Review Article - Interventional Cardiology (2011) Volume 3, Issue 2
Cardiac shock-wave therapy in the treatment of refractive angina pectoris
- Corresponding Author:
- Amir Lerman
Division of Cardiovascular Diseases, Mayo Clinic Rochester
200 First Street SW, Rochester, MN 55905, USA
E-mail: lerman.amir@mayo.edu
Abstract
Keywords
advanced coronary disease, angiogenesis, extracorporeal shock-wave therapy, nonrevascularizable patient
As a result of the improvements in both pharmacologic and revascularization therapies life expectancy for patients with coronary artery disease (CAD) has greatly increased. This means that patients with more extensive CAD are now living longer, so many of them will develop myocardial ischemia and clinical angina that are not amenable to traditional revascularization therapy [1–4].
Patients with severe, symptomatic, chronic CAD have been described as having intractable angina, end-stage CAD or have been called ‘no option’ patients. However, despite being considered a therapy-resistant condition, refractory angina is a more appropriate term because a considerable number of new therapeutic methods are now available. The Canadian Cardiovascular Society (CCS) has recently defined refractory angina as a persistent (more than 3 months), painful condition, characterized by chest pain caused by coronary insufficiency in the presence of CAD, which cannot be controlled by a combination of medical therapy, angioplasty and coronary bypass surgery [5]. It is necessary to ensure that revascularization is unfeasible, medical therapy is optimal and that other causes of chest pain are excluded before establishing the diagnosis of refractory angina [5,6].
Currently, few data are available on the number of nonrevascularizable patients. Estimates have been obtained from both population surveys and catheterization laboratory registries. A Swedish survey in 1994–1995 demonstrated that approximately 10% of patients referred for coronary angiography because of stable angina were rejected for revascularization despite having severe symptoms [7]. In the late 1990s, a single tertiary referral center series reported that 12% of the patients referred for symptomatic CAD were not amenable to percutaneous coronary intervention or coronary artery bypass graft [8]. A more recent study conducted in Minnesota (MN, USA) found 6.7% of patients with coronary lesions >70% and no revascularization options despite optimal medical therapy [9]. In absolute numbers it would represent more than 100,000 new patients/year in the USA and 30,000–50,000 new patients/year in Europe who will probably fulfil the diagnosis of refractory angina [1,2,6].
The outcome of these patients continues to be controversial. The controlled trials with alternative therapies conducted in patients with refractory angina demonstrated (in their control arms) that annual mortality rates varied from 3 to 21%, whereas coronary event rates (including acute coronary syndromes and hospitalization for angina) varied from 11 to 69% [10–18]. In contrast to these trials, in a large series of 1066 patients with refractory angina followed for a median of 3.5 years only 126 patients died (11.8%), and only 58 of these deaths were from cardiovascular causes (5.4%) [19]. Data from 200 patients treated at our institution confirm this low rate (2% cardiac mortality per year) [20], and the recent study mentioned above also found a low mortality (15% at 3 years), although this was significantly higher than the mortality in patients completely revascularized (6.6% at 3 years) [9].
Since it has emerged that the mortality of patients with refractory angina patients is less than was considered previously, it may be speculated that the primary target of the incoming therapies for this growing population should focus on relieving chest pain and improving quality of life, which is significantly impaired compared with revascularized patients [21], and not necessarily the reduction of cardiovascular events and/or ischemia. It must also be noted that costs attributable to cardiovascular hospitalizations, outpatient visits, procedures, and medications are very high in these patients [22,23], so new treatments are required to help to reduce the high economic burden of refractory angina.
Current treatment possibilities in refractory angina
It should be stated that it is of the utmost importance that all patients who are considered for these therapeutic measures are first subjected to careful optimization of medical treatment (including novel drugs with new antianginal mechanism such as ivabradine [24–26] and ranolazine [27–29]), optimum cardiac rehabilitation and evaluation concerning psychosocial pain determinants. Secondary causes of angina, such as anemia, poorly controlled hypertension, thyroid dysfunction or arrhythmias, should also be identified and treated accordingly.
When evaluating new methods of treatment for refractory angina, one should consider the following points: clinical efficacy, scientific documentation, feasibility (i.e., accessibility, costs, need of compliance and follow-up) and adverse reactions. As demonstrated previously, given the likely low mortality of these patients, any novel approach should always have low complication rates.
▪ Enhanced external counterpulsation
Enhanced external counterpulsation (EECP) produces externally what an intra-aortic balloon pump does internally. Three pairs of pneumatic blood pressures cuffs are placed on the calves, lower and upper thighs. An electrocardiographic trigger is used to sequentially inflate the cuffs, starting at the calves, during onset of diastole and rapidly deflate before systole. This systematic diastolic pressure augmentation is presumed to increase coronary perfusion pressure and venous return, and to decrease ventricular afterload [30]. EECP reduces symptoms of angina and nitrate use, improves exercise tolerance and enhances quality of life of the patients with refractory angina; most of these effects are sustained for at least 3 years [31–35]. Side effects are minimal and include leg pain and bruising. Few contraindications exist, but significant aortic regurgitation, aortic aneurysm and arrhythmias that may interfere with triggering of the EECP system should be ruled our before starting therapy. The use of EECP is limited by the need for 35 days to complete the treatment [30].
▪ Neuromodulation
Spinal cord stimulation (SCS) is a recognized valid therapeutic option in Europe for the treatment of refractory angina [6]. The device has an epidural lead inserted at C7–T1 level, a subcutaneous wire and a pulse generator implanted in the left lower abdominal area. The therapy is self-administered; the patient receives stimulation for 1 h, three times daily, and can activate the device whenever angina is felt. Fewer angina attacks, reduced nitrate consumption, and improved CCS class and quality of life have been described with its use [18,36–39]. The main complications include infections (1%) and lead migration or fracture (7.8%) [39]. Pain due to acute coronary syndromes has not been demonstrated to be blocked in the studies and it is probable that with SCS it continues to cause typical symptoms despite stimulation; however, special care is required with pacemakers and implantable defibrillators as SCS could interfere with some of their functions [40–43].
▪ Cell therapy
Stem cell therapy is currently being investigated as a new therapeutic option for patients with chronic ischemia who are ineligible for revascularization. In a small randomized placebo-controlled study, myocardial injection has been demonstrated to be safe and to be associated with a modest but statistically significant improvement in myocardial perfusion, left ventricular ejection fraction (LVEF), exercise capacity and CCS class [44]. This technique is still in the experimental stages, and further studies are required to assess long-term results and efficacy for reducing morbidity and mortality.
