Review Article - Interventional Cardiology (2023)
Brief overview of lipoprotein (a) in atherosclerosis processes: Measurements and therapeutic strategies
- Corresponding Author:
- Anastasia V. Poznyak
Institute for Atherosclerosis Research, Osennyaya 4-1-207, 121609 Moscow, Russia
E-mail:tehhy_85@mail.ru - Alexander N. Orekhov
Laboratory of Angiopathology, Institute of General Pathology and Pathophysiology, 8 Baltiiskaya Street, Moscow 125315, Russia
E-mail: a.h.opexob@gmail.com
Received date: 06-Mar-2023, Manuscript No. FMIC-23-90710; Editor assigned: 08-Mar-2023, PreQC No. FMIC-23-90710 (PQ); Reviewed date: 22-Mar-2023, QC No. FMIC-23-90710; Revised date: 29-Mar-2023, Manuscript No. FMIC-23-90710 (R); Published date: 07-Apr-2023, DOI: 10.37532/1755- 5310.2023.15(S16).395
Abstract
There is ample evidence confirming Lipoprotein (a) pathogenicity. As an independent cardiovascular risk factor, Lp(a) can aggravate Cardiovascular Disease (CVD) in various ways contributing to the development of atherosclerosis, thrombosis, and inflammation. In this review, we will summarize currently available knowledge on Lp(a) physiological and biochemical properties underlying its impact on CVD, as well as describe the established mechanisms through which Lp(a) is involved in atherogenesis and CVD progression. We will also look at various measures aimed at Lp(a) management and alleviation of associated cardiovascular risk. Different Lp(a) measurement methods will be considered. In the last section of the paper, we compare existing therapeutic strategies for Lp(a) reduction and compare them by such parameters as clinical efficacy in decreasing CVD risk, availability, tolerability, and safety
Keywords
Atherosclerosis; Lipids; Lipoprotein A; ApoA; Lipid measurement; Atherosclerotic indexes
Abbreviations
Lp(a): Lipoprotein A; ApoA: Apolipoprotein A; CVD: Cardiovascular Disease: CHD: Coronary Heart Disease; LDL: Low-Density Lipoprotein; ApoB: Apolipoprotein B; LDL-R: Low-Density Lipoprotein Receptor; LDL-C: Low-Density Lipoprotein Cholesterol; PCSK9: Proprotein Convertase Subtilisin/Kexin type 9; FH: Familial Hypercholesterolemia; SMC: Smooth Muscle Cell; EC: Endothelial Cell; e-ACA: epsilon-Aminocaproic Acid; sLBS: supported Lipid Bilayers; OSE: Oxidation- Specific Epitope; oxPL: Oxidized Phospholipids; CAM: Cell Adhesion Molecules; HUVEC: Human Umbilical Vein Endothelial Cells; ASCVD: Atherosclerotic Cardiovascular Disease; LA: Lipoprotein Apheresis; CAD: Coronary Artery Disease; MACE: Major Adverse Cardiovascular Event; SOC: Standard of Care; ASO: Antisense Oligonucleotides; VLDL: Very Low-Density Lipoprotein
Introduction
After its discovery in 1963, Lipoprotein (a) was believed to have little significance. This changed when plasma Lp(a) level appeared to be an independent causal risk factor for CVD. The plasma Lp(a) level was also associated with myocardial infarction, ischemic stroke, chronic kidney disease, and peripheral vascular disease [1]. According to recent studies, approximately 20-25% of the population have an increased Lp(a) level (greater than 30-50 mg/dL), which contributes to increased CVD risk. Some recent pieces of evidence allow us to treat Lp(a) as the strongest genetic risk factor for CHD and the only monogenic risk factor for aortic stenosis [2].
Despite the topic of apolipoproteins in the scope of atherosclerosis and CVS seeming to be depleted, it is too soon to dismiss these molecules. In this review, we analyzed the complexity of Lp(a) role in atherosclerosis. One of the main manifestations of this is the differences in the mechanisms of action of drugs, one way or another used to treat atherosclerosis and affect Lp(a). In this regard, the need arises again to further reassess the possibilities of measuring Lp(a) levels, as well as to develop indices that would reflect the complexity of the pathogenesis of atherosclerosis and differences in Lp(a) levels due to natural differences in the population (gender, ethnicity, age, etc.).
Features of the structure and application of Lp(a) as a target for therapeutic intervention have already been described many times, including recent reviews, such as papers by Likozar et al. [3], by Saeed et al. [4], by Schwartz and Ballantyne [5], and by Di Fusco et al [6]. These comprehensive reviews cover the effects of Lp(a) in various cardiovascular pathologies and provide data on potential treatment options. All these manuscripts describe Lp(a) structure in details and analyze the background of lipoprotein a molecule, its functions, and synthesis. In our review, we paid more attention to atherosclerosis, rather than other cardiovascular pathologies. We summarized current knowledge on the mechanisms by which Lp(a) is involved in atherogenesis, underlying the wide range of the connections between the disease and the molecule. To find this information, we used PubMed and Google Scholar databases. We mostly obtained papers published in recent five years, including older ones when it was necessary. To find initial publications, we used keywords, such as “lipoprotein a”, “lipoprotein a in atherosclerosis’, “lipoprotein a in thrombosis”, etc.
Literature Review
Lipoprotein (a) structure
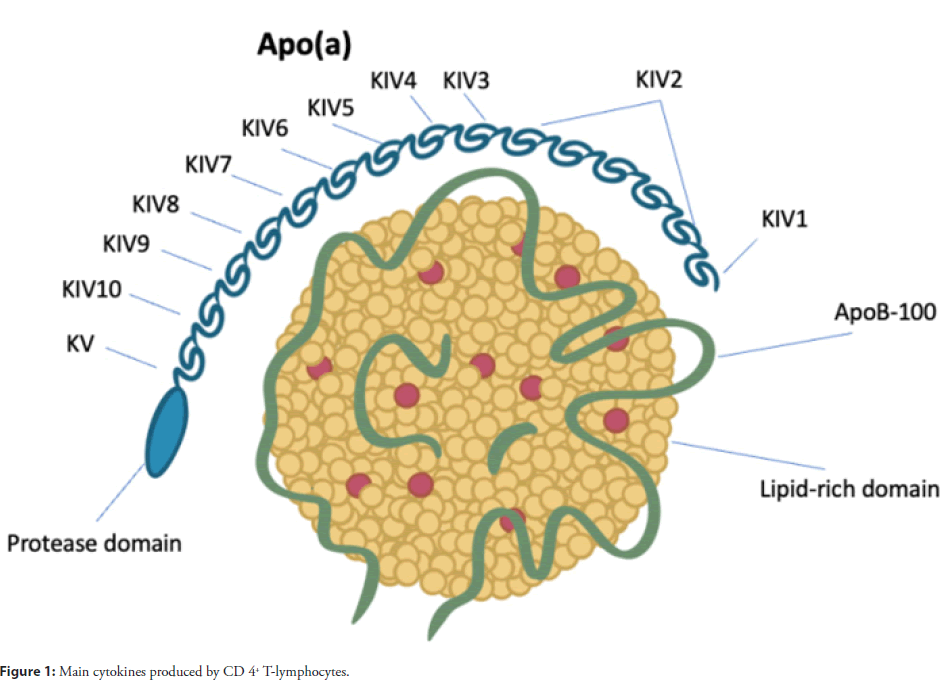
The atherogenic and pro-thrombotic effect of lipoprotein (a) (Lp(a)) leading to aggravation of ischemic CVD is largely due to its structure. This particle is similar to Low-Density Lipoprotein (LDL). It is cholesterol-laden as well; however, it contains one more plasminogen-like glycoprotein, apoLipoprotein (a) (ApoA), which is covalently bound to apolipoprotein B (ApoB), and thus it is identified as a unique lipoprotein fraction [7]. While plasminogen contains five various kringle shaped protein structures (I through V) as well as a protease region, ApoA includes one plasminogenlike kringle V structure, several plasminogen-like kringle IV structures (type 1 to 10), and an inactive protease region [8]. The size of the isoform depends on the amount of Kringle IV type 2 (KIV2) structures and is negatively associated with the amount of the protein produced in the liver, presumably because intracellular processing and degradation of larger isoforms takes longer time. See schematic Lp(a) structure in Figure 1.
Thus, isoform size is also negatively associated with Lp(a) levels in plasma, and in most subjects elevated Lp(a) levels are only observed for small isoforms. Usually, Lp(a) concentrations are presented as Lp(a) total mass in mg/dL. Another option that has recently become more popular is to report particle number in nmol/L [9]. The third and less common alternative is Lp(a) cholesterol mass. The differences between these options should be considered when analyzing the results.
