Review Article - Interventional Cardiology (2009) Volume 1, Issue 1
Catheter ablation for the treatment of persistent atrial fibrillation
- Corresponding Author:
- Sébastien Knecht
Service de Cardiologie, CHU Brugmann, Place Van Gehuchten, 4, 1020 Bruxelles, Belgique
Tel: +32 2 477 26 79
Fax: +32 2 477 26 79
E-mails: sebastien.knecht@ chu-brugmann.be
Abstract
Keywords
atrial fibrillation, catheter ablation, complex fractionated atrial electrogram, linear lesion, persistent, pulmonary vein
Atrial fibrillation (AF) is the most frequent human arrhythmia. Its prevalence of 2% in the general population reaches 5.9% in people over 65 years of age, and approximately 10% in those more than 80 years old [1]. AF is associated with an increased risk of all-cause mortality, heart failure and stroke [1,2] and it is also responsible for approximately a third of all hospitalizations with cardiac rhythm disturbance [3]. As a consequence, AF constitutes a major socioeconomic and healthcare problem.
Although no difference in mortality has been proven using antiarrhythmic medication [4], a rhythm- or a rate-control strategy have both still to be envisaged based on patient’s symptoms [5]. If a rhythm-control strategy is preferred, the first step still consists of trying at least one antiarrhythmic drug [3]. Nevertheless, different studies have clearly shown the superiority of catheter ablation compared with pharmacological therapy for reducing AF burden [6,7], although mortality trials have not yet been performed. Therefore, an invasive ablative strategy has to be envisaged if anti-arrhythmic medications fail, which is a relatively frequent occurance, especially in the context of persistent AF following cardioversion [8].
This review will focus on the understanding of the mechanisms that have led to the birth of catheter ablation for AF, and in particular persistent AF. We will also review the techniques, results, consequences and risks of this emerging therapy.
Electrophysiological mechanisms of AF based on early ablative experiences
In the 1990s, early attempts at curing AF with catheter ablation by a percutaneous approach were inspired by the surgical Maze technique and its subsequent modifications [9]. These early attempts were based upon the ‘multiple wavelet’ hypothesis, proposed by Moe [10], with contributory experimental work by Allessie [11]. The hypothesis was that by compartmentalizing the atria with the linear lesions, the critical mass of atrial tissue would be reduced so that re-entrant wavelets could not exist. Schwartz was the first to try to replicate bi-atrial surgical linear approaches, with a high procedural success rate but at the cost of unacceptable complications [12]. Other authors reported inefficacy of linear lesions applied in the right atrium (RA) that were similar to those performed during surgical procedures [13]. Those poor results emphasize that linear lesions are only one of several elements of the puzzle.
In the late 1990s, Haïssaguerre et al. demonstrated the pivotal role of the pulmonary veins (PVs) in triggering paroxysmal AF [14], which resulted in attempts at treating focal sources instead of compartmentalizing the atria [15]. By mapping the atria it was seen that paroxysmal AF was triggered by ectopic beats originating from the PVs, and that by electrically isolating the PVs, AF was eliminated [16]. Other reports also demonstrated the importance of the PVs for AF perpetuation through automatic or re-entrant mechanisms [17,18]. A ‘venous wave hypothesis’ has therefore been proposed as the main electrophysiological mechanism of paroxysmal AF, implicating the PVs as the exclusive sources of ‘venous waves/drivers’ maintaining the atria in fibrillation [19]. For persistent AF, sources outside the PVs have also been evidenced [20–22].
Catheter ablation of persistent AF: importance of a stepwise & multifaceted approach
During persistent AF, catheter ablation progressively targets all structures potentially contributing to initiation and maintenance of AF: the PVs, left atria (LA) tissue, linear ablation of LA roof and mitral isthmus, and RA. Each region is ablated following a sequential approach until AF termination; the impact of ablation is assessed by measurement of AF cycle length (AFCL) in both appendages. Each step is accompanied by an increase in AFCL until conversion of AF directly to sinus rhythm or more often to multiple atrial tachycardias (ATs) that are then systematically ablated [23–25].
This sequential approach has resulted in unprecedented success in maintaining sinus rhythm in the medium term with recovery of atrial mechanical function in patients with longstanding persistent AF [26]. Termination of AF occurs in 82 to 87% [23,27], with 95% of the patients in sinus rhythm at 1 year [24] and 90% after more than 2 years [25]; however, a second procedure is needed in approximately 50% of the patients, mainly for AT [25].
Crucial importance of the AFCL
The AFCL can be reliably monitored during the procedure by averaging 30 consecutive cycles at the left and right atrial appendages, which display unambiguous high voltage and reproducible electrograms [28]. Various early studies have shown that AFCL correlates with the local refractory period, that it shortens in parallel with the duration of AF and that drugs may affect it [29,30]. However, AFCL prolongation during ablation at remote sites [31] is evidence that it is not only due to the local refractory period. In fact, a study based upon advanced computer simulation showed that the AFCL as measured in the LA appendage represents the sum of all fibrillatory activities converging to this area [28]. The higher the number of elements participating in the AF process, the shorter the AFCL and the more complex the ablation.
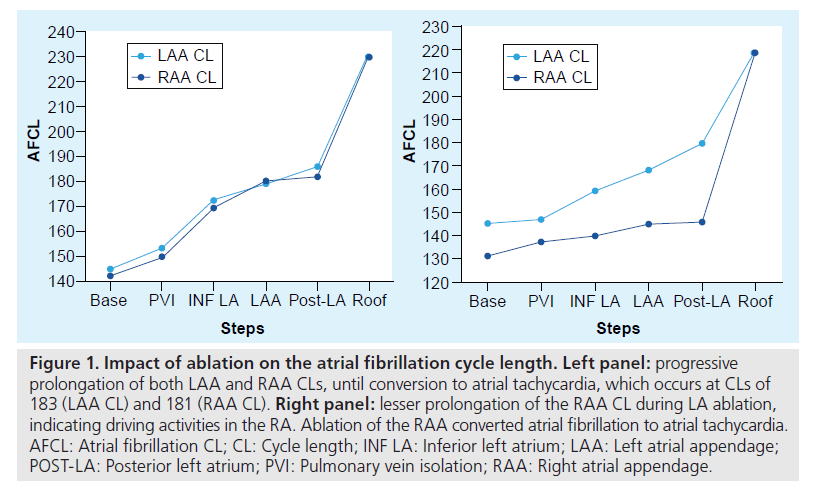
The impact of ablation of each region can therefore be followed and estimated by monitoring the AFCL (Figure 1). After each step of ablation, a gradual prolongation of the AFCL is observed [23]. Conversion to sinus rhythm or atrial tachycardia usually occurs when the AFCL reaches 180 and 200 ms in patients off drugs. If AF persists during ablation of the LA despite a prolonged LA appendage cycle length, a lesser prolongation of the RA appendage cycle length would suggest that the RA may contain elements that participate in the AF process [28].
