Review Article - Interventional Cardiology (2022) Volume 14, Issue 2
Cardiac involvement and outcomes in patients with COVID-19 infection
- Corresponding Author:
- Leonardo A. Sechi
Department of Medicine,
Clinica Medica, University of Udine, 33100, Udine, Italy,
E-mail: sechi@uniud.it
Received date: 10-Feb-2022, Manuscript No. FMIC-22-54055; Editor assigned: 14-Feb-2022, PreQC No. FMIC-22-54055 (PQ); Reviewed date: 28-Feb-2022, QC No. FMIC-22-54055; Revised date: 03-Mar-2022, Manuscript No. FMIC-22-54055 (R); Published date: 10-Mar-2022, DOI: 10.37532/1755-5310.2022.14(2).460
Abstract
Cardiac involvement can be a prominent feature in patients hospitalized with SARS-CoV-2 infection and is associated with a greater risk of death. Moreover, clear evidence indicates that outcomes of COVID-19 infection are closely related with pre-existing cardiovascular conditions. A broad spectrum of clinical manifestations has been described during SARS-CoV-2 infection and multiple mechanisms could be involved in the pathogenesis of cardiac involvement including hypoxia, direct viral injury, cytokine storm, generalized endothelitis, and sympathetic activation. Biochemical and instrumental testing including cardiac imaging studies have been performed in these patients reporting different types of cardiac dysfunction. In this narrative review we overview the clinical spectrum of cardiac involvement with the related short-term outcomes of COVID-19 infection and the biochemical and instrumental findings that could be part of the diagnostic workup that might allow timely therapeutic interventions.
Keywords
Sudden cardiac death • Biomarkers; Echocardiography • Electrocardiography • Magnetic resonance imaging • SARS-CoV-2 •Short-term outcome
Introduction
Since its recognition in December 2019, the Coronavirus Disease 2019 (COVID-19) has rapidly spread globally causing a pandemic and representing a public health emergency of international concern [1]. COVID-19 is caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) that predominantly infects the lungs, causing interstitial pneumonia and severe Acute Respiratory Distress Syndrome (ARDS) [2]. Cardiovascular implications of infection, however, could be particularly relevant and are closely related to disease severity and progression [3].
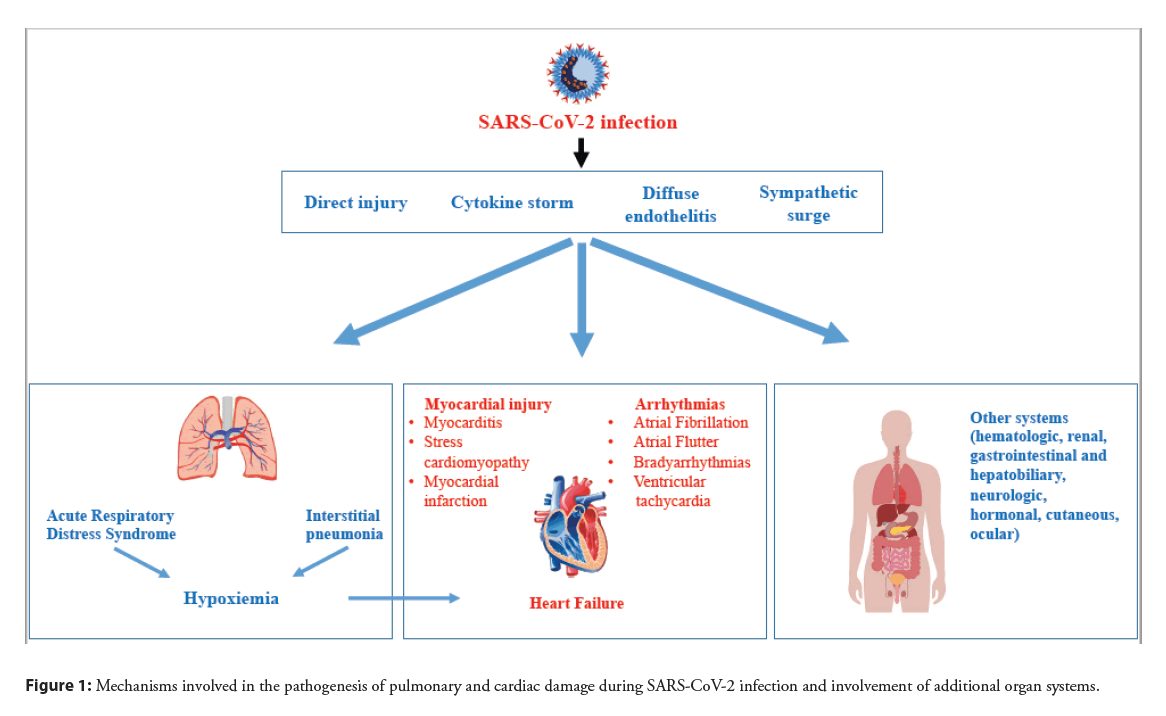
SARS-CoV-2 Spike (S) protein binds to Angiotensin-Converting Enzyme II (ACE2) expressed on surface of pulmonary cells thereby explaining viral tropism for the lung. The S1 subunit of S protein binds to the cell surface receptor while the S2 subunit mediates the fusion between the viral and cellular membranes. This process requires S protein priming by host cell proteases which entails S protein cleavage at the boundary of S1 and S2 proteins or within the S2 subunit [4]. SARS-CoV-2 uses Transmembrane Serine Protease 2 (TMPRSS2) and the endosomal cysteine proteases cathepsin B and L (CatB/L) for S protein priming [5]. After SARS-CoV-2 infection, the related condition may occur with different severity, ranging from asymptomatic to fatal disease. The most common presentation in severely ill patients is bilateral interstitial pneumonia with moderate to severe oxygen