. Med. J. 117, 1825–1829 (2004).
- Kadoglou NP, Daskalopoulou SS, Perrea D et al.: Matrix metalloproteinases and diabetic vascular complications. Angiology 56(2), 173–189 (2005).
- Kadoglou NP, Liapis CD: Matrix metalloproteinases: contribution to pathogenesis, diagnosis, surveillance and treatment of abdominal aortic aneurysms. Curr. Med. Res. Opin. 20(4), 419–432 (2004).
- Cipollone F, Fazia ML, Iezzi A et al.: Association between prostaglandin E receptor subtype EP4 overexpression and unstable phenotype in atherosclerotic plaques in human. Arterioscler. Thromb. Vasc. Biol. 25(9), 1925–1931 (2005).
- Cipollone F, Fazia ML, Iezzi A et al.: Balance between PGD synthase and PGE synthase is a major determinant of atherosclerotic plaque instability in humans. Arterioscler. Thromb. Vasc. Biol. 24(7), 1259–1265 (2004).
- Cipollone F, Fazia ML, Iezzi A et al.: Blockade of the angiotensin II type 1 receptor stabilizes atherosclerotic plaques in humans by inhibiting prostaglandin E2-dependent matrix metalloproteinase activity. Circulation 109(12), 1482–1488 (2004).
- Cipollone F, Prontera C, Pini B et al.: Overexpression of functionally coupled cyclooxygenase-2 and prostaglandin E synthase in symptomatic atherosclerotic plaques as a basis of prostaglandin E(2)- dependent plaque instability. Circulation 104(8), 921–927 (2001).
- Podrez EA, Schmitt D, Hoff HF et al.: Myeloperoxidase-generated reactive nitrogen species convert LDL into an atherogenic form in vitro. J. Clin. Invest. 103, 1547–1560 (1999).
- Naruko T, Ueda M, Haze K et al.: Neutrophil infiltration of culprit lesions in acute coronary syndromes. Circulation 106, 2894–2900 (2002).
- Buffon A, Biasucci LM, Liuzzo G et al.: Widespread coronary inflammation in unstable angina. N. Engl. J. Med. 347, 5–12. (2002).
- Sugiyama S, Okada Y, Sukhova GK et al.: Macrophage myeloperoxidase regulation by granulocyte macrophage colony-stimulating factor in human atherosclerosis and implications in acute coronary syndromes. Am. J. Pathol. 158, 879–891 (2001).
- Anderson JL, Adams CD, Antman EM et al.: ACC/AHA 2007 guidelines for the management of patients with unstable angina/non ST-elevation myocardial infarction. Circulation 116, E148–E304 (2007).
- Thygesen K, Alpert JS, White HD et al.: Universal definition ofmyocardial infarction. Circulation 116, 2634–2653 (2007).
- Morrow DA, Cannon CP, Jesse RL et al.: National Academy of Clinical Biochemistry Laboratory Medicine Practice guidelines: clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Circulation 115, E356–E375 (2007)
- Meuwese MC, Stroes ES, Hazen SL et al.: Serum myeloperoxidase levels are associated with the future risk of coronary artery disease in apparently healthy individuals: the EPIC-Norfolk Prospective Population Study. J. Am. Coll. Cardiol. 50, 159–165 (2007).
- Baldus S, Heeschen C, Meinertz T et al.: Myeloperoxidase serum levels predict risk in patients with acute coronary syndromes. Circulation 108, 1440–1445 (2003).
- Mocatta TJ, Pilbrow AP, Cameron VA et al.: Plasma concentrations of myeloperoxidase predict mortality after myocardial infarction. J. Am. Coll. Cardiol. 49, 1993–2000 (2007).
- Morrow DA, Sabatine MS, Brennan ML et al.: Concurrent evaluation of novel cardiac biomarkers in acute coronary syndrome: myeloperoxidase and soluble CD40 ligand and the risk of recurrent ischaemic events in TACTICS-TIMI 18. Eur. Heart J. 29, 1096–1102 (2008).
- Brennan ML, Penn MS, Van Lente F et al.: Prognostic value of myeloperoxidase in patients with chest pain. N. Engl. J. Med. 349, 1595–1604 (2003).
- Ringleb PA, Allenberg J, Bruckmann H et al.; SPACE Collaborative Group: 30 day results from the SPACE trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non-inferiority trial. Lancet 368, 1239–1247 (2006).
- Mas JL, Chatellier G, Beyssen B; EVA-3S Investigators: Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N. Engl. J. Med. 355, 1660–1671 (2006).
- Ederle J, Dobson J, Featherstone RL et al.; International Carotid Stenting Study Investigators: Carotid artery stenting compared with endarterectomy in patients with symptomatic carotid stenosis (International Carotid Stenting Study): an interim analysis of a randomised controlled trial. Lancet 375, 985–997 (2010).