Figure 1: Impact of ablation on the atrial fibrillation cycle length. Left panel: progressive prolongation of both LAA and RAA CLs, until conversion to atrial tachycardia, which occurs at CLs of 183 (LAA CL) and 181 (RAA CL). Right panel: lesser prolongation of the RAA CL during LA ablation, indicating driving activities in the RA. Ablation of the RAA converted atrial fibrillation to atrial tachycardia. AFCL: Atrial fibrillation CL; CL: Cycle length; INF LA: Inferior left atrium; LAA: Left atrial appendage; POST-LA: Posterior left atrium; PVI: Pulmonary vein isolation; RAA: Right atrial appendage.
Importantly, the surface ECG AFCL (manually measured by making a mean from ten unambiguous fibrillatory waves on lead V1) has also been shown to be a clinically useful pre-ablation tool [32]. Indeed, among 90 patients ablated for persistent AF, the surface ECG AFCL was the only independent predictor of AF termination (p < 0.01) and predicted clinical success of persistent AF ablation. The statistical cut-off as determined by the ROC curve was calculated at 142 ms [32].
Pulmonary vein isolation
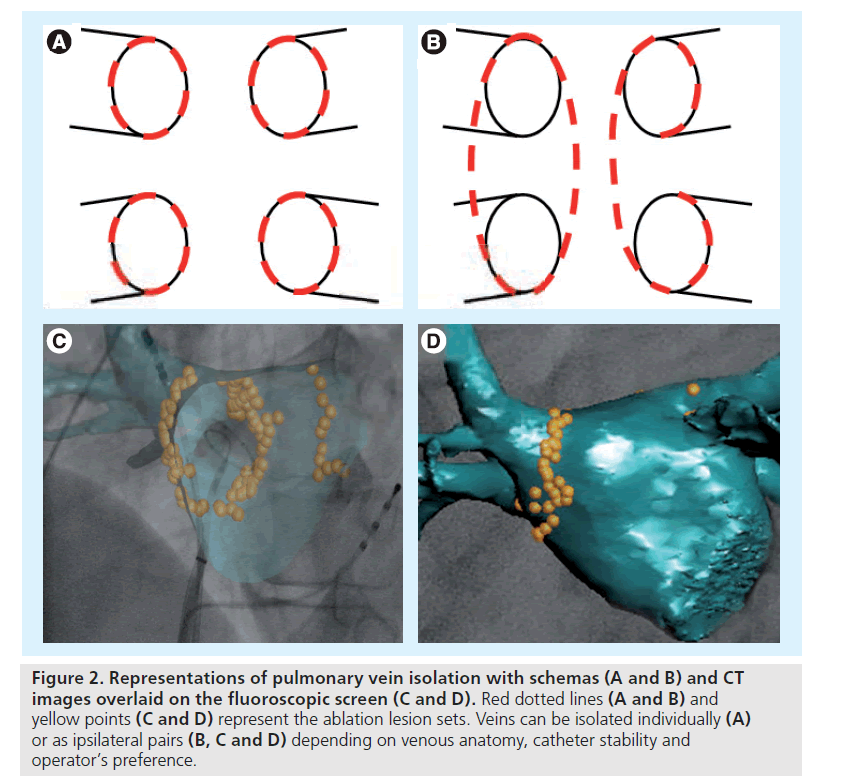
Pulmonary vein isolation (either antral, ostial or circumferential) invariably results in a better clinical prognosis in patients with paroxysmal as compared with persistent AF [23,33–36]. Despite these poor results when used as a standalone strategy, PV isolation is still performed as the initial ablation step in all patients with persistent AF, because absence of PV isolation can lead to arrhythmia recurrence due to triggering foci [37]. A circumferential catheter is used to map and guide ablation of the PVs, which can be isolated individually or as ipsilateral pairs depending on venous anatomy, issues with catheter stability and the operator’s preference (Figure 2). In all cases, ablation has to be performed at least 0.5 to 1 cm apart from the PV ostia to avoid the risk of PV stenosis. For all veins, isolation is assessed by either electrical elimination or dissociation of the PV potentials [38].
Figure 2: Representations of pulmonary vein isolation with schemas (A and B) and CT images overlaid on the fluoroscopic screen (C and D). Red dotted lines (A and B) and yellow points (C and D) represent the ablation lesion sets. Veins can be isolated individually (A) or as ipsilateral pairs (B, C and D) depending on venous anatomy, catheter stability and operator’s preference.
Complex fractionated atrial electrograms
▪ Electrophysiological mechanisms
Complex fractionated atrial electrograms (CFAE) are defined as electrograms displaying more than two deflections that are fractionated or have a short cycle length (<120 ms), in its maximal form giving continuous electrical activity. The mechanisms underlying such fractionated potentials are still disputed.
Important progress has been made since the first report from Cosio describing fragmentation in zones of slow interatrial conduction produced by extrastimulation in patients with AF [39]. Jaïs et al. proposed that CFAE represents the ultimate degree of temporal asynchrony and demonstrated a heterogeneous distribution during paroxysmal AF, mostly in the posterior LA and septum [40]. Following this, Konings et al. showed that fractionation may be caused by asynchronous activation of local muscle bundles, due to tissue anisotropy and the presence of insulating collagenous septa between atrial muscle bundles [41]. From these studies, fractionation may represent zones of colliding wavefronts or pivoting points between different wavelets participating in the AF process. These areas of slow conduction could shorten the wavelength of the wandering wavelets, thereby increasing the number that can coexist in the atria and the complexity of AF. Rostock et al. reported that occurrence of CFAE was associated with prior acceleration of the AFCL and that the duration of CFAE was inversely correlated with the preceding AFCL [42]. Consequently, a given region may harbor apparently normal potentials during slow AFCL episodes, while fractionation may be observed after acceleration of the AFCL. Kalifa et al. analyzed the relationship between local frequency, AF wave propagation and electrogram fractionation during sustained AF in the posterior LA of the isolated sheep heart [43]. They showed that sites where most fractionation occurs were located at the margin of the more rapid areas. Fractionation would arise from slow conduction at the outer limit of the region displaying the higher frequency and the most regular activity.
All of the above studies reinforce the concept that fractionation/CFAE is a manifestation of either active re-entrant mechanisms, or passive slow conduction with a tight functional relationship with local cycle length. Therefore, location of CFAE may sometimes not represent a critical region of AF perpetuation but a consequence of close faster activity.
On the other hand, the autonomic nervous system is also thought to be implicated in the mechanism of fractionation, by release of acetylcholine from the ganglionated plexi, which results in a shortening of the action potential and effective refractory period [44–46]. Acetylcholine administration has been shown to be capable of inducing AF and a spatial correlation between ganglionated plexi and CFAE localization has been observed [46,47].