desaturation and hypoxia [6]. In this context, lung damage may begin with “silent hypoxia” [7] subsequently worsening to a severe respiratory failure [8]. Severely ill patients may develop Respiratory Failure (RF) and Acute Respiratory Distress Syndrome (ARDS), requiring prompt admission to Intensive Care Units (ICU). While pulmonary impairment is the most evident clinical manifestation of COVID-19 infection, other organs can be involved as a consequence of diffuse endothelitis [9].In fact, hematologic, cardiovascular, renal, gastrointestinal and hepatobiliary, hormonal, neurologic, ocular, and cutaneous involvements have been reported in patients with COVID-19 infection [9,10].
Cardiovascular involvement can be a prominent feature in COVID-19 infection and is associated with a worse prognosis [3,11] with higher death rate [12]. Furthermore, clinical data indicate that the susceptibility to SARS-CoV-2 infection and outcomes of COVID-19 are closely associated with pre-existing cardiovascular diseases [11,13]. On one hand, cardiac injury could indirectly result from a systemic inflammatory response causing an unrestrainable cytokine storm [14]. Systemic inflammation increases shear stress in the coronary arteries due to increased coronary blood flow and this mechanism could lead to plaque rupture resulting in acute myocardial infarction. In addition, systemic inflammation promotes a prothrombotic status, inducing an additional risk for cardiovascular events [15]. On the other hand, because viral localization was detected in the heart [16], SARS-CoV-2 might directly act on cardiomyocytes and coronary vessels [17] prompting hypoxia and myocardial changes. Hypoxia due to acute respiratory illness and increased cardiometabolic demand can impair the myocardial oxygen demand-supply balance resulting in acute myocardial injury (Figure 1). All these mechanisms might be more impactful in subjects with preexisting cardiovascular conditions. In fact, observational studies conducted by the Italian Society of Hypertension in thousands of hospitalized patients with COVID-19 infection have clearly demonstrated that presence of cardiovascular risk factors predicts ICU admission [18] and death [19], and that absence of cardiovascular comorbidities is associated with more frequent healing [20]. This narrative review summarizes the clinical spectrum of cardiac manifestations that have been reported in patients hospitalized with COVID-19 infection with the related outcomes, outlines the mechanisms that potentially contribute to cardiac damage in these patients, and examines the biochemical and instrumental findings that might be part of a diagnostic workup aimed at providing prompt supportive care.
Figure 1: Mechanisms involved in the pathogenesis of pulmonary and cardiac damage during SARS-CoV-2 infection and involvement of additional organ systems.
The Clinical Spectrum
Cardiac involvement in COVID-19 infection is heterogeneous, ranging from right heart overload due to pulmonary embolism to myocarditis with heart failure, acute coronary syndromes, and sustained arrhythmias with cardiac arrest. Most of these complications occur within the first two weeks from presentation [21].