▪ Other therapies
In contrast with EECP, SCS and stem cell therapy, disappointing results were obtained with gene therapy, percutaneous and transmyocardial laser revascularization. Opposite to their promising results in small trials, larger randomized studies demonstrated no associated benefit with their use [11,15,45–47]. One of the potential failings of these therapies may be due to their invasive approach and the potential higher risk–benefit ratio; for example, in one trial, a significantly higher 30‑day myocardial infarction incidence was observed in patients receiving percutaneous myocardial laser revascularization compared with patients in the placebo group [11], and procedure- related deaths have even been reported in some studies [13,15].
Cardiac shock-wave therapy
▪ History of shock-wave therapy
High-energy extracorporeal shock-wave therapy (ESWT) was introduced for medical use 30 years ago as a treatment for ureteral stones [48]. ESWT has changed the treatment of urolithiasis, and even today remains the primary treatment for most uncomplicated upper urinary tract calculi [49]. ESWT has also been therapeutically applied in bile ductus [50], pancreatic [51] and salivary stones [52].
The ‘destroyer-use’ of high-energy shock waves (SWs) is different from the more recent ‘regenerative-use’ of low-energy SWs. Based on the incidental finding of iliac bone thickening in patients undergoing lithotripsy [53], low-energy ESWT has been developed as a treatment standard or alternative therapy for a variety of orthopadic and soft tissue diseases, in several kinds of chronic tendinopathies and muscular pathologies [54–56]. The observed immediate increase in blood flow due to local vasodilation and the formation of new capillaries in the treated tissue [57–59] has led to its more promising application in cardiovascular medicine as a possible therapy for patients with refractory angina.
▪ Generation of SWs in cardiac SW therapy
Shock waves consist of acoustic energy that can be transmitted in a liquid medium and focused with precision of several millimeters to any intended treatment area inside the body. The energy density describes the maximum amount of acoustical energy that is transmitted per pulse, and varies among different uses of SWs, from 0.09 mJ/mm2 in cardiology [60] up to 0.9–1.8 mJ/mm2 in lithotripsy [61].
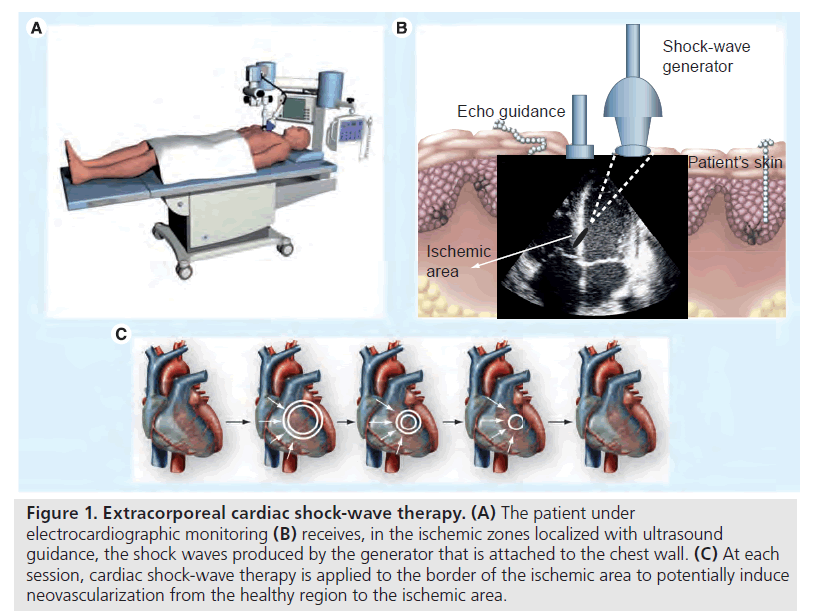
Shock waves can be artificially generated by discharge of a high-voltage spark under water. Cardiac SW therapy (CSWT) is performed using a SW generator system designed to address the clinical anatomical requirements of the chest cavity. A cardiac ultrasound imaging system is used to locate the treatment area with documented ischemia. SWs are then delivered via a special applicator through the anatomical acoustic window to the treatment area under electrocardiographic R-wave gating. Several treatment sessions are required. At each session, SWs are delivered to the border of the ischemic area, to potentially induce neovascularization from the healthy area to the ischemic area. For optimal therapy, the treatment zones are divided into target spots corresponding to the size of the focal zone of the SW applicator (Figure 1). At least two CSWT generators are available today: MODULITH® SLC (Storz Medical AG, Taegerwilen, Switzerland) and Cardiospec (Medispec, Germantown, MD, USA).
Figure 1: Extracorporeal cardiac shock-wave therapy. (A) The patient under electrocardiographic monitoring (B) receives, in the ischemic zones localized with ultrasound guidance, the shock waves produced by the generator that is attached to the chest wall. (C) At each session, cardiac shock-wave therapy is applied to the border of the ischemic area to potentially induce neovascularization from the healthy region to the ischemic area.