- Heider P, Poppert H, Wolf O et al.: Fibrinogen and high-sensitive C-reactive protein as serologic predictors for perioperative cerebral microembolic lesions after carotid endarterectomy. J. Vasc. Surg. 46, 449–454 (2007)
- Gröschel K, Ernemann U, Larsen J et al.: Preprocedural C-reactive protein levels predict stroke and death in patients undergoing carotid stenting. Am. J. Neuroradiol. 28, 1743–1746 (2007).
- Abe Y, Sakaguchi M, Furukado S et al.: Interleukin-6 release after carotid artery stenting and periprocedural new ischemic lesions. J. Cereb. Blood Flow Metab. 30, 857–863 (2010).
- Aronow HD, Shishehbor M, Davis DA et al.: Leukocyte count predicts microembolic Doppler signals during carotid stenting. A link between inflammation and embolization. Stroke 36, 1910–1914 (2005).
- Reiff T, Stingele R, Eckstein HH et al.: Stent-protected angioplasty in asymptomatic carotid artery stenosis vs. endarterectomy: SPACE2 – a three-arm randomised-controlled clinical trial. Int. J. Stroke 4, 294–299 (2009).
- McDermott MM, Guralnik JM, Corsi A et al.: Patterns of inflammation associated with peripheral arterial disease: the InCHIANTI study. Am. Heart J. 150, 276–281 (2005).
- Allison MA, Criqui MH, McClelland RL et al.: The effect of novel cardiovascular risk factors on the ethnic-specific odds for peripheral arterial disease in the Multi-Ethnic Study of Atherosclerosis (MESA). J. Am. Coll. Cardiol. 48, 1190–1197 (2006).
- Libra M, Signorelli SS, Bevelacqua Y et al.: Analysis of G(-174)C IL-6 polymorphism and plasma concentrations of inflammatory markers in patients with Type 2 diabetes and peripheral arterial disease. J. Clin. Pathol. 59, 211–215 (2006).
- Fiotti N, Giansante C, Ponte E et al.: Atherosclerosis and inflammation. Patterns of cytokine regulation in patients with peripheral arterial disease. Atherosclerosis 145, 51–60 (1999).
- Danielsson P, Truedsson L, Eriksson KF, Norgren L: Inflammatory markers and IL-6 polymorphism in peripheral arterial disease with and without diabetes mellitus. Vasc. Med. 10, 191–198 (2005).
- Ziegler S, Kudlacek S, Luger A, Minar E: Osteoprotegerin plasma concentrations correlate with severity of peripheral artery disease. Atherosclerosis 182, 175–180 (2005).
- DePalma RG, Hayes VW, Cafferata HT et al.: Cytokine signatures in atherosclerotic claudicants. J. Surg. Res. 111, 215–221 (2003).
- Nylaende M, Kroese A, Stranden E et al.: Markers of vascular inflammation are associated with the extent of atherosclerosis assessed as angiographic score and treadmill walking distances in patients with peripheral arterial occlusive disease. Vasc. Med. 11, 21–28 (2006).
- Hoogeveen RC, Morrison A, Boerwinkle E et al.: Plasma MCP-1 level and risk for peripheral arterial disease and incident coronary heart disease: Atherosclerosis Risk in Communities Study. Atherosclerosis 183, 301–307 (2005).
- Girn HR, Orsi NM, Homer-Vanniasinkam S: An overview of cytokine interactions in atherosclerosis and implications for peripheral arterial disease. Vasc. Med. 12, 299–309 (2007).
- Tayebjee MH, Tan KT, MacFadyen RJ, Lip GY: Abnormal circulating levels of metalloprotease 9 and its tissue inhibitor 1 in angiographically proven peripheral arterial disease: relationship to disease severity. J. Intern. Med. 257, 110–116 (2005).
- Fung ET, Wilson AM, Zhang F et al.: A biomarker panel forperipheral arterial disease. Vasc. Med. 13, 217–224 (2008).
- Snyder N, Nguyen A, Gajula L et al.: The APRI may be enhanced by the use of the FIBROSpect II in the estimation of fibrosis in chronic hepatitis C. Clin. Chim. Acta 381, 119–23 (2007).
- Paik S, Shak S, Tang G et al.: A multigene assay to predict recurrence of tamoxifentreated, node-negative breast cancer. N. Engl. J. Med. 351, 2817–2826 (2004).
- Wang TJ, Gona P, Larson MG et al.: Multiple biomarkers for the prediction of first major cardiovascular events and death. N. Engl. J. Med. 355, 2631–2639 (2006).
▪ Defines the term ‘biomarker’.
▪ Characteristics of the ‘ideal’ biomarker.
▪ The Stary histological classification of the atherosclerotic plaque.
▪ The significance of the use of a panel of biomarkers.