Lipoprotein (a) functions
Physiology: Apo(a) is primarily synthesized in the liver. However, there is still a lot of debate regarding the exact production site of Lp(a). While some studies suggest that the assembly site is extracellular-either on the surface of hepatocytes or in the circulation-other studies point to an intracellular Apo(a) synthesis [10]. Apo(a) is tethered to Apo B-100 by a specific disulfide bond within the KIV-9 domain. Although plasma levels of Lp(a) are mainly determined by Apo(a) synthesis, thorough understanding of all the mechanisms involved in Lp(a) catabolism is required in order to develop new strategies for improving Lp(a) clearance [11]. Lp(a) catabolism is believed to be predominantly dependent on the liver, although part of the clearance takes place in the kidneys. There are many receptor types involved in the Lp(a) clearance pathways, including the LDL-Receptor (LDL-R) [12]. Still, the exact involvement of LDL-R in Lp(a) clearance remains unclear, despite certain homology between Lp(a) and HDL. The research has shown somewhat controversial results. While in vitro studies reported that Lp(a) clearance was based on binding to human HepG2 hepatoma cells and fibroblasts via the LDL-R, other experiments demonstrated that patients with Familial Hypercholesterolemia (FH) had the same Lp(a) clearance rates as controls. In accordance with catabolic studies, circulating Lp(a) levels increased together with LDL-R levels as a result of statin administration [13,14].
By contrast, trials of Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor evolocumab, which inhibits LDL-R degradation, demonstrated a decrease in Lp(a) correlating with LDL-cholesterol (LDL-C) lowering [15]. In a more recent study involving patients with high Lp(a) levels treated with statins, another PCSK9 inhibitor alirocumab was shown to enhance Lp(a) catabolism by 28% [16]. Although the results of some studies suggested that Lp(a) reduction as a result of treatment with PCSK9 inhibitors involved LDL-R, in vitro studies clearly demonstrated that PCSK9 inhibition lowered hepatic Apo(a) synthesis. It is worth mentioning that Lp(a) catabolism was increased by PCSK9 inhibitors only when used in combination with statins [17]. This finding suggests that Lp(a) competes with LDL tethering on LDL-R only when LDL concentrations are kept low. Lower affinity of Lp(a) particles for the LDL-R compared to LDL fraction further supports this theory. Furthermore, Lp(a) clearance by other receptors from the LDL-R family could also be improved as a result of PCSK9 inhibition [18]. This notion is strongly supported by the results of evolocumab trials in patients with homozygous FH since a considerable decrease in Lp(a) levels (by 20%) took place despite the lack of functional LDL-R. Therefore, the exact role of LDL-R in Lp(a) catabolism remains unclear, although recently discovered gain-of-function mutation in the LDLR gene may shed light on this matter [19].
Pathogenicity
Obviously, plasma Lp(a) levels vary between individuals. They are determined genetically and are little or not affected by diet and environment [20].
There are three main ways in which Lp(a) may be pathogenic: It may induce atherosclerosis, promote inflammation, and lead to thrombosis [21]. The most common Lp(a) measuring method is via monoclonal anti-Apo(a) antibody, which allows to identify of the Lp(a) levels. In clinical practice enzyme immunoassay (ELISA) is also widely used. However, the ratio of mass to molar concentration varies individually due to significant individual differences in the molecular weight of Apo(a) particles [22]. As a result, it is difficult to find a standard method for Lp(a) measurement. Furthermore, specific ethnic and risk populations present with abnormal Lp(a) levels which cannot be determined clinically. In general, median Lp(a) concentrations depend on race/ ethnicity and can be affected by certain conditions. Lp(a) levels vary between undetectable values to over 1,000 mg/dL [23]. Many studies have demonstrated that elevated levels of Lp(a) in plasma (50 mg/dL) are an independent predictor and risk factor for CVD [24].
Atherosclerosis
The Apo(a) in Lp(a) contributes to the development of atherosclerosis by promoting Lp(a) retention as well as accumulation of Smooth Muscle Cells (SMC) and vascular endothelial cells, which affects endothelial function and leads to chemotactic activation of macrophages and monocytes. Lp(a) attaches to vascular tissues through a strong lysine binding site in Apo(a), resulting in contraction and increased permeability of the endothelial layer through rhoa/rho kinase/mypt1-related pathways. Furthermore, Apo(a) can be involved in the concentration-dependent SMC rejection throughout migration assays as a result of rhoa/rho kinase and integrin αvβ3 action. Lp(a) binding to macrophages by means of high-affinity VLDL-R-induced endocytosis, leads to foam cell formation and accumulation of cholesterol [25].
Thrombosis: Lp(a) can contribute to thrombosis through various mechanisms, the main being obstruction of fibrinolysis. Production of active plasmin can be impaired as a result of the homology between plasminogen and Apo(a). At the same time, Lp(a) binds to fibrin, thereby competing with plasminogen and impeding thrombolysis mediated by plasmin. The Apo(a) can also obstruct platelet accumulation as it replaces fibrinogen and binds to integrin αIIbβ3, inhibiting plasminogen activation by means of urokinase-type plasminogen activator action [26].
It was shown that Apo(a) and Lp(a) can directly suppress the activation of tPA-mediated plasminogen on the surface of monocytes, macrophages, and ECs [27]. The crucial role in this effect appeared to be played by the lysine binding ability of Apo(a) because treatment with the lysine analog, e-aminocaproic acid (e-ACA), or perturbation of the sLBS could cancel this effect [28]. Lp(a) was suggested to inhibit plasmin formation by promoting the expression of plasminogen activator inhibitors by ECs and human monocytes [29]. Thus, Lp(a) treatment of ECs was shown to reduce the expression of tPA. Various atherothrombotic processes, i.e., angiogenesis, cell migration, and mural thrombus formation can be mediated by the inhibition of pericellular plasminogen activation [30]. In the same way, Lp(a)/Apo(a) can attenuate fibrinolysis via suppression of tPA-mediated plasminogen activation on the surface of fibrin clots. Competitive inhibition of the conversion of Glu-plasminogen to its more easily activated form Lys-plasminogen by Lp(a)/Apo(a) has been shown to inhibit plasminogen activation [31]. In addition, it has been shown in vitro that Apo(a) is able to inhibit the binding of plasminogen to its substrate fibrin. Due to the formation of a quaternary complex with plasminogen, tPA, and fibrin, Apo(a) can inhibit the process of fibrinolysis [32]. In vivo experiments on rabbit jugular vein models and transgenic Apo(a) mice showed that the rate of clot destruction in such models is lower than in wild-type samples [33].
In addition to the mechanism of competitive binding to plasminogen and plasmin substrates, there are data on other potential mechanisms. In the study of rs3798220 SNP LPA, the rate of fibrinolysis for mutant Apo(a) containing fibrin clots was lower than for clots treated with wild-type Apo(a) [34]. The results of this study did not show differences in the ability of mutant Apo(a) to inhibit plasmin formation, however, the authors observed the ability of Apo(a) to modulate the architecture of fibrin clots, so that they could resist lysis.
In addition to plasminogenrelated mechanisms, the involvement of Apo(a) in thrombosis has been shown to increase thrombosis in a Plg -/- mouse model [35]. In addition, Lp(a) has been shown to potentially bind to an inhibitor of the tissue factor pathway and inactivate it, enhancing platelet aggregation. Since Lp(a) can also induce tissue factor expression on macrophages, these two effects may result in uncontrolled activation of the tissue factor-mediated coagulation cascade upon atherosclerotic plaque rupture [36].
Inflammation: Lp (a) is prone to oxidation. As a result of this process, the Oxidation-Specific Epitope (OSE) is formed. OSE consists predominantly of apoptotic cells and oxidized LDL, sterols and phospholipids, contributing to both inflammation and atherogenesis [37].
Certain pathogenic properties of Lp(a) are related to the lysine binding sites in some of the KIV Apo(a) domains. For instance, there are certain sites in KIV10 that allow for the covalent binding of pro-inflammatory oxidized Phospholipids (oxPLs) [38]. Being one of the main oxPL transporters, Lp(a) has high oxPL content. Oxidized phospholipids promote inflammation and are also involved in the development of atherosclerosis [39].
The pro-inflammatory effect of Lp(a) is also based on its ability to induce inflammatory cytokines. Apo(a) promotes macrophages to express monocyte chemotactic protein, TNF-α, and interleukin-8. Furthermore, as mentioned above, Lp(a) can directly trigger the chemotaxis of monocytes and attract them to the endothelium by various mechanisms [40,41].