▪ Cardiac SW therapy results
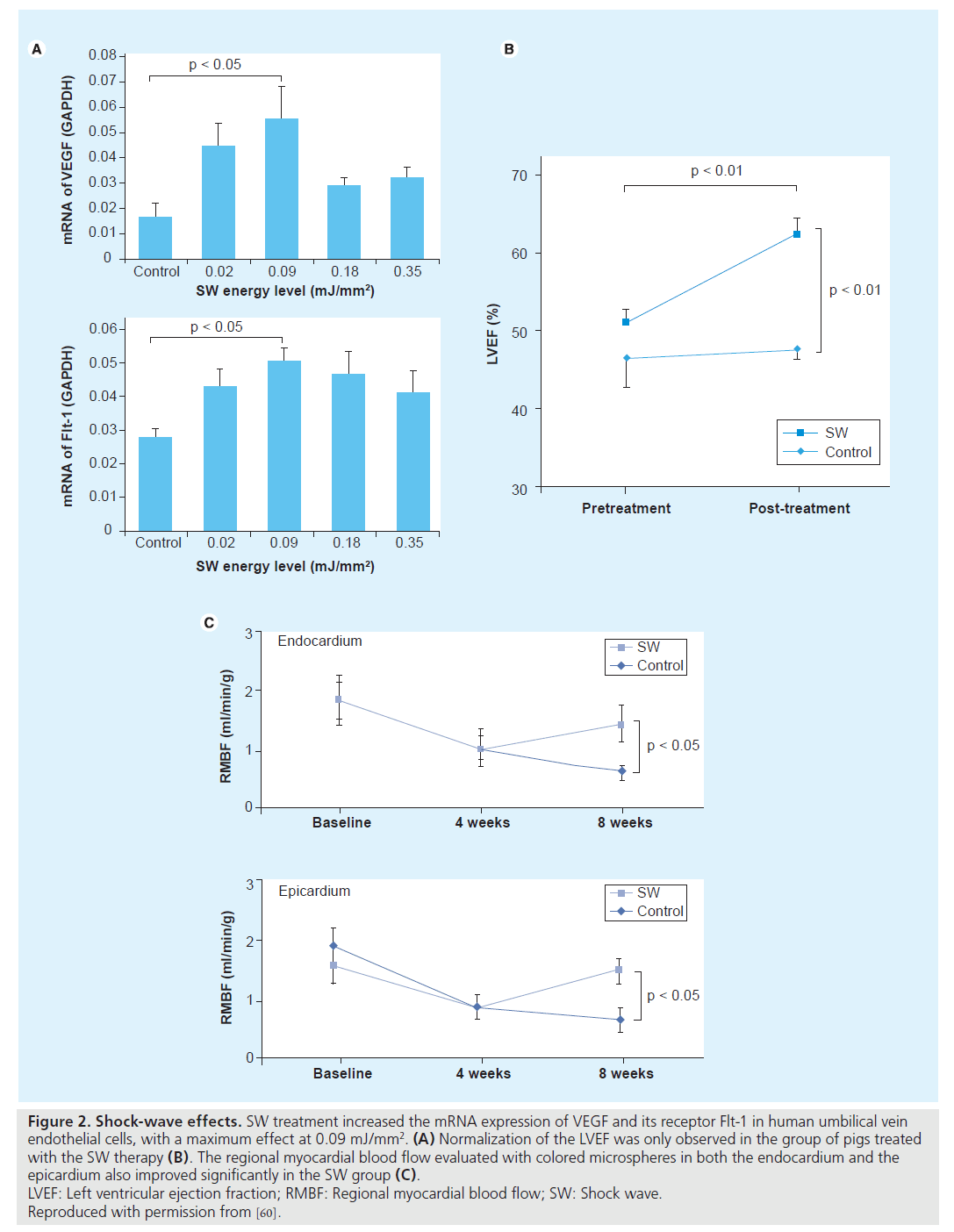
Nishida et al. published the first study to examine the effects of CSWT that could induce angiogenesis in the heart [60]. Initially, they treated human umbilical vein endothelial cells with SW at different energy levels, and found that CSWT significantly upregulated mRNA expression of VEGF and its receptor Flt-1, with a maximum effect noted at 0.09 mJ/ mm2. Subsequently, they performed an experiment in a porcine model of chronic myocardial ischemia without causing myocardial infarction. On the basis of the in vitro experiment, they applied a low-energy SW (0.09 mJ/mm2) to nine spots in the ischemic region (200 shots/ spot). Patients treated with CSWT threetimes per week were compared with the control group, who received the same anesthesia procedures three times a week but without the CSWT. A total of 4 weeks after the CSWT, patients who received SW therapy, but not the control group, had a marked development of coronary collateral vessels in the ischemic region and a significantly increased number of visible coronary arteries in the region and this was associated with a complete and significant recovery of the ejection fraction only in the SW group. The CSWT also significantly normalized the regional myocardial blood flow, increased capillary density and upregulated VEGF expression in the ischemic myocardium in vivo (Figure 2). Owing to the promising results in animal studies, Fukumoto et al. started a small, non-placebo-controlled clinical trial of CSWT in nine patients with refractory angina [62]. A CSWT consisting of 200 shots/spot at 0.09 mJ/mm2 for 20–40 spots each time, three times a week/series, was applied over the ischemic area, confirmed by dipyridamole stress thallium scintigraphy, and repeated up to three series at 0 and 1, 3 or 6 months depending on the results of their evaluation. A significant relief of the symptoms, as evaluated by CCS class score and the use of nitroglycerin (NTG), was confirmed after the CSWT. In addition, a clear improvement in myocardial perfusion, evaluated by dipyridamole stress thallium scintigraphy, was observed in the ischemic myocardium, but only where SW were applied. It should be noted that the anti-ischemic effects of CSWT started at 3 months after the therapy and persisted for at least 12 months (end of the follow-up period).
Figure 2: Shock-wave effects. SW treatment increased the mRNA expression of VEGF and its receptor Flt-1 in human umbilical vein
endothelial cells, with a maximum effect at 0.09 mJ/mm2. (A) Normalization of the LVEF was only observed in the group of pigs treated
with the SW therapy (B). The regional myocardial blood flow evaluated with colored microspheres in both the endocardium and the
epicardium also improved significantly in the SW group (C).
LVEF: Left ventricular ejection fraction; RMBF: Regional myocardial blood flow; SW: Shock wave.
Reproduced with permission from [60].
Khattab et al. in Germany shared their initial experience with CSWT applied in ten patients with refractory angina 1 year later [63]. All ten patients had evidence of myocardial ischemia on exercise Tc99 SPECT perfusion scan. They were subjected to nine sessions (three cycles, with the same energy and amount of shocks described previously [60]) over 3 months. A significant reduction in symptoms (CCS class at baseline was 3.3 ± 0.5 and at follow-up was 1.0 ± 1.3) and myocardial perfusion improvement was reported. Myocardial perfusion improved again only in the ischemic areas treated with CSWT.
Recently, the same group from Japan has published a small, unique double-blind placebo- controlled trial in eight patients with refractory angina [64]. Patients were treated with one series of placebo and CSWT in a double-bind and crossover manner with an interval of 3 months. One series of CSWT (200 shoots/spot at 0.09 mJ/mm2 for 40–60 spots per session) comprised three sessions per week. Follow-up was limited to 3 months. CSWT, but not placebo, significantly improved symptoms, NTG use and 6-min walking distance. Furthermore, the LVEF and left ventricular stroke volume, evaluated by MRI, improved significantly only after CSWT.
Currently, to the best of our knowledge, there are few other published data about the application of CSWT in patients with refractory angina. There are some isolated cases reported in journals [65] and small cohorts of patients presented at congresses [66–73]. It should be mentioned that in all of these studies, a positive effect of CSWT in clinical symptoms was shown and an improvement in myocardial perfusion – assessed by PET or SPECT – was demonstrated.
