Research Article - International Journal of Clinical Rheumatology (2018) Volume 13, Issue 4
Body image in lupus: Is it disease activity, physical function, depression, pain, fatigue, sleep, fibromyalgia or stress?
- *Corresponding Author:
- Meenakshi Jolly
Rush University Medical Center
1611 W Harrison St, Ste 510, Chicago, Il 60612, USA
E-mail: Meenakshi_Jolly@rush.edu
Abstract
Patients with systemic lupus erythematosus (SLE) frequently experience poor body image (BI), an important issue, though not well researched or understood thus far. BI is perception of one’s own body. The effects of disease activity, damage, sleep, stress, pain, fatigue, function, medications, depression and fibromyalgia (FM) on BI in SLE are not known.
Objective: We aimed to evaluate the relative role of the specific variables listed above on BI in SLE patients.
Methods: SLE patients receiving rheumatology care at two academic medical centers were recruited. Each patient completed questionnaire assessments evaluating target variables and BI. Disease activity was evaluated using SELENA-SLEDAI. Multivariate regression analyses including stepwise modeling were conducted with BI as the dependent variable for all patients and for patients with and without FM.
Results: 115 SLE patients participated. Mean (SD) age was 40.1 (13.8) years. For all patients and patients without FM, depression (β -1.7, p 0.02), stress (β -1.8, p 0.05), ACR malar rash (β -13.5, p 0.03), and steroid dose (β -0.4, p 0.04) were found to be independent predictors of BI, and explained 54% of BI variance. On stepwise regression modelling, scores for depression (β -2.2, p <0.001), stress (β -1.6, p 0.05), and disease activity (β -1.5, p 0.005) were found to be predictive of poor BI in the whole group, and similar results were noted among those without fibromyalgia. Malar rash presence as defined in the ACR classification criteria for SLE (β -10.3, p 0.04) was most predictive of poor BI among patients without fibromyalgia. Of the modifiable variables among those without FM, depression and stress had similar contributions to BI, followed by disease activity. In patients with fibromyalgia, depression (β -3.6, p 0.002) alone was associated with BI.
Conclusions: Depression, stress, and disease activity are important predictors of BI in SLE patients. Malar rash is a risk for poor BI among those without FM. Attention to depression and stress concurrently with control of disease is suggested among SLE patients with poor BI.
Keywords
lupus, body image, systemic lupus erythematosus
Introduction
Body Image (BI) in the broadest sense describes how a person views his or her own body, regardless of its actual appearance [1]. This includes perceptions, thoughts and feelings about function, competence, size and esthetics. The term itself may be applied to many different aspects of body-related issues including awareness of the body, perceptions, and affects [2]. BI is also connected to one’s functioning and therefore is influenced by one’s overall health [3]. Much of the previous research that has been done on BI has been done in patients with eating disorders or cancer. Specifically studies done on cancer patients have shown that poor body image negatively influences quality of life [4]. Other studies have also shown that women are more likely than men to be affected by body image concerns [5].
Systemic Lupus Erythematosus (SLE) is a multi-systemic chronic autoimmune inflammatory condition. It has been shown that quality of life in SLE patients is similar to or worse than many other chronic medical conditions such as congestive heart failure, diabetes mellitus, and depression [6]. The disease is known to have a predilection for young women. Also, there are significant physical disease manifestations of rashes, alopecia, and adverse effects of medication use (stretch marks, weight gain), in addition to physical (function, sleep, pain, fatigue), emotional (stress, anxiety, depression) and social health issues which may all contribute to poor body image in patients [7-11]. Therefore, there is considerable value in studying body image in this particular population. However, few studies exist on body image in SLE patients [7-9,12]. Focus of studies in SLE has traditionally included understanding of pathogenesis and clinical trials evaluating treatment options for SLE. Patient reported symptoms and concerns as the main outcome of interest in studies has not been the major focus of studies in SLE, despite symptoms such as fatigue being very common and severe.