Myocardial injury
In patients with COVID-19 infection prevalence of myocardial injury, as defined by elevation of troponin levels during SARSCoV- 2 infection, ranges from 10% to 46% [22]. Clinical conditions associated with myocardial injury are myocarditis, stress cardiomyopathy, and acute myocardial infarction. Pathogenesis is multifactorial and it relates to imbalance of oxygen supplydemand, direct viral myocardial invasion, inflammation, coronary plaque rupture, microvascular thrombosis and adrenergic stress [23]. In a study by Guo, et al. 28% of 187 hospitalized patients with COVID-19 infection who had elevated troponin T exhibited activated inflammatory biomarkers including leukocytosis, lymphocytopenia, C-reactive protein, pro-calcitonin and D-dimer, suggesting that myocardial injury was likely related to myocarditis due to viral infection [24]. Occurrence of malignant tachyarrhythmias in the setting of troponin elevation could also rise the suspicion of underlying myocarditis [25]. Similarly to ARDS, fulminant myocarditis could result from SARS-CoV-2- related changes in ACE2 with accumulation of angiotensin II [26], although the massive systemic cytokine storm is surely involved [14,27].
Stress-induced cardiomyopathy has also been associated with COVID-19 infection [28], but mechanisms are not fully understood. Three leading factors that have been reported in SARSCoV- 2 infection should be considered in this context, including an overactive immune response, increased activity of the sympathetic nervous system, and presence of microvascular dysfunction [29].
Last, Acute Myocardial Infarction (AMI) has been associated with SARS-CoV-2 infection and patients with S-T Elevation Myocardial Infarction (STEMI) and concomitant COVID-19 have increased in-hospital mortality [30]. Because of increased myocardial oxygen consumption and decreased oxygen supply, coronary involvement during COVID-19 infection could lead to type 2 AMI, whereas type 1 AMI that is associated with thrombotic coronary occlusion is less frequent [31]. In this context, SARS-CoV-2 damages endothelial cells causing activation of the hemostatic cascade and complement system, thereby leading to coronary thrombus formation.
Myocardial injury has emerged also as a rare side effect of RNA vaccines against COVID-19. Retrospective data of myocarditis in vaccinated persons were detected by two groups of investigators in Israeli population after receipt of the BNT162b2 mRNA vaccine (Pfizer-BioNTech) [32,33]. These two studies had an important limitation in the lack of endomyocardial biopsy and therefore diagnosis of myocarditis could be only suspected. Also and most important, in both studies patients had favorable outcome with a benign course of disease.
Arrhythmia
Arrhythmias are common in patients with COVID-19 infection. Myocardial hypoxia, metabolic abnormalities and neurohormonal and inflammatory activation could explain occurrence of arrhythmia in patients with or without prior cardiovascular disease [34]. Wang, et al. reported 16.7% incidence of arrhythmia in an outcome study of 138 Chinese patients with COVID-19 [11]. In this study, arrhythmia required transfer to an ICU in 44% of patients. In a meta-analysis of 19 observational studies including 21653 hospitalized patients with COVID-19, prevalence of Atrial Fibrillation (AF) was 15% among Europeans and 11% among Americans [35]. In patients with severe COVID-19 prevalence of AF was 6-fold that of patients with milder disease. Many additional observational studies have reported elevated incidence of all sort of arrhythmias in patients hospitalized with COVID-19 [36,37].
Heart failure
Acutely Decompensated Heart Failure (ADHF) is a frequent complication of COVID-19 infection and is associated with a higher risk of death in the short-term. In a systematic review and meta-analysis, Zuin, et al. found that about one out of five patients develops ADHF as a complication of SARS-CoV-2 infection [38]. Consistently, in a follow-up study of 3080 patients with COVID-19, an elevated incidence of ADHF was reported [39]. In this study, patients with previous history of chronic heart failure (HF, n=153%) were more prone to develop ADHF, whereas among 77 patients (2.5%) who were diagnosed with new onset HF the majority (78%) had no previous history of disease.
Multiple mechanisms could contribute to cause HF in patients with COVID-19 infection and many important triggers including myocardial ischemia, sympathetic activation, renal impairment with volume overload, increased metabolic demand and thrombotic activity could prime worsening of cardiac function [40]. SARS-CoV-2 infection leads to formation of viral inclusions with subsequent attraction of inflammatory cells [41] and subsequent release of cytokines, such as interleukin-6 (IL- 6), interleukin-1β (IL-1β), Tumor Necrosis Factor-α (TNF-α), Monocyte Chemoattractant Protein-1 (MCP-1), and many others. These cytokines increase the tissue levels of Matrix Metalloproteinases (MMPs), Protease-Activated Receptor (PAR) and Nitric Oxide Synthase (NOS) thereby causing severe oxidative stress, cardiomyocyte apoptosis, and tissue edema. Concomitant inflammatory changes in the lung stimulate further recruitment of pro-inflammatory cells initiating a vicious cycle [42].
