Review Article - Imaging in Medicine (2011) Volume 3, Issue 1
Carotid plaque imaging with FDG-PET and ultrasound
Martin Græbe†1, Henrik Sillesen1, Andreas Kjær1 & Liselotte Højgaard11Rigshospitalet, University of Copenhagen, Copenhagen, Denmark
- Corresponding Author:
- Martin Græbe
Department of Vascular Surgery
Rigshospitalet, University of Copenhagen
Blegdamsvej 9, 2100 Copenhagen Ø, Denmark
Tel: +45 3545 3545
Fax: +45 3545 2303
E-mail: martin.graebe@rh.regionh.dk
Abstract
Stroke and other thromboembolic events in the brain are often due to carotid artery atherosclerosis, and atherosclerotic plaques with inflammation are considered particularly vulnerable with an increased risk of becoming symptomatic. At present, however, patients are selected for carotid surgical intervention on the basis of the degree of stenosis alone, and not the vulnerability or inflammation of the lesion. During the past decade, research, using PET with the glucose analog tracer 18F-fluor-deoxy-glucose, has been implemented for identifying increased tracer uptake in symptomatic carotid plaques, and tracer uptake has been shown to correlate with plaque inflammation and vulnerability. These findings imply that FDGPET might hold the promise for a new and better diagnostic test to identify patients eligible for carotid endarterectomy. The rationale for developing diagnostic tests based on molecular imaging with FDG-PET, as well as methods for simple clinical PET approaches, are discussed.
Keywords
atherosclerosis • carotid • FDG • inflammation • PET • ultrasound • vulnerable plaque
Carotid artery atherosclerosis
Cardiovascular disease is the leading cause of morbidity and mortality worldwide, and stroke alone is the third leading cause of death in the USA [1,2]. The majority (~85%) of all strokes are ischemic, following thromboembolic events, and the main cause is believed to be atherosclerosis [2,3]. Approximately a fifth of all stroke patients get a ‘warning sign’ within 90 days before the stroke, enabling aggressive prophylactic treatment in this short time period [4,5]. If carotid artery stenosis is present in such patients with nonfatal warning symptoms, including transitory ischemic attack, ipsilateral minor stroke or transitory ocular symptoms, the risk of recurrence is higher [6–8]. The benefit of carotid endarterectomy in these patients was established in large trials conducted in the 1990s. The symptomatic patients were randomized to either medical treatment alone or carotid endarterectomy, showing that the operation reduced the risk of future stroke by 50% [9,10].
Asymptomatic carotid artery atherosclerosis is of course also a risk factor for ipsilateral cerebral thromboembolic events, and primary prevention, medical or surgical, should be considered in such patients. However, the annual risk of stroke in asymptomatic patients with carotid artery stenosis is low, at least when comparing the event rates to the risk of perioperative death or stroke when performing carotid endarterectomy [11,12]. Furthermore, the stroke rate has decreased in recent years following the introduction of aggressive medical treatments (e.g., antiplatelet, statin and hypertension treatment) and improving impact of public attention to reduce risk factors in the industrialized countries (e.g., decreasing smoking and increasing physical activity) [1,2]. Current annual risk of ipsilateral ischemic symptoms in asymptomatic patients with significant carotid artery stenosis (>50%) is in the region of 1% per year [12–14]. In comparison, preoperative and 6 weeks’ postoperative risk of stroke or death following endarterectomy has recently been estimated to be 4.9% on average, and risk of death alone is 1.4% [15].
Identifying a subgroup of patients with carotid artery plaque who are at high risk (i.e., higher than 4.9%) of suffering a stroke despite best medical treatment would help stratifying asymptomatic patients in the selection for primary surgical treatment. In the same way, symptomatic patients, where risk of recurrence may be low despite the warning signs, currently eligible for surgery might benefit from a new risk stratification tool used to abstain from surgical intervention, thus eliminating the risk of surgical complications.
Patient selection for intervention
In the selection of patients with carotid artery stenosis eligible for endarterectomy, only the degree of stenosis and time passed since symptoms are used today as risk stratification parameters, and there is an ongoing debate as to whether asymptomatic patients should be offered surgical intervention at all [16,17]. For decades, cardiovascular interventions have been targeted against large atherosclerotic lesions with the degree of stenosis as a risk marker. The hemodynamic significant stenosis, where luminal narrowing caused turbulence or eventually low flow, was believed to be the main site of thrombus formation. This assumption was inevitable, as witnessed by the high efficacy of coronary stenting. However, in the majority of patients presenting with symptoms of cardiac ischemia, significant stenoses could not be identified [18] and it has repeatedly been shown, that although revascularization in patients with coronary artery disease was efficient in bringing relief of symptoms, long-term survival was not improved [19–21]. Post-mortem studies revealed that the majority of lethal plaques had low-grade stenosis whereas they, independently of size, shared similar molecular and structural characteristics [22].
Several histopathological traits of these vulnerable plaques at risk of giving symptoms have been identified and investigations of surgically removed plaques from patients with symptoms of cerebral ischemia have shown that the pathophysiology of vulnerable carotid plaques is similar to that of the coronary plaques [23,24]. Symptoms occur either as sudden thrombosis at the site where the vulnerable plaque occludes the vessel, or because a thrombus is released from its origin, embolizing and occluding a downstream vessel. The latter pathogenesis is typical for the high flow carotid artery, where only approximately 3% of patients presenting with cerebral symptoms (not including severe stroke or deaths) have an occluded carotid artery [10].
Identification and differentiation of vulnerable plaques (i.e., atherosclerotic lesions at high risk of thromboembolic events) is important in all vascular beds in order to intensify and specify selection criteria for primary and secondary treatment. This article is focused on carotid artery plaques, as these plaques are readily accessible for both imaging and excision. However, results from recent studies underscore that 18F-f luor-deoxy-glucose (FDG) PET is likely to be used in all vascular beds, identifying, for example, coronary vulnerable plaques or vulnerable patients in general [25,26].
Histopathology of vulnerable plaques
The vulnerable plaque is defined as an atherosclerotic lesion at risk of giving symptoms [27]. These plaques are characterized by a thin fibrous cap surrounding a lipid core with scattered presence of necrotic debris or hemorrhages. As the core increases in size and the fibrous cap continually weakens, the plaque is destabilized. If the cap eventually ruptures, the highly thrombogenic core material will be exposed to the bloodstream, causing thrombus formation [23,27]. Dense inflammatory cell infiltration, primarily by monocytes and macrophages, is present in the vulnerable plaque. Degradation of the caps’ extracellular matrix is a pivotal step in the process of destabilizing the plaque and this effect is propagated by the release of proteolytic enzymes, such as matrix metalloproteinases and cathepsins, from the macrophages [28–31]. The surface molecule CD68 is specifically and constitutively expressed by macrophages and CD68 is therefore traditionally used as a marker of inflammation in tissue analyses of surgically removed plaques [32].
Imaging of the vulnerable plaque
The histopathological characteristics of the vulnerable plaque comprise two entities, the structural and the molecular, that can be targeted with modern imaging modalities. These entities are distinctly different from the plaque burden, or the degree of stenosis, which may be assessed using conventional digital subtraction angiography, CT angiography, MRI angiography or ultrasound duplex imaging.
Regarding plaque structure, B-mode ultrasonography, CT and MRI has been used in prospective studies to confirm that certain morphological features of carotid plaques predict focal cardiovascular disease [33–35]. Furthermore, these modalities have been used in detection of drug-modifying effects on plaque composition over time [36–38]. With molecular imaging, MRI and FDG-PET are of particular interest, as both modalities have shown to be able to depict increased inflammatory processes in vulnerable plaques certified by immunohistochemical and gene expression analyses [28,39–41]. Again, this feature has been used for demonstrating drug-modifying effects over time in patients receiving statins [42,43]. Other imaging modalities are emerging for both structural and molecular imaging of atherosclerosis, but to our knowledge, none, including the above mentioned, have reached clinical decision making. Randomized trials, incorporating plaque morphology with degree of stenosis as a risk stratification tool before surgical intervention, are lacking.
