Review Article - Interventional Cardiology (2011) Volume 3, Issue 4
Catheter ablation of atrial fibrillation: an update for 2011
- Corresponding Author:
- Erik Wissner
Department of Cardiology, AsklepiosKlinik St. Georg
Lohmuehlenstrasse 5, 20099 Hamburg, Germany
Tel: +49 401 818 858 844
Fax: +49 401 818 854 435
E-mail: e.wissner@asklepios.com
Abstract
Keywords
atrial fibrillation, catheter ablation, contact force, longstanding persistent, magnetic navigation, magnetic resonance, paroxysmal, remotecontrolled
Since the first report on the pivotal role of the pulmonary veins (PVs) in the initiation of atrial fibrillation (AF), catheter ablation for the treatment of AF has undergone major refinements [1]. If the PVs are targeted for ablation, PV isolation (PVI) marks the cornerstone of an ablative strategy as set forth by the international consensus document on catheter ablation for AF [2]. Although longterm results in patients with paroxysmal AF are encouraging, outcome data in patients with persistent or longstanding persistent AF highlight the need for improvement. Alternative energy sources and new technologies under development may overcome current limitations in transmural lesion formation, heralding a new era in catheter ablation of AF. This article will focus on recent developments and new technologies currently under investigation.
Current status of catheter ablation for atrial fibrillation
Several smaller trials suggest superiority of catheter ablation over drug-based therapy in maintaining freedom from AF, while a meta-analysis on antiarrhythmic drug use versus catheter ablation concluded that an interventional approach demonstrated higher eff icacy and lower rates of complications [3–6]. The ongoing RAAFT 2 (First Line Radiofrequency Ablation versus Antiarrhythmic Drugs for Atrial Fibrillation Treatment [101]) trial compares catheter ablation with antiarrhythmic medication as first-line treatment, enrolling 400 patients in a randomized fashion. To date it is unknown whether catheter ablation decreases mortality. An observational study comparing patients with AF with a case-matched cohort of patients without AF demonstrated that mortality was similar between the catheter ablation group and those without AF [7]. However, mortality was significantly increased in patients with AF not undergoing catheter ablation at 1- and 3-year follow-up. The randomized, multicenter CABANA (Catheter Ablation versus Antiarrhythmic Drug Therapy for Atrial Fibrillation [102]) trial will prospectively test the hypothesis of whether PVI is superior to pharmacological therapy in lowering mortality. The CABANA trial will enroll 3000 patients followed ≥2 years. First results are expected in 2015.
▪ Paroxysmal atrial fibrillation
Recently, data on 5-year follow-up results became available reporting on the outcome of patients undergoing ablation for paroxysmal and persistent or longstanding persistent AF [8–10] . Analyzing the publication by Ouyang et al. three critical messages need to be appreciated. First, a median of one (range 1–3) procedure was necessary to achieve stable sinus rhythm (SR) in 79.5% of patients, while an additional 13% reported improvement in clinical symptoms. Second, contrary to previous reports on the short- and mid-term results of PVI, demonstrating plateauing in the incidence of AF recurrence over time, the follow-up results reported by Ouyang et al. demonstrate a continuous rate of recurrence up to 60 months following the procedure [8]. Third, analyzing the natural course of AF, there is a steady rate of transition from paroxysmal to persistent AF of approximately 15% per year [11]. Ouyang et al. reported only four out of 161 (1.2%) patients progressing to chronic AF over a 5-year follow-up period [8]. Haissaguerre et al. published their 5-year follow-up data on patients undergoing catheter ablation of AF [10]. More than a third of enrolled patients suffered from persistent or longstanding persistent AF. The fairly high number of patients with advanced AF may explain the low single-procedure success rate. After 1, 2 and 5 years of follow-up, only 40, 37 and 29% of patients were free from recurrent arrhythmias, respectively. After a median of two procedures per patient, the arrhythmiafree survival rate increased to 87, 81 and 63% at 1, 2 and 5 years. Subgroup analysis revealed that patients with longstanding persistent AF were almost twice as likely to experience arrhythmia recurrence than patients with paroxysmal or persistent AF. Hence, in view of the sobering data on the results of catheter ablation in patients in the advanced stages of the disease, a preferred strategy may be an early ablative approach to prevent transition into chronic forms of AF. Furthermore, continuation of proper anticoagulation in patients with a higher CHADS2-VASc score is of particular importance, owing to the steady rate of late AF recurrences noted during longterm follow-up.