Involvement of the right heart has also been demonstrated in many studies conducted in hospitalized patients with COVID-19 infection [43]. Right HF or dysfunction can occur in subjects with pre-existing diseases (i.e. chronic obstructive pulmonary disease, obstructive sleep apnea, pulmonary hypertension) or as a new onset condition in critically ill patients [44-46]. Acute right HF caused by acute pulmonary hypertension associated with acute pulmonary embolism (acute cor pulmonale) or Adult Respiratory Distress Syndrome (ARDS) has been reported in patients with COVID-19 infection [47,48]. In a retrospective study of 870 hospitalized patients with COVID-19 infection, 17% had Left Ventricular (LV) dysfunction (LV ejection fraction <50%) and 29% had Right Ventricular (RV) dysfunction (RV free wall strain >20%) [49]. In a study of 120 COVID-19 patients, those who died had higher pulmonary artery systolic pressure, dilated right heart chambers, and impaired RV function as compared to survivors [50]. To underline the relevance of right ventricular involvement in COVID-19, a multivariate analysis reported that RV dilatation was the only cardiac factor significantly associated with mortality [51].
Laboratory Tests and Cardiac Imaging
Cardiac involvement is associated with an increased risk of death in patients with COVID-19 infection. This is why timely detection of heart damage is important for a better care of these patients. In this view, assessment of cardiac biomarkers and use of cardiac imaging should be appropriately included in the diagnostic workup of COVID-19 (Table 1).
| Clinical manifestation | What to do | What to look for |
|---|---|---|
| Myocarditis | Biomarkers | Increase in TnI, CRP, BNP/NT-proBNP, IL-6, serum ferritin |
| ECG | Changes S-T tract/T wave | |
| Echocardiography | Ventricular wall motion changes | |
| MRI | Myocardial edema, wall motion changes, T1/T2 weighed signal changes, LGE | |
| Ischemia | Biomarkers | Increase in TnI |
| ECG | Changes S-T tract/T wave, Q wave | |
| Echocardiography | Ventricular wall motion changes | |
| Angiography | Coronary thrombosis | |
| Arrhythmia/Atrial fibrillation | ECG | Characterization of arrhythmia |
| Echocardiography | Cardiac valves function | |
| Holter ECG | ||
| Heart failure | Biomarkers | Increase in TnI and BNP/NT-proBNP |
| Echocardiography | Changes in systolic/diastolic function |
Abbreviations: BNP: Brain Natriuretic Peptide; CRP: C-Reactive Protein; ECG: Electrocardiogram; IL-6: Interleukin-6; LGE: Late Gadolinium Enhancement; MRI: Magnetic Resonance Imaging; NT-proBNP: N-Terminal fragment of pro-Brain Natriuretic Peptide; TnI: Troponin I.
Table 1: Proposed algorithm for assessment of cardiac complications in patients hospitalized with COVID-19 infection.
Biomarkers
Because of the cardiac involvement of SARS-CoV-2 infection and its relevance for patients’ prognosis, early monitoring of cardiac damage and inflammatory status with use of biomarkers in hospitalized patients has been recommended. This approach could have specific relevance in patients with COVID-19 infection and preexisting cardiovascular conditions [52]. Increased circulating levels of troponins and brain-type natriuretic peptide demonstrate cardiac involvement in many patients with COVID-19 infection [24] and elevated levels of C-Reactive Protein (CRP) suggest occurrence of inflammation [53]. In a retrospective multicenter cohort study of 191 patients with COVID-19 infection, patients who died had higher levels of troponins and inflammatory biomarkers than survivors and these levels were associated with higher rates of HF. Similarly, in another smaller retrospective study COVID-19 patients who died had higher levels of troponin, myoglobin, CRP, IL-6 and serum ferritin than survivors [3].
Electrocardiography
Abnormal ECG tracings have been reported in many patients with COVID-19 and troponin elevation. In a retrospective study of 756 COVID-19 patients, premature atrial beats were found in 7.7%, premature ventricular beats in 3.4%, AF or atrial flutter in 5.6%, right bundle branch block in 7.8%, left bundle branch block in 1.5%, and abnormalities of repolarization in more than 40% [54]. In a much larger study, Musikantow, et al. reported incidence of AF of 10% in patients with COVID-19 infection without previous history of atrial arrhythmias. Incidence of AF was comparable to that of patients with influenza infection, suggesting that atrial arrhythmia is not specific to COVID-19 but might be associated to disease severity [55]. Another retrospective analysis of ECG data of COVID-19 patients demonstrated that right bundle branch block and poor R wave progression assessed upon in hospital admission are associated with worse clinical course [56].