Morphological identification of the vulnerable plaque using ultrasound
Ultrasound remains the primary and often only diagnostic tool used when investigating the carotid arteries. In a fast, cheap and reliable manner, the bifurcation can be screened for the presence of atherosclerotic lesions using a combination of grayscale B-mode imaging and color duplex. If plaques are present, the degree of stenosis is determined using Doppler for assessment of absolute flow velocities [44,45]. Accessibility for surgical intervention can be established at the same time and most surgeons master the technique themselves.
One of the most thoroughly investigated structural predictors of cerebral events in patients with carotid artery plaques is plaque ultrasonographic echogenicity. Different tissue components reflect the echoes of ultrasound beams differently and plaque morphology can be characterized with high-resolution B-mode images [46–51]. Plaque echogenicity is typically classified as either echolucent or echorich, but combined with the description of specific texture analyses, the classifications become somewhat confusing. The vulnerable plaque has predominantly been reported to be heterogeneous, but a homogeneous echolucent plaque might represent a vulnerable plaque with a large lipid core [45–47,49–56]. These conflicting classifications reflect the visual and subjective nature of ultrasound plaque morphology classification, and lack of reproducibility and interobserver diversity has limited its use in clinical decision making [52,55,57].
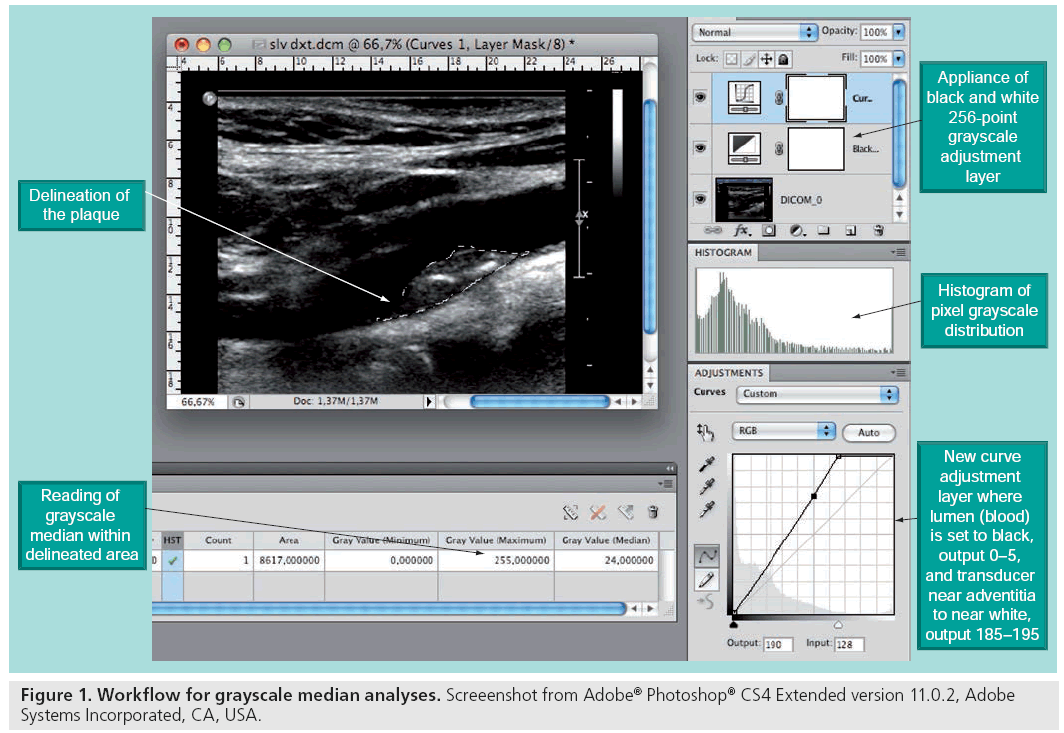
With modern ultrasound scanners, recordings of ultrasound images can be stored digitally for computer-assisted quantitative analysis of the morphologic components of the plaque (Figure 1). This method has proved to increase the specificity of the echo classification, and the reproducibility has improved greatly [57,58]. It has been shown that echolucent plaques are associated with both an increased presence of macrophages and histological features that resemble that of the vulnerable plaques [51,54,56,59], and a large amount of research linking ultrasonographic morphology to risk of stroke has been published [11,47,53].
There are, in our opinion, two major reasons why ultrasound morphology assessment has not reached standardized usage in clinical decision making. First, a diagnostic tool needs to be fast and available. This is not the case for the computer analyses with current time-consuming selection and transfer of digital images to manual offline post-processing software. Second, the ability to truly identify patients at risk using ultrasound alone carries only a moderate specificity for the vulnerable plaque. In the Asymptomatic Carotid Stenosis and Risk of Stroke (ACSRS) study [11], semiquantitative assessment of plaque echogenicity and texture was implemented using visual evaluation of digitally standardized B-mode images. In 905 asymptomatic patients with stenosis greater than 70%, two subgroups with different risk profiles were identified by these analyses: In 181 patients, the annual risk of an ipsilateral event was 0.14%, whereas in the other group of 724 patients, the risk was 2%. Thus, using present state of the art, ultrasound may be able to identify those not at risk, rather than identifying the truly high-risk patients.
Molecular identification of the vulnerable plaque using PET
With the introduction of high-resolution molecular imaging for clinical indications, a novel strategy of targeting molecular activity can be implemented using PET scanning. It has repeatedly been suggested that molecular imaging can improve the specificity in identification of plaques with significantly higher risk of giving symptoms [60,61], and PET with the tracer FDG might be the new modality for identification of the deleterious inflammatory characteristics of the vulnerable plaque. Observations of focal FDG uptake in arterial walls were first reported in oncology PET studies and often in parenthetical sentences describing artefacts or pitfall observations that could mimic malignancies [62,63]. In 2001, the potential clinical usage of FDG-PET in imaging of atherosclerosis was recognized in humans as observations of increasing arterial focal FDG uptake were linked to increasing age [64].
In 2002, an ex vivo experiment in endarterectomized carotid specimens from eight symptomatic patients showed that FDG accumulated in macrophages. Furthermore, this study showed in vivo that the uptake was higher in symptomatic plaques compared with contralateral asymptomatic plaques, and the authors suggested that FDG-PET “could be used to predict the risk of future plaque rupture, and therefore to target surgery to high-risk carotid stenosis regardless of angiographic appearance” [65].
Since then, this hypothesis has been addressed by only a few human studies [40,66,67], as most research has focused on overall arterial FDG uptake (atherosclerotic burden for identification of vulnerable patients) rather than specific outcome of increased focal plaque uptake. FDG uptake has been suggested as a surrogate marker for medical treatment efficacy [42] or as a monitoring tool for overall cardiovascular risk assessment [68–72], but to date no prospective studies have been published concerning specific assessment of single plaque outcome in relation to FDG uptake. The prevalence of high FDG uptake in asymptomatic plaques is thus unknown, as is the prognostic value of increased plaque uptake in both asymptomatic and symptomatic patients. However, studies from symptomatic plaques support the idea that FDG-PET reflects inflammation and vulnerability, and pave the way for further prospective studies. The finding of a positive correlation between CD68 and FDG uptake have been reproduced using both immunohistochemical [40,65,73] and gene expression analyses [28,74], and our recent results have shown that PET FDG uptake in carotid artery plaques correlates positively with gene expression of known markers of vulnerability [28].
The following sections describe the specific procedures and considerations needed for practical conduction and evaluation of present and future carotid PET/CT. The recommendations are mainly based on own experiences.