▪ Longstanding persistent atrial fibrillation
To date, it is unclear which strategy serves best the patient with longstanding persistent AF defined as continuous AF lasting more than 12 months. Complex fractionated atrial electrograms (CFAE) are thought to play an important role in the maintenance of AF. Nademanee et al. reported high procedural success rate when solely targeting CFAE for the treatment of AF, while a similar study by Oral et al. could not corroborate these findings [12,13]. Three meta-analyses concluded that CFAE ablation provided additional benefit to PV ablation alone in patients with persistent AF [14–16]. However, interpretation of results should be carefully weighed owing to: various definitions being used to characterize CFAE; different end points of procedural success; and differences in performing PV ablation. Furthermore, targeting CFAE will result in longer procedure and fluoroscopy times, as well as a higher number of energy applications with the potential for significant collateral damage.
Tilz et al. reported on the outcome of an individualized ablative strategy in this patient cohort, including PVI and, if SR could not be achieved with electrical cardioversion, ablation of complex fractionated atrial electrograms [17]. If PVI was the sole ablative strategy, stable SR during follow-up was noted in merely 43% of patients. Overall, 63% of patients were in SR after a mean of 1.7 ± 0.8 procedures including a combination of PVI, linear lesions and CFAE ablation. The role of additional CFAE ablation in patients with longstanding persistent AF is currently under investigation. The Alster- Lost-AF (Ablation at St. Georg Hospital for Long-Standing Persistent Atrial Fibrillation [103]) study nears completion of enrollment comparing PVI and PVI plus CFAE ablation within the left atrium (LA), coronary sinus and right atrium. Primary end point is freedom from AF over a 1-year follow-up period.
▪ Recent update of the international guidelines for the treatment of atrial fibrillation
The 2011 American College of Cardiology Foundation/American Heart Association/ Heart Rhythm Society focused update on the management of AF considers catheter ablation as a Class IA indication in patients without significant structural heart disease refractory to antiarrhythmic drug therapy [18]. By contrast, the 2011 update on the guidelines for the management of AF by the European Society of Cardiology lists catheter ablation as a class IIa recommendation in patients refractory to antiarrhythmic drug therapy [19]. Catheter ablation may be considered first-line therapy in a select group of patients with paroxysmal AF and no significant underlying structural heart disease if an experienced operator performs the procedure (class IIb indication).
Simplified approach to pulmonary vein isolation
▪ Cryoballoon ablation
The randomized, multicenter STOP AF (Sustained Treatment of Paroxysmal Atrial Fibrillation) [104] trial, presented at the 2010 American College of Cardiology scientific sessions and the 2010 Heart Rhythm meeting, was designed to compare the efficacy of PVI using cryoballoon ablation with antiarrhythmic drug therapy. Following 245 patients for 1 year, nearly 70% in the ablation arm were free from recurrent AF without the use of antiarrhythmic medication. This compared favorably to the medication-only group with 7.3% of patients still in SR. Commercially available in Europe since 2005, the results of the STOP AF study led to US FDA approval of the cryoballoon catheter in the USA in late 2010. Of note, in the STOP AF trial, acute procedural success was defined as isolation of ≥three PVs, a definition not previously used in other trials. In addition, during the 3-month blanking period, 19% of patients in the intervention arm underwent a redo procedure using cryoenergy. Several European trials have shown that cryoballoon ablation is a viable option for patients with paroxysmal or short-lasting persistent AF [20,21]. Right phrenic nerve palsy was the most common complication in these trials, while the STOP AF study reported a higher rate of 11.2%. Using the smaller 23-mm balloon may result in distal positioning of the balloon catheter within the right superior PV ostium, thereby increasing the risk for inadvertent phrenic nerve palsy. This complication is minimized with the bigger 28-mm balloon allowing for a more antral position along the right superior PV ostium.
▪ Laser balloon ablation
An alternative technology based on the use of laser energy, the Endoscopic Ablation System (Cardiofocus, MA, USA) combines balloon technology with direct visualization of intracardiac tissue through use of an endoscopic camera. A laser beam allows point-by-point lesion creation after a compliant balloon is wedged into the PV facilitating direct balloonto- tissue contact. As opposed to currently available ablation systems, the novel catheter design permits direct visualization of LA tissue, enabling the operator for the first time to apply lesions in plain sight. Initial results in patients with paroxysmal AF are encouraging, demonstrating successful acute isolation in 99% of PVs and a 1-year success rate of 60% after a single procedure off antiarrhythmic medication [22]. The incidence of esophageal thermal lesions was similar (18 vs 16%), however, the severity of thermal lesions (ulcerations) was more pronounced in the laser balloon group [23].