Echocardiography
Early assessment of cardiac function in patients with COVID-19 infection could be easily performed with use of Transthoracic Echocardiography (TTE). TTE is a first-line imaging modality for assessment, and it is an essential bedside tool, allowing noninvasive quantification of cardiac structure and function in patients hospitalized in isolated wards [57]. Initial studies conducted in patients who were hospitalized for COVID-19 infection reported greater left and right ventricular dimensions, reduced LV ejection fraction, and more frequent pericardial effusion in patients with severe disease [58]. In a prospective international survey of 1216 patients with COVID-19 infection, echocardiographic abnormalities were found in 55% of patients, mostly related to LV (39%) and RV (33%) abnormalities, AMI (3%), myocarditis (3%), and Takotsubo cardiomyopathy (2%) [59]. In this study, however, 74% of patients had preexisting cardiac disease and severe cardiac functional impairment was detected in less than 15% of patients. These data suggest that clinically relevant cardiac involvement is relatively unusual in patients with COVID-19 infection, mostly occurring in patients with severe disease and very often related to pre-existing cardiac abnormalities.
Because the fatality rate of COVID-19 is relatively low, most patients survive infection leaving open a question about its possible cardiac sequelae. In a short-term follow-up study, patients who had been hospitalized with COVID-19 were compared to controls who were matched for demographics and comorbidities [60]. No considerable structural or functional differences with controls were found in the heart of survivors of COVID-19 infection, nor differences were observed between COVID-19 patients who recovered from mild/moderate or severe disease. Separate followup analysis with comparison of COVID-19 survivors with or without prior biochemical evidence of cardiac injury (increased troponin) did not show structural or functional cardiac differences [61].These echocardiographic data suggest that patients who recover from COVID-19 infection do not have considerable cardiac sequelae.
Magnetic resonance imaging
Cardiac Magnetic Resonance Imaging (MRI) has received little attention during the COVID-19 pandemic due to the infectious risk of these patients and long duration of image acquisition. This is why data obtained with cardiac MRI during the acute phase of COVID-19 infection are limited to single case reports, and most of the literature focuses on the post-recovery phase. Ojha, et al. analyzed data of 34 studies including 199 patients with COVID-19 infection and reported cardiac MRI changes indicative of myocarditis in 40% [62]. The findings of this study, however, are limited by the high heterogeneity of patients in terms of comorbidity and severity of disease. Two cross-sectional cardiac MRI studies have investigated the heart of patients with COVID-19 infection in the early recovery phase. In a first study, cardiac MRI was performed in 100 patients a mean of 71 days after diagnosis, reporting increased LV volumes and reduced LV ejection fraction, associated with evidence of ongoing myocardial inflammation in comparison to controls [63]. Another study reported on cardiac MRI of 26 young athletes 4 of whom had imaging data suggestive of myocarditis and 8 of possible prior myocardial injury [64]. It is important to notice that the majority of patients included in these two studies were not hospitalized and were asymptomatic or only mildly symptomatic, suggesting that their clinical conditions were not severe. This observation raises an important question on what could be the meaning, in terms of clinical relevance, of the cardiac MRI changes that were observed in these patients.
Another cardiac MRI study by Kotecha, et al. reported evidence of myocardial injury in patients with prior severe COVID-19 infection associated with troponin elevation [65]. After a median of 68 days from diagnosis, three different patterns of injury were observed: myocarditis-like scars in 26% of patients, infarction/ ischemia in 22%, and mixed pattern in 6%. Data of cardiac MRI obtained in COVID-19 survivors have been recently examined in a systematic analysis of 16 observational studies that included 2954 patients [66]. In this analysis, detection of cardiac MRI abnormalities was extremely variable: from 0% to 73% of patients had raised T1-weighed images, from 2% to 60% had raised T2- weighed images, and myocardial/pericardial Late Gadolinium Enhancement (LGE) ranged from 4% to 100%. This analysis highlights the striking heterogeneity of cardiac MRI findings in patients who recover from SARS-CoV-2 infection, once again raising an important question about the functional relevance of these MRI changes. Notably, this systematic analysis did not include any clinical information on patients with COVID-19 infection, further limiting the possibility to attribute any clinical meaning to the cardiac MRI findings obtained in this context.