Endothelial dysfunction and vascular remodeling: Initial atherosclerotic stages include endothelial dysfunction, which is one of the key events in the disease pathogenesis. Lp(a) can participate in the initial atherogenic processes via its Apo(a) component in a way quite like the role of oxidized LDL [42]. Acquiring the dysfunctional state, ECs become to act pro-inflammatory. The expression of CAMs is known to attract proinflammatory cells to the vessel wall. Studies have shown that the Lp(a) treatment of HUVEC causes the overexpression of E-Selectin, VCAM1, and ICAM1 [43]. A similar effect was demonstrated for LDL and oxLDL [44]. Then, the ICAM1-stimulating effect of Lp(a) in HUVEC appeared to be caused solely by Apo(a), but not by Lp(a) [45].
It was also shown that the lysine-dependent binding of Lp(a) to b-integrin Mac-1 on THP-1 cells can stimulate EC-monocyte adhesion. This, in turn, led to the overexpression of tissue factors by monocytes [46].
Moreover, the treatment of HUVEC with recombinant Apo(a) (r(ApoA)) resulted in a notable increase in the permeability of HUVEC monolayers [47]. This can happen due to the induction of actin cytoskeleton and intercellular adhesion junctions’ changes. After r-Apo(a) treatment in HUVEC, synthesis and secretion of prostaglandin E2 were also increased because of the stimulation of nuclear translocation of b-catenine and enhanced expression of cyclooxygenase-2 [48].
One of the critical roles in the process of atherogenesis is played by the migration of ECs and VSMCs, which is important for the remodeling that occurs inside the vessel and leads to the formation of a fibrous cap in a mature plaque [49]. Lp(a)/Apo(a) can stimulate the growth and migration of ECs by inhibiting the amount of transforming growth factor-b (TGF-b) generated by HUVEC [50]. A relationship was shown between this effect and the binding of lysine to sLBS in KIV10 Apo(a) by binding to the aVb3 integrin on the cell surface. Although, Lp(a) can suppress in vitro EC tube formation by inhibiting the plasminogen activation system on the cell surface [51]. Similarly, Apo(a) stimulated the migration and proliferation of SMCs both in vitro and in mice. This was due to a TGF-β inhibition-dependent mechanism that determines the role of the KIV9 domain and its ability to inhibit the formation of pericellular plasmin [52]. In rabbits expressing recombinant human Apo(a), an increased rate of SMC proliferation was noted. Competitive inhibition of activation of pericellular plasminogen activation on the surface of ECs, monocytes, and macrophages in vitro has also been shown in a KIV10 and KV-dependent manner [53]. Notably, in coronary atheromas of patients, 90 and 31% of macrophages and SMCs, respectively, were found together with Lp(a) [54]. Taken together, these data underscore the ability of Lp(a)/Apo(a) to affect vascular endothelial and smooth muscle cell populations in a pro-atherogenic manner. The ability of Lp(a) to stimulate inflammatory conditions in SMCs, ECs, monocytes, and macrophages both in vitro and in vivo by initiating various cell signaling processes suggests that Lp(a) may promote proinflammatory phenotypes in the vasculature-key steps in the initiation of atherosclerotic formation. Plaques [55,56].
Lipoprotein measurements
Median Lp(a) concentrations reported by different epidemiologic studies vary a lot. The results can be affected by well-known ethnic differences. However, the data may also vary depending on the type of assay used. Reporting Lp(a) concentrations as total mass concentrations (i.e., mg/dL) is the most common practice as well as using various immunoassays, like immunoturbidimetric, which are relying on antigen-antibody interaction [57]. However, recently it has become more common to report particle number (i.e., nmol/L) in such assays as the enzyme-linked immunosorbent assay and the immunoturbidimetric assay. The third and far less common option is to report Lipoprotein (a) cholesterol (Lp(a)-C), based on highperformance liquid chromatography or electrophoresis. However, this method does not work for high-throughput analysis [58].
Irrespective of the methodology used, a common difficulty has been the lack of reference material or a calibrator to the results could be traced, the target value being preferably reported as particle number, since the size and content of the particles may vary. Traceability to a single common reference value would allow us to compare results from different assays and establish threshold values indicating increased risk. Regular deviations by 13 mg/dL (total mass) have been observed at a mean Lp(a) concentration of 57 mg/dL when applying 17 various immunoturbidimetric assays without a common calibrator. The reported mean concentration variations in comparable populations may be also due to the difference between fresh vs. long-term frozen material [59]. The Copenhagen General Population Study reported that the 80th percentile of the concentration distribution for fresh samples was equal to 41 mg/dL, and 33 mg/dL for samples stored on average 7 years at -80 Celsius. In addition, the measurement bias may be related to Apo(a) size in some assays, which may also influence the reported results [60].
The isoform-related bias may take place in assays where the Lp(a) measurement is based on polyclonal antibodies directed at Apo(a), as these methodologies imply that the degree of antibody binding and the signal will not be affected by the size of the Apo(a) isoform. However, substantial isoform-related measurement deviations of >100% positive and >50% negative bias have been observed [61]. Therefore, assays involving monoclonal antibodies directed at Apo(a) domains that do not vary are theoretically preferable. However, their development and implementation may be difficult, especially when it comes to high throughput.
Thus, it turns out that the most commercially reasonable method is to use polyclonal antibodies and try and reduce the isoformrelated bias as much as possible, for example, by using latexparticle- enhanced immunoassays or calibrators where large apoLipoprotein (a) isoforms are used for calibration points with a lower concentration and small isoforms for calibration points with higher concentration [62]. Noteworthy, the latter approach may result in inaccurate measurements in case the patient does not demonstrate the assumed inverse correlation between Lp(a) levels and Apo(a) isoform size. However, there is little probability that this will lead to serious medical consequences since presumably few patients will reach the threshold Lp(a) values for the initiation of Lp(a) treatment and corresponding decisions will be made based on other factors as well [63].
Thus, Lp(a) measurements should be preferably carried out using fresh samples. The assays should be well-validated, i.e., it should be documented that the method provides acceptable accuracy, precision, and linearity. Furthermore, the assay should be traceable to an internationally established calibrator, such as the WHO SRM 2B primary reference material, which allows making sure that there will be common cut-offs for high concentrations [64]. In cases where there is no traceability, percentile cut points for the cohort concentration distribution should be documented together with the absolute values in order to establish at least some comparability with other measurements. In addition, the assay provider should report minimal Apo(a) isoform-related measurement bias. Currently, there is consensus, that reporting particle number (i.e., nmol/L) should be used rather than the traditionally reported total mass (mg/dL). Still, isoform-related measurement bias affects all assays using polyclonal antibodies to a certain degree. As a result, only the approximate value may be provided. Therefore, there is still some debate around the transitioning to nmol/L, and whether commercially available, high-throughput assays not prone to isoform-dependent bias should be developed prior to the transitioning [65].
Lp(a) lowering therapies
It has been vastly reported that Lp(a) may increase risk of Atherosclerotic Cardiovascular Disease (ASCVD); however, randomized clinical trials data are lacking that would demonstrate that lower Lp(a) levels are beneficial for the cardiovascular system. In a study using genetic methods, Emdin et al. estimated how Lp(a) reduction could be clinically beneficial [66]. For each standard deviation reduction in Lp(a) of about 28 mg/dl, the risk of stroke, peripheral vascular disease, coronary heart disease, aortic stenosis, and heart failure was reduced by 13%, 31%, 29%, 37%, and 17%, respectively. Using the data from a vast perspective European study, Afshar et al. estimated the effect of Lp(a) lowering and discovered that keeping Lp(a) below 50 mg/ dL decreased the prevalence of aortic stenosis cases and myocardial infarction cases by approximately 14% and 7%, respectively [67]. It means that Lp(a) lowering may significantly contribute to reducing cardiovascular disease. As demonstrated by a Mendelian randomized study, Lp(a) lowering by 100 mg/ dL reduces the CVD risk to the same degree as LDL-C lowering by 38.67 mg/ dL; at the same time, lower Lp(a) seems to be clinically beneficial regardless of the variation in LDL-C concentrations. Lamina et al. in another Mendelian randomized study showed that it would be necessary to reduce Lp(a) by 65.7 mg/dL to potentially achieve the effect similar to a 38.67 mg/dL reduction of LDL-C on clinical outcomes [68].