18F-fluor-deoxy-glucose
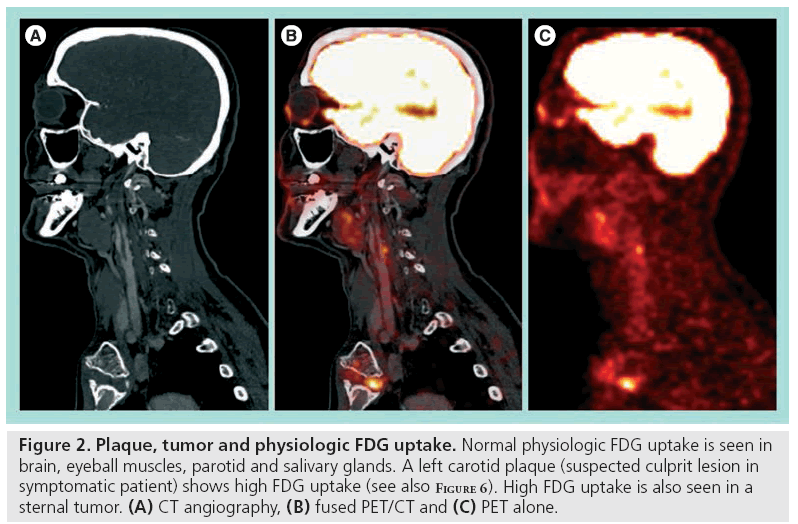
18F-fluor-deoxy-glucose is a glucose analog labelled with fluorine-18 (18F) and tissue FDG uptake reflects glucose metabolism. Tissue areas with relatively high metabolic activity will show a relatively increased FDG uptake. Normal high physiologic uptake in the fasting and resting patient is seen in the brain, salivary glands, thyroid gland, heart, liver, kidneys and urinary tracts as FDG is cleared by the kidneys. Muscles might show uptake when activated (seen in neck musculature with head movements or in vocal cords when talking) and focal uptake is clearly visualized in tumors and inflammation (Figure 2) [75]. A tracer with higher specificity for the molecular processes in the vulnerable plaque would be preferable and several novel radionuclide candidates have been presented [76–78]. However, availability of FDG, being the most common tracer used in clinical PET studies for oncologic indications, grossly follows availability of the PET scanners, and this is a major advantage when considering future clinical implementation of a new diagnostic test.
Figure 2: Plaque, tumor and physiologic FDG uptake. Normal physiologic FDG uptake is seen in brain, eyeball muscles, parotid and salivary glands. A left carotid plaque (suspected culprit lesion in symptomatic patient) shows high FDG uptake (see also Figure 6). High FDG uptake is also seen in a sternal tumor. (A) CT angiography, (B) fused PET/CT and (C) PET alone.
PET/CT image quality & resolution
The quality and resolution of the final reconstructed PET image is dependent on several factors, some of which are fixed, and some of which can be manipulated. Current spatial resolution of PET is in the range of 4 to 5 mm, dependent on the positron range, which is the distance an emitted positron travels before annihilating. The median positron range for 18F is 2.3 mm [79]. Other fixed variables are specific to the scanner used, such as the amount, type and density of detection crystals. Scanning procedures might have great impact on the final results, emission time and reconstruction settings, and timing of acquisition can also be subjected to changes. Increasing time of emission recording will increase the amount of counts registered in small structures such as the carotid plaque, but at the same time, noise is increased. Reconstruction settings, such as number of iterations or the filter applied in the computerized reconstruction process, also have an impact on the different quantification methods of FDG uptake. Finally, the timing of acquisition might be the single most important parameter for final image quality and quantification of plaque tracer uptake [80,81].
Visual evaluation of FDG uptake with differentiation between high- and low-uptake plaques is not considered possible when using early 1-h acquisition as background activity in blood, and other structures at this point will blur the images. The partial volume effect, or spill-in of FDG uptake from adjacent pixels, can, to a small degree, be altered by changing reconstruction protocols or emission timing, but standardized quantification methods of the tracer uptake is necessary to identify inflammation (discussed later). In late 3-h acquisition, plaque-specific tracer uptake is clearly visualized, and the partial volume effect may have changed to a spill-out effect, where FDG uptake in the small structures are underestimated. Quantification of FDG uptake at this time point has proven to correlate to the amount of inflammation [81].
Anatomical coregistering
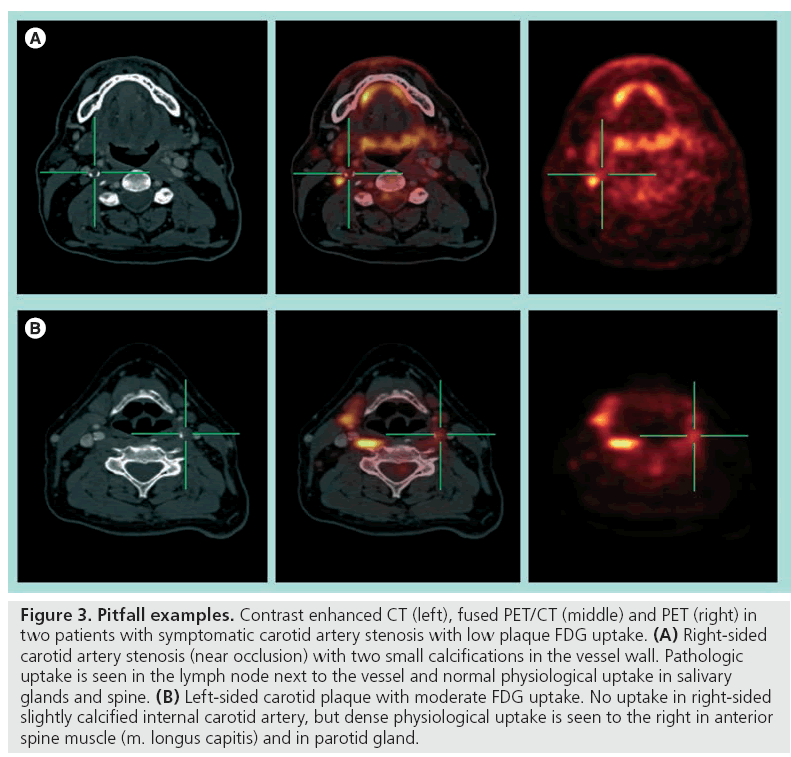
Fusion with a contrast-enhanced CT or MRI angiography is necessary for exact anatomical localization of the molecular imaging provided by the PET scanning. Standalone PET for carotid artery plaque identification is not recommended as many pericarotid structures can exhibit both physiologic and pathophysiologic uptake (Figure 3). The new integrated PET/CT scanners facilitate image fusion of the two modalities with minimal risk of patient movement artefacts. With the introduction of high-speed 64 multislice CT scanners, as well as the possibility of ECG gating, the FDG uptake can even be correlated to exact intraplaque morphology.
Figure 3: Pitfall examples. Contrast enhanced CT (left), fused PET/CT (middle) and PET (right) in two patients with symptomatic carotid artery stenosis with low plaque FDG uptake. (A) Right-sided carotid artery stenosis (near occlusion) with two small calcifications in the vessel wall. Pathologic uptake is seen in the lymph node next to the vessel and normal physiological uptake in salivary glands and spine. (B) Left-sided carotid plaque with moderate FDG uptake. No uptake in right-sided slightly calcified internal carotid artery, but dense physiological uptake is seen to the right in anterior spine muscle (m. longus capitis) and in parotid gland.
A low radiation CT should precede the PET scan for attenuation correction, and after the PET acquisition, a contrast-enhanced, highresolution CT arteriography should be made for exact localizations of vessels and plaques. The reason for the two levels of CT scan is that the presence of contrast influences the attenuation correction [82]. CT attenuation correction is based on linear correction according to pixel Hounsfield units (HU). Bone has a photoelectric attenuation of 511 keV photons different from soft tissue and, therefore, a separate scaling factor is used in the linear correction for pixels with HU above a certain threshold (e.g., 300 HU). If this threshold is reached within the arteries during the arteriography, the CT attenuation correction will erroneously consider the artery to be bone, and the PET signal from the arteries will subsequently be erroneously corrected. In this context, it should be noted that a possible influence of plaque calcification on attenuation correction is present [83]. Until specific studies have addressed this potential pitfall, it is recommended that the uncorrected PET scan is always reconstructed and viewed together with the corrected images.
Patient management
PET/CT scanning using a hybrid PET/CT scanner is recommended, with at least a 16-slice CT. Scanning should be performed 1–3 h (see following discussion) after intravenous injection of 400 MBq FDG, with the head placed in a head holder, dental prosthesis removed, to avoid CT artefacts, and arms aligned to the side. The patient should have fasted for at least 6 h before the injection, and serum blood glucose levels should be less than 8 mmol/l. We have not observed carotid near brown fat FDG uptake in our cohort of patients, but data from one animal study suggested that brown fat (and not atherosclerosis) was the main site of periarterial FDG uptake [84]. To reduce tracer uptake in brown fat, the patient should be kept in warm surroundings before and after injection [85]. Furthermore, in order to reduce tracer uptake in other pericarotid structures, mainly muscles, the patient should rest at least 10 min before injection and oral instructions should be given before this period to avoid any talking before the injection. The use of sedatives is not recommended, as adverse effects of excessive talking, nausea or paradox effects, such as agitation, often in elderly patients, have been experienced in our cohort using benzodiazepine. Emission in 3D for at least 4 min per frame is recommended [81,86].