In previous work, we reported poor body image in patients with SLE as compared to age and gender matched non-SLE controls [9], and that it was modifiable [13]. Existent studies have thus far not evaluated the role of sleep, pain, fatigue and stress in body image in SLE or their relative contribution(s) to poor body image in SLE. Building upon previous work in body image in SLE, this study was undertaken to specifically evaluate the role that sleep, stress, depression, fatigue, pain, physical health, disease activity, damage, and medication (steroids in particular) play in body image in SLE patients. As these variables may be interconnected, we probed the magnitude of their relative contribution, independently, towards body image in SLE, to facilitate identification of potential targets for intervention. Our hypothesis was that all of the chosen variables would contribute to body image in the anticipated direction; for example poor sleep would be associated with worse body image. As fibromyalgia, which is commonly seen in patients with SLE, is associated with pain, sleep, depression and stress, independent of SLE, we undertook secondary analysis stratifying by presence or absence of a diagnosis of fibromyalgia.
Methods
The study was approved by the Institutional Review Board. Patients were recruited from Rush University Medical Center and John H. Stroger Hospital from August 2014 to December 2016. Eligibility criteria included age ≥ 18 years and meeting the American College of Rheumatology classification criteria for SLE [14]. Subjects were also required to be able to read and understand English as all the questionnaires were in English. The goals of the study were explained to the participants, and they each provided written informed consent prior to participation. They were provided with a packet of surveys to be completed before leaving the office on the day of their visit.
The following information was obtained from each participant through patient self-report: demographic data (age, gender, ethnicity, age at SLE diagnosis, education, marital status). Patients then completed a self-administered set of questionnaires, which included: LupusPro [15] (includes Body Image in Lupus Scale-BILS [14]), depression (Patient Health Questionnaire (PHQ)-9) [16], pain (pain inventory), fatigue (FACIT Fatigue Scale Version 4) [17,18], stress (Perceived Stress Scale (PSS)-4) [19], insomnia (Insomnia severity index) [20], and fibromyalgia self-reported tender point quantitative assessment questionnaire. These tools are further described below.
In addition, assessments of disease activity (SELENA-SLE Disease Activity Index) [21], and damage (Systemic Lupus International Collaborating Clinics-American College of Rheumatology-SLICC-ACR) [22] were made by two independent physicians at each visit. The participants’ medical charts were reviewed for ACR classification criteria for SLE met (e.g. ACR malar rash), disease and serological characteristics, current use (and dose) of corticosteroids and other immunosuppressant medications, and comorbidities (including fibromyalgia).
Measurement tools
LupusPro v 1.8
LupusPRO is a 49-item disease specific questionnaire evaluating aspects of healthrelated (HRQOL) and non-health-related (Non-HRQOL) quality of life in SLE patients. HRQOL domains include Lupus Symptoms, Medications, Cognition, Procreation, Physical Health (PH), Emotional Health, Fatigue, Sleep, Pain and Body Image. Non-HRQOL domains are Desires-Goals, Coping, Social Support and Satisfaction with Care. The tool has fair psychometric properties [23]. Scores range from 0 to 100 with higher scores associated with better quality of life [15].
Body image in lupus screen (BILS)
BILS is a five item self-reported tool that forms the body image domain of the LupusPRO. BILS has been validated against other body image measures in SLE patients. Scores range from 0 to 100 with higher scores associated with better quality of life [24].
Patient health questionnaire (PHQ-9)
PHQ-9 was used to measure depression. This is a nine item self-assessment of severity of depression symptoms over the past two weeks. Scores range from 0 to 27. Score of 5-9 implies mild depression, 10-14 moderate depression, 15-19 moderately severe depression and 20-27 as severe depression [16].
FACIT-fatigue
The Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-Fatigue) was administered to participants to measure fatigue. This is a 13-item patient reported questionnaire that assesses the effect of fatigue on function and daily life in the past seven days. Total score ranging from 0 to 52. Higher scores are indicative of less fatigue. The FACIT-Fatigue has been validated in SLE patients [17,18].
Perceived stress scale (PSS-4)
Participant perception of stress was measured using the Perceived Stress Scale-4 (PSS). This four item questionnaire assesses how unpredictable, overloaded, and uncontrollable participants have felt in the past month. Total score ranging from 0-16. Higher scores are associated with higher level of stress. An average score for a college age person is 5-6 [19].