Lp(a) levels in plasma primarily depend on genetic factors, so they cannot be substantially lowered by exercise, diet and other such interventions and drug therapy is necessary to reduce them. Studies have demonstrated that first-line statins lowering LDL-C slightly increase Lp(a) [69]. Other LDL-C-lowering drugs, like ApoB-100 Antisense Oligonucleotides (ASO) and PCSK9 inhibitors have been demonstrated to lower Lp(a) levels, but only by 20%-30%, so clinical benefits are limited. Currently, the only method proved by RCT and observational studies to significantly reduce both cardiovascular events and Lp(a) levels is lipoprotein apheresis [70]. It is recommended by British and German guidelines; however, it is expensive and invasive, which limits its clinical application. At the same time, recently conducted phase 2 clinical trials have proved that Apo(a) ASO IONIS-Apo(a)-LRX effectively reduces Lp(a) levels and has good tolerance, so in the future it may be a promising medication for elevated Lp(a) management [71]. We summarized various therapeutic approaches and their mechanisms in Table 1.
| Therapy approach | Mechanism of action on Lp(a) | Effect on Lp(a) level | References |
|---|---|---|---|
| PCSK9 Inhibitors+Statins | LDL-R mediated Lp(a) clearance | n/a | [41] |
| PCSK9 Inhibitors | Inhibiting Lp(a) synthesis | -20% to 60% | [42,43] |
| Lipoprotein Apheresis | Removes Lp(a) from blood | -70% | [47] |
| IONIS-APO(a)RX | Targeted suppression of Apo(a) expression via ASO | -35% to 80% | [54] |
| Mipomersen | Inhibiting apoB synthesis. | -25% | [63] |
| Lomitapide | Inhibiting microsomal triglyceride transfer protein. | -17% | [64] |
| Niacin | Unknown | -31% | [67] |
| Anacetrapib | Unknown | -15% to 40% | [75] |
Table 1: Therapeutic approaches targeting Lp(a) and their mechanisms.
PCSK9 inhibitors: It is traditionally believed that the Lp(a) lowering action of PCSK9 inhibitors (PCSK9i) is based on enhancing LDL-R mediated Lp(a) clearance. However, it has recently been found that PCSK9 inhibitors lower Lp(a) in two ways. When used alone, PCSK9 inhibitors lower Lp(a) by inhibiting Lp(a) synthesis. When used together with statins, PCSK9 inhibitors lower Lp(a) by the acceleration of Lp(a) catabolism mediated by increased activity of LDL-Rs [72]. Clinical trials demonstrated LDL-C reduction by approximately 55% and Lp(a) reduction by ~30% (an approximate 2 to 1 ratio) due to PCSK9 inhibitors. Nevertheless, LDL-C and Lp(a) response to PCSK9 inhibitors is not always concordant: In patients who initially had elevated Lp(a), Lp(a) can lower by up to 60% [73]. A FOURIER trial demonstrated that in patients with ASCVD, increased levels of Lp(a) were associated with MI, emergency revascularization, and cardiovascular death regardless of LDL-C levels, and evolocumab reduced Lp(a) levels by 26.9% [74]. At the same time, the PCSK9i appeared to be more effective in patients who had higher initial Lp(a) levels. The analysis of the ODYSSEY OUTCOMES trial showed that alirocumab reduced Lp(a) concentrations as well as cardiovascular events [75]. Having adjusted for cholesterol levels, individuals with higher initial Lp(a) demonstrated a substantially greater reduction of MACE events and Lp(a) concentrations. However, it is still unclear whether the reduction in cardiovascular events during PCSK9 inhibitors therapy was associated with the Lp(a) decrease or with a further LDL-C decrease due to statins. In another, multicenter RCT study, 129 patients with NCEP high risk, mean LDL-C 3.7 (standard deviation 1.0) mmol/L, and median Lp(a) 200.0 (interquartile range 155.5-301.5) nmol/L were randomized to monthly evolocumab 420 mg subcutaneously or placebo [76].
Evolocumab reduced Lp(a) by 13.9% and LDL-C by 60.7% compared to the placebo. However, computed tomography/18Ffluorodeoxyglucose positron-emission tomography has not revealed any significant alterations in artery wall inflammation in evolocumab patients vs. placebo. To sum it up, further research is necessary to better understand the impact of PCSK9 inhibitors on Lp(a) reduction, the scale of the reduction as well as possible beneficial clinical applications.
Lipoprotein apheresis: Common lipid-lowering therapy currently used does not significantly lower Lp(a) levels. Lipoprotein Apheresis (LA) is clinically recommended for patients with progressive ASCVD and high Lp(a) who do not respond to optimal therapy. 120 patients with progressive Coronary Heart Disease (CHD) and Lp(a) ≥ 60 mg/dL already receiving statins in maximum tolerable doses were allocated for a longitudinal multicenter cohort study where they received a combination of statins and LA [77]. The results demonstrated that the median Lp(a) level was lowered by 73%, from 112 to 30 mg/dL (P<0.0001), while the amount of MACE cases per year was reduced per patient from 1.056 to 0.144. Another prospective multicenter study investigated LA effects for two years in 170 patients with progressive Coronary Artery Disease (CAD) and increased Lp(a) levels. major adverse cardiac events (MACE) incidence was found to reduce by more than 75% compared to two previous years and a 5-year followup confirmed a lasting effect of LA on the MACE prevention in individuals with CHD in progress and elevated Lp(a) [78].
In 2017, the LA effect was evaluated on patients with hyperlipoproteinemia-complicated CAD in a randomized, prospective trial. The study included 20 patients with LDL-C<4 mmol/L, Lp(a)>50 mg/dL, and refractory angina who randomly received either weekly LA or sham surgery for three months [79]. It was found that the LA group compared to the controls demonstrated significant reduction of LDL-C, plasma Lp(a), ApoB-100, HDL cholesterol, and triglyceride levels, together with significant improvement of carotid atheroma burden, myocardial perfusion reserve, angina symptoms, six-minute walk test, and overall life quality. Nevertheless, since lipoprotein apheresis also improved other lipid profile components, LDL-C in particular, the clinical benefits could not be specifically attributed to Lp(a) reduction. A Russian clinical study investigating effects of specific Lp(a) apheresis on the regression of coronary atherosclerosis in patients with high Lp(a) and stable CAD demonstrated that in 18 months the specific Lp(a) apheresis lowered Lp(a) by 73% as well as improved minimal lumen diameter and coronary stenosis percentage [80]. Thus, Lp(a) ≥ 60 mg/dL is considered by British and German Guidelines to be the cutoff point for lipoprotein apheresis as a preventive measure against recurrence of CVD. LA clinical application is only limited by its costly, time-consuming and invasive nature.
IONIS: APO(a)-RX and APO(a)-LRX: Lp(a)-lowering therapies described above are not specific to Lp(a) and sometimes not efficient enough. Since the Lp(a) particle includes the Apo(a) as the characteristic component, it is possible to learn whether reduction of Lp(a) brings cardiovascular benefits using drugs that would specifically target Apo(a) [81]. The first medication to decrease Lp(a) specifically is Apo(a) ASO. In the blood, hepatocytes take up subcutaneously injected Apo(a) ASO which binds directly in the nucleus to Apo(a) mRNA. It leads to the antisense: mRNA complex destruction by RNase H1 and targeted suppression of Apo(a) synthesis, which subsequently reduces Lp(a) concentrations in plasma. IONIS-APO(a)RX, previously referred to as ISIS-APO(a)RX is an ASO medicine of second generation [82]. It was evaluated in Phase 1 of a clinical trial on 47 healthy subjects 18 to 65 y. o. who had body mass index <32 kg/m2 and Lp(a) concentration in plasma ≥ 25 nmol/L (10 mg/dL) [83]. They were randomized so that 37 patients received IONIS-APO(a)RX in a single dose of 50 mg, 100 mg, 200 mg, or 400 mg, or 6 doses of 100 mg, 200 mg, or 300 mg with the total dose of 600 mg, 1200 mg, or 1800 mg, while ten participants were administered placebo. It was found that in a single-dose group (ISIS-APO(a)RX 50 to 400 mg) Lp(a) concentration was not decreased by day 30; at the same time, the six-dose group (ISIS-APO(a)RX 100 mg, 200 mg, or 300 mg) demonstrated reduced concentrations of Lp(a) by 39.6%, 59.0%, and 77.8% respectively, depending on the dosage. Moreover, both groups demonstrated a significant decrease in OxPL-Apo(a) and OxPL-ApoB-100 concentrations in plasma and good dose tolerance.