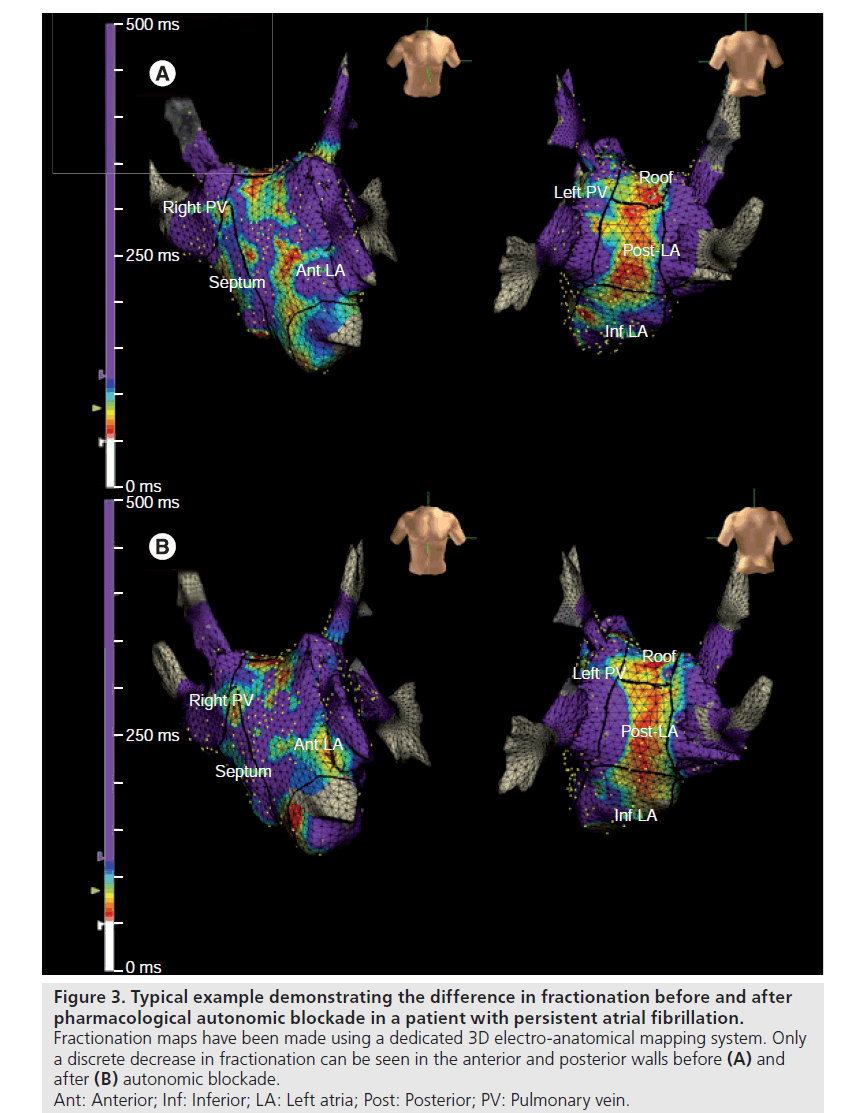
We have evaluated the impact of pharmacological autonomic blockade on CFAE [Sébastien Knecht, Unpublished Data]. Autonomic blockade was achieved with intravenous injection of propanolol and atropine sulphate in 29 consecutive patients during AF. 3D maps of the fractionation degree were made before and after autonomic blockade using the Ensite Navx® system (Figure 3). We showed that CFAE as a proportion of all atrial electrogram samples were indeed significantly reduced after autonomic blockade, but only for paroxysmal AF (and not persistent AF). Furthermore, fractionation only decreased in patients with a significant prolongation of the AFCL, suggesting that the effect on CFAE is mediated by a prolongation of the AFCL.
Figure 3: Typical example demonstrating the difference in fractionation before and after pharmacological autonomic blockade in a patient with persistent atrial fibrillation. Fractionation maps have been made using a dedicated 3D electro-anatomical mapping system. Only a discrete decrease in fractionation can be seen in the anterior and posterior walls before (A) and after (B) autonomic blockade. Ant: Anterior; Inf: Inferior; LA: Left atria; Post: Posterior; PV: Pulmonary vein.
Clinical results
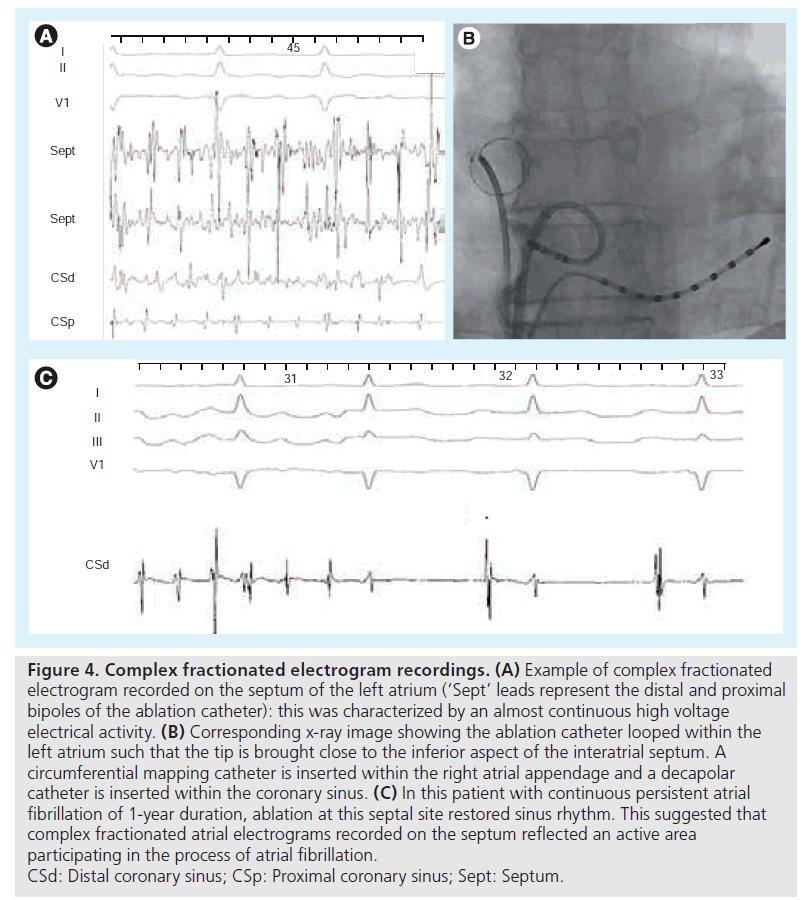
Nademanee was the first to exclusively target CFAE in both atria in patients with paroxysmal and persistent AF [48]. He reported maintenance of sinus rhythm of 91% at 1 year, with an average of 1.2 procedures per patient, with most patients being treated with antiarrhytmic drugs. On the other hand, another group reported only modest short-term efficacy (57% persistent AF patients) with ablation of persistent AF only guided by CFAE [49]. In this latter study, a significant number of patients developed AT, finally necessitating PV isolation or LA linear lesions to be controlled. Results from other groups also confirmed that the addition of CFAE ablation to PV isolation provides an increased clinical success rate (see example in Figure 4) [50], but at the cost of numerous subsequent iatrogenic ATs [23,24,27].