Pain inventory
The pain inventory is a 5 item questionnaire assessing the presence of pain in the last week. If present, the individual rates levels of pain on a ten point Likert scale, where zero indicates “no pain” and ten indicates “pain as bad as you can imagine,” specific to “pain as its worst in the last week,” “pain at its least in the last week,” “pain on average,” and “how much pain you have right now.” Total pain score ranging from zero to 40. Higher scores symbolize greater levels of pain.
Insomnia severity index
To measure degree of insomnia, the Insomnia Severity Index (ISI) was used. This seven-item questionnaire addresses the presence of insomnia in the past two weeks. Total score ranging from 0-28. A score of less than seven indicates no clinical insomnia, while a score above 15 indicates clinical insomnia with increasing severity as scores increase [20].
Statistical analyses
The analysis was performed using SPSS 16.0 software (SPSS Inc., Chicago, IL USA). Descriptive statistics for the whole group were obtained for demographics and disease characteristics. Steroid use was analyzed as current use (yes/no) and current daily prednisone dose in milligrams. Spearman correlational analyses were performed the whole group to guide which variables to include in the multivariate regression modeling for body image. Variables found to be predictive of body image were then entered into three different multivariate regression models (all patients, non-fibromyalgia and fibromyalgia patients) with body image as the dependent variable. Stepwise regression analyses were performed to identify parsimonious models that explained the most variance in body image. P<0.05 was considered significant.
Results
115 patients with SLE were enrolled in the study. The baseline demographics of our study participants are summarized in Table 1. The mean age was 40.1 ± 13.74 years. Greater than ninety percent of participants were women. The majority of the patients were African American (52.2%). More than 90% of participants had high school education or higher. Over half of the participants identified as single (53%) while 25% were married. Everyone met at least four ACR criteria for SLE (5.41 ± 1.44). Thirteen percent had concomitant depression and 14.8% had fibromyalgia on chart review. The participants were on a variety of medications to treat SLE, with hydroxychloroquine and prednisone being most frequently used by 73.9% and 60.9% respectively. Mean (SD) BILS score was 74.48 (27.68). Descriptives for other variables of interest are shown in Table 1.
| Number of Patients | 115 |
| Age, years (Mean ± SD) | 40.1 ± 13.75 |
| Duration of SLE, years (Mean ± SD) | 8.15 ± 6.57 |
| Sex | |
| Female | 104 (90.4) |
| Male | 11(9.6) |
| Ethnicity | |
| African American | 60 (52.2) |
| White | 27 (23.5) |
| Hispanic | 13 (11.3) |
| Asian | 6 (5.2) |
| Other | 9 (7.8) |
| Meeting ≥4 ACR criteria | 115 (100) |
| Comorbidities | |
| Hypertension | 35 (30.4) |
| Diabetes | 5 (4.3) |
| Depression | 15 (13) |
| Fibromyalgia | 17 (14.8) |
| Medication use, current | |
| Hydroxychloroquine | 85 (73.9) |
| Prednisone | 70 (60.9) |
| Mycophenolate Mofetil | 25 (21.7) |
| Azathioprine | 12 (10.4) |
| Methotrexate | 7 (6.1) |
| Cyclophosphamide | 3 (2.6) |
| Current daily prednisone dose (Mean ± SD) | 11.5 (15.6) |
| PGA VAS (Mean ± SD) | 0.60 ± 0.54 |
| Total SSLEDAI (Mean ± SD) | 4.8 ± 4.07 |
| Total SDI (Mean ± SD) | 0.67 ± 1.10 |
| LupusPRO Physical Health (Mean ± SD) | 79.7 (25.8) |
| LupusPro BILS (Mean ± SD) | 74.5 (27.7) |
| PSS score (Mean ± SD) | 6.2 (3.5) |
| Pain score (Mean ± SD) | 13.0 (12.7) |
| PHQ-9 score (Mean ± SD) | 7.2 (6.3) |
| FACIT fatigue score (Mean ± SD) | 20.3 (13.0) |
| ISI score (Mean ± SD) | 9.4 (7.6) |
The numbers within parenthesis are percentage.