Discussion
Cardiac involvement during COVID-19 infection might be considered as part of disease, in particular in those patients with severe infection who develop systemic inflammatory response. When it is present, cardiac damage occurs just after lung involvement and is associated with an increased risk of death. As outlined in this narrative article, cardiac involvement is highly heterogeneous ranging from inflammatory myocarditis with left HF, acute coronary syndromes, and sustained arrhythmias to right HF due to pulmonary embolism. Most of these complications follow lung involvement and occur a few days after presentation. This is why early detection of cardiac involvement during the acute phase of COVID-19 infection would be extremely important for a better care. Timely assessment of cardiac biomarkers and appropriate use of imaging techniques that can be readily applied to patients with COVID-19 infection become fundamental to identify those patients who have developed or are at risk of developing cardiac complications. Measurement of troponins, brain-type natriuretic peptide, CRP and other proinflammatory molecules and cytokines would provide important information. Similarly, repeated ECG registration and use of bedside TTE should be integral part of the diagnostic workup of patients with COVID-19 infection in order to appropriately establish cardiac supportive care when needed. There is now considerable interest in the characterization of residual/persistent cardiac damage in survivors of COVID-19 infection. This is because cardiac abnormalities may persist after clearance of SARS-CoV-2 infection, or even arise in a chronic stage of disease. Therefore assessment of cardiac structure and function in patients with COVID-19 infection who recover from acute disease would be of great relevance. Many studies have addressed this issue in the short and long-term after acute COVID-19 infection. Studies conducted with TTE indicate that patients who recover from COVID-19 infection do not have significant structural or functional cardiac involvement. This is true even when patients with biochemical evidence of cardiac injury detected during the acute phase of infection are examined. Conversely, a highly heterogeneous picture has been reported with use of cardiac MRI, and findings obtained with this technique in cumulative analyses are extremely variable. This raises some important questions about the functional/clinical relevance of the MRI changes that have been detected in survivors of COVID-19 infection. This issue needs to be further investigated in appropriately designed MRI studies that collect detailed information on the clinical status of patients. Thus, with a few exceptions, available evidence would suggest that patients who recover from COVID-19 do not have considerable cardiac sequelae and meet a favorable outcome.
Conclusion
In conclusion, current knowledge on the involvement of the heart during SARS-CoV-2 infection, both in the acute phase and after recovery, has to be considered just preliminary. In the future, more extensive and well organized studies will help to gain better understanding of this important aspect of this infection that has represented an unprecedented challenge for the entire healthcare community.
References
- World Health Organization (WHO). Pneumonia of unknown cause-China
- Huang C, Wang Y, Xingwang L, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 395(10223): 497-506 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 46(5): 846-848 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Hirano T, Murakami M. COVID-19: A new virus, but a familiar receptor and cytokine release syndrome. Immunity. 52(5): 731-733 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 181(2): 271-280.e8 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Serebrovska ZO, Chong EY, Serebrovska TV, et al. Hypoxia, HIF-1α, and COVID-19: From pathogenic factors to potential therapeutic targets. Acta Pharmacol Sin. 41(12): 1539-1546 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Simonson TS, Baker TL, Banzett RB, et al. Silent hypoxaemia in COVID‐19 patients. J Physiol. 599(4): 1057-1065 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Haberecker M, Schwarz EI, Steiger P, et al. Autopsy-based pulmonary and vascular pathology: Pulmonary endotheliitis and multi-organ involvement in COVID-19 associated deaths. Respiration. 101(2): 155-165 (2022).
[CrossRef] [Google Scholar] [PubMed]
- Wadman M, Couzin-Frankel J, Kaiser J, et al. A rampage through the body. Science. 368(6489): 356-360 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Gupta A, Madhavan MV, Sehgal K, et al. Extrapulmonary manifestations of COVID-19. Nat Med. 26(7): 1017-1032 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 323(11): 1061-1069 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Mozzini C, Cicco S, Setti A, et al. Spotlight on cardiovascular scoring systems in COVID-19: Severity correlations in real-world setting. Curr Probl Cardiol. 