Figure 4: Complex fractionated electrogram recordings. (A) Example of complex fractionated electrogram recorded on the septum of the left atrium (‘Sept’ leads represent the distal and proximal bipoles of the ablation catheter): this was characterized by an almost continuous high voltage electrical activity. (B) Corresponding x-ray image showing the ablation catheter looped within the left atrium such that the tip is brought close to the inferior aspect of the interatrial septum. A circumferential mapping catheter is inserted within the right atrial appendage and a decapolar catheter is inserted within the coronary sinus. (C) In this patient with continuous persistent atrial fibrillation of 1-year duration, ablation at this septal site restored sinus rhythm. This suggested that complex fractionated atrial electrograms recorded on the septum reflected an active area participating in the process of atrial fibrillation. CSd: Distal coronary sinus; CSp: Proximal coronary sinus; Sept: Septum.
▪ Linear lesions
The most common LA linear lesions consist of the roof line that connects the two superior PVs [51] and the mitral line that joins the mitral annulus to the PV either anteriorly or laterally [52,53]. The observed therapeutic efficacy of these linear lesions drawn during AF may be related to interruption of wavelet and macro-re-entrant tachycardias, alteration of autonomic innervation, atrial debulking or an effect on local complex electrograms.
A recent study highlights that although PV isolation and electrogram-based ablation without linear lesions may be effective for terminating persistent AF in a significant number of patients, macro-re-entrant AT requiring LA linear ablation is very likely to occur during the overall followup period [27]. In this study, 96% of the patients ultimately required a roofline and 86% a mitral line after a mean follow-up of 2 years, despite attempts to avoid LA linear lesions. These data suggest that at least the roofline (which is safer compared with the mitral isthmus line) could be used in the case of AF persistence after PV isolation and CFAE ablation. This study also confirmed the high risk of AT recurrence in cases of incomplete conduction block at LA lines.
▪ Right atrium
Early work by several groups investigated the utility of RA linear lesions, with or without additional LA linear lesions with only modest success in patients with either paroxysmal and persistent AF [9,13]. This does not mean, however, that the RA does not contribute to AF. There is accumulating evidence that in a subset of patients, possibly up to 20% of patients with long-lasting persistent AF, the right atrium plays a vital role in the perpetuation of AF [54].
AT: the hidden menace
In the stepwise approach the end point of ablation is restoration of sinus rhythm with confirmation of PV isolation and electrically confirmed block of any linear lesion performed; however, sinus rhythm is rarely restored directly and in more than 70% of patients, AF terminates by conversion to AT [23]. Those may also appear late after the healing process of ablation [24,27]. They are multiple in number and mechanisms and add significantly to the complexity of ablation. They are considered as the last step of persistent and long-standing AF ablation (during the initial procedure or during follow-up), and results of their mapping and ablation will achieve either subsequent success or failure of the procedure for patients. Mechanisms of AT after an AF ablation varies with the ablation approach. While focal origins from reconnected PVs are more usual using segmental PV isolation [37,55], macro-re-entrant mechanisms are more frequent after an anatomical approach [56,57], and ‘small circuits’ (corresponding to localized re-entries) are common after a stepwise approach [58].
Although 3D electroanatomical mapping systems may assist in mapping ATs, using these technologies are often impractical because of AT instability or multiple ATs that each require mapping. For this reason, a deductive diagnostic electrophysiological approach has recently been validated [59]; this prospective study also high-lighted the dominant role of localized re-entry as a novel mechanism of AT.
Other strategies & procedural end points for persistent AF ablation
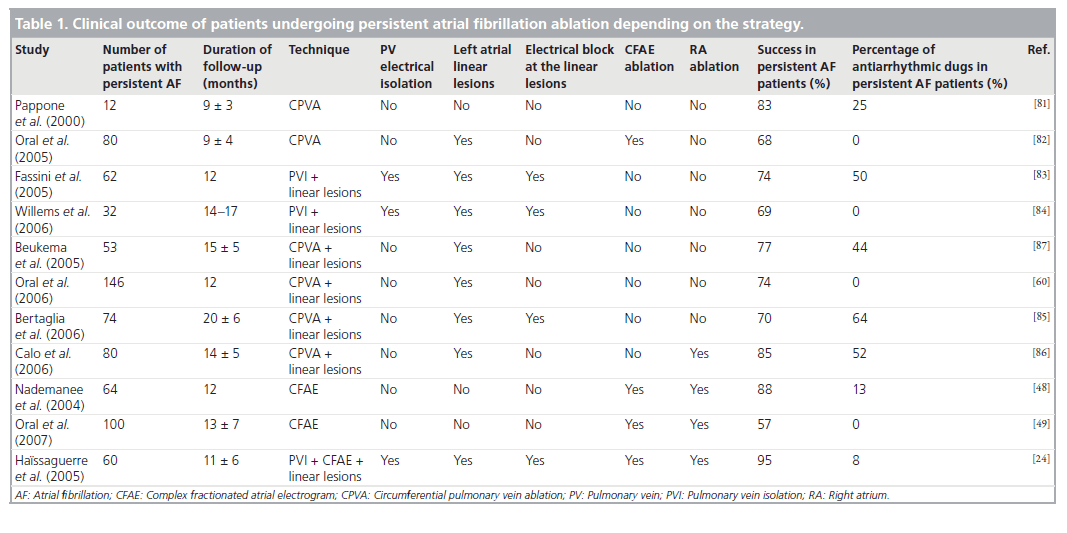
Different investigators have evaluated other strategies and results for ablation of persistent AF. These studies have been summarized i▪ Table 1. Overall, the proposed techniques also include PV isolation (more or less proximally), CFAE ablation, linear lesions, ablation at the RA or combined techniques. More importantly, procedural end points may differ depending on the operators. In addition, it is important to emphasize that while for CFAE ablation and ablation at the RA there is no clear technical end point during ablation, electrical isolation of the PVs as well as bidirectional electrical conduction block at linear lesions are essential for the global success of the procedure [8,27,37,55].
Table 1: Clinical outcome of patients undergoing persistent atrial fibrillation ablation depending on the strategy.
Concerning the procedural end point of the ablation procedure itself, some authors prefer to perform a standardized lesion set; however, it appears that AF termination by catheter ablation is associated with the best clinical outcome [25]. Therefore, the question can be raised whether all patients with persistent AF should be induced at the beginning of the procedure or not. Indeed, AF inducibility at the end of the procedure has a low specificity and is not indicated for persistent AF [60]; however, if the procedural end point is to terminate AF, then the arrhythmia should be induced before starting ablation in case of the presence of sinus rhythm, although this requires further prospective trials.