SD: Standard deviation, ACR: America College of Rheumatology, VAS: Visual Analog Scale, SSLEDAI: SELENA-SLEDAI, SDI: SLICC/ACR Damage Index, BILS: Body Image in Lupus Screen, PSS: Perceived Stress Scale, PHQ-9: Patient Health Questionnaire-9, FACIT: Functional Assessment of Chronic Illness Therapy, ISI: Insomnia Severity Index
Table 1. Description of the study participants.
Correlational analysis for body image (BILS) with ACR classification criteria, disease activity, damage, medications, tender point count and patient reported health status (physical health, fatigue, depression, pain, insomnia, stress) revealed significant (or at least a trend) of association with 18 variables. These variables were ACR criteria (total number met, ACR-discoid and malar rash), medications (current steroid or hydroxychloroquine use, current prednisone dose), disease activity (PGA; total SSLEDAI; SLEDAI items of rash, alopecia, arthritis), FACIT-fatigue, insomnia score, PHQ score, total pain score, total PSS score and physical health.
The group was analyzed as a whole and stratified groups by fibromyalgia diagnosis status, with these eighteen variables in the multivariate model for body image (Table 2).
| Predictor Variable | All patients (n=115); R2=0.54 | (-)FMS (n=98); R2=0.55 | ||||
|---|---|---|---|---|---|---|
| β | 95%CI | p | β | 95%CI | p | |
| PGA | 7 | -8.98, 22.9 | 0.385 | 6.92 | -10.0, 23.8 | 0.416 |
| SSLEDAI total | -0.952 | -3.03, 1.13 | 0.364 | -0.988 | -3.17, 1.20 | 0.369 |
| SSLEDAI rash | -4.81 | -15.6, 6.01 | 0.378 | -4.24 | -15.8, 7.30 | 0.465 |
| SSLEDAI alopecia | -3.44 | -17.9, 11.0 | 0.635 | -5.05 | -20.1, 9.97 | 0.504 |
| SSLEDAI arthritis | 1.433 | -14.4, 17.3 | 0.857 | -0.902 | -19.4, 17.6 | 0.922 |
| ACR discoid rash | -4.05 | -15.9, 7.83 | 0.499 | -2.32 | -15.1, 10.4 | 0.522 |
| ACR malar rash | -13.5 | -25.6, -1.43 | 0.029 | -15.5 | -28.6, -2.34 | 0.021 |
| PRO PH | 0.073 | -0.200, 0.347 | 0.594 | -0.015 | -0.313, 0.283 | 0.921 |
| Sleep | -0.256 | -1.19, 0.679 | 0.587 | -0.055 | -0.868, -0.020 | 0.924 |
| FACIT | 0.278 | -0.362, 0.919 | 0.389 | 0.179 | -0.502, 0.861 | 0.6 |
| PHQ | -1.72 | -3.11, -0.326 | 0.016 | -1.86 | -3.34, -0.379 | 0.015 |
| Pain | -0.433 | -1.11, 0.246 | 0.208 | -0.503 | -1.22, 0.212 | 0.164 |
| PSS | -1.78 | -3.52, -0.043 | 0.045 | -2.05 | -4.08, 0.012 | 0.049 |
| Self-reported tender points | 0.035 | -1.4, 1.53 | 0.963 | |||
| Steroid use | 5.16 | -7.49, 17.8 | 0.419 | 8.2 | -5.71, 22.1 | 0.243 |
| Steroid dose | -0.427 | -0.835, -0.019 | 0.04 | -0.444 | -0.868, -0.020 | 0.04 |
FMS: Fibromyalgia; PGA: Physical Global Activity; SSLEDAI: SELENA-SLE Disease Activity Index; PRO PH = LupusPRO physical Health domain; FACIT: Functional Assessment of Chronic Illness Therapy Fatigue; PHQ: Patient Health Questionnaire; PSS: Perceived Stress Scale
Table 2. Multivariate Regression Analyses for Body Image.