Wide differences exist between national thresholds of acceptable radiation exposure in medical imaging. The exact carcinogenic risk of a single low radiation dose exposure is not known, but patients should be informed about the potential risks. A combined PET/CT of the thorax, neck and head is estimated to be 16 mSv when using 400 MBq FDG. This dose corresponds to 1/80 of the lethal radiation doses received by Chernobyl fire fighters, eight-times the normal yearly Danish background radiation dose or 400-times the dose of cosmic radiation received during a one-way Copenhagen–Newark flight [87–89]. Other groups have reported good results using a conceivable lesser amount of FDG [67].
Reconstruction of acquisition data
Reconstructions should be made using the noncontrast (and low radiation) CT for attenuation correction using a backprojection algorithm or the ordered subset–expectation maximation algorithm [28,86]. We have used the latter in analyses of emission time and filter setting changes in final images and recommend reconstructions to be made with eight iterations and four subsets, 3 mm axial slice thickness, 3 mm Gaussian filtering and a fixed pixel value of 256 × 256 × 55.
The reconstructed images should be exported in DICOM-format to a viewing station for image analysis. Primary identification of plaques should be performed using the high-resolution contrast-enhanced CT-scans and oval regions of interest (ROIs) should be fitted around the carotid artery containing both vessel wall and lumen, including the plaque (present as luminal filling defect on contrast images) [81,86]. Quantification of plaque FDG uptake should be measured in all axial slices containing the plaque and averaged to a single value expressing the entire plaque [28,40]. In the same way, if a background (blood) activity is desired for the target to background quantification method, consecutive intraluminal ROIs in the jugular vein should be drawn in five to six slices and averaged. The reason for recommending this kind of ROI placement and averaging, as opposed to identifying and delineating the plaque alone or selecting single slices with high FDG uptake areas alone, is primarily because this simple method has been shown to yield a positive correlation between FDG uptake and plaque macrophage presence [28,40,74]. Furthermore, this method corresponds to the analyses performed on a daily basis on PET scans from oncologic indications, and is thus considered easily clinically implementable.
After placement of ROIs on the high-resolution CT, the ROIs can be transferred to the attenuation-corrected PET scans for readings of FDG uptake. Automatic fusion (which is easy with the hybrid scanners using absolute slice ID) of PET and CT should be checked and adjusted by visual inspection aligning parotid glands, cerebrum, spinal cord, and other high-uptake landmarks in sagittal, coronal and axial planes.
Timing of the PET/CT
In the pioneer study from 2002 it was noted that late acquisition of atherosclerotic plaque FDG uptake showed a better contrast between target and background, as the luminal blood activity diminished with time [65,67]. Most prospective studies published since then have, with reference to these first observations, used PET acquisition times from 90 to 180 min after FDG injection. This conflicts with current clinical practice in oncology PET scans, where acquisitions are typically made 45–60 min after FDG injection. If an early acquisition could be used for PET imaging of atherosclerosis, it would provide a protocol that could easily be implemented in the current daily clinical workflow, and it would favor both staff and patients. Radioactive exposure time to staff and surroundings from injected patients awaiting the PET scan would diminish and the patient waiting time during the fast, which for the elderly can be quite a challenge, would also improve. Furthermore, an early acquisition protocol would give access to a large amount of retrospective data due to the many PET scans performed all over the world for oncology indications. The partial volume effect is the main concern when choosing time of acquisition. Partial volume correction can be applied with advanced image analyses [90], but with emphasis on clinical usability of the PET/CT, such analyses are probably only useful in preclinical research and future improvement of the method, identifying the ‘right’ quantification method.
Quantification of FDG uptake
Many studies published concerning atherosclerosis and FDG uptake have been observational in design and used visual evaluation of plaque uptake, whereas some groups have preferred to quantitate the uptake [40,64–72]. From a clinical perspective, choice of a reproducible and reliable quantification method is paramount in order to identify threshold values stratifying patients into different risk groups. Prospective studies that investigate the suspected coincidence between inflammation in carotid plaques and cerebrovascular events are still awaited and recommendation on proper quantification of the FDG uptake therefore relies on the basic theory; that vulnerability (i.e., risk of an event from the investigated plaque) is a function of macrophage abundance. In this context, the maximum standardized uptake value (SUVmax) and a target to background ratio (TBR), using venous blood pool activity as background and plaque activity as target, have been shown to correlate to macrophage extent in carotid artery plaques in 3-h PET acquisitions [28,31,40,74]. The use of these quantification methods in late acquisitions is thus recommended.
The SUV corrects for injected dose, decay and patient weight is calculated using the formula:
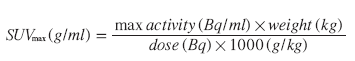
Mean (SUVmean) or maximum pixel activity (SUVmax) can be read within the ROIs, and averaging all ROIs for each plaque the FDG uptake can be quantified as either an average SUVmax or as a target to background ratio (TBR):
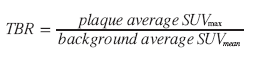
Necessity of a strict protocol with fixed reconstruction algorithms, timing of acquisition, and timing of emission is emphasized. This might be evident in prospective studies, but it should be noted as a caveat when collecting data for retrospective studies, as well as in comparisons of absolute quantification measures from different prospective studies carried out by different research groups.
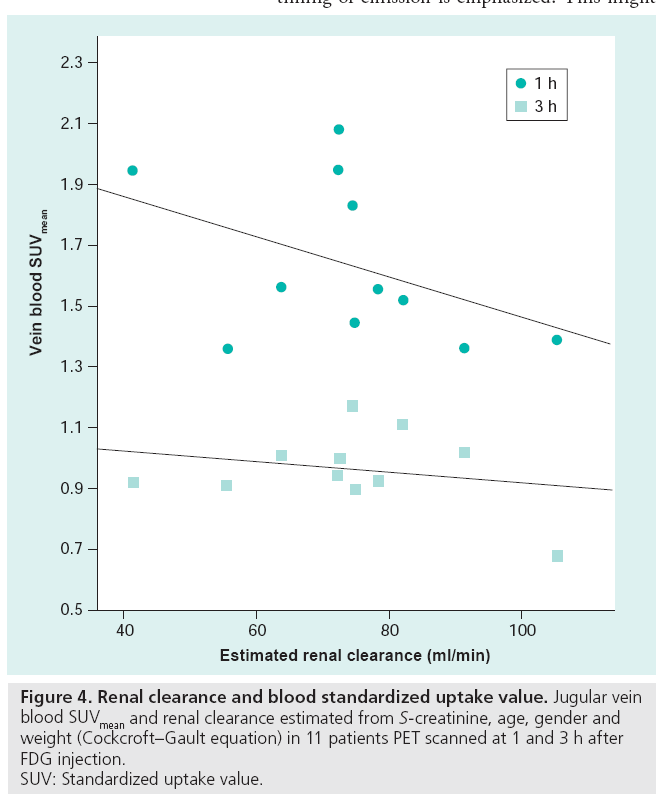
The TBR, using venous blood as a background, is highly reproducible and it is suggested that this quantification method is used in 90-min acquisitions for atherosclerosis therapy trials [90,91]. However, vein blood FDG content is a function of tissue metabolism, injected dose (decay corrected by using SUV) and renal clearance (Figure 4), and when an early 1-h acquisition is used, this quantification method is not recommended [81]. It can be speculated that a background with normal physiological uptake reflecting normal tissue consumption of glucose might be a better parameter than blood, used for normalizing the plaque uptake. A single study comparing plaque FDG uptake with histology has been conducted with early acquisition. Acquisitions were made 30–45 min after injection and a TBR was used to quantify tracer uptake. Instead of using the venous blood pool SUV as background, ‘normal’ arterial vessel wall FDG uptake was used, and the results showed a very close correlation between plaque CD68 staining and FDG uptake [73]. Being an inflammatory disease, atherosclerosis exhibits some degree of inflammation at all times and as overall arterial FDG uptake has been shown to be dynamic with repeated scans [69], this quantification method might be very prone to misinterpretation. Further studies using histological verification of different quantification methods at different acquisition times are warranted, and it is suggested that liver or lung tissue FDG uptake could be used as a reference in quantification using ratios, as these areas are consistent in FDG uptake over time [92].