These clinical results are supported by animal experiments conducted in pigs in a model of acute phase of acute myocardial infarction (AMI). A significant recovery of LVEF and reduction in left ventricular end-diastolic volume was described at 4 weeks after the CSWT in the SW group compared with the control group. In addition, regional myocardial blood flow and the number of capillaries in the border zone of the infarction were significantly improved in the SW group. These beneficial effects were only observed when the CSWT was started 3 days after AMI, but not if started 4 weeks later [74]. Similar results were recently obtained in pigs subjected to 90-min ischemia and reperfusion [75]. In a different rodent model, direct epicardial SWs applied 4 weeks after AMI induced significant angiogenesis and improved ventricular function compared with the control group [76].
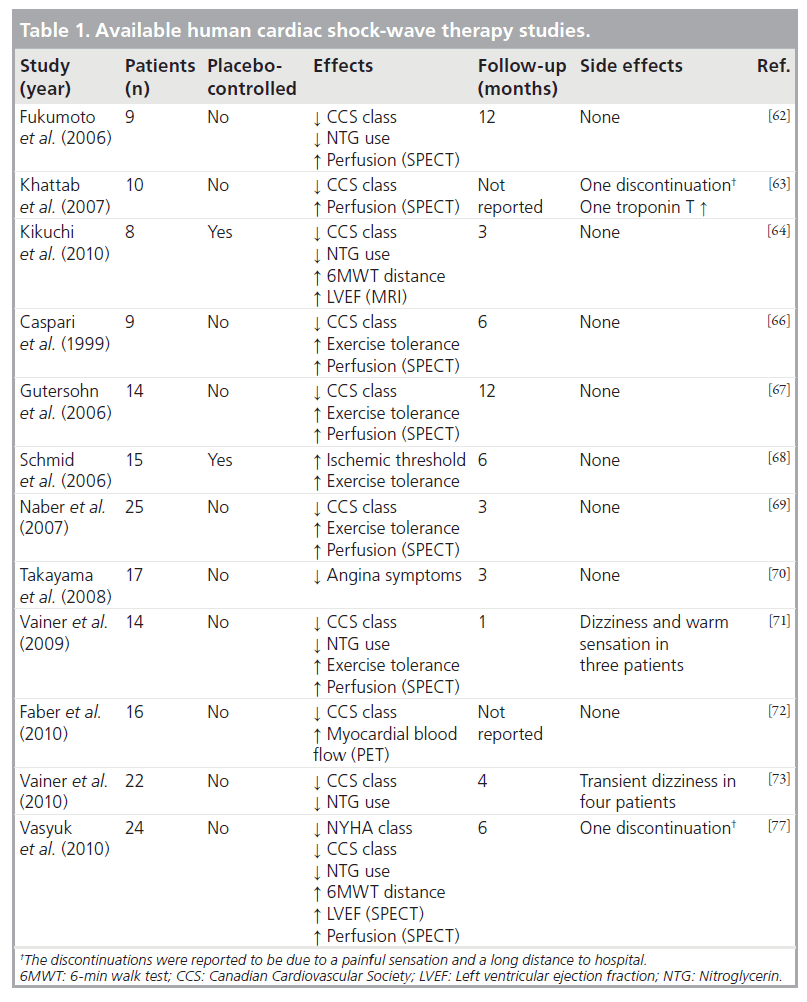
Taking into account these previous experimental studies, Vasyuk et al. have recently conducted a first study in patients with ischemic heart failure due to documented myocardial infarction (at least 6 months before inclusion) [77]. A total of 24 stable patients with systolic dysfunction (LVEF <40%) and no planned revascularization received CSWT (nine sessions with 100 shocks per spot per session at 0.09 mJ/mm2 energy level in hibernated or ischemic segments detected by dobutamine stress echocardiography) and were followed for 6 months. CSWT significantly decreased New York Heart Association class, CCS class and NTG use per week. Similarly, an improvement in 6-min walk test and quality of life was reported. The authors also found a significant increase in LVEF and in perfusion scores after the CSWT. The available human data are summarized in Table 1.
▪ Possible mechanisms of action
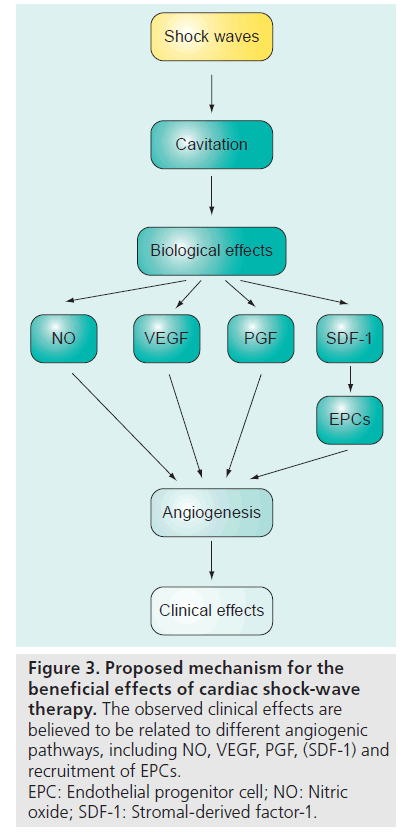
Although the precise mechanisms remain to be elucidated, two major effects may contribute to the aforementioned observations: immediate vasodilatation, and the induction of neovascularization in the treated tissue, which probably accounts for the observed long-term effects. It has been described that SW may induce tissue cavitation [78], generating highly localized physical forces that could produce localized stress on cell membranes. This would lead to a variety of biochemical effects, including: shear stress on cell membranes [79], hyperpolarization and Ras activation [80], an increase in nitric oxide synthesis [75,81–83], an upregulation of VEGF, its receptor Flt-1 and PGF [60,76,84–86], in addition to an enhance expression of stromal-derived factor-1 [87]. Another potential cellular mechanism may involve the recruitment of progenitor cells to the site of the ischemia undergoing CSWT [87–89]. Thus, we can conclude that there are probably multiples angiogenic pathways involved in the beneficial effects of CSWT (Figure 3).
Figure 3: Proposed mechanism for the beneficial effects of cardiac shock-wave therapy. The observed clinical effects are believed to be related to different angiogenic pathways, including NO, VEGF, PGF, (SDF-1) and recruitment of EPCs. EPC: Endothelial progenitor cell; NO: Nitric oxide; SDF-1: Stromal-derived factor-1.