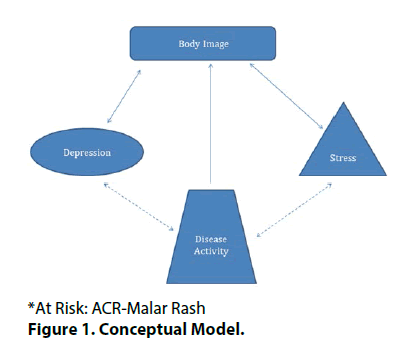
Based on the results, our conceptual model is shown in Figure 1. When looking at the group as a whole, the variables that were independently associated with lower body image were depression, ACR malar rash, daily steroid dose, and stress (Table 2). The largest contribution was from ACR malar rash. These 18 variables explained 54% of variance in body image for the combined group. Similar results with depression, ACR malar rash, daily steroid dose, and stress were noted when the analyses were limited to SLE patients without a diagnosis of fibromyalgia. The largest contribution was again by ACR malar rash. The full model explained 55% of variance in body image among those without fibromyalgia. There were only a small group of patients in this cohort who had a diagnosis of fibromyalgia (n=17), and in this group, a full model with 18 variables was not achievable.
Stepwise multivariate regression models were performed to identify parsimonious models that explained the maximum variance in body image for the whole study group as well as those without and with fibromyalgia, the results of which are summarized in Table 3. Depression, stress, and total SSLEDAI disease activity score were inversely associated with body image (R2 parsimonious model 3=0.46). The magnitude of inverse association with body image was highest for depression. Disease activity and stress contributed similarly towards poor body image.
| All patients (n=115) | (-) FMS (n=86) | (+) FMS (n=17) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | 95%CI | p | R2 | β | 95%CI | p | R2 | β | 95%CI | p | R2 | ||
| Model 1 | PHQ | -2.7 | -3.47, -1.94 | 0 | 0.365 | -2.74 | -3.58, -1.89 | 0 | 0.352 | -3.61 | -5.47, -1.74 | 0.002 | 0.713 |
| Model 2 | PHQ | -0.558 | -3.20, -1.65 | 0 | 0.429 | -2.62 | -3.43, -1.81 | 0 | 0.425 | ||||
| Total SLEDAI | -1.64 | -2.70, -0.586 | 0.003 | -1.73 | -2.84, -0.618 | 0.003 | |||||||
| Model 3 | PHQ | -2.19 | -3.04, -1.34 | 0 | 0.455 | -2.12 | -3.03, -1.21 | 0 | 0.46 | ||||
| Total SLEDAI | -1.51 | -2.56, -0.465 | 0.005 | -1.58 | -2.67, -0.489 | 0.005 | |||||||
| PSS | -1.55 | -3.08, -0.027 | 0.046 | -1.8 | -3.44, -0.154 | 0.032 | |||||||
| Model 4 | PHQ | -2.1 | -2.98, -1.21 | 0 | 0.492 | ||||||||
| Total SLEDAI | -1.37 | -2.45, -0.285 | 0.014 | ||||||||||
| PSS | -2.01 | -3.62, -0.395 | 0.015 | ||||||||||
| ACR Malar Rash | -10.3 | -19.8, -0.720 | 0.035 | ||||||||||
FMS: Fibromyalgia; PRO PH: LupusPRO physical Health domain; SSLEDAI: SELENA-SLE Disease Activity Index; PHQ: Patient Health Questionnaire
Table 3. Multivariate stepwise regression models.
Among patients without a diagnosis of fibromyalgia, depression, stress, total SSLEDAI disease activity score, and ACR malar rash had inverse association with body image (R2 parsimonious model 4=0.49). The highest magnitude of association with poor body image was with ACR-Malar Rash among those without fibromyalgia. Of the modifiable variables, depression and stress had similar contribution to poor body image, followed by disease activity (Table 3).
Among the 17 SLE patients with fibromyalgia diagnosis, stepwise regression modelling identified depression as the predictor of body image, with 71% explanation in variance of body image (Table 3).
Discussion
Systemic lupus erythematosus predominantly affects young women, and body image is an important issue experienced by patients with SLE, as we have previously reported [13,24-26]. This domain is now included in disease targeted patient reported outcome tools: LupusQoL [25] and LupusPRO [15]. In this study, a history of SLE-related malar rash was a significant risk factor for poor body image, apart from depression, stress and disease activity. While the history of malar rash is unmodifiable, the rash may be ameliorated by therapy and cosmetics, suggesting a possible intervention.