46(5): 100819 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 395(10223): 507-513 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Fajgenbaum DC, June CH. Cytokine storm. N Engl J Med. 383(23): 2255-2273 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 135(23): 2033-2040 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 395(10229): 1054-1062 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N Engl J Med. 383(2): 120-128 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Iaccarino G, Grassi G, Borghi C, et al. Gender differences in predictors of intensive care units admission among COVID-19 patients: The results of the SARS-RAS study of the Italian society of hypertension. PLoS ONE. 15(10): e0237297 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Iaccarino G, Grassi G, Borghi C, et al. Age and multimorbidity predict death among COVID-19 patients: Results of the SARS-RAS study of the Italian society of hypertension. Hypertension. 76(2): 366-372 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Mancusi C, Grassi G, Borghi G, et al. Determinants of healing among patients with coronavirus disease 2019: The results of the SARS-RAS study of the Italian society of hypertension. J Hypertens. 39(2): 376-380 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Kunal S, Madan M, Tarke C, et al. Emerging spectrum of post-COVID-19 syndrome. Postgrad Med J. postgradmedj-2020-139585 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Habets MAW, Sturkenboom HN, Tio RA, et al. How often and to what extent do admitted COVID-19 patients have signs of cardiac injury? Neth Heart J. 29(S1): 5-12 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Sandoval Y, Januzzi JL, Jaffe AS. Cardiac troponin for assessment of myocardial injury in COVID-19: JACC review topic of the week. J Am Coll Cardiol. 76(10): 1244-1258 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 5(7): 811-818 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Chen C, Zhou Y, Wang DW. SARS-CoV-2: A potential novel etiology of fulminant myocarditis. Herz. 45(3): 230-232 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Chang WT, Toh HS, Liao C-T, et al. Cardiac involvement of COVID-19: A comprehensive review. American J Med Sci. 361(1): 14-22 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Cicco S, Cicco G, Racanelli V, et al. Neutrophil Extracellular Traps (NETs) and Damage-Associated Molecular Patterns (DAMPs): Two potential targets for COVID-19 treatment. Mediators Inflamm. 2020: 7527953 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Shah RM, Shah M, Shah S, et al. Takotsubo syndrome and COVID-19: Associations and implications. Curr Probl Cardiol. 46(3): 100763 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Salah HM, Mehta JL. Takotsubo cardiomyopathy and COVID-19 infection. Eur Heart J Cardiovasc Imaging. 21(11): 1299-1300 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Saad M, Kennedy KF, Imran H, et al. Association between COVID-19 diagnosis and in-hospital mortality in patients hospitalized with ST-segment elevation myocardial infarction. JAMA. 326(19): 1940-1952 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Trivi M, Lalor N, Spaletra P, et al. Acute myocardial infarction in patients recovering from COVID-19 pneumonia. Medicina (B Aires). 80 Suppl 6: 97-99 (2020).
[Google Scholar] [PubMed]
- Witberg G, Barda N, Hoss S, et al. Myocarditis after COVID-19 vaccination in a large health care organization. N Engl J Med. 385(23): 2132-2139 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Mevorach D, Anis E, Cedar N, et al. Myocarditis after BNT162b2 mRNA vaccine against COVID-19 in Israel. N Engl J Med. 385(23): 2140-2149 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Driggin E, Madhavan MV, Bikdeli B, et al. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J Am Coll Cardiol. 75(18): 2352-2371 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Li Z, Shao W, Zhang J, et al. Prevalence of atrial fibrillation and associated mortality among hospitalized patients with COVID-19: A systematic review and meta-analysis. Front Cardiovasc Med. 8: 720129 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Bhatla A, Mayer MM, Adusumalli S, et al. COVID-19 and cardiac arrhythmias. Heart Rhythm. 17(9): 1439-1444 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Merino JL, Caro J, Rey JR, et al. Cardiac arrhythmias in COVID-19: Mechanisms, outcomes and the potential role of proarrhythmia. Europace. 23(S3): euab116.115 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Zuin M, Rigatelli G, Bilato C, et al. Heart failure as a complication of COVID-19 infection: Systematic review and meta-analysis. Acta Cardiol. 1-7 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Rey JR, Caro-Codòn J, Rosillo SO, et al. Heart failure in COVID‐19 patients: Prevalence, incidence and prognostic implications. Eur J Heart Fail. 22(12): 2205-2215 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Bader F, Manla Y, Atallah B, et al. Heart failure and COVID-19. Heart Fail Rev. 26(1): 1-10 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Bavishi C, Bonow RO, Trivedi V. et al. Special article-Acute myocardial injury in patients hospitalized with COVID-19 infection: A review. Prog Cardiovasc Dis. 63(5): 682-689 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Adeghate EA, Eid N, Singh J. Mechanisms of COVID-19-induced heart failure: A short review. Heart Fail Rev. 26(2): 363-369 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Lan Y, Liu W, Zhou Y. Right ventricular damage in COVID-19: Association between myocardial injury and COVID-19. Front