▪ Adverse effects
No significant side effects or symptoms were reported during the CSWT [62,64–67,69]. Some of the patients referred experienced transient dizziness and warm sensation [71,73] and in one case the procedure was not completed due to the painful sensation [63].
Only in one case was a slight troponin T elevation found [63]; in the rest of the cases, elevation of myocardial enzymes in animal and human studies was not observed [60,65,66,68–70,73,77]. Currently, there is no available information about human histology, but in rats no histologic signs of myocardial damage, including cellular infiltrates, extravasates, edema, cell membrane, or cell nuclei damage and hypertrophy, were observed [76]. In human cardiac MR studies, no increase in scar tissue volume was demonstrated following CSWT in humans [71,73].
The CSWT therapy was delivered under electrocardiographic R-wave gating, and no significant arrhythmias were reported during it. However, attention should be paid in patients with pacemakers or implantable defibrillators, as there are not sufficient safety data.
▪ Advantages
A major advantage of CSWT over other angiogenic strategies (cell therapy, gene therapy and laser revascularization) is the fact that it is non-invasive and can be performed as an outpatient treatment. It can be repeated easily as no surgery, anesthesia or catheter intervention is required.
As described previously, the prognosis of these patients seems to be better than previously thought, so it is important to highlight that no major adverse effects have been reported with its use. In the absence of long-term follow-up, it suggests an important positive benefit/risk balance of this approach.
▪ Limitations
As treatment area needs are localized, the patients without an adequate echocardiographic window (e.g., pulmonary disease, breast prothesis or overweight) cannot receive this therapy, and the safe use of CSWT in patients with pacemakers or implantable defibrillators is still not defined, but beyond these points no other technical limitations are described. However, as the number of sessions to observe the benefits is high and variable, some concern has been raised regarding the adherence of patients to CSWT, similar to EECP therapy.
It is important to note that CSWT is still an experimental therapy, with very few patients currently being treated with this modality around the world, compared with other strategies used in refractory angina, and the longterm prognosis still remains unknown. Besides, as there is a lack of large placebo-controlled studies, it might be possible that the short-term reduction in symptoms observed were mediated by a placebo effect. Thus, there is a requirement to perform multicentric, randomized and/ or placebo-controlled studies, with long-term follow-ups.
Conclusion
Cardiac SW therapy is a novel approach that might play an important role in the treatment of patients with refractory angina. It seems to reduce the symptoms and to improve the quality of life. As it is a noninvasive therapy and no significant adverse effects have been reported with its use, it emerges as an attractive option for these patients. However, there is still little experience with the use of this technique compared with EECP and SCS, so more data deriving from randomized and placebo-controlled trials are required before its widespread use can be implemented.
Future perspective
In the future, the development of new percutaneous or surgical revascularization techniques and novel antianginal drugs could allow some of those patients suffering from refractory angina to become asymptomatic. However, the continued aging of the population, the emerging epidemic of diabetes and the improvement in survival after acute cardiovascular events will increase the prevalence of this medical condition.
It is also probable that, owing to the mentioned development in cardiovascular medicine, the mortality of those patients continues to decline; at that point, the new therapies should focus even more on relieving symptoms and improving the quality of life.
Cardiac SW therapy is a promising therapy. If the previous data and its angiogenic effects are confirmed, SWs may play a potential role in other conditions of ischemic heart disease, such as bridge to transplantation and diastolic dysfunction; more research into these potential indications is warranted. The possibility of a noninvasive revascularization is exciting, as is the absence of significant side effects with its use.
Executive summary
The patient with refractory angina
▪ Refactory angina is a growing problem, as patients with more extensive coronary disease live longer.
▪ More than 100,000 new patients are diagnosed per year in the USA and 30,000–50,000 in Europe.
▪ Patients appear to have a relatively low mortality, but significant quality of life impairment.
Current treatment possibilities
▪ Before making the diagnosis of refractory angina, always ensure that patient is under optimal medical therapy.
▪ Enhanced external counterpulsation and spinal cord stimulation have proved to relieve symptoms. Few side effects and contraindications have been reported.
▪ Cell therapy is still in the experimental stages.
▪ Gene therapy, percutaneous and transmyocardial laser revascularization have demonstrated disappointing results.
Shock waves
▪ Shock waves have been used in medicine for 30 years.
▪ The energy applied in cardiovascular medicine (0.09 mJ/mm2) is much lower than in other specialties.
▪ The probable angiogenic effects of shock waves are mediated by nitric oxide, VEGF, PGF and stromal derived factor-1.
Cardiac shock-wave therapy in refractory angina
▪ Cardiac shock-wave therapy has demonstrated promising results: it reduces angina symptoms and improves myocardial perfusion in the ischemic areas.
▪ Cardiac shock-wave therapy is a noninvasive outpatient treatment. No significant side effects have been reported with its use.
▪ Cardiac shock-wave therapy is still an experimental therapy with low numbers of patients treated worldwide. There is a lack of randomized, placebo-controlled studies.
Financial & competing interests disclosure
Amir Lerman serves as a national principal investigator for the study in the USA that is supported by Medispec. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- Lloyd-Jones D, Adams RJ, Brown TM et al.:Heart disease and stroke statistics – 2010update: a report from the American HeartAssociation. Circulation 121, E46–E215(2010).
- Jolicoeur EM, Granger CB, Henry TD et al.:Clinical and research issues regarding chronicadvanced coronary artery disease: part I:contemporary and emerging therapies.Am. Heart J. 155, 418–434 (2008).
- Jolicoeur EM, Ohman EM, Temple R et al.:Clinical and research issues regarding chronicadvanced coronary artery disease part II: trialdesign, outcomes, and regulatory issues.Am. Heart J. 155, 435–444 (2008).
- Kiernan TJ, Boilson BA, Sandhu GS et al.:Nonrevascularizable coronary artery diseasefollowing coronary artery bypass graftsurgery: a population-based study in OlmstedCounty, Minnesota. Coron. Artery Dis. 20,106–111 (2009).
- McGillion M, L’Allier PL, Arthur H et al.:Recommendations for advancing the care ofCanadians living with refractory anginapectoris: a Canadian Cardiovascular Societyposition statement. Can. J. Cardiol. 25,399–401 (2009).
- Mannheimer C, Camici P, Chester MR et al.:The problem of chronic refractory angina;report from the ESC Joint Study Group onthe Treatment of Refractory Angina. Eur.Heart J. 23, 355–370 (2002).