To make uptake by hepatocytes faster and more specific, a ligandconjugated form of IONIS-APO(a)RX was developed, IONISAPO( a)-LRX (AKCER-APO(a)-LRX with N-Acetylgalactosamine complex (GalNAc 3) modified molecule [84]. Resultantly, the drug potency was more than 30 times increased. Safety and efficacy of IONIS-APO(a)-LRX was assessed in Phase 1 of a clinical experiment on healthy individuals who had Lp(a) levels above 75 nmol/L. It was found that IONIS-APO(a)-LRX in multiple doses (10 mg, 20 mg, or 40 mg) injected subcutaneously 6 times during a three-week period decreased Lp(a) concentrations in plasma by 66%, 80%, and 92% (P<0.05), respectively, and tolerability was good. Results of Phase 2b of another sham-controlled study in 286 individuals with elevated Lp(a) levels ( ≥ 60 mg/dL) and established cardiovascular disease have recently been reported at the AHA Scientific Sessions [85]. The trial assessed IONISAPO( a)-LRX safety and efficacy; the patients were randomized to be administered IONIS-APO(a)-LRX 20 mg weekly, 20 mg biweekly, 20 mg monthly, 40 mg monthly, or 60 mg monthly for half a year to a year. The percentage change from the initial level at six months was chosen as the primary efficacy endpoint; two secondary efficacy endpoints included the number of subjects whose Lp(a) lowered to the target value (below 50 mg/dL) and the average percentage change of OxPL-Apo(a), OxPL-ApoB-100, ApoB-100, and LDL-C from the baseline level at six months. It was found that IONIS-APO(a)-LRX 20 mg administered weekly, 20 mg biweekly, 20 mg monthly, 40 mg monthly, and 60 mg monthly, reduced Lp(a) from baseline by 80%, 58%, 35%, 56%, and 72%, correspondingly. The percentage of subjects whose Lp(a) achieved the targeted level was 97.7%, 64.6%, 25%, 62.5%, and 80.9%, respectively, without serious adverse events in any group. The ability of IONIS-APO(a)-LRX to reduce the risk of CVD in elevated Lp(a) patients is the area that warrants further research [86].
Postmenopausal estrogen replacement: Within the frames of the Heart and Estrogen Replacement Study, it was reported that a combination of progestin plus estrogen decreased Lp(a) and CVD in women after menopause with high Lp(a) levels. The results were both clinically and statistically significant when compared with the placebo group [87]. However, the analyses were made post hoc and without further testing in Women’s Health Initiative trials or elsewhere. Consequently, estrogen replacement cannot be advised as therapy preventing cardiovascular disease for this type of patients, as potential benefits need to be confirmed.
Antisense oligonucleotide targeting Lp(a) mRNA: The only treatment that specifically reduces Apo(a) expression as well as Lp(a) synthesis by the liver is nucleotide-based therapy. Second generation of ASO targeting LPA mRNA was developed and tested. The first prototype was APO(a)-Rx; in two-phase trials it demonstrated significant Lp(a) reduction and good tolerability. Its ligand-conjugated version, APO(a)- LRx, has a triantennary Acetylgalactosamine moiety (GalNAc3) allowing liver-targeting through rapid clearance by the hepatic asialoglycoprotein receptor [88]. APO(a)-LRx demonstrated a 30 times higher potency than APO(a)-Rx with a ten-fold smaller dose to reach similar Lp(a)- lowering action in a phase 1/2 trial involving healthy subjects with Lp(a)>75 nmol/L. Phase 2 study including patients with increased Lp(a) (over 60 mg/dL) and established CVD receiving SOC therapy showed that APO(a)-LRx in highest doses (20 mg weekly and 60 mg every 4 weeks) produced 80% and 72% Lp(a) reduction, respectively [89]. The drug demonstrated good tolerability and safety, and the most frequent side effects were reactions at the injection site. Recently a pivotal Phase 3 study was designed called Lp(a)HORIZON (NCT04023552) that enrolled 7680 subjects with diagnosed CVD and Lp(a) ≥ 70 mg/ dL for 80 mg APO(a)-LRx injections or placebo monthly for 4 to 5 years [90]. The trial that began in December 2019 is planned to complete in April 2024. It will be the only placebo-controlled study apart from MultiSELECT trial of LA that will show whether significant Lp(a) reduction can lead to positive clinical outcomes in CV patients [91].
Besides ASO, under development now are small interfering RNA (siRNA)-based medications aimed at Lp(a) specifically. In 2020, a phase 2 trial (NCT04270760) started to continue till April 2023 [92]. Its aim is to evaluate the Lp(a) percent change in patients with Lp(a) above 150 nmol/L and with established atherosclerosis receiving AMG 890 (olpasiran) therapy. Another phase 1 trial (NCT04606602) with SLN360 is evaluating olpasiran tolerability and safety [93]. SiRNA may be potentially more patient-compliable compared to ASO as they may be injected less frequently.
Mipomersen and lomitapide: Mipomersen and lomitapide are known to reduce liver expression of Apo-B that contains LDL, VLDL, and lipoproteins. Mipomersen is used in liverfocused treatment, while lomitapide also reduces the secretion of chylomicrons by the intestine. Mipomersen is an antisense oligonucleotide inhibitor targeted to apolipoprotein B mRNA in humans and causing duplex degradation by ribonuclease H [94]. In patients with Familial Hypercholesterolemia (FH), it is reported to lower Lp(a) by 21% to 30%, however, its mechanism is uncertain and may be driven by ApoB synthesis suppression and subsequent reduction in VLDL secretion. Lomitapide inhibits microsomal triglyceride transfer protein in the intestines and the liver, thus reducing newly synthesized ApoB lipidation and promoting its degradation. It was reported to reduce Lp(a) by 19% in 56 weeks, but without further reduction [95]. Both drugs cause serious adverse effects, like hepatic steatosis and liver enzyme increase, so in the future, they will hardly be indicated for specific Lp(a) reduction. Lomitapide has been approved for CV risk and LDL-C reduction in homozygous patients with FH. Mipomersen is not available in the United States [96].
Niacin: Niacin lowers Lp(a) and improves the lipogram results (decreases TG, increases HDL-C, and decreases LDL-C). Its effect is dose-dependent; niacin in doses of 1500-3000 mg leads to 20%- 40% reduction in Lp(a) [97]. Formerly, it was recommended by 2010 EAS guidelines as a Lp(a)-lowering drug, but not any longer due to the results of AIM-HIGH [98], and HPS2-THRIVE [99], trials which found neutral cardiovascular outcomes. In the AIMHIGH trial, a niacin extended-release form in doses of 1500–2000 mg/day was used for LDL-C reduction together with 40–80 mg/ day of simvastatin and, in some patients, with 10 mg/day of ezetimibe [98]. It was reported that Lp(a) reduction was 21% vs. 6% in the placebo group, however, CV risk was not reduced, though it had been predicted by initial Lp(a) and on-treatment Lp(a) concentrations. A larger HPS2-THRIVE clinical trial involving 25,673 patients studied whether using a combination of niacin and laropiprant together with statin therapy with or without the addition of ezetimibe would improve cardiovascular outcomes in patients with high-risk and CVD history [99]. Unfortunately, Lp(a) was evaluated only in 3978 patients from China and the UK whose baseline and follow-up measurements had been provided.
Niacin provided an overall Lp(a) reduction by 31% with great variety depending on the baseline concentration of Lp(a); at that, participants with the lowest baseline concentration of Lp(a) demonstrated a stronger Lp(a) reduction. However, the trial did not ascertain how this Lp(a) reduction could be translated into CV risk decrease. Niacin impact in patients with elevated Lp(a) has never been specifically clinically investigated in a randomized trial, so necessary data are lacking.
CETP inhibitors (CETPi): CETP inhibitors have either been declined regulatory approval (like anacetrapib) or were unsuccessful because of doubtful cardiovascular benefit and adverse effects, like evacetrapib or torcetrapib [100]. However, one of CETP inhibitors, dalcetrapib, is still being tested for secondary prevention in the current Dal-GenE study involving patients with a certain favourable genotype [101]. According to the dal-OUTCOMES study, dalcetrapib showed marginal effect on Lp(a) in subjects who’ve had acute coronary syndrome [102]. The only CETPi which showed positive results in phase 3 of a trial, was anacetrapib, reducing Lp(a) concentrations by 25% in subjects with diagnosed CVD, according to the REVEAL trial [71]. Previously the drug had been demonstrated to reduce Lp(a) by 32% in patients with homozygous familial hypercholesterolemia in the REALIZE study [103], and by 36% in subjects with elevated cardiovascular risk, according to the DEFINE trial [104]. Another study on a Japanese cohort of patients with dyslipidemia receiving statin treatment also showed a 48% reduction in Lp(a) after anacetrapib administration for half a year [105].
Unlike PCSKi, anacetrapib suppresses Lp(a) synthesis by 40% rather than enhances its clearance, resulting in a subsequent decrease in Lp(a) levels by 34%-39%. Still, no proof has been shown that anacetrapib-induced Lp(a) decrease results in lower CV risk [106]. The understanding of the drug’s potential in CVD risk reduction could be complemented by a post hoc analysis. As a result of a dou btful medical effect and safety concerns provoked by its long half-life in tissues, anacetrapib has not been approved. However, a more recent drug with a similar effect on Lp(a) levels, obicetrapib, has been demonstrated to reduce Lp(a) levels by 35% in 3 months in the TULIP phase 2 study and is still being investigated [107].