The use of SUVmax is also highly reproducible [86,90], and using this method for specific plaque uptake analysis, an early acquisition protocol seems feasible [81]. This assumption is strengthened by the growing number of retrospective studies using SUV to show associations between overall cardiovascular risk and arterial wall FDG uptake [42,72,80,93,94]. We found that quantification of FDG uptake in late acquisitions using both TBR and SUVmax showed a close correlation to the key markers of plaque vulnerability. Thus, both methods can be used in late acquisitions, and in our set-up, the late acquisitions were used as ‘gold standards’. The interindividual relative uptake values were preserved using SUVmax, whereas TBR failed to conserve the individual findings over time. The increase seen over time in SUVmax values for some plaques was associated with high inflammatory activity and the results are comparable to timely changes observed in cancer lesions [92,95,96].
Clinical implementation
Several problems in assessing ultrasonographic plaque morphology have prevented this imaging method from reaching the level of clinical decision making. The 2D nature of the ultrasound image makes quantification of echolucent areas in the heterogeneous plaques difficult, and the shadowing from calcified portions of plaques hamper the investigation. Furthermore, it is difficult to report findings objectively [57,58]. At present, the method is not sensitive enough as a standalone investigation of vulnerability to complement the degree of stenosis in stratifying patients at high stroke-risk to endarterectomy. With the introduction of computer-assisted image normalization and grayscale pixel analyses (Figure 1), the reproducibility of ultrasound imaging has been greatly enhanced. Ongoing trials will determine risk of stroke assessed by novel dedicated software for plaque texture and echogenecity analyses, and incorporation of such software in the scanners for immediate diagnosis provision is anticipated, as it would ease the workflow in future clinical use [97,98].
Since the first observations of FDG uptake in atherosclerotic lesions, it has repeatedly been suggested that molecular imaging could provide a noninvasive measure of activity, hence risk of cardiovascular events, for individual plaques. As reflected by the overwhelming number of reviews suggestive of this implication (including the present article) compared with the lower number of prospective studies actually evaluating the possibility, the path from an apparent lifesaving idea to clinical everyday life seems long. Several obstacles in bringing FDG-PET studies of atherosclerosis from research to clinical trials, and eventually clinical use, are present. An often-met point of scepticism of PET is the apparent lack of availability of this imaging method. However, an estimated need for 38,000 scans in Denmark in 2010, predicted in a report from The National Board of Health, compared with the actual number of 1100 scans performed in 2002 in Denmark, reveals that the number of PET scans increases dramatically for oncology indications. Being the number one cause of mortality in industrialized countries, with cancer in second place, cardiovascular disease should be able to draw the same benefits of molecular imaging as oncology. The capacity of current PET centers is ever increasing, as is the number of new centers, and future studies evaluating the use of PET in atherosclerotic disease are anticipated. Another scepticism of PET is the nature of inflammatory activity in atherosclerosis; plaques are believed to exhibit some degree of inflammation at all times, and although some do become symptomatic, the vast majority of plaques will probably ‘burn out’, calcify by time, and only give rise to symptoms related to flow-limiting properties, if any. If this is true, only FDG-PET in patients with recent symptoms is of interest, as asymptomatic disease in theory at any given timepoint could be in the build-up or in the stabilizing phase of plaque evolution. Further studies are needed to elucidate the natural history of plaque evolution, and paradoxically, FDG-PET could be considered as the perfect tool for monitoring plaque inflammation over time in such studies.
Finally, the ease of the scanning procedure for the patient as well as the physician is often questioned. As described, this fear has been overcome in oncology, and considering the possible severity of cardiovascular disease outcome, we believe that the radiation burden of the imaging method is acceptable.
Future perspective
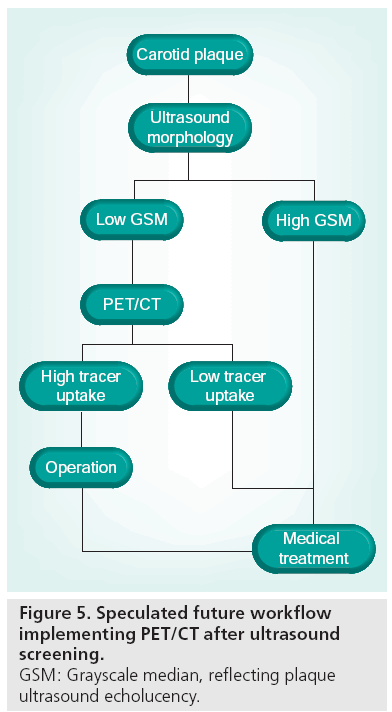
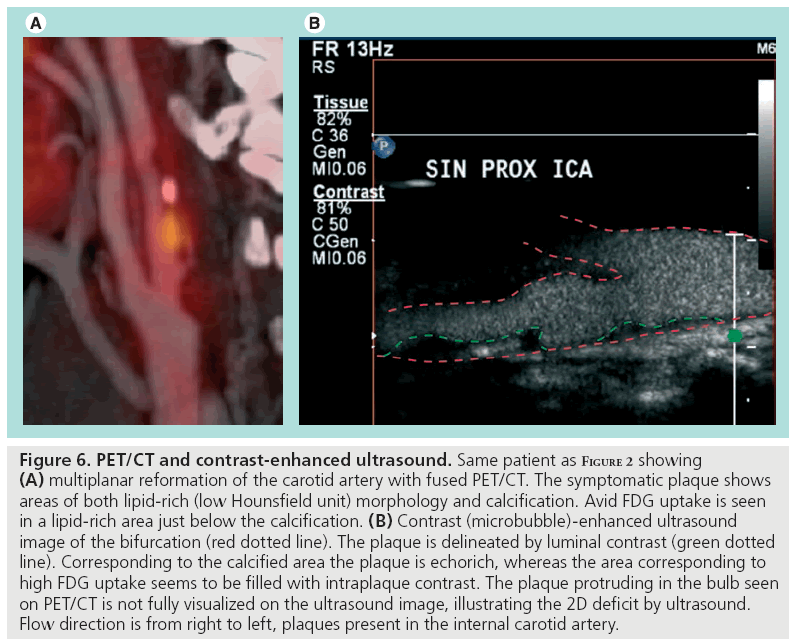
We have shown that echorich plaques tend to show low FDG uptake, whereas echolucent plaques exhibit a wide range of FDG uptake values [74]. Thus, although morphological traits of vulnerability are present, the plaque may or may not be metabolically active. In screening programs, plaque ultrasonographic morphology assessment could be used for stratification of patients eligible for further advanced imaging rather than for the final risk stratification. PET might be used in this context as a supplement to ultrasound later in the diagnostic hierarchy (Figure 5). A prospective follow-up study with PET/CT of patients with carotid artery stenosis would be of interest and initiation of population-based ultrasound screening of the carotids in elderly (currently not implemented in Denmark as asymptomatic patients are not offered surgery) would enhance the possibility of conducting such a study. We speculate that the clinical role of PET will be established within the next decade. However, we also acknowledge that technological development in the context of medical imaging is thriving, and that more sensitive or specific imaging modalities for detection of high-risk plaques might evolve during this decade. For example, imaging of plaque neovascularization seems promising. A single animal study has shown that intraplaque microvascularization and FDG uptake is positively correlated [99], and together with contrastenhanced ultrasound [48,100,101], this specific feature of vulnerable plaques might be targeted with high specificity using the two imaging modalities in conjunction (Figure 6). Development of realtime 3D ultrasound imaging and microbubble contrast-enhanced imaging might also improve morphological assessment of risk parameters. Even if FDG-PET is to be surpassed by more clever methods of implementing plaque inflammation and morphology in clinical risk assessment, we believe that the method in the next decade will be used as a research tool during the transition to less costly imaging modalities developed to become more specific in the identification of inflammation in vulnerable plaques.