Body image is influenced by a variety of factors, many of which are relevant in SLE. These include, but are not limited to, visible changes in appearance in the form of rashes, hair loss and loss of teeth. Side effects of medications such as steroids, used to treat SLE, may also lead to weight gain, acne, moon facies, buffalo hump, facial hair and stretch marks. Functional (physical, emotional and social) and role limitations, stress, fatigue, pain, depression, and insomnia may add to the picture. Other factors may include unpredictable flares, damage, need for long term ongoing medical care, loss of control over body, need for long term medications, hospitalizations, adverse impact on vocation, relationships, and reproductive health [26]. In one third of patients with SLE, fibromyalgia may be comorbid. Since patients are often young, they may not have the coping skills, support or financial resources to deal with the disease. Greater than 80% of SLE patients perceived “changes in appearance” as an unmet need [27]. All of these factors can manifest as poorer body image. In one study comparing BILS of SLE and controls [24], in 53 non-SLE controls the mean (SD) was 81.2 (20.3), while the scores were significantly lower among 233 patients with SLE (Mean (SD) 71.7 (26.9)).
In our previous work, association of depression, overall health status, and cutaneous stigmata of SLE with poor BI in SLE was noted. Body image in SLE is potentially modifiable [21] through non-pharmacologic behavioral interventions. In the current study, significant expanded on previous work by inclusion of other important variables (sleep, pain, fatigue, stress, tender point counts) and exploration of the body image models stratified by fibromyalgia diagnosis.
Despite eighteen candidate predictor variables for body image on univariate analysis, only four were independently associated with body image on multivariate analysis. Further refining the model led to identification of depression as the major independent predictor of poor body image in all the three groups (all, non-fibromyalgia, and fibromyalgia). Furthermore, stress and disease activity were associated with body image in SLE patients (all and non-fibromyalgia patient groups). The biggest contributors to poor body image were depression, stress and history of having a malar rash from SLE. Directions of these associations were as hypothesized in the study methods. Solid lines show the associations that were tested. Hatched lines reflect plausible associations that were not evaluated herein. Of note, the direction of associations may be bidirectional as shown in the Figure, and need to be further evaluated in a longitudinal study.
Our results confirm that in addition to controlling disease activity, the treatment of SLE patients should include the identification of those at high risk of poor body image, depression, and stress. Disease and Care management models [28] could be developed for SLE, that include evaluation and addressal of BI, among others.
The availability of self-administered survey instruments for use during routine care can facilitate such screening. Strategies to address body image, depression and stress may include active listening, support, education, appropriate referrals and resources, self-management resources, behavioral interventions and medications. Concurrent attention to depression, stress and disease activity is suggested among those at risk for or with poor body image.
Limitations of this study include its cross sectional study design, which precludes the assignment of cause and effect. Secondly, the multivariate analysis for the SLE patient group with fibromyalgia was not possible with the full model because of the sample size and the large number of variables in the model.
There are several strengths to this study. First, it is the largest study to-date evaluating body image in SLE patients. It is also the only study on body image in SLE to examine the role of a comprehensive list of variables on BI and stratified by underlying diagnosis of fibromyalgia. BI was measured using a tool specifically designed from and for SLE patients. All the measurement tools used within the study performed well psychometrically. Future directions include
• Exploring the validity of the models in a longitudinal setting and
• Designing and testing of interventions guided by the results of this study, with the goal of improving body image and overall health outcomes among SLE patients.
Conflict of interest
The authors declare that they have no conflicts of interest.
Funding
This study was supported through funding from The Brewer Foundation.
References
- Alleva J, Sheeran P, Webb T, et al. A meta-analytic review of stand-alone interventions to improve body image. PLoS One. 10(9), e0139177 (2015).
- Scheffers M, van Busschbach JT, Bosscher RJ, et al. Body image in patients with mental disorders: characteristics, associations with diagnosis and treatment outcome. Comp. Psych. 74, 53-70 (2017).
- Pope CG, Pope HG, Menard W. Clinical features of muscle dysmorphia among males with body dysmorphic disorder. Body. Image. 2(4), 395-400 (2005).
- Annunziata M, Giovannini L, Muzzatti B. Assessing the body image: relevance, application and instruments for oncological settings. Support. Care. Cancer. 20(5), 901-907 (2012).