Discussion and Conclusion
In this paper, we have reviewed the existing knowledge on Lp(a). Some of the pathogenic properties of Lp(a) have been described as well as its involvement in the promotion and aggravation of CVD. Studies have shown that Lp(a) can contribute to CVD in several different ways. Therefore, Lp(a) may be a potential therapeutic target in CVD treatment.
Various Lp(a) management methodologies have been compared. While Lp(a) measurement is currently mostly carried out via immunoassays, and the results are reported as total mass concentrations (i.e., mg/dL), reporting particle number (i.e., nmol/L) may become a more beneficial strategy. The main challenge currently affecting Lp(a) assessment is the lack of an internationally established calibrator, which indicates a need for more research in this area. Also, the lack of generally accepted measurement methods, as well as the understanding of the meaning of raw measurements allow suggesting that the Lp(a) measurements can be used as a basis for some index calculations. At least the triglyceride-glucose index and atherogenic index of plasma, and various ratios are used to assess the risks for cardiovascular disease. The wide range of implications of Lp(a) in atherosclerosis illustrate the relevance of this molecule in the scope of CVD prediction.
The last section of the paper is dedicated to Lp(a) lowering methods. Despite the lack of established and commonly accepted treatment practices involving Lp(a), numerous studies indicate that its reduction may be an up-and-coming strategy in CVD treatment and prevention. Thus, there is no doubt that this problem should be further investigated. This means that we should improve our understanding of mechanisms of Lp(a) level lowering, and develop more effective approaches.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author Contributions
Writing-original draft preparation, A.V.P.; Writing-review and editing, V.N.S., I.I.E., I.I.N., N.A.G., M.A.P., A.N.O.
Funding
This research was funded by Russian Science Foundation, grant number 23-45-00031.
References
- Nordestgaard BG, Langsted A. Lipoprotein (a) as a cause of cardiovascular disease: Insights from epidemiology, genetics, and biology. J Lipid Res. 57(11): 1953-1975 (2016).
- Kronenberg F. Lipoprotein (a). Handb Exp Pharmacol. 270: 201-232 (2022).
- Likozar AR, Zavrtanik M, Sebestjen M, et al. Lipoprotein (a) in atherosclerosis: From pathophysiology to clinical relevance and treatment options. Ann Med. 52(5): 162-177 (2020).
- Saeed A, Kinoush S, Virani SS, et al. Lipoprotein (a): Recent updates on a unique lipoprotein. Curr Atheroscler Rep. 23(8): 41 (2021).
- Schwartz GG, Ballantyne CM. Existing and emerging strategies to lower Lipoprotein (a). Atherosclerosis. 349: 110-122 (2022).
- Di Fusco SA, Arca M, Scicchitano P, et al. (2022). Lipoprotein (a): A risk factor for atherosclerosis and an emerging therapeutic target. Heart. 109(1): 18-25.
- Ugovšek S, Šebeštjen M. Lipoprotein (a) the crossroads of atherosclerosis, atherothrombosis and inflammation. Biomolecules. 12(1): 26 (2021).
[Crossref] [Google Scholar] [PubMed]
- Ye Q, Rahman MN, Koschinsky ML, et al. High-resolution crystal structure of apolipoprotein (a) kringle IV type 7: Insights into ligand binding. Protein Sci. 10(6): 1124-1129 (2001).
[Crossref] [Google Scholar] [PubMed]
- Lamon-Fava S, Marcovina SM, Albers JJ, et al. Lipoprotein (a) levels, apo(a) isoform size, and coronary heart disease risk in the framingham offspring study. J Lipid Res. 52(6): 1181-1187 (2011).
[Crossref] [Google Scholar] [PubMed]
- McCormick SP. Lipoprotein (a): Biology and clinical importance. Clin Biochem Rev. 25(1): 69-80 (2004).
- Hoover-Plow J, Huang M. Lipoprotein (a) metabolism: Potential sites for therapeutic targets. Metabolism. 62(4): 479-491 (2013).
- Feingold KR. Introduction to lipids and lipoproteins. (2021).
- Romagnuolo R, Scipione CA, Boffa MB, et al. Lipoprotein (a) catabolism is regulated by proprotein convertase subtilisin/kexin type 9 through the low density lipoprotein receptor. J Biol Chem. 290(18): 11649-11662 (2015).
[Crossref] [Google Scholar] [PubMed]
- Clark JR, Gemin M, Youssef A, et al. Sortilin enhances secretion of apolipoprotein (a) through effects on apolipoprotein B secretion and promotes uptake of lipoprotein (a). J Lipid Res. 63(6): 100216 (2022).
[Crossref] [Google Scholar] [PubMed]
- Raal FJ, Giugliano RP, Sabatine MS, et al. PCSK9 inhibition-mediated reduction in Lp(a) with evolocumab: An analysis of 10 clinical trials and the LDL receptor's role. J Lipid Res. 57(6): 1086-1096 (2016).
[Crossref] [Google Scholar] [PubMed]
- Watts GF, Chan DC, Pang J, et al. PCSK9 Inhibition with alirocumab increases the catabolism of lipoprotein (a) particles in statin-treated patients with elevated lipoprotein (a). Metabolism. 107: 154221 (2020).
[Crossref] [Google Scholar] [PubMed]
- Reyes-Soffer G, Pavlyha M, Ngai C, et al. Effects of PCSK9 inhibition with alirocumab on lipoprotein metabolism in healthy humans. Circulation. 135(4): 352-362 (2017).
[Crossref] [Google Scholar] [PubMed]
- Romagnuolo R, Scipione CA, Boffa MB, et al. Lipoprotein (a) catabolism is regulated by proprotein convertase subtilisin/kexin type 9 through the low density lipoprotein receptor. J Biol Chem. 290(18): 11649-1162 (2015).
[Crossref] [Google Scholar] [PubMed]
- Thedrez A, Blom DJ, Ramin-Mangata S, et al. Homozygous familial hypercholesterolemia patients with identical mutations variably express the ldlr (low-Density Lipoprotein Receptor): Implications for the efficacy of evolocumab. Arterioscler Thromb Vasc Biol. 38(3): 592-598 (2018).
[Crossref] [Google Scholar] [PubMed]
- Kostner KM, Kostner GM. Factors affecting plasma lipoprotein (a) levels: Role of hormones and other nongenetic factors. Semin Vasc Med. 4(2): 211-214 (2004).
[Crossref] [Google Scholar] [PubMed]
- Orsó E, Schmitz G. Lipoprotein (a) and its role in inflammation, atherosclerosis and malignancies. Clin Res Cardiol Suppl. 12(S1): 31-37 (2017).
[Crossref] [Google Scholar] [PubMed]
- Liu T, Yoon WS, Lee SR, et al. Recent updates of lipoprotein (a) and cardiovascular disease. Chonnam Med J. (1): 36-43 (2021).
- Cegla J, France M, Marcovina SM, et al. Lp(a): When and how to measure it. Ann Clin Biochem. 58(1): 16-21 (2021).
- Le Bras A. Lipoprotein (a) is an independent predictor of CVD. Nat Rev Cardiol. 15(12): 727 (2018).
[Crossref] [Google Scholar] [PubMed]
- Jawi MM, Frohlich J, Chan SY, et al. Lipoprotein (a) the insurgent: A new insight into the structure, function, metabolism, pathogenicity, and medications affecting lipoprotein (a) molecule. J Lipids. 2020: 3491764 (2020).
[Crossref] [Google Scholar] [PubMed]
- Anglés-Cano E, Hervio L, Rouy D, et al. Effects of lipoprotein (a) on the binding of plasminogen to fibrin and its activation by fibrin-bound tissue-type plasminogen activator. Chem Phys Lipids. 67-68: 369-380 (1994).
[Crossref] [Google Scholar] [PubMed]
- Boffa MB, Koschinsky ML. Lipoprotein (a): Truly a direct prothrombotic factor in cardiovascular disease? J Lipid Res. 57(5): 745-757 (2016).
- Wu TP, Padmanabhan K, Tulinsky A, et al. The refined structure of the epsilon-aminocaproic acid complex of human plasminogen kringle 4. Biochemistry. 30(43): 10589-10594 (1991).
- Romagnuolo R, Marcovina SM, Boffa MB, et al. Inhibition of plasminogen activation by apo (a): Role of carboxyl-terminal lysines and identification of inhibitory domains in apo (a). J Lipid Res. 55(4): 625-634 (2014).
- Akanji AO, Abdullah A, Tahzeeb S, et al. Lipoprotein (a), tissue plasminogen activator and plasminogen activator inhibitor 1 levels in hyperlipidaemic patients in Kuwait. Eur J Clin Invest. 27(5): 386 (11978).