Cardiovasc Med. 8: 606318 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Lazzeri C, Bonizzoli M, Batacchi S, et al. Echocardiographic assessment of the right ventricle in COVID -related acute respiratory syndrome. Intern Emerg Med. 16(1): 1-5 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Cicco S, Castellana G, Marra L, et al. Galectin-3 and neutrophil-to-lymphocyte ratio are indicative of heart remodelling and disease severity in patients with obstructive sleep apnoea. Sleep Med. 82: 117-124 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Zhang L, Wang B, Zhou J, et al. Bedside focused cardiac ultrasound in COVID-19 from the Wuhan epicenter: The role of cardiac point-of-care ultrasound, limited transthoracic echocardiography, and critical care echocardiography. J Am Soc Echocardiogr. 33(6): 676-682 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Creel-Bulos C, Hockstein M, Amin N, et al. Acute cor pulmonale in critically Ill patients with COVID-19. N Engl J Med. 382(21): e70 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Huette P, Beyls C, Guilbart M, et al. Acute Cor Pulmonale in COVID-19-Related ARDS: Improvement with Almitrine infusion. JACC Case Rep. 2(9): 1311-1314 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Karagodin I, Singulane CC, Moodward GM, et al. Echocardiographic correlates of in-hospital death in patients with acute COVID-19 infection: The World Alliance Societies of Echocardiography (WASE-COVID) study. J Am Soc Echocardiogr. 34(8): 819-830 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Li Y, Li H, Zhu S, et al. Prognostic value of right ventricular longitudinal strain in patients with COVID-19. JACC Cardiovasc Imaging. 13(11): 2287-2299 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Argulian E, Sud K, Vogel B, et al. Right ventricular dilation in hospitalized patients with COVID-19 infection. JACC Cardiovasc Imaging. 13(11): 2459-2461 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Tajbakhsh A, Hayat SMG, Taghizadeh H, et al. COVID-19 and cardiac injury: Clinical manifestations, biomarkers, mechanisms, diagnosis, treatment, and follow up. Expert Rev of Anti Infect Ther. 19(3): 345-357 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Singhal T. A review of coronavirus disease-2019 (COVID-19). Indian J Pediatr. 87(4): 281-286 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Mccullough SA, Goyal P, Krishnan U, et al. Electrocardiographic findings in coronavirus disease-19: Insights on mortality and underlying myocardial processes. J Card Fail. 26(7): 626-632 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Musikantow DR, Turagam MK, Sartori S, et al. Atrial fibrillation in patients hospitalized with COVID-19: Incidence, predictors, outcomes, and comparison to influenza. JACC Clin Electrophysiol. 7(9): 1120-1130 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Pepe M, Napoli G, Brindicci G. et al. Prognostic value of 12-leads admission electrocardiogram in low-risk patients hospitalized for Covid-19. Minerva Med. (2021).
[CrossRef] [Google Scholar] [PubMed]
- Cicco S, Vacca A, Cariddi C, et al. Imaging evaluation of pulmonary and non-ischaemic cardiovascular manifestations of COVID-19. Diagnostics (Basel). 11(7): 1271 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Barman HA, Atici A, Tekin EA, et al. Echocardiographic features of patients with COVID-19 infection: A cross-sectional study. Int J Cardiovasc Imaging. 37(3): 825-834 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Dweck MR, Bularga A, Hahn RT, et al. Global evaluation of echocardiography in patients with COVID-19. Eur Heart J Cardiovasc Imaging. 21(9): 949-958 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Sechi LA, Colussi G, Bulfone L, et al. Short-term cardiac outcome in survivors of COVID-19: A systematic study after hospital discharge. Clin Res Cardiol. 110(7): 1063-1072 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Catena C, Colussi G, Bulfone L, et al. Echocardiographic comparison of COVID-19 patients with or without prior biochemical evidence of cardiac injury after recovery. J Am Soc Echocardiogr. 34(2): 193-195 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Ojha V, Mansi V, Niraj P, et al. Cardiac magnetic resonance imaging in coronavirus disease 2019 (COVID-19): A systematic review of cardiac magnetic resonance imaging findings in 199 patients. J Thorac Imaging. 36(2): 73-83 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Puntmann VO, Carerj L, Wieters I, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol. 5(11): 1265-1273 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Rajpal S, Tong MS, Borchers J, et al. Cardiovascular magnetic resonance findings in competitive athletes recovering from COVID-19 infection. JAMA Cardiol. 6(1): 116-118 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Kotecha T, Knight DS, Razvi Y, et al. Patterns of myocardial injury in recovered troponin-positive COVID-19 patients assessed by cardiovascular magnetic resonance. Eur Heart J. 42(19): 1866–1878 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Hassani NS, Talakoob H, Karim H, et al. Cardiac magnetic resonance imaging findings in 2954 COVID-19 adult survivors: A comprehensive systematic review. J Magn Reson Imaging. (2021).
[CrossRef] [Google Scholar] [PubMed]