- Bernstein SJ, Brorsson B, Aberg T,Emanuelsson H, Brook RH, Werko L:Appropriateness of referral of coronaryangiography patients in Sweden.SECOR/SBU Project Group. Heart 81,470–477 (1999).
- Mukherjee D, Bhatt DL, Roe MT, Patel V,Ellis SG: Direct myocardial revascularizationand angiogenesis – how many patients mightbe eligible? Am. J. Cardiol. 84, 598–600, A8(1999).
- Williams B, Menon M, Satran D et al.:Patients with coronary artery disease notamenable to traditional revascularization:prevalence and 3‑year mortality. Catheter.Cardiovasc. Interv. 75, 886–891 (2010).
- Oesterle SN, Sanborn TA, Ali N et al.:Percutaneous transmyocardial laserrevascularization for severe angina: thePACIFIC randomised trial. Potential ClassImprovement From IntramyocardialChannels. Lancet 356, 1705–1710 (2000).
- Leon MB, Kornowski R, Downey WE et al.:A blinded, randomized, placebo-controlledtrial of percutaneous laser myocardialrevascularization to improve angina symptomsin patients with severe coronary disease. J. Am.Coll. Cardiol. 46, 1812–1819 (2005).
- Allen KB, Dowling RD, Fudge TL et al.:Comparison of transmyocardialrevascularization with medical therapy inpatients with refractory angina. N. Engl. J.Med. 341, 1029–1036 (1999).
- Kastrup J, Jorgensen E, Ruck A et al.: Directintramyocardial plasmid vascular endothelialgrowth factor-A165 gene therapy in patientswith stable severe angina pectoris.A randomized double-blind placebocontrolledstudy: the Euroinject One trial.J. Am. Coll. Cardiol. 45, 982–988 (2005).
- McNab D, Khan SN, Sharples LD et al.:An open label, single-centre, randomized trialof spinal cord stimulation vs. percutaneousmyocardial laser revascularization in patientswith refractory angina pectoris: the SPiRiTtrial. Eur. Heart J. 27, 1048–1053 (2006).
- Simons M, Annex BH, Laham RJ et al.:Pharmacological treatment of coronary arterydisease with recombinant fibroblast growthfactor-2: double-blind, randomized,controlled clinical trial. Circulation 105,788–793 (2002).
- Frazier OH, March RJ, Horvath KA:Transmyocardial revascularization with acarbon dioxide laser in patients with end-stagecoronary artery disease. N. Engl. J. Med. 341,1021–1028 (1999).
- Henry TD, Annex BH, McKendall GR et al.:The VIVA trial: Vascular Endothelial GrowthFactor in Ischemia for Vascular Angiogenesis.Circulation 107, 1359–1365 (2003).
- Andrell P, Yu W, Gersbach P et al.: Long-termeffects of spinal cord stimulation on anginasymptoms and quality of life in patients withrefractory angina pectoris – results from theEuropean Angina Registry Link Study(EARL). Heart 96, 1132–1136 (2010).
- Henry TD, Satran D, Johnson RJ et al.:Natural history of patients with refractoryangina. J. Am. Coll. Cardiol. 47, 231A (2006).
- Barsness GW, Lennon RJ, Tesmer L:Refractory angina is associated with excessivechest pain but not excessive cardiac mortality.J. Am. Coll. Cardiol. 47, 374A (2006).
- Andrell P, Ekre O, Grip L et al.: Fatality,morbidity and quality of life in patients withrefractory angina pectoris. Int. J. Cardiol.147(3), 377–382(2009).
- McGillion MH, Croxford R, Watt-Watson J,Lefort S, Stevens B, Coyte P: Cost of illnessfor chronic stable angina patients enrolled in aself-management education trial. Can. J.Cardiol. 24, 759–764 (2008).
- Arnold SV, Morrow DA, Lei Y et al.:Economic impact of angina after an acutecoronary syndrome: insights from theMERLIN-TIMI 36 trial. Circ. Cardiovasc.Qual. Outcomes 2, 344–353 (2009).
- Borer JS, Fox K, Jaillon P, Lerebours G:Antianginal and antiischemic effects ofivabradine, an I(f ) inhibitor, in stable angina:a randomized, double-blind, multicentered,placebo-controlled trial. Circulation 107,817–823 (2003).
- Koster R, Kaehler J, Meinertz T: Treatment ofstable angina pectoris by ivabradine in everyday practice: the REDUCTION study. Am.Heart J. 158, E51–E57 (2009).
- Tardif JC, Ponikowski P, Kahan T: Efficacyof the I(f ) current inhibitor ivabradine inpatients with chronic stable angina receivingb-blocker therapy: a 4‑month, randomized,placebo-controlled trial. Eur. Heart J. 30,540–548 (2009).
- Chaitman BR, Skettino SL, Parker JO et al.:Anti-ischemic effects and long-term survivalduring ranolazine monotherapy in patientswith chronic severe angina. J. Am. Coll.Cardiol. 43, 1375–1382 (2004).
- Chaitman BR, Pepine CJ, Parker JO et al.:Effects of ranolazine with atenolol,amlodipine, or diltiazem on exercise toleranceand angina frequency in patients with severechronic angina: a randomized controlled trial.JAMA 291, 309–316 (2004).
- Stone PH, Gratsiansky NA, Blokhin A,Huang IZ, Meng L: Antianginal efficacy ofranolazine when added to treatment withamlodipine: the ERICA (Efficacy ofRanolazine in Chronic Angina) trial. J. Am.Coll. Cardiol. 48, 566–575 (2006).
- Manchanda A, Soran O: Enhanced externalcounterpulsation and future directions: stepbeyond medical management for patientswith angina and heart failure. J. Am. Coll.Cardiol. 50, 1523–1531 (2007).
- Arora RR, Chou TM, Jain D et al.: Themulticenter study of enhanced externalcounterpulsation (MUST-EECP): effect ofEECP on exercise-induced myocardialischemia and anginal episodes. J. Am. Coll.Cardiol. 33, 1833–1840 (1999).
- Shah SA, Shapiro RJ, Mehta R, Snyder JA:Impact of enhanced external counterpulsationon Canadian Cardiovascular Society anginaclass in patients with chronic stable angina: ameta-analysis.Pharmacotherapy 30, 639–645(2010).
- Loh PH, Cleland JG, Louis AA et al.: Enhancedexternal counterpulsation in the treatment ofchronic refractory angina: a long-term follow-upoutcome from the International EnhancedExternal Counterpulsation Patient Registry.Clin. Cardiol. 31, 159–164 (2008).