▪ Multielectrode ablation catheter
In order to simplify PVI, new catheters have been developed combining the characteristics of a spiral mapping and ablation catheter. Without aid of an electroanatomical mapping system, the decapolar Pulmonary Vein Ablation Catheter (PVAC, Medtronic Ablation Frontiers, CA, USA) facilitates duty-cycled unipolar and bipolar radiofrequency current delivery at the individual PV ostium. Unipolar energy is applied between the individual electrodes and the dispersive electrode located at the patient’s back, while bipolar duty-cycled energy is delivered between individual electrodes. The combination of unipolar and bipolar radiofrequency current facilitates deployment of complete circular lesions. In paroxysmal AF, results have been promising with 83% of treated patients free of recurrent AF over a 6-month follow-up period [24]. Scharf et al. reported on the use of PVAC as well as two additional duty-cycled catheters specifically developed for ablation along the interatrial septum (MASC; Medtronic Ablation Frontiers) and left atrium (MAAC; Medtronic Ablation Frontiers) in patients with longstanding persistent AF [25]. Freedom from AF off antiarrhythmic drugs after 20 ± 4 months of follow-up was 45%. Randomized studies with longer follow-up are needed to define the place of this new technology amongst established ablation tools. The European randomized, multicenter CLARITY AF (CARTO 3 System-guided RF Ablation Using the Thermocool Catheter versus Fluoroscopy-guided RF Using the Pulmonary Vein Ablation Catheter in Subjects with Paroxysmal Atrial Fibrillation [105]) trial is comparing use of the electroanatomical mapping system CARTO 3 (Biosense Webster Inc.) and the Navistar ThermoCool catheter (Biosense Webster Inc.) with fluoroscopically guided PVI using the PVAC ablation catheter. First results are expected in 2012.
Two separate studies investigated the impact of AF ablation using irrigated radiofrequency energy, the cryoballoon catheter and the multielectrode catheter on the incidence of silent cerebral embolizations. Both studies independently found a significantly higher rate of silent embolization on postprocedural MRI in those patients treated with the multielectrode catheter (37.5 and 38.9%, respectively). The clinical ramifications at this point are unknown [26,27].
The quest for permanent lesion formation
In patients with paroxysmal AF, recurrence after PVI is common and typically due to reconduction of previously isolated PVs [28]. Hence, achieving permanent lesion transmurality without need for repeat ablation procedures poses a great challenge. Importantly, improvement in lesion formation needs to be accomplished without an increase in periprocedural complications. Specifically, avoidance of esophageal damage is warranted. In the past, alternative technologies such as high intensity focused ultrasound seemed promising; however, during vigorous clinical evaluation an unacceptably high rate of collateral damage was noted [29]. In summary, there is an urgent need for improvement in the electrophysiologist’s ablative armamentarium.
▪ Contact force sensing technology
Using radiofrequency current, lesion formation will depend on several factors currently measured in the electrophysiology laboratory, such as catheter tip-to-tissue temperature, power and duration of energy application. While these parameters can be directly measured during radiofrequency current delivery, assessment of wall contact, that is, contact force between catheter tip and tissue, is not as straightforward. It has been shown previously that changes in impedance and electrogram amplitude correlate poorly with tissue–electrode contact [30]. The importance of proper contact force becomes obvious when considering that insufficient contact will result in suboptimal lesion formation. In turn, excessive contact force may result in collateral tissue damage, for example, cardiac perforation or esophageal injury [31].
A novel contact force sensor embedded into the distal tip of a standard 7 F steerable, radiofrequency ablation catheter (TactiCath, Endosense SA, Geneva, Switzerland) allows real-time assessment of contact force during energy delivery. The tri-axial force sensor has a resolution and sensitivity of 1 g, offering real-time information on lateral and axial forces applied during radiofrequency energy delivery. To compensate for cardiac cycledependent variations in tissue-to-electrode tip contact force, the concept of force-time integral measurement has been introduced [32]. Ultimately, the correct force-power combination will aid the electrophysiologist in lesion creation, preventing complications such as steam pops and perforation. In order to assess the impact of contact force measurement in clinical practice, the international, multicenter EFFICAS studies will evaluate use of contact force assessment during ablation of paroxysmal AF. Unique to the protocol is a second-look procedure. A total of 3 months following wide-area circumferential PVI, all patients undergo redo trans-septal puncture and assessment for potential gaps along previously placed circular lesion sets irrespective of symptom status or documented recurrence. Results of EFFICAS I and II will be available later in 2011. Concurrently, the TOCCASTAR (TactiCath Contact Force Ablation Catheter Study for Atrial Fibrillation [106]) trial will assess use of contact force during AF ablation with an estimated enrolment of 400 patients in the USA and Europe.