Figure 6: PET/CT and contrast-enhanced ultrasound. Same patient as Figure 2 showing (A) multiplanar reformation of the carotid artery with fused PET/CT. The symptomatic plaque shows areas of both lipid-rich (low Hounsfield unit) morphology and calcification. Avid FDG uptake is seen in a lipid-rich area just below the calcification. (B) Contrast (microbubble)-enhanced ultrasound image of the bifurcation (red dotted line). The plaque is delineated by luminal contrast (green dotted line). Corresponding to the calcified area the plaque is echorich, whereas the area corresponding to high FDG uptake seems to be filled with intraplaque contrast. The plaque protruding in the bulb seen on PET/CT is not fully visualized on the ultrasound image, illustrating the 2D deficit by ultrasound. Flow direction is from right to left, plaques present in the internal carotid artery.
Conclusion
FDG-PET is a promising method for noninvasive characterization of high-risk atherosclerotic plaques. High plaque FDG uptake is associated with molecular markers of inflammation and vulnerability, and associations between ultrasonographic plaque echolucency, PET FDG uptake and histopathologic findings in surgically removed carotid plaques from patients with recent symptoms suggest a basis for further risk stratification improving patient selection for surgical intervention. Prospective protocols elucidating the role of FDG-PET and ultrasound are under way, and future randomized trials are needed to establish the clinical usability of PET/CT.
Financial & competing interests disclosure
Original research presented has been conducted with regional ethics review board approval and funding for the specific research has been obtained from The Danish Heart Foundation, The Research Fund of Rigshospitalet (Denmark) and the John and Birthe Meyer Foundation (Denmark). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- Lloyd-Jones D, Adams RJ, Brown TM et al.: Heart disease and stroke statistics – 2010 update: a report from the American Heart Association. Circulation 121(7), E46–E215 (2010).
- Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V: Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol. 8(4), 355–369 (2009).
- Mohr JP, Albers GW, Amarenco P et al.: American heart association prevention conference. IV. Prevention and rehabilitation of stroke. Etiology of stroke. Stroke 28(7), 1501–1506 (1997).
- Rothwell PM, Buchan A, Johnston SC: Recent advances in management of transient ischaemic attacks and minor ischaemic strokes. Lancet Neurol. 5(4), 323–331 (2006).
- Rothwell PM, Giles MF, Chandratheva A et al.: Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (express study): a prospective population-based sequential comparison. Lancet 370(9596), 1432–1442 (2007).
- Coull AJ, Lovett JK, Rothwell PM: Population based study of early risk of stroke after transient ischaemic attack or minor stroke: implications for public education and organisation of services. BMJ (Clin. Res. Ed.) 328(7435), 326 (2004).
- Fairhead JF, Mehta Z, Rothwell PM: Population-based study of delays in carotid imaging and surgery and the risk of recurrent stroke. Neurology 65(3), 371–375 (2005).
- Ois A, Gomis M, Rodriguez-Campello A et al.: Factors associated with a high risk of recurrence in patients with transient ischemic attack or minor stroke. Stroke 39(6), 1717–1721 (2008).
- Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. North American symptomatic carotid endarterectomy trial collaborators. N. Engl. J. Med. 325(7), 445–453 (1991).
- Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet 351(9113), 1379–1387 (1998).
- Nicolaides AN, Kakkos SK, Griffin M et al.: Effect of image normalization on carotid plaque classification and the risk of ipsilateral hemispheric ischemic events: results from the Asymptomatic Carotid Stenosis and Risk of Stroke study. Vascular 13(4), 211–221 (2005).
- Halliday A, Mansfield A, Marro J et al.: Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet 363(9420), 1491–1502 (2004).
- Spence JD, Tamayo A, Lownie SP, Ng WP, Ferguson GG: Absence of microemboli on transcranial doppler identifies low-risk patients with asymptomatic carotid stenosis. Stroke 36(11), 2373–2378 (2005).
- Marquardt L, Geraghty OC, Mehta Z, Rothwell PM: Low risk of ipsilateral stroke in patients with asymptomatic carotid stenosis on best medical treatment: a prospective, population-based study. Stroke 41(1), E11–E17 (2010).
- Rerkasem K, Rothwell PM: Temporal trends in the risks of stroke and death due to endarterectomy for symptomatic carotid stenosis: an updated systematic review. Eur. J. Vasc. Endovasc. Surg. 37(5), 504–511 (2009).
- Schneider PA, Naylor AR: Transatlantic debate. Asymptomatic carotid artery stenosis – medical therapy alone versus medical therapy plus carotid endarterectomy or stenting. Eur. J. Vasc. Endovasc. Surg. 40(2), 274–281 (2010).
- Liapis CD, Bell PR, Mikhailidis D et al.: ESVS guidelines. Invasive treatment for carotid stenosis: indications, techniques. Eur. J. Vasc. Endovasc. Surg. 37(4 Suppl.), 1–19 (2009).
- Ambrose JA, Tannenbaum MA, Alexopoulos D et al.: Angiographic progression of coronary artery disease and the development of myocardial infarction. J. Am. Coll. Cardiol. 12(1), 56–62 (1988).
- Boden WE: Interpreting the COURAGE trial. It takes courage to alter our belief system. Cleve. Clin. J. Med. 74(9), 623–625, 629–633 (2007).
- Katritsis DG, Ioannidis JP: Percutaneous coronary intervention versus conservative therapy in nonacute coronary artery disease: a meta-analysis. Circulation 111(22), 2906–2912 (2005).
- Kwok YS, Kim C, Heidenreich PA: Medical therapy or coronary artery bypass graft surgery for chronic stable angina: an update using decision analysis. Am. J. Med. 111(2), 89–95 (2001).
- Falk E, Shah PK, Fuster V: Coronary plaque disruption. Circulation 92(3), 657–671 (1995).
- Redgrave JN, Lovett JK, Gallagher PJ, Rothwell PM: Histological assessment of 526 symptomatic carotid plaques in relation to the nature and timing of ischemic symptoms: the Oxford plaque study. Circulation 113(19), 2320–2328 (2006).
- Spagnoli LG, Mauriello A, Sangiorgi G et al.: Extracranial thrombotically active carotid plaque as a risk factor for ischemic stroke. JAMA 292(15), 1845–1852 (2004).
- Saam T, Rominger A, Wolpers S et al.: Association of inflammation of the left anterior descending coronary artery with cardiovascular risk factors, plaque burden and pericardial fat volume: a PET/CT study. Eur. J. Nucl. Med. Mol. Imaging 37(6), 1203–1212 (2010).
- Rogers IS, Nasir K, Figueroa AL et al.: Feasibility of FDG imaging of the coronary arteries: comparison between acute coronary syndrome and stable angina. JACC Cardiovasc. Imaging 3(4), 388–397 (2010).
- Virmani R, Burke AP, Farb A, Kolodgie FD: Pathology of the vulnerable plaque. J. Am. Coll. Cardiol. 47(8 Suppl.), C13–C18 (2006).
- Graebe M, Pedersen SF, Borgwardt L, Højgaard L, Sillesen H, Kjaer A: Molecular pathology in vulnerable carotid plaques: correlation with [18]-fluorodeoxyglucose positron emission tomography (FDG-PET). Eur. J. Vasc. Endovasc. Surg. 37(6), 714–721 (2009).
- Chen J, Tung CH, Mahmood U et al.: In vivo imaging of proteolytic activity in atherosclerosis. Circulation 105(23), 2766–2771 (2002).
- Lutgens SPM, Cleutjens KBJM, Daemen MJAP, Heeneman S: Cathepsin cysteine proteases in cardiovascular disease. FASEB J. 21(12), 3029–3041 (2007).
- Pedersen SF, Graebe M, Fisker Hag AM, Højgaard L, Sillesen H, Kjaer A: Gene expression and 18FDG uptake in atherosclerotic carotid plaques. Nucl. Med. Commun. 31(5), 423–429 (2010).
- Holness CL, Simmons DL: Molecular cloning of CD68, a human macrophage marker related to lysosomal glycoproteins. Blood 81(6), 1607–1613 (1993).
- Gronholdt ML, Nordestgaard BG, Schroeder TV, Vorstrup S, Sillesen H: Ultrasonic echolucent carotid plaques predict future strokes. Circulation 104(1), 68–73 (2001).
- Eesa M, Hill MD, Al-Khathaami A et al.: Role of CT angiographic plaque morphologic characteristics in addition to stenosis in predicting the symptomatic side in carotid artery disease. AJNR Am. J. Neuroradiol. 31(7), 1254–1260 (2010).