- Feric NT, Boffa MB, Johnston SM, et al. Apolipoprotein (a) inhibits the conversion of glu-plasminogen to lys-plasminogen: A novel mechanism for lipoprotein (a)-mediated inhibition of plasminogen activation. J Thromb Haemost. 6(12): 2113-2120 (2008).
- Hancock MA, Boffa MB, Marcovina SM, et al. Inhibition of plasminogen activation by lipoprotein (a): Critical domains in apolipoprotein (a) and mechanism of inhibition on fibrin and degraded fibrin surfaces. J Biol Chem. 278(26): 23260-23269 (2003).
- Zabriskie MS, Wang C, Wang S, et al. Apolipoprotein E knockout rabbit model of intracranial atherosclerotic disease. Animal Model Exp Med. 3(2): 208-213 (2020).
- Lu W, Cheng YC, Chen K, et al. Evidence for several independent genetic variants affecting lipoprotein (a) cholesterol levels. Hum Mol Genet. 24(8): 2390-2400 (2015).
- Sha J, McCullough B, Hart E, et al. Apo(a) promotes thrombosis in a vascular injury model by a mechanism independent of plasminogen. J Thromb Haemost. 3(10): 2281-2289 (2005).
- Lampsas S, Xenou M, Oikonomou E, et al. Lipoprotei (a) in atherosclerotic diseases: From pathophysiology to diagnosis and treatment. Molecules. 28(3): 969 (2023).
- Binder CJ, Papac-Milicevic N, Witztum JL, et al. Innate sensing of oxidation-specific epitopes in health and disease. Nat Rev Immunol. 16(8): 485-497 (2016).
[Crossref] [Google Scholar] [PubMed]
- Handa JT, Tagami M, Ebrahimi K, et al. Lipoprotein (A) with an intact lysine binding site protects the retina from an age-related macular degeneration phenotype in mice (an american ophthalmological society thesis). Trans Am Ophthalmol Soc. 113: T5 (2015).
- Berliner JA, Leitinger N, Tsimikas S, et al. The role of oxidized phospholipids in atherosclerosis. J Lipid Res. 50: S207-S212 (2009).
[Crossref] [Google Scholar] [PubMed]
- Deshmane SL, Kremlev S, Amini S, et al. Monocyte Chemoattractant Protein-1 (MCP-1): An overview. J Interferon Cytokine Res. 29(6):313-326 (2009).
[Crossref] [Google Scholar] [PubMed]
- Kany S, Vollrath JT, Relja B, et al. Cytokines in inflammatory disease. Int J Mol Sci. 20(23): 6008 (2019).
[Crossref] [Google Scholar] [PubMed]
- Gimbrone MA Jr, Garcia-Cardena G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res. 118(4): 620-636 (2016).
- Kheradmandi M, Ackers I, Burdick MM, et al. Targeting dysfunctional vascular endothelial cells using immunoliposomes under flow conditions. Cell Mol Bioeng. 13(3): 189-199 (2020).
- Zhu H, Xia M, Hou M, et al. Ox-LDL plays dual effect in modulating expression of inflammatory molecules through LOX-1 pathway in human umbilical vein endothelial cells. Front Biosci. 10: 2585-2594 (2005).
- Riches K, Porter KE. Lipoprotein (a): Cellular effects and molecular mechanisms. Cholesterol. 2012: 923289 (2012).
- Mussbacher M, Salzmann M, Brostjan C, et al. Cell type-specific roles of nf-κb linking inflammation and thrombosis. Front Immunol. 10: 85 (2019).
- Cho T, Jung Y, Koschinsky ML, et al. Apolipoprotein (a), through its strong lysine-binding site in KIV(10'), mediates increased endothelial cell contraction and permeability via a Rho/Rho kinase/MYPT1-dependent pathway. J Biol Chem. 283(45): 30503-30512 (2008).
[Crossref][Google Scholar ][PubMed]
- Cho T, Romagnuolo R, Scipione C, et al. Apolipoprotein (a) stimulates nuclear translocation of β-catenin: A novel pathogenic mechanism for lipoprotein (a). Mol Biol Cell. 24(3): 210-221 (2013).
- Jaminon A, Reesink K, Kroon A, et al. The role of vascular smooth muscle cells in arterial remodeling: Focus on calcification-related processes. Int J Mol Sci. 20(22): 5694 (2019).
- Liu L, Craig AW, Meldrum HD, et al. Apolipoprotein (a) stimulates vascular endothelial cell growth and migration and signals through integrin alphaVbeta3. Biochem J. 418(2): 325-336 (2009).
- Liu L, Boffa MB, Koschinsky ML, et al. Apolipoprotein (a) inhibits in vitro tube formation in endothelial cells: Identification of roles for Kringle V and the plasminogen activation system. PLoS One. 8(1): e52287 (2013).
- O'Neil CH, Boffa MB, Hancock MA, et al. Stimulation of vascular smooth muscle cell proliferation and migration by apolipoprotein (a) is dependent on inhibition of transforming growth factor-beta activation and on the presence of kringle IV type 9. J Biol Chem. 279(53): 55187-55195 (2004).
- Ichikawa T, Unoki H, Sun H, et al. Lipoprotein (a) promotes smooth muscle cell proliferation and dedifferentiation in atherosclerotic lesions of human apo (a) transgenic rabbits. Am J Pathol. 160(1): 227-236 (2002).
- Allahverdian S, Chaabane C, Boukais K, et al. Smooth muscle cell fate and plasticity in atherosclerosis. Cardiovasc Res. 114(4): 540-550 (2018).
- Konii H, Sato K, Kikuchi S, et al. Stimulatory effects of cardiotrophin 1 on atherosclerosis. Hypertension. 62(5): 942-950 (2013).
- Susser LI, Rayner KJ. Through the layers: How macrophages drive atherosclerosis across the vessel wall. J Clin Invest. 132(9): e157011 (2022).
- Brandt EJ, Mani A, Spatz ES, et al. Lipoprotein (a) levels and association with myocardial infarction and stroke in a nationally representative cross-sectional US cohort. J Clin Lipidol. 14(5): 695-706 (2020).
[Crossref] [Google Scholar] [PubMed]
- Contois JH, Nguyen RA, Albert AL, et al. Lipoprotein (a) particle number assay without error from apolipoprotein (a) size isoforms. Clin Chim Acta. 505: 119-124 (2020).
[Crossref] [Google Scholar] [PubMed]
- Wyness SP, Genzen JR. Performance evaluation of five lipoprotein (a) immunoassays on the roche cobas c501 chemistry analyzer. Pract Lab Med. 25: e00218 (2021).
[Crossref] [Google Scholar] [PubMed]
- Enas EA, Varkey B, Dharmarajan TS, et al. Lipoprotein (a): An independent, genetic, and causal factor for cardiovascular disease and acute myocardial infarction. Indian Heart J. 71(2): 99-112 (2019).
[Crossref] [Google Scholar] [PubMed]
- Marcovina SM, Navabi N, Allen S, et al. Development and validation of an isoform-independent monoclonal antibody-based ELISA for measurement of lipoprotein (a). J Lipid Res. 63(8): 100239 (2022).
[Crossref] [Google Scholar] [PubMed]
- Ruscica M, Sirtori CR, Corsini A, et al. Lipoprotein (a): Knowns, unknowns and uncertainties. Pharmacol Res.173: 105812 (2021).
- Handhle A, Viljoen A, Wierzbicki AS, et al. Elevated Lipoprotein (a): Background, current insights and future potential therapies. Vasc Health Risk Manag. 17: 527-542 (2021).
- Yeang C, Witztum JL, Tsimikas S. Novel method for quantification of lipoprotein (a)-cholesterol: Implications for improving accuracy of LDL-C measurements. J Lipid Res. 62: 100053 (2021).
- Marcovina SM, Albers JJ. Lipoprotein (a) measurements for clinical application. J Lipid Res. 57(4): 526-537 (2016).
- Emdin CA, Khera AV, Natarajan P, et al. Phenotypic characterization of genetically lowered human lipoprotein (a) levels. J Am Coll Cardiol. 68(25): 2761-2772 (2016).
- Afshar M, Pilote L, Dufresne L, et al. Lipoprotein (a) interactions with low-density lipoprotein cholesterol and other cardiovascular risk factors in premature Acute Coronary Syndrome (ACS). J Am Heart Assoc. 5(4): e003012 (2016).
- Lamina C. Mendelian randomization: Principles and its usage in Lp(a) research. Atherosclerosis. 349: 36-41 (2022).
- Saeedi R, Frohlich J. Lipoprotein (a), an independent cardiovascular risk marker. Clin Diabetes Endocrinol. 2: 7 (2016).