The SmartTouch contact force catheter by Biosense Webster, Inc. uses electromechanical deformation of a spring situated at the distal tip of the catheter for assessment of local contact force. The catheter integrates seamlessly with the CARTO 3 electroanatomical mapping system. It received CE mark status and is undergoing clinical assessment in several centers throughout Europe. To date, no clinical research studies have been performed.
An alternative method of contact force assessment analyzes local catheter tip-to-tissue impedance. Utilizing a three-terminal model, the electrical coupling index provides feedback about local electrical catheter tip-to-tissue contact. The system (EnSite Contact, St. Jude Medical, MN, USA) interfaces with the EnSite NavX (St. Jude Medical) electroanatomical mapping system. First animal and human studies have demonstrated its clinical utility during mapping and ablation [33,34].
▪ Novel in-tissue temperature assessment
Factors that influence lesion formation include resistive and conductive heating, as well as convective cooling by blood and irrigation flow. Current catheter designs provide information about temperature rise at the tissue–electrode interface. However, the extent of conductive heating within the deeper layers of myocardial tissue, which is commonly higher than at the surface level, is not ref lected using current technology. Hence, the operator lacks important information about the true rise of in-tissue temperature, thereby increasing the risk of tissue overheating and steam pop formation once temperature reaches 100°C. Moreover, despite an adequate rise in catheter tip-totissue temperature above 50°C, true in-tissue temperature may still be suboptimal resulting in poor quality lesions.
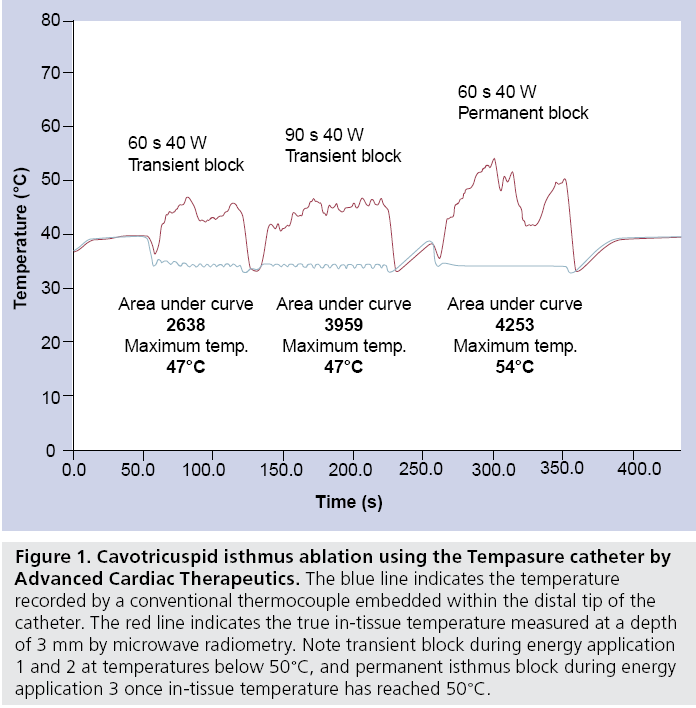
Rea l-t ime in-t i s sue temperat u re measurement using microwave radiometry (Tempasure, Advanced Cardiac Therapeutics, CA, USA) is a novel concept that may overcome these limitations (Figure 1). Embedded in a standard ablation catheter, the integrated catheter tip design incorporates circuitry that allows temperature assessment at a depth of 3 mm. In addition, the Tempasure catheter provides information on quality of continuous contact during ablation, the approximate lesion volume, and thickness of the targeted tissue. Tested in an animal model, the first human trial (ACT Catheter System in Atrial Flutter) is underway assessing its feasibility in patients with atrial flutter.