Predictors of success
When AF can be terminated by catheter ablation (without drug or external cardioversion), only 5% of these patients will recur [25]; however almost 50% will have AT recurrence, which is often more symptomatic than AF [24,25]. Thankfully, in experienced centers, catheter ablation of subsequent ATs is associated with a high success rate [59]. Other important predictors of success have been reported by investigators: pre-existing LA scarring [61], voltage abatement [62], the percentage of the LA ablated, or vagal denervation [63] or electrical block at linear lesions [27].
More practically, the main predictors of success before the ablation procedure are the following: AFCL [28], duration of continuous AF [25,27,64], history of hypertension and LA dimensions [65].
We have evaluated the clinical predictors of success in 90 consecutive patients with persistent AF followed over 2 years [32]. The duration of AF, LA dimensions, presence of structural heart disease and the surface ECG AF cycle length were assessed prior to ablation and analyzed with respect to long-term clinical outcome. The surface ECG AFCL was manually measured from ten unambiguous fibrillatory waves on lead V1 (minimal voltage >0.01 mV) that were not fused with QRST segments at a paper speed of 50 mm/s and a gain setting of 20, 40 or 80 mm/mV. Long-term maintenance of sinus rhythm was associated with a shorter duration of continuous AF (p < 0.0001), a longer surface ECG AFCL (p < 0.001) and a smaller LA (p < 0.05) compared with those with recurrent arrhythmia. In multivariate analysis the surface ECG AFCL and the AF duration predicted clinical success of persistent AF ablation (p < 0.01 and p < 0.05, respectively). Furthermore, using a ROC curve, the optimal cut-off for the AFCL as a predictor of AF termination was 142 msec with a specificity and sensitivity of 92.9 and 69.7%, respectively. On the other hand, the optimal cut-off point for the duration of continuous AF was 21 months for AF termination (specificity 92.9% and sensitivity 61.8%). The combined cut-off using a surface ECG AFCL was greater than 142 msec and a duration of continuous AF of less than 21 months had 100.0% specificity in predicting procedural termination of AF (sensitivity: 39.5%; positive predictive value: 100.0%; negative predictive value: 23.3%).
Related complications & potential benefits
The most frequent complications related to catheter ablation of persistent AF occur in 1–2% of patients and mainly include stroke, which is fortunately rare, and pericardial tamponade [66]. Stroke or transient ischemic attacks are mainly the consequences of thrombi adherent to catheters and sheaths, endocardial disruption from the ablation lesions and air passing through trans-septal sheaths. Anticoagulation is used to prevent thrombi forming, but this increases the risk of pericardial tamponade due to ooze from the ablated tissue. Other possible complications are PV stenosis, which has dramatically reduced with a more proximal ablation strategy compared with the initial reports of isolation within the vein [67], atrio–esophageal fistula (exceedingly rare but almost always fatal) [68] and phrenic nerve paralysis, which patients almost always recover from [69].
On the other hand, catheter ablation of persistent AF has shown promising results concerning improved morbidity [70,71] and quality of life [72,73] (especially in patients with pre-existing heart failure [70,74]), but also potential benefits in term of mortality [71,75]. A nonrandomized study comparing catheter ablation versus medical therapy patients showed that patients treated with catheter ablation were approximately half as likely to die during the follow-up period and half as likely to have a stroke or other major adverse cardiovascular event as those treated medically [71]. Another study showed that, after catheter ablation of symptomatic persistent AF in high-risk patients, patients remaining in sinus rhythm had a significant benefit in terms of mortality compared with patients recurring AF [75].
Indications for persistent AF ablation
The latest guidelines published by the American College of Cardiology (ACC), the American Heart Association (AHA), and the European Society of Cardiology (ESC) societies have recommended not to differentiate between patients on the basis of the duration of AF [8]. Briefly, patients are considered for ablation in cases of symptomatic recurrent AF despite failure of at least one anti-arrhythmic drug, electrical cardioversion or both.
Importantly, as mentioned earlier, one has to consider that catheter ablation for patients with a very long duration persistent AF (especially >5 years), a very short cycle length on a 12-lead ECG, and extremely dilated LA have a very poor chance of clinical success.
Of note, data have suggested that patients with heart failure (NYHA II or more) or evidence of left ventricular dysfunction without an alternative explanation have the most to gain from catheter ablation [70], even if the success rate is lower compared with patients with normal left ventricular function. It has been suggested that asymptomatic patients with AF-related thromboembolism should be treated with catheter ablation [76], even though there have been no trials that have reported a reduction in events post ablation, due to the large number of patients that would need to be recruited. Therefore the ACC/AHA/ESC recommendations for anticoagulation remain the same following catheter ablation [8].
Future perspective
In order to make progress in our understanding of the mechanisms underlying persistent AF, improved mapping tools are required to allow the identification of the precise electro-physiological substrate. Indeed, although the presence of CFAE could indicate the most favorable sites, there is currently no accurate mapping technology that has been shown to be clinically effective in differentiating active from passive areas of activation. There have been some investigations about analysis of the dominant frequency, to try and determine the areas with the highest frequency of activation; however, these have been disappointing for persistent AF [77].
There is room for improvement in ablation technology. There are a number of ‘single shot’ catheters that have been developed using a variety of energy sources: for example, radiofrequency [78], high-intensity focused ultrasound [79] and cryothermal balloons [80]. Currently, only limited data are available on the efficacy and safety profile of such catheters. One major limitation of such technology is the variation in pulmonary venous anatomy. Furthermore, these catheters are very specific to pulmonary vein isolation; and although this could be appropriate for most paroxysmal AF patients, the need for several different catheters is mandatory in persistent AF, thereby increasing the cost and limiting their applicability.
Conclusion
Since the initial report that AF was triggered from ectopic beats within the PVs, we have learnt a tremendous amount about the underlying physiopathology of AF. Catheter ablation of persistent AF is undergoing a massive expansion because of very promising results, and persistent AF patients can now look forward to a cure for their arrhythmia and the morbidity and mortality risk that this brings. Although catheter ablation of persistent AF is a difficult procedure, advances in catheter design and identification of areas that actively participate in the AF process should increase the number of patients that can receive treatment.
Executive summary
▪ Atrial fibrillation (AF) is the most frequent human arrhythmia and is associated with an increased risk of all-cause mortality, heart failure and stroke. Many studies have clearly shown the superiority of catheter ablation compared with pharmacological therapy in reducing AF burden; however, no randomized controlled trials have yet been performed to assess whether this translates into a reduction in all-cause mortality.
▪ During persistent AF, catheter ablation progressively targets all structures, potentially contributing to initiation and maintenance of AF: the pulmonary veins, left atria (LA) tissue, linear ablation of the LA roof and mitral isthmus, and the right atrium.
▪ Each region is ablated following a sequential approach until AF termination, and the impact of ablation is assessed by measurement of AF cycle length in both appendages.