- Feldman AM, Silver MA, Francis GS et al.:Enhanced external counterpulsation improvesexercise tolerance in patients with chronicheart failure. J. Am. Coll. Cardiol. 48,1198–1205 (2006).
- Urano H, Ikeda H, Ueno T, Matsumoto T,Murohara T, Imaizumi T: Enhanced externalcounterpulsation improves exercise tolerance,reduces exercise-induced myocardial ischemiaand improves left ventricular diastolic fillingin patients with coronary artery disease.J. Am. Coll. Cardiol. 37, 93–99 (2001).
- Hautvast RW, DeJongste MJ, Staal MJ,van Gilst WH, Lie KI: Spinal cordstimulation in chronic intractable anginapectoris: a randomized, controlled efficacystudy. Am. Heart J. 136, 1114–1120 (1998).
- Eddicks S, Maier-Hauff K, Schenk M,Muller A, Baumann G, Theres H: Thoracicspinal cord stimulation improves functionalstatus and relieves symptoms in patients withrefractory angina pectoris: the first placebocontrolledrandomised study. Heart 93,585–590 (2007).
- Borjesson M, Andrell P, Lundberg D,Mannheimer C: Spinal cord stimulation insevere angina pectoris – a systematic reviewbased on the Swedish Council on Technologyassessment in health care report on longstandingpain. Pain 140, 501–508 (2008).
- Taylor RS, De Vries J, Buchser E, DejongsteMJ: Spinal cord stimulation in the treatmentof refractory angina: systematic review andmeta-analysisof randomised controlled trials.BMC Cardiovasc. Disord. 9, 13 (2009).
- Sanderson JE, Ibrahim B, Waterhouse D,Palmer RB: Spinal electrical stimulation forintractable angina – long-term clinicaloutcome and safety. Eur. Heart J. 15, 810–814(1994).
- Andersen C, Hole P, Oxhoj H: Does painrelief with spinal cord stimulation for anginaconceal myocardial infarction? Br. Heart J.71, 419–421 (1994).
- Eckert S, Horstkotte D: Management ofangina pectoris: the role of spinal cordstimulation. Am. J. Cardiovasc. Drugs 9,17–28 (2009).
- Enggaard TP, Andersen C, Scherer C: Spinalcord stimulation for refractory angina inpatients implanted with cardioverterdefibrillators: five case reports. Europace 12,1336–1337 (2010).
- van Ramshorst J, Bax JJ, Beeres SL et al.:Intramyocardial bone marrow cell injectionfor chronic myocardial ischemia:a randomized controlled trial. JAMA 301,1997–2004 (2009).
- Stone GW, Teirstein PS, Rubenstein Ret al.: A prospective, multicenter,randomized trial of percutaneoustransmyocardial laser revascularization inpatients with nonrecanalizable chronic totalocclusions. J. Am. Coll. Cardiol. 39,1581–1587 (2002).
- Saririan M, Eisenberg MJ: Myocardial laserrevascularization for the treatment ofend-stage coronary artery disease. J. Am. Coll.Cardiol. 41, 173–183 (2003).
- Henry TD, Grines CL, Watkins MW et al.:Effects of Ad5FGF-4 in patients with angina:an analysisof pooled data from the AGENT-3and AGENT-4 trials. J. Am. Coll. Cardiol. 50,1038–1046 (2007).
- Chaussy C, Brendel W, Schmiedt E:Extracorporeally induced destruction ofkidney stones by shock waves. Lancet 2,1265–1268 (1980).
- Preminger GM, Tiselius HG, Assimos DGet al.: 2007 Guideline for the management ofureteral calculi. Eur. Urol. 52, 1610–1631(2007).
- Tandan M, Reddy DN, Santosh D et al.:Extracorporeal shock wave lithotripsy of largedifficult common bile duct stones: efficacyand analysisof factors that favor stonefragmentation. J. Gastroenterol. Hepatol. 24,1370–1374 (2009).
- Parsi MA, Stevens T, Lopez R, Vargo JJ:Extracorporeal shock wave lithotripsy forprevention of recurrent pancreatitis caused byobstructive pancreatic stones. Pancreas. 39,153–155 (2010).
- Capaccio P, Torretta S, Pignataro L:Extracorporeal lithotripsy techniques forsalivary stones. Otolaryngol. Clin. North Am.42, 1139–1159 (2009).
- Haupt G, Haupt A, Ekkernkamp A, GeretyB, Chvapil M: Influence of shock waves onfracture healing. Urology 39, 529–532(1992).
- Zelle BA, Gollwitzer H, Zlowodzki M,Buhren V: Extracorporeal shock wavetherapy: current evidence. J. Orthop. Trauma24(Suppl. 1) S66–S70 (2010).
- Sems A, Dimeff R, Iannotti JP:Extracorporeal shock wave therapy in thetreatment of chronic tendinopathies. J. Am.Acad. Orthop. Surg. 14, 195–204 (2006).
- Manganotti P, Amelio E: Long-term effect ofshock wave therapy on upper limb hypertoniain patients affected by stroke. Stroke 36,1967–1971 (2005).
- Young SR, Dyson M: The effect oftherapeutic ultrasound onangiogenesis. Ultrasound Med. Biol. 16,261–269 (1990).
- Wang CJ, Huang HY, Pai CH: Shockwave-enhanced neovascularization at thetendon-bone junction: an experiment indogs. J. Foot Ankle Surg. 41, 16–22(2002).
- Song J, Qi M, Kaul S, Price RJ: Stimulationof arteriogenesis in skeletal muscle bymicrobubble destruction with ultrasound.Circulation 106, 1550–1555 (2002).
- Nishida T, Shimokawa H, Oi K et al.:Extracorporeal cardiac shock wave therapymarkedly ameliorates ischemia-inducedmyocardial dysfunction in pigs in vivo.Circulation 110, 3055–3061 (2004).
- Seitz C, Fajkovic H, Waldert M et al.:Extracorporeal shock wave lithotripsy in thetreatment of proximal ureteral stones: doesthe presence and degree of hydronephrosisaffect success? Eur. Urol. 49, 378–383(2006).
- Fukumoto Y, Ito A, Uwatoku T et al.:Extracorporeal cardiac shock wave therapyameliorates myocardial ischemia in patientswith severe coronary artery disease. Coron.Artery Dis. 17, 63–70 (2006).