- Hatsukami TS, Yuan C: MRI in the early identification and classification of high-risk atherosclerotic carotid plaques. Imaging Med. 2(1), 63–75 (2010).
- Boussel L, Arora S, Rapp J et al.: Atherosclerotic plaque progression in carotid arteries: monitoring with high-spatialresolution MR imaging – multicenter trial. Radiology 252(3), 789–796 (2009).
- Uehara M, Funabashi N, Mikami Y, Shiina Y, Nakamura K, Komuro I: Quantitative effect of atorvastatin on size and content of non-calcified plaques of coronary arteries 1 year after atorvastatin treatment by multislice computed tomography. Int. J. Cardiol. 130(2), 269–275 (2008).
- Makris GC, Lavida A, Nicolaides AN, Geroulakos G: The effect of statins on carotid plaque morphology: a LDL-associated action or one more pleiotropic effect of statins? Atherosclerosis 213(1), 8–20 (2010).
- Trivedi RA, Mallawarachi C, U-King-Im JM et al.: Identifying inflamed carotid plaques using in vivo USPIOenhanced MR imaging to label plaque macrophages. Arterioscler. Thromb. Vasc. Biol. 26(7), 1601–1606 (2006).
- Tawakol A, Migrino RQ, Bashian GG et al.: In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J. Am. Coll. Cardiol. 48(9), 1818–1824 (2006).
- Kerwin WS, O’Brien KD, Ferguson MS, Polissar N, Hatsukami TS, Yuan C: Inflammation in carotid atherosclerotic plaque: a dynamic contrast-enhanced MR imaging study. Radiology 241(2), 459–468 (2006).
- Tahara N, Kai H, Ishibashi M et al.: Simvastatin attenuates plaque inflammation: evaluation by fluorodeoxyglucose positron emission tomography. J. Am. Coll. Cardiol. 48(9), 1825–1831 (2006).
- Tang TY, Howarth SP, Miller SR et al.: The atheroma (Atorvastatin Therapy: Effects on Reduction of Macrophage Activity) study. Evaluation using ultrasmall superparamagnetic iron oxide-enhanced magnetic resonance imaging in carotid disease. J. Am. Coll. Cardiol. 53(22), 2039–2050 (2009).
- Polak JF: Carotid ultrasound. Radiol. Clin. North Am. 39(3), 569–589 (2001).
- Tahmasebpour HR, Buckley AR, Cooperberg PL, Fix CH: Sonographic examination of the carotid arteries. Radiographics 25(6), 1561–1575 (2005).
- Reilly LM, Lusby RJ, Hughes L, Ferrell LD, Stoney RJ, Ehrenfeld WK: Carotid plaque histology using real-time ultrasonography. Clinical and therapeutic implications. Am. J. Surg. 146(2), 188–193 (1983).
- Carotid artery plaque composition – relationship to clinical presentation and ultrasound b-mode imaging. European carotid plaque study group. Eur. J. Vasc. Endovasc. Surg. 10(1), 23–30 (1995).
- Aburahma AF, Kyer PD 3rd, Robinson PA, Hannay RS: The correlation of ultrasonic carotid plaque morphology and carotid plaque hemorrhage: clinical implications. Surgery 124(4), 721–726; discussion 726–728 (1998).
- Belcaro G, Laurora G, Cesarone MR et al.: Ultrasonic classification of carotid plaques causing less than 60% stenosis according to ultrasound morphology and events. J. Cardiovasc. Surg. (Torino) 34(4), 287–294 (1993).
- Gray-Weale AC, Graham JC, Burnett JR, Byrne K, Lusby RJ: Carotid artery atheroma: Comparison of preoperative B-mode ultrasound appearance with carotid endarterectomy specimen pathology. J. Cardiovasc. Surg. (Torino) 29(6), 676–681 (1988).
- Gronholdt ML, Nordestgaard BG, Bentzon J et al.: Macrophages are associated with lipid-rich carotid artery plaques, echolucency on b-mode imaging, and elevated plasma lipid levels. J. Vasc. Surg. 35(1), 137–145 (2002).
- El-Barghouty N, Geroulakos G, Nicolaides A, Androulakis A, Bahal V: Computer-assisted carotid plaque characterisation. Eur. J. Vasc. Endovasc. Surg. 9(4), 389–393 (1995).
- Mathiesen EB, Bonaa KH, Joakimsen O: Echolucent plaques are associated with high risk of ischemic cerebrovascular events in carotid stenosis: the Tromsø study. Circulation 103(17), 2171–2175 (2001).
- Russell DA, Wijeyaratne SM, Gough MJ: Changes in carotid plaque echomorphology with time since a neurologic event. J. Vasc. Surg. 45(2), 367–372 (2007).
- Sztajzel R: Ultrasonographic assessment of the morphological characteristics of the carotid plaque. Swiss Med. Wkly 135(43–44), 635–643 (2005).
- Tegos TJ, Sohail M, Sabetai MM et al.: Echomorphologic and histopathologic characteristics of unstable carotid plaques. AJNR Am. J. Neuroradiol. 21(10), 1937–1944 (2000).
- Sabetai MM, Tegos TJ, Nicolaides AN, Dhanjil S, Pare GJ, Stevens JM: Reproducibility of computer-quantified carotid plaque echogenicity: can we overcome the subjectivity? Stroke 31(9), 2189–2196 (2000).
- Fosse E, Johnsen SH, Stensland-Bugge E et al.: Repeated visual and computer-assisted carotid plaque characterization in a longitudinal population-based ultrasound study: the Tromsø study. Ultrasound Med. Biol. 32(1), 3–11 (2006).
- Grogan JK, Shaalan WE, Cheng H et al.: B-mode ultrasonographic characterization of carotid atherosclerotic plaques in symptomatic and asymptomatic patients. J. Vasc. Surg. 42(3), 435–441 (2005).
- Nighoghossian N, Derex L, Douek P: The vulnerable carotid artery plaque: current imaging methods and new perspectives. Stroke 36(12), 2764–2772 (2005).
- Rudd JH, Davies JR, Weissberg PL: Imaging of atherosclerosis – can we predict plaque rupture? Trends Cardiovasc. Med. 15(1), 17–24 (2005).
- Hanif MZ, Ghesani M, Shah AA, Kasai T: F-18 fluorodeoxyglucose uptake in atherosclerotic plaque in the mediastinum mimicking malignancy: another potential for error. Clin. Nucl. Med. 29(2), 93–95 (2004).
- Vesselle HJ, Miraldi FD: FDG PET of the retroperitoneum: normal anatomy, variants, pathologic conditions, and strategies to avoid diagnostic pitfalls. Radiographics 18(4), 805–823; discussion 823–804 (1998).
- Yun M, Yeh D, Araujo LI, Jang S, Newberg A, Alavi A: F-18 FDG uptake in the large arteries: a new observation. Clin. Nucl. Med. 26(4), 314–319 (2001).
- Rudd JHF, Warburton EA, Fryer TD et al.: Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation 105(23), 2708–2711 (2002).
- Arauz A, Hoyos L, Zenteno M, Mendoza R, Alexanderson E: Carotid plaque inflammation detected by 18F-fluorodeoxyglucose-positron emission tomography. Pilot study. Clin. Neurol. Neurosurg. 109(5), 409–412 (2007).
- Davies JR, Rudd JH, Fryer TD et al.: Identification of culprit lesions after transient ischemic attack by combined 18F fluorodeoxyglucose positron-emission tomography and high-resolution magnetic resonance imaging. Stroke 36(12), 2642–2647 (2005).
- Basu S, Zhuang H, Alavi A: Imaging of lower extremity artery atherosclerosis in diabetic foot: FDG-PET imaging and histopathological correlates. Clin. Nucl. Med. 32(7), 567–568 (2007).
- Ben-Haim S, Kupzov E, Tamir A, Frenkel A, Israel O: Changing patterns of abnormal vascular wall F-18 fluorodeoxyglucose uptake on follow-up PET/CT studies. J. Nucl. Cardiol. 13(6), 791–800 (2006).
- Ben-Haim S, Kupzov E, Tamir A, Israel O: Evaluation of 18F-FDG uptake and arterial wall calcifications using 18F-FDG PET/CT. J. Nucl. Med. 45(11), 1816–1821 (2004).