▪ This stepwise and multifaceted approach is associated with the best reported success rate, with more than 90% of patients maintaining stable sinus rhythm after more than 2 years of follow-up.
▪ Importantly, following an initial ablation for AF a second procedure is often needed for atrial tachycardia.
▪ The main predictors of success before the ablation procedure are a long AF cycle length (>142 ms as measured on the 12-lead ECG in V1) and a short duration of continuous AF (<21 months).
▪ Although some developments are still required to improve the ablation technology and electrophysiological mapping, AF ablation is now included in the international guidelines as a reasonable alternative to pharmacological therapy to prevent recurrent AF in symptomatic patients.
Financial & competing interests disclosure
Sébastien Knecht has served on the advisory board of St Jude Medical and has received lecture fees from Biosense-Webster, Philips Medical Systems and St Jude Medical. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D: Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation 98(10), 946–952 (1998).
- Stewart S, Hart C, Hole D, McMurray J: A population-based study of the long-term risks associated with atrial fibrillation: 20‑year follow-up of the Renfrew/Paisley study. Am. J. Med. 113(5), 432–435 (2002).
- Fuster V, Ryden LE, Cannom DS et al.: ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committeeto Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation), developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation 114(7), E257–E354 (2006).
- Van Gelder IC, Hagens VE, Bosker HA et al.: A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N. Engl. J. Med. 347(23), 1834–1840 (2002).
- Hagens VE, Ranchor AV, Van Sonderen E et al.: Effect of rate or rhythm control on quality of life in persistent atrial fibrillation. Results from the Rate Control Versus Electrical Cardioversion (RACE) study. J. Am. Coll. Cardiol. 43(2), 241–247 (2004).
- Pappone C, Augello G, Sala S et al.: A randomized trial of circumferential pulmonary vein ablation versus antiarrhythmic drug therapy in paroxysmal atrial fibrillation: the APAF Study. J. Am. Coll. Cardiol. 48(11), 2340–2347 (2006).
- Jais P, Cauchemez B, Macle L et al.: Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 Study. Circulation 118(24), 2498–2505 (2008).
- Calkins H, Brugada J, Packer DL et al.: HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: Recommendations for Personnel, Policy, Procedures and Follow-Up: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA) and the European Cardiac Arrhythmia Society (ECAS); in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), and the Society of Thoracic Surgeons (STS). Endorsed and Approved by the governing bodies of the American College of Cardiology, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, and the Heart Rhythm Society. Heart Rhythm 4(6), 816–861 (2007).
- Haissaguerre M, Jais P, Shah DC et al.: Right and left atrial radiofrequency catheter therapy of paroxysmal atrial fibrillation. J. Cardiovasc. Electrophysiol. 7(12), 1132–1144 (1996).
- Moe G, Abildskov J: Atrial fibrillation as a self-sustained arrhythmia independant of focal discharge. Am. J. Med. 113, 359–364 (1959).
- Allessie M, Lammers W, Bonke F, Hollen L: Experimental evaluation of Moe’s multiple wavelet hypothesis of atrial fibrillation. In: Cardiac Electrophysiology and Arrhythmias. Zipes D, Jalife J (Eds). Grune & Stratton, New York, NY, USA, 265–275 (1985).
- Schwartz J, Pellersels G, Silvers J: A catheter-based curative approach to atrial fibrillation in humans. Circulation 90, I-335 (1993).
- Jais P, Shah DC, Takahashi A, Hocini M, Haissaguerre M, Clementy J: Long-term follow-up after right atrial radiofrequency catheter treatment of paroxysmal atrial fibrillation. Pacing Clin. Electrophysiol. 21(11 Pt 2), 2533–2538 (1998).
- Haissaguerre M, Jais P, Shah DC et al.: Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N. Engl. J. Med. 339(10), 659–666 (1998).
- Haissaguerre M, Jais P, Shah DC et al.: Catheter ablation of chronic atrial fibrillation targeting the reinitiating triggers. J. Cardiovasc. Electrophysiol. 11(1), 2–10 (2000).
- Haissaguerre M, Jais P, Shah DC et al.: Electrophysiological end point for catheter ablation of atrial fibrillation initiated from multiple pulmonary venous foci. Circulation 101(12), 1409–1417 (2000).
- Jais P, Hocini M, Macle L, Choi KJ et al.: Distinctive electrophysiological properties of pulmonary veins in patients with atrial fibrillation. Circulation 106(19), 2479–2485 (2002).
- Hocini M, Ho SY, Kawara T et al.: Electrical conduction in canine pulmonary veins: electrophysiological and anatomic correlation. Circulation 105(20), 2442–2448 (2002).
- Haissaguerre M, Sanders P, Hocini M, Jais P, Clementy J: Pulmonary veins in the substrate for atrial fibrillation: the ‘venous wave’ hypothesis. J. Am. Coll. Cardiol. 43(12), 2290–2292 (2004).
- Knecht S, O’Neill MD, Matsuo S et al.: Focal arrhythmia confined within the coronary sinus and maintaining atrial fibrillation. J. Cardiovasc. Electrophysiol. 18(11), 1140–1146 (2007).
- Haissaguerre M, Hocini M, Sanders P et al.: Localized sources maintaining atrial fibrillation organized by prior ablation. Circulation 113(5), 616–625 (2006).
- Rostock T, Rotter M, Sanders P et al.: Fibrillating areas isolated within the left atrium after radiofrequency linear catheter ablation. J. Cardiovasc. Electrophysiol. 17(8), 807–812 (2006).
- Haissaguerre M, Sanders P, Hocini M et al.: Catheter ablation of long-lasting persistent atrial fibrillation: critical structures for termination. J. Cardiovasc. Electrophysiol. 16(11), 1125–1137 (2005).
- Haissaguerre M, Hocini M, Sanders P et al.: Catheter ablation of long-lasting persistent atrial fibrillation: clinical outcome and mechanisms of subsequent arrhythmias. J. Cardiovasc. Electrophysiol. 16(11), 1138–1147 (2005).
- O’Neill MD, Wright M, Knecht S et al.: Long-term follow-up of persistent atrial fibrillation ablation using termination as a procedural endpoint. Eur. Heart J. 30(9), 1105–1112 (2009).
- Takahashi Y, O’Neill MD, Hocini M et al.: Effects of stepwise ablation of chronic atrial fibrillation on atrial electrical and mechanical properties. J. Am. Coll. Cardiol. 49(12), 1306–1314 (2007).
- Knecht S, Hocini M, Wright M et al.: Left atrial linear lesions are required for successful treatment of persistent atrial fibrillation. Eur. Heart J. 29, 2359–2366 (2008).
- Haissaguerre M, Lim K-T, Jacquemet V et al.: Atrial fibrillatory cycle length: computer simulation and potential clinical importance. Europace 9(Suppl. 6), vi64–vi70 (2007).