- Khattab AA, Brodersen B,Schuermann-Kuchenbrandt D et al.:Extracorporeal cardiac shock wave therapy:first experience in the everyday practice fortreatment of chronic refractory anginapectoris. Int. J. Cardiol. 121, 84–85(2007).
- Kikuchi Y, Ito K, Ito Y et al.: Double-blindand placebo-controlled study of theeffectiveness and safety of extracorporealcardiac shock wave therapy for severeangina pectoris. Circ. J. 74, 589–591(2010).
- Prinz C, Lindner O, Bitter T et al.:Extracorporeal cardiac shock wave therapyameliorates clinical symptoms and improvesregional myocardial blood flow in a patientwith severe coronary artery disease andrefractory angina. Case Report Med. 2009,639594 (2009).
- Caspari GH, Erbel R: Revascularization withextracorporeal cardiac shock wave therapy:first clinical results. Circulation100(Suppl. 19), 84 (1999).
- Gutersohn A, Marlinghaus E: Comparision ofcardiac shock wave therapy and percutanousmyocardial laser revascularization therapy inendstage CAD patient with refractory angina.Eur. Heart J. 27(Suppl. 1), 351 (2006).
- Schmid J, Capoferro M, Schepis T et al.:Extracorporeal shock wave for therapy ofrefractory angina pectoris: the shock trial.Eur. Heart J. 27(Suppl. 1), 351–352 (2006).
- Naber C, Ebralidze T, Lammers S, Hakim G,Erbel R: Non invasive cardiac angiogenesisshock wave therapy increases perfusion andexercise tolerance in endstage CAD patients.Eur. J. Heart Fail. (Suppl. 6), 71 (2007).
- Takayama T, Saito S, Hirayama A et al.:Investigation into the effectiveness of shockwave treatment for angina pectoris patientspost-bypass surgery. Eur. Heart J.29(Suppl. 1), 200 (2008).
- Vainer J, Habets J, Lousberg A, Braat S,Schalla S, Waltenberger J: Cardiac shockwavetherapy ameliorates ischemia and symptoms inpatients with end-stage coronary artery diseaseand chronic refractory angina pectoris. Eur.Heart J. 30(Suppl. 1), 740 (2009).
- Faber L, Lindner O, Prinz C et al.: Echoguidedextracorporeal shock wave therapy forrefractory angina improves regional myocardialblood flow as assessed by PET imaging. J. Am.Coll. Cardiol. 55, A120 (2010).
- Vainer J, Habets J, Lousberg A, Brans B,Schalla S, Waltenberger J: Cardiac shockwavetherapy in patients with end-stage coronaryartery disease and chronic refractory anginapectoris – mild term results. Eur. Heart J.31(Suppl.), 198 (2010).
- Uwatoku T, Ito K, Abe K et al.:Extracorporeal cardiac shock wave therapyimproves left ventricular remodeling afteracute myocardial infarction in pigs. Coron.Artery Dis. 18, 397–404 (2007).
- Ito Y, Ito K, Shiroto T et al.: Cardiac shockwave therapy ameliorates left ventricularremodeling after myocardial ischemiareperfusioninjury in pigs in vivo. Coron.Artery Dis. 21, 304–311 (2010).
- Zimpfer D, Aharinejad S, Holfeld J et al.:Direct epicardial shock wave therapyimproves ventricular function and inducesangiogenesis in ischemic heart failure.J. Thorac. Cardiovasc. Surg. 137, 963–970(2009).
- Vasyuk YA, Hadzegova AB, Shkolnik ELet al.: Initial clinical experience withextracorporeal shock wave therapy intreatment of ischemic heart failure. Congest.Heart Fail. 16(5), 226–230 (2010).
- Apfel RE: Acoustic cavitation: a possibleconsequence of biomedical uses of ultrasound.Br. J. Cancer (Suppl. 5), 140–146 (1982).
- Maisonhaute E, Prado C, White PC,Compton RG: Surface acoustic cavitationunderstood via nanosecond electrochemistry.Part III: shear stress in ultrasonic cleaning.Ultrason. Sonochem. 9, 297–303 (2002).
- Wang FS, Wang CJ, Huang HJ, Chung H,Chen RF, Yang KD: Physical shock wavemediates membrane hyperpolarization andRas activation for osteogenesis in human bonemarrow stromal cells. Biochem. Biophys. Res.Commun. 287, 648–655 (2001).
- Gotte G, Amelio E, Russo S, Marlinghaus E,Musci G, Suzuki H: Short-time nonenzymaticnitric oxide synthesis froml-arginine and hydrogen peroxide induced byshock waves treatment. FEBS Lett. 520,153–155 (2002).
- Mariotto S, Cavalieri E, Amelio E et al.:Extracorporeal shock waves: from lithotripsyto anti-inflammatory action by NOproduction. Nitric Oxide 12, 89–96 (2005).
- Mariotto S, de Prati AC, Cavalieri E,Amelio E, Marlinghaus E, Suzuki H:Extracorporeal shock wave therapy ininflammatory diseases: molecular mechanismthat triggers anti-inflammatory action. Curr.Med. Chem. 16, 2366–2372 (2009).
- Reher P, Doan N, Bradnock B, Meghji S,Harris M: Effect of ultrasound on theproduction of IL-8, basic FGF and VEGF.Cytokine 11, 416–423 (1999).
- Gutersohn A, Caspari G: Shock wavesupregulates vascular endothelial growth factorm-RNA in human umbilical vascularendothelial cells. Circulation 102(Suppl.),18 (2000).
- Yoshida J, Ohmori K, Takeuchi H et al.:Treatment of ischemic limbs based on localrecruitment of vascular endothelial growthfactor-producing inflammatory cells withultrasonic microbubble destruction. J. Am.Coll. Cardiol. 46, 899–905 (2005).
- Aicher A, Heeschen C, Sasaki K, Urbich C,Zeiher AM, Dimmeler S: Low-energy shockwave for enhancing recruitment of endothelialprogenitor cells: a new modality to increaseefficacy of cell therapy in chronic hind limbischemia. Circulation 114, 2823–2830(2006).
- Sheu JJ, Sun CK, Chang LT et al.: Shockwave-pretreated bone marrow cells furtherimprove left ventricular function aftermyocardial infarction in rabbits. Ann. Vasc.Surg. 24, 809–821 (2010).
- Chavakis E, Koyanagi M, Dimmeler S:Enhancing the outcome of cell therapy forcardiac repair: progress from bench to bedsideand back. Circulation 121, 325–335 (2010).