- Bural GG, Torigian DA, Chamroonrat W et al.: Quantitative assessment of the atherosclerotic burden of the aorta by combined FDG-PET and CT image analysis: a new concept. Nucl. Med. Biol. 33(8), 1037–1043 (2006).
- Bural GG, Torigian DA, Chamroonrat W et al.: FDG-PET is an effective imaging modality to detect and quantify age-related atherosclerosis in large arteries. Eur. J. Nucl. Med. Mol. Imaging 35(3), 562–569 (2007).
- Font MA, Fernandez A, Carvajal A et al.: Imaging of early inflammation in low-to-moderate carotid stenosis by 18-FDG-PET. Front. Biosci. 14, 3352–3360 (2009).
- Graebe M, Pedersen SF, Højgaard L, Kjaer A, Sillesen H: 18FDG PET and ultrasound echolucency in carotid artery plaques. JACC Cardiovasc. Imaging 3(3), 289–295 (2010).
- Schulthess GKV: Clinical Molecular Anatomic Imaging PET, PET/CT, and SPECT/CT. (Rev.). Lippincott Williams & Wilkins, Philadelphia, PA, USA (2003).
- Breyholz HJ, Wagner S, Faust A et al.: Radiofluorinated pyrimidine-2,4,6-triones as molecular probes for noninvasive MMPtargeted imaging. ChemMedChem 5(5), 777–789 (2010).
- Liu Y, Abendschein D, Woodard GE et al.: Molecular imaging of atherosclerotic plaque with (64)CU-labeled natriuretic peptide and pet. J. Nucl. Med. 51(1), 85–91 (2010).
- Nahrendorf M, Keliher E, Panizzi P et al.: 18F-4v for PET-CT imaging of VCAM-1 expression in atherosclerosis. JACC Cardiovasc. Imaging 2(10), 1213–1222 (2009).
- Levin CS, Hoffman EJ: Calculation of positron range and its effect on the fundamental limit of positron emission tomography system spatial resolution. Phys. Med. Biol. 44(3), 781–799 (1999).
- Wu YW, Kao HL, Chen MF et al.: Characterization of plaques using 18F-FDG PET/CT in patients with carotid atherosclerosis and correlation with matrix metalloproteinase-1. J. Nucl. Med. 48(2), 227–233 (2007).
- Graebe M, Borgwardt L, Højgaard L, Sillesen H, Kjaer A: When to image carotid plaque inflammation with FDG PET/CT. Nucl. Med. Commun. 31(9), 773–779 (2010).
- Berthelsen AK, Holm S, Loft A, Klausen TL, Andersen F, Højgaard L: PET/CT with intravenous contrast can be used for PET attenuation correction in cancer patients. Eur. J. Nucl. Med. Mol. Imaging 32(10), 1167–1175 (2005).
- Mehta AS, Mehta A, Laymon C, Blodgett T: Calcified lymph nodes causing clinically relevant attenuation correction artifacts on PET/CT imaging. J. Radiol. Case Reports 4(2), 31–37 (2010).
- Laurberg JM, Olsen AK, Hansen SB et al.: Imaging of vulnerable atherosclerotic plaques with FDG-micropet: no FDG accumulation. Atherosclerosis 192(2), 275–282 (2007).
- Paidisetty S, Blodgett TM: Brown fat: atypical locations and appearances encountered in PET/CT. AJR Am. J. Roentgenol. 193(2), 359–366 (2009).
- Silvera SS, Aidi HE, Rudd JH et al.: Multimodality imaging of atherosclerotic plaque activity and composition using FDG-PET/CT and MRI in carotid and femoral arteries. Atherosclerosis 207(1), 139–143 (2009).
- UNSCEAR 2000. The United Nations Scientific Committee on the Effects of Atomic Radiation. Health Phys. 79(3), 314 (2000).
- Herzog BA, Wyss CA, Husmann L et al.: First head-to-head comparison of effective radiation dose from low-dose 64-slice CT with prospective ECG-triggering versus invasive coronary angiography. Heart 95(20), 1656–1661 (2009).
- Bagshaw M: Cosmic radiation in commercial aviation. Travel Med. Infect. Dis. 6(3), 125–127 (2008).
- Izquierdo-Garcia D, Davies JR, Graves MJ et al.: Comparison of methods for magnetic resonance-guided [18-F]fluorodeoxyglucose positron emission tomography in human carotid arteries: reproducibility, partial volume correction, and correlation between methods. Stroke 40(1), 86–93 (2009).
- Rudd JH, Myers KS, Bansilal S et al.: 18Fluorodeoxyglucose positron emission tomography imaging of atherosclerotic plaque inflammation is highly reproducible: implications for atherosclerosis therapy trials. J. Am. Coll. Cardiol. 50(9), 892–896 (2007).
- Chin BB, Green ED, Turkington TG, Hawk TC, Coleman RE: Increasing uptake time in FDG-PET: standardized uptake values in normal tissues at 1 versus 3 h. Mol. Imaging Biol. 11(2), 118–122 (2009).
- Paulmier B, Duet M, Khayat R et al.: Arterial wall uptake of fluorodeoxyglucose on PET imaging in stable cancer disease patients indicates higher risk for cardiovascular events. J. Nucl. Cardiol. 15(2), 209–217 (2008).
- Rominger A, Saam T, Wolpers S et al.: 18F-FDG PET/CT identifies patients at risk for future vascular events in an otherwise asymptomatic cohort with neoplastic disease. J. Nucl. Med. 50(10), 1611–1620 (2009).
- Hustinx R, Smith RJ, Benard F et al.: Dual time point fluorine-18 fluorodeoxyglucose positron emission tomography: a potential method to differentiate malignancy from inflammation and normal tissue in the head and neck. Eur J. Nucl. Med. 26(10), 1345–1348 (1999).
- Kim DW, Jung SA, Kim CG, Park SA: The efficacy of dual time point F-18 FDG PET imaging for grading of brain tumors. Clin. Nucl. Med. 35(6), 400–403 (2010).
- Muntendam P, Mccall C, Sanz J, Falk E, Fuster V: The Bioimage study: novel approaches to risk assessment in the primary prevention of atherosclerotic cardiovascular disease – study design and objectives. Am. Heart J. 160(1), 49–57 E41 (2010).
- Kakkos SK, Stevens JM, Nicolaides AN et al.: Texture analysis of ultrasonic images of symptomatic carotid plaques can identify those plaques associated with ipsilateral embolic brain infarction. Eur. J. Vasc. Endovasc. Surg. 33(4), 422–429 (2007).
- Calcagno C, Cornily JC, Hyafil F et al.: Detection of neovessels in atherosclerotic plaques of rabbits using dynamic contrast enhanced MRI and 18F-FDG PET. Arterioscler. Thromb. Vasc. Biol. 28(7), 1311–1317 (2008).
- Ten Kate GL, Sijbrands EJ, Valkema R et al.: Molecular imaging of inflammation and intraplaque vasa vasorum: a step forward to identification of vulnerable plaques? J. Nucl. Cardiol. 17(5), 897–912 (2010).
- Staub D, Patel MB, Tibrewala A et al.: Vasa vasorum and plaque neovascularization on contrast-enhanced carotid ultrasound imaging correlates with cardiovascular disease and past cardiovascular events. Stroke 41(1), 41–47 (2010).
• • Mandatory reading for the understanding of the concept of the vulnerable plaque.
• • First to describe feasibility of using FDG-PET in coronary plaque inflammation assessment.
• Update on the concept of the vulnerable plaque. Compare with [22].
• • First to show association between plaque gene expression of vulnerability markers and FDG uptake.
• • Promising results for using ultrasonographic plaque morphology assessment in detection of high-risk plaques.
• • First to provide histopathologic evidence of the association between plaque inflammation and FDG uptake.
• Illustrate a possible role for PET in future cardiovascular studies: monitoring of drug efficacy on atherosclerosis.
• • High significance for current hypotheses regarding usability of FDG-PET for detecting culprit atherosclerotic lesions.
• • Describes how FDG-PET might be used to identify culprit lesions not associated to the carotid artery.
• Association between plaque morphology and molecular biology combined.
• Reproducibility study of carotid plaque FDG uptake with promising results for clinical implementation.
• Ongoing trial. Results expected to further elucidate the role of PET and ultrasound for risk assessment.
• Ongoing trial. Results expected to further elucidate the role of ultrasound for risk assessment.