- Kim K-B, Rodefeld MD, Schuessler RB, Cox JL, Boineau JP: Relationship between local atrial fibrillation interval and refractory period in the isolated canine atrium. Circulation 94(11), 2961–2967 (1996).
- Wang Z, Page P, Nattel S: Mechanism of flecainide’s antiarrhythmic action in experimental atrial fibrillation. Circ. Res. 71(2), 271–287 (1992).
- Haissaguerre M, Sanders P, Hocini M et al.: Changes in atrial fibrillation cycle length and inducibility during catheter ablation and their relation to outcome. Circulation 7, 7 (2004).
- Matsuo S, Lellouche N, Wright M et al.: Clinical predictors of termination and clinical outcome of catheter ablation for persistent atrial fibrillation. J. Am. Coll. Cardiol. 54, 788–795 (2009).
- Ouyang F, Bansch D, Ernst S et al.: Complete isolation of left atrium surrounding the pulmonary veins: new insights from the double-Lasso technique in paroxysmal atrial fibrillation. Circulation 110(15), 2090–2096 (2004).
- Pappone C, Radinovic A, Manguso F et al.: Atrial fibrillation progression and management: a 5‑year prospective follow-up study. Heart Rhythm 5(11), 1501–1507 (2008).
- Marrouche NF, Martin DO, Wazni O et al.: Phased-array intracardiac echocardiography monitoring during pulmonary vein isolation in patients with atrial fibrillation: impact on outcome and complications. Circulation 107(21), 2710–2716 (2003).
- Kanagaratnam L, Tomassoni G, Schweikert R et al.: Empirical pulmonary vein isolation in patients with chronic atrial fibrillation using a three-dimensional nonfluoroscopic mapping system: long-term follow-up. PACE 24(12), 1774–1779 (2001).
- Gerstenfeld EP, Callans DJ, Dixit S et al.: Mechanisms of organized left atrial tachycardias occurring after pulmonary vein isolation. Circulation 110(11), 1351–1357 (2004).
- Haissaguerre M, Shah DC, Jais P et al.: Electrophysiological breakthroughs from the left atrium to the pulmonary veins. Circulation 102(20), 2463–2465 (2000).
- Cosio F, Palacios J, Vidal J, Cocina E, Gomez-Sanchez M, Tamargo L: Electrophysiologic studies in atrial fibrillation: slow conduction of premature impulses: a possible manifestation of the background for reentry. Am. J. Cardiol. 51(1), 122–130 (1983).
- Jais P, Haissaguerre M, Shah DC, Chouairi S, Clementy J: Regional disparities of endocardial atrial activation in paroxysmal atrial fibrillation. Pacing Clin. Electrophysiol. 19(11 Pt 2), 1998–2003 (1996).
- Konings KTS, Smeets JLRM, Penn OC, Wellens HJJ, Allessie MA: Configuration of unipolar atrial electrograms during electrically induced atrial fibrillation in humans. Circulation 95(5), 1231–1241 (1997).
- Rostock T, Rotter M, Sanders P et al.: High-density activation mapping of fractionated electrograms in the atria of patients with paroxysmal atrial fibrillation. Heart Rhythm 3(1), 27–34 (2006).
- Kalifa J, Tanaka K, Zaitsev AV et al.: Mechanisms of wave fractionation at boundaries of high-frequency excitation in the posterior left atrium of the isolated sheep heart during atrial fibrillation. Circulation 113(5), 626–633 (2006).
- Schauerte P, Scherlag BJ, Pitha J et al.: Catheter ablation of cardiac autonomic nerves for prevention of vagal atrial fibrillation. Circulation 102(22), 2774–2780 (2000).
- Lemery R, Birnie D, Tang ASL, Green M, Gollob M: Feasibility study of endocardial mapping of ganglionated plexuses during catheter ablation of atrial fibrillation. Heart Rhythm 3(4), 387–396 (2006).
- Scherlag BJ, Yamanashi W, Patel U, Lazzara R, Jackman WM: Autonomically induced conversion of pulmonary vein focal firing into atrial fibrillation. J. Am. Coll. Cardiol. 45(11), 1878–1886 (2005).
- Sharifov OF, Zaitsev AV, Rosenshtraukh LV et al.: Spatial distribution and frequency dependence of arrhythmogenic vagal effects in canine atria. J. Cardiovasc. Electrophysiol. 11(9), 1029–1042 (2000).
- Nademanee K, McKenzie J, Kosar E et al.: A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J. Am. Coll. Cardiol. 43(11),2044–2053 (2004).
- Oral H, Chugh A, Good E et al.: Radiofrequency catheter ablation of chronic atrial fibrillation guided by complex electrograms.Circulation 115(20), 2606–2612 (2007).
- Verma A, Novak P, Macle L et al.: A prospective, multicenter evaluation of ablating complex fractionated electrograms (CFEs) during atrial fibrillation (AF) identified by an automated mapping algorithm: acute effects on AF and efficacy as an adjuvant strategy. Heart Rhythm 5(2),198–205 (2008).
- Hocini M, Jais P, Sanders P et al.: Techniques, evaluation, and consequences of linear block at the left atrial roof in paroxysmal atrial fibrillation: a prospective randomized study. Circulation 112(24),3688–3696 (2005).
- Jais P, Hocini M, Hsu LF et al.: Technique and results of linear ablation at the mitral isthmus. Circulation 110(19), 2996–3002(2004).
- Sanders P, Jais P, Hocini M et al.: Electrophysiologic and clinical consequences of linear catheter ablation to transect the anterior left atrium in patients with atrial fibrillation.Heart Rhythm 1(2), 176–184 (2004).
- Wright M, Haissaguerre M, Knecht S et al.: State of the art: catheter ablation of atrial fibrillation. J. Cardiovasc. Electrophysiol.19(6), 583–592 (2008).
- Ouyang F, Antz M, Ernst S et al.: Recovered pulmonary vein conduction as a dominant factor for recurrent atrial tachyarrhythmias after complete circular isolation of the pulmonary veins: lessons from double Lassotechnique. Circulation 111(2), 127–135 (2005).
- Mesas CE, Pappone C, Lang CC et al.: Left atrial tachycardia after circumferential pulmonary vein ablation for atrial fibrillation: electroanatomic characterization and treatment. J. Am. Coll. Cardiol. 44(5),1071–1079 (2004).
- Pappone C, Manguso F, Vicedomini G et al.: Prevention of iatrogenic atrial tachycardia after ablation of atrial fibrillation: a prospective randomized study comparing circumferential pulmonary vein ablation with a modified approach. Circulation 110(19),3036–3042 (2004).
- Deisenhofer I, Estner H, Zrenner B et al.: Left atrial tachycardia after circumferential pulmonary vein ablation for atrial fibrillation: incidence, electrophysiological characteristics, and results of radiofrequency ablation.Europace 8(8), 573–582 (2006).