- Moumné L, Marie AC, Crouvezier N, et al. Oligonucleotide therapeutics: From discovery and development to patentability. Pharmaceutics. 14(2): 260 (2022).
- Tsimikas S, Karwatowska-Prokopczuk E, Gouni-Berthold I, et al. Lipoprotein (a) reduction in persons with cardiovascular disease. N Engl J Med. 382(3): 244-255 (2020).
- Cho KH, Hong YJ. Proprotein convertase subtilisin/kexin type 9 inhibition in cardiovascular disease: Current status and future perspectives. Korean J Intern Med. 35(5): 1045-1058 (2020).
- Shapiro MD, Minnier J, Tavori H, et al. Relationship between low-density lipoprotein cholesterol and lipoprotein (a) lowering in response to pcsk9 inhibition with evolocumab. J Am Heart Assoc. 8(4): e010932 (2019).
- Giugliano RP, Pedersen TR, Park JG, et al. Clinical efficacy and safety of achieving very low LDL-cholesterol concentrations with the PCSK9 inhibitor evolocumab: A prespecified secondary analysis of the FOURIER trial. Lancet. 390(10106): 1962-1971 (2017).
- Ray KK, Colhoun HM, Szarek M, et al. Effects of alirocumab on cardiovascular and metabolic outcomes after acute coronary syndrome in patients with or without diabetes: A prespecified analysis of the ODYSSEY OUTCOMES randomised controlled trial. Lancet Diabetes Endocrinol. 7(8): 618-628 (2019).
- Stiekema LCA, Stroes ESG, Verweij SL, et al. Persistent arterial wall inflammation in patients with elevated lipoprotein (a) despite strong low-density lipoprotein cholesterol reduction by proprotein convertase subtilisin/kexin type 9 antibody treatment. Eur Heart J. 40(33): 2775-2781 (2019).
- Greco MF, Sirtori CR, Corsini A, et al. Lipoprotein (a) lowering-from lipoprotein apheresis to antisense oligonucleotide approach. J Clin Med. 9(7): 2103 (2020).
- Zhu L, Fang Y, Gao B, et al. Effect of an increase in Lp(a) following statin therapy on cardiovascular prognosis in secondary prevention population of coronary artery disease. BMC Cardiovasc Disord. 22(1): 474 (2022).
- Khan TZ, Hsu LY, Arai AE, et al. Apheresis as novel treatment for refractory angina with raised lipoprotein (a): A randomized controlled cross-over trial. Eur Heart J. 38(20): 1561-1569 (2017).
- Pokrovsky SN, Afanasieva OI, Safarova MS, et al. Specific Lp(a) apheresis: A tool to prove lipoprotein (a) atherogenicity. Atheroscler Suppl. 30: 166-173 (2017).
- Korneva VA, Kuznetsova TY, Julius U, et al. Modern approaches to lower lipoprotein (a) concentrations and consequences for cardiovascular diseases. Biomedicines. 9(9): 1271 (2021).
- Gagliardi M, Ashizawa AT. The challenges and strategies of antisense oligonucleotide drug delivery. Biomedicines. 9(4): 433 (2021).
- Jubran A, Zetser A, Zafrir B, et al. Lipoprotein (a) screening in young and middle-aged patients presenting with acute coronary syndrome. Cardiol J. 26(5): 511-518 (2019).
- Baker BF, Xia S, Partridge W, et al. Integrated assessment of phase 2 data on galnac3-conjugated 2'-o-methoxyethyl-modified antisense oligonucleotides. Nucleic Acid Ther. (2022).
- Viney NJ, van Capelleveen JC, Geary RS, et al. Antisense oligonucleotides targeting apolipoprotein (a) in people with raised lipoprotein (a): Two randomised, double-blind, placebo-controlled, dose-ranging trials. Lancet. 388(10057): 2239-2253 (2016).
- Shlipak MG, Simon JA, Grady D, et al. Renal insufficiency and cardiovascular events in postmenopausal women with coronary heart disease. J Am Coll Cardiol. 38(3): 705-711 (2001).
- Gareri C, Polimeni A, Giordano S, et al. Antisense oligonucleotides and small interfering RNA for the treatment of dyslipidemias. J Clin Med. 11(13): 3884 (2022).
- Duarte Lau F, Giugliano RP. Lipoprotein (a) and its significance in cardiovascular disease: A review. JAMA Cardiol. 7(7): 760-769 (2022).
- Nicholls SJ, Bubb KJ. The Riskier Lipid: What is on the HORIZON for lipoprotein (a) and should there be Lp(a) screening for all?. Curr Cardiol Rep. 23(8): 97 (2021).
- Hohenstein B, Julius U, Lansberg P, et al. Rationale and design of MultiSELECt: A european multicenter study on the effect of lipoprotein (a) elimination by lipoprotein apheresis on cardiovascular outcomes. Atheroscler Suppl. 30: 180-186 (2017).
- O'Donoghue ML, Rosenson RS, Gencer B, et al. Small interfering RNA to reduce lipoprotein (a) in cardiovascular disease. N Engl J Med. 387(20): 1855-1864 (2022).
- Nissen SE, Wolski K, Balog C, et al. Single ascending dose study of a short interfering RNA targeting lipoprotein (a) production in individuals with elevated plasma lipoprotein (a) levels. JAMA. 327(17): 1679-1687 (2022).
- Rader DJ, Kastelein JJ. Lomitapide and mipomersen: Two first-in-class drugs for reducing low-density lipoprotein cholesterol in patients with homozygous familial hypercholesterolemia. Circulation. 129(9): 1022-1032 (2017).
- Cuchel M, Meagher EA, du Toit Theron H, et al. Efficacy and safety of a microsomal triglyceride transfer protein inhibitor in patients with homozygous familial hypercholesterolaemia: A single-arm, open-label, phase 3 study. Lancet. 381(9860): 40-46 (2013).
- Norata GD, Ballantyne CM, Catapano AL, et al. New therapeutic principles in dyslipidaemia: Focus on LDL and Lp(a) lowering drugs. Eur Heart J. 34(24): 1783-1789 (2013).
- Schandelmaier S, Briel M, Saccilotto R, et al. Niacin for primary and secondary prevention of cardiovascular events. Cochrane Database Syst Rev. 6(6): CD009744 (2017).
- Probstfield JL, Boden WE, Anderson T, et al. Cardiovascular outcomes during extended follow-up of the AIM-HIGH trial cohort. J Clin Lipidol. 12(6): 1413-1419 (2018).
- Toth PP, Murthy AM, Sidhu MS, et al. Is HPS2-THRIVE the death knell for niacin? J Clin Lipidol. 9(3): 343-350 (2015).
- Tall AR, Rader DJ. Trials and tribulations of CETP inhibitors. Circ Res. 122(1): 106-112 (2018).
- Tardif JC, Dubé MP, Pfeffer MA, et al. Study design of Dal-GenE, a pharmacogenetic trial targeting reduction of cardiovascular events with dalcetrapib. Am Heart J. 222: 157-165 (2020).
- Schwartz GG, Olsson AG, Ballantyne CM, et al. Rationale and design of the dal-OUTCOMES trial: Efficacy and safety of dalcetrapib in patients with recent acute coronary syndrome. Am Heart J. 158(6): 896-901.e3 (2009).
- Varnai P, Davé A, Farla K, et al. The evidence reveal study: Exploring the use of real-world evidence and complex clinical trial design by the european pharmaceutical industry. Clin Pharmacol Ther. 110(5): 1180-1189 (2021).
- Gurrell R, Iredale P, Evrard A, et al. Pronounced antiseizure activity of the subtype-selective GABAA positive allosteric modulator darigabat in a mouse model of drug-resistant focal epilepsy. CNS Neurosci Ther. 28(11): 1875-1882 (2022).
- Gaughan E, Quinn T, Bruce A, et al. Evaluation of new or repurposed treatments for COVID-19: Protocol for the phase Ib/IIa DEFINE trial platform. BMJ Open. 11(12): e054442 (2021).
- Yamashita S, Arai H, Bujo H, et al. Probucol trial for secondary prevention of atherosclerotic events in patients with coronary heart disease (PROSPECTIVE). J Atheroscler Thromb. 28(2): 103-123 (2021).
- Filippatos TD, Kei A, Elisaf MS, et al. Anacetrapib, a new CETP Inhibitor: The new tool for the management of dyslipidemias? Diseases. 5(4): 21 (2017).
- Vyletelová V, Nováková M, Pašková Ľ, et al. Alterations of hdl's to pihdl's proteome in patients with chronic inflammatory diseases, and hdl-targeted therapies. Pharmaceuticals (Basel). 15(10): 1278 (2022).