- Pruzinsky T, Cash TF. Understanding body images. In: Cash TF, Pruzinsky T, editors. Body image. New York: Yuildford Press. pp, 3-12 (2002).
- Jolly M. How does quality of life of patients with systemic lupus erythematous compare with that of other common chronic illnesses? J. Rheumatol. 32, 1706-1708 (2005).
- Hale ED, Treharne GJ, Norton Y, et al. Concealing the evidence: the importance of appearance concerns for patients with systemic lupus erythematosus. Lupus. 15(8), 532-540 (2006).
- Monaghan SM, Sharpe L, Denton F, et al. Relationship between appearance and psychological distress in rheumatic diseases. Arthritis. Rheum. 57(2), 303-309 (2007).
- Jolly M, Pickard AS, Mikolaitis RA, et al. Body image in patients with systemic lupus erythematosus. Int. J. Behav. Med. 19(2), 157-164 (2012).
- Fonseca R, Bernardes M, Terroso G, et al. Silent burdens in disease: fatigue and depression in SLE. Autoimmune. Dis. 2014, 1-9 (2014).
- Waldheim E, Elkan AC, Pettersson S, et al. Health-related quality of life, fatigue, and mood in patients with SLE and high levels of pain compared to controls and patients with low levels of pain. Lupus. 22(11), 1118-1127 (2013).
- Cornwell CJ, Schmitt MH. Perceived health status, self-esteem and body image in women with rheumatoid arthritis or systemic lupus erythematosus. Res. Nurs. Health. 13(2), 303-309 (2007).
- Jolly M, Peters KF, Mikolaitis RA, et al. Body image intervention to improve health outcomes in lupus. J. Clin. Rheumatol. 20(8): 403-410 (2014).
- Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematous. Arthritis. Rheum. 40(9), 1725 (1997).
- Jolly M, Pickard S, Block JA, et al. Disease-specific patient reported outcome tools for systemic lupus erythematosus. Semin. Arthritis. Rheum. 42(1), 56-65 (2012).
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J. Gen. Intern. Med. 16(9), 606-613 (2001).
- Lai JS, Beaumont JL, Ogale S, et al. Validation of the functional assessment of chronic illness therapy-fatigue scale in patients with severely active systemic lupus erythematosus, participating in a clinical trial. J. Rheum. 38(4), 672-679 (2011).
- Kosinski M, Gajria K, Fernandes AW, et al. Qualitative validation of the FACIT-Fatigue scale in systemic lupus erythematosus. Lupus. 22(5), 422-430 (2013).
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J. Health. Soc. Behav. 24(4), 385-396 (1983).
- Bastien C, Valliéres A, Morin C. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep. Med. 2(4), 297-307 (2001).
- Bombardier C, Gladman DD, Urowitiz MB, et al. Derivation of the SLEDAI: a disease activity index for lupus patients. Arthritis. Rheum. 35, 630-640 (1992).
- Gladman DD, Urowitz MB. The SLICC/ACR damage index: progress report and experience in the field. Lupus. 8(8), 632-637 (1999).
- Jolly M, Gandhi N, Nevares A, et al. Validation of Lupuspro V1.8, disease targeted patient reported outcome for systemic lupus erythematosus [abstract]. Arthritis Rheumatol. 67(10) (2015).
- Jolly M, Pickard AS, Sequeira W, et al. A brief assessment tool for body image in systemic lupus erythematosus. Body. Image. 9(2), 279-284 (2012).
- Jolly M, Pickard AS, Wilke C, et al. Lupus-specific health outcome measure for US patients: the LupusQoL-US version. Ann. Rheum. Dis. 69(1), 29-33 (2010).
- Jolly M. Body image issues in Rheumatology. In: Cash T, Smolak L, ed. Body Image, 2nd ed. New York, NY: 2012.
- Moses N, Wiggers J, Nicholas C, et al. Prevalence and correlates of perceived unmet needs of people with systemic lupus erythematosus. Patient. Educ. Couns. 57(1), 30-38 (2005).
- Ciccone MM, Aquilino A, Cortese F, et al. Feasibility and effectiveness of a disease and care management model in the primary health care system for patients with heart failure and diabetes (Project Leonardo). Vasc. Health. Risk. Manag. 6, 297-305 (2010).