- Jaïs P, Matsuo S, Knecht S et al.: A deductive mapping strategy for atrial tachycardia following atrial fibrillation ablation: importance of localized reentry. J. Cardiovasc.Electrophysiol. 20(5), 480–491 (2009).
- Oral H, Pappone C, Chugh A et al.: Circumferential pulmonary-vein ablation for chronic atrial fibrillation. N. Engl. J. Med.354(9), 934–941 (2006).
- Verma A, Wazni OM, Marrouche NF et al.: Pre-existent left atrial scarring in patients undergoing pulmonary vein antrum isolation: an independent predictor of procedural failure. J. Am. Coll. Cardiol. 45(2), 285–292(2005).
- Pappone C, Oreto G, Rosanio S et al.: Atrial electroanatomic remodeling after circumferential radiofrequency pulmonary vein ablation: efficacy of an anatomic approach in a large cohort of patients with atrial fibrillation. Circulation 104(21),2539–2544 (2001).
- Pappone C, Santinelli V, Manguso F et al.: Pulmonary vein denervation enhances long-term benefit after circumferential ablation for paroxysmal atrial fibrillation.Circulation 109(3), 327–334 (2004).
- Matsuo S, Lim K-T, Haissaguerre M: Ablation of chronic atrial fibrillation. HeartRhythm 4(11), 1461–1463 (2007).
- Berruezo A, Tamborero D, Mont L et al.: Pre-procedural predictors of atrial fibrillation recurrence after circumferential pulmonary vein ablation. Eur. Heart J. 28(7), 836–841(2007).
- Cappato R, Calkins H, Chen SA et al.: Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation 111(9),1100–1105 (2005).
- Packer DL, Keelan P, Munger TM et al.: Clinical presentation, investigation, and management of pulmonary vein stenosis complicating ablation for atrial fibrillation.Circulation 111(5), 546–554 (2005).
- Pappone C, Oral H, Santinelli V et al.: Atrio-esophageal fistula as a complication of percutaneous transcatheter ablation of atrial fibrillation. Circulation 109(22), 2724–2726(2004).
- Sacher F, Monahan KH, Thomas SP et al.: Phrenic nerve injury after atrial fibrillation catheter ablation: characterization and outcome in a multicenter study. J. Am. Coll.Cardiol. 47(12), 2498–2503 (2006).
- Hsu LF, Jais P, Sanders P et al.: Catheter ablation for atrial fibrillation in congestive heart failure. N. Engl. J. Med. 351(23),2373–2383 (2004).
- Pappone C, Rosanio S, Augello G et al.: Mortality, morbidity, and quality of life after circumferential pulmonary vein ablation for atrial fibrillation: outcomes from a controlled nonrandomized long-term study. J. Am. Coll.Cardiol. 42(2), 185–197 (2003).
- Pürerfellner H, Martinek M, Aichinger J, Nesser HJ, Kempen K, Janssen JPG: Quality of life restored to normal in patients with atrial fibrillation after pulmonary vein ostial isolation. Am. Heart J. 148(2), 318–325(2004).
- Weerasooriya R, Jaïs P, Hocini M et al.: Effect of catheter ablation on quality of life of patients with paroxysmal atrial fibrillation. Heart Rhythm 2(6), 619–623(2005).
- Khan MN, Jais P, Cummings J et al.: Pulmonary-vein isolation for atrial fibrillation in patients with heart failure. N. Engl. J. Med.359(17), 1778–1785 (2008).
- Nademanee K, Schwab MC, Kosar EM et al.: Clinical outcomes of catheter substrate ablation for high-risk patients with atrial fibrillation. J. Am. Coll. Cardiol. 51(8),843–849 (2008).
- Oral H, Chugh A, Ozaydin M et al.: Risk of thromboembolic events after percutaneous left atrial radiofrequency ablation of atrialfibrillation. Circulation 114(8), 759–765 (2006).
- Sanders P, Berenfeld O, Hocini M et al.: Spectral analysis identifies sites of highfrequency activity maintaining atrial fibrillation in humans. Circulation 112(6),789–797 (2005).
- Meissner A, Plehn G, Bracht MV et al.: First Experiences for pulmonary vein isolation with the high-density mesh ablator (HDMA), a novel mesh electrode catheter for both mapping and radiofrequency delivery in a single unit. J. Cardiovasc. Electrophysiol.20(4), 359–366 (2009).
- Nakagawa H, Antz M, Wong T et al.: Initial experience using a forward directed, high-intensity focused ultrasound balloon catheter for pulmonary vein antrum isolation in patients with atrial fibrillation. J. Cardiovasc. Electrophysiol. 18(2), 136–144(2007).
- Sarabanda AV, Bunch TJ, Johnson SB et al.: Efficacy and safety of circumferential pulmonary vein isolation using a novel cryothermal balloon ablation system. J. Am. Coll. Cardiol. 46(10), 1902–1912(2005).
- Pappone C, Rosanio S, Oreto G et al.: Circumferential radiofrequency ablation of pulmonary vein ostia: a new anatomic approach for curing atrial fibrillation. Circulation 102(21), 2619–2628(2000).
- Oral H, Chugh A, Good E et al.: Randomized comparison of encircling and nonencircling left atrial ablation for chronic atrial fibrillation. Heart Rhythm 2(11),1165–1172 (2005).
- Fassini G, Riva S, Chiodelli R et al.: Left mitral isthmus ablation associated with PV Isolation: long-term results of a prospective randomized study. J. Cardiovasc. Electrophysiol. 16(11),1150–1156 (2005).
- Willems S, Klemm H, Rostock T et al.: Substrate modification combined with pulmonary vein isolation improves outcome of catheter ablation in patients with persistent atrial fibrillation: a prospective randomized comparison. Eur. Heart J. 27(23), 2871–2878(2006).
- Bertaglia E, Stabile G, Senatore G et al.: Long-term outcome of right and left atrial radiofrequency ablation in patients with persistent atrial fibrillation. Pacing Clin.Electrophysiol. 29(2), 153–158 (2006).
- Calò L, Lamberti F, Loricchio ML et al.: Left atrial ablation versus biatrial ablation for persistent and permanent atrial fibrillation: a prospective and randomized study. J. Am. Coll. Cardiol. 47(12),2504–2512 (2006).
- Beukema WP, Elvan A, Sie HT, Misier AR, Wellens HJ. Successful radiofrequency ablation in patients with previous atrial fibrillation results in a significant decrease in left atrial size. Circulation 112(14),2089–2095 (2005).
▪▪ Guidelines for the management of patients with atrial fibrillation (AF).
▪▪ Key paper for the clinical management of AF.
▪▪ Guidelines for catheter and surgical ablation of AF.
▪▪ Key paper for persistent AF ablation.
▪ Key paper for the role of linear lesions for persistent AF ablation.
▪ Key paper for the role of complex fractionated atrial electrograms in AF ablation.
▪ Key paper for AF ablation in the context of heart failure.