Figure 1: Cavotricuspid isthmus ablation using the Tempasure catheter by Advanced Cardiac Therapeutics. The blue line indicates the temperature recorded by a conventional thermocouple embedded within the distal tip of the catheter. The red line indicates the true in-tissue temperature measured at a depth of 3 mm by microwave radiometry. Note transient block during energy application 1 and 2 at temperatures below 50°C, and permanent isthmus block during energy application 3 once in-tissue temperature has reached 50°C.
Remote-controlled catheter ablation of atrial fibrillation
▪ Remote magnetic navigation and ablation
It has previously been shown that AF ablation using magnetic navigation (Stereotaxis, MO, USA) is feasible [10,35–37]. Compared to conventional manual radiofrequency ablation, remote-controlled magnetic PVI reduces operator radiation exposure by one third. This is achieved by limiting the operator’s time spent alongside the patient, since mapping and ablation is performed from the control room. However, total procedure times are still longer when compared with conventional manual PVI. In order to analyze the time needed for individual procedural steps during magnetic ablation, patients were prospectively enrolled to manual or remote magnetic ablation. Preliminary findings confirm previous data in that isolation of the right inferior PV is particularly challenging and time consuming [36]. In part, this is explained by the close anatomical relationship between site of trans-septal puncture and inferior margin of the right inferior PV ostium. The magnetic ablation catheter embeds three magnets at its distal end and in order to allow free range of movement all three magnets need to extend beyond the distal end of the trans-septal sheath. If shorter distance to the target PV prohibits full catheter extension outside the trans-septal sheath, remote-controlled ablation becomes challenging and time consuming.
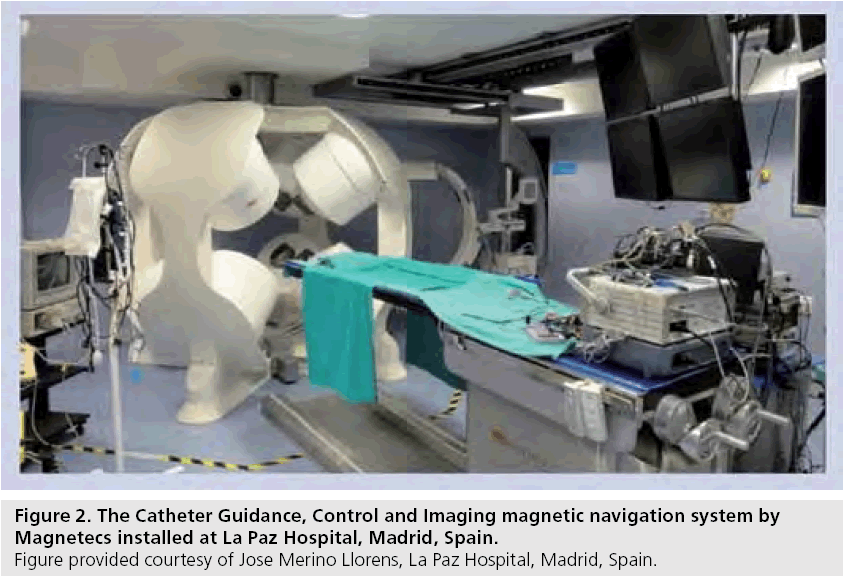
The Catheter Guidance, Control and Imaging (CGCI; Magnetecs, CA, USA) system is an alternative system for remote magnetic mapping and ablation (Figure 2). Currently only installed at La Paz Hospital in Madrid, Spain, it promises precise, real-time catheter maneuverability within a magnetic field of 1.5 Tesla. Compared to Stereotaxis, it does not require magnetic shielding of the examination room, however, due to its significant weight, reinforcement of the floor structure is necessary. The system allows for real-time catheter movement and integrates with EnSite NavX. Human clinical studies are currently underway to assess the system’s mapping ability [107]. To date, there are only animal studies reporting on catheter ablation using CGCI.
▪ Remote robotic navigation and ablation
There has been reasonable experience using the Sensei robotic navigation system (Hansen Medical, CA, USA) for AF ablation [38–40]. A 14 F steerable outer and 10.5 F inner sheath, facilitating advancement of virtually any conventional catheter, permit remote catheter manipulation by a pull-wire mechanism. Catheter navigation from the control room is accomplished via a 3D joystick. The system is portable and requires no special requisites for installment. Nonrandomized trials demonstrated that fluoroscopy times are lower when using the robotic system, while success rates for AF ablation were comparable to manual ablation. Three prospective studies are currently underway enrolling patients to compare robotic with manually guided AF ablation. It will be important to prove that robotic navigation does not increase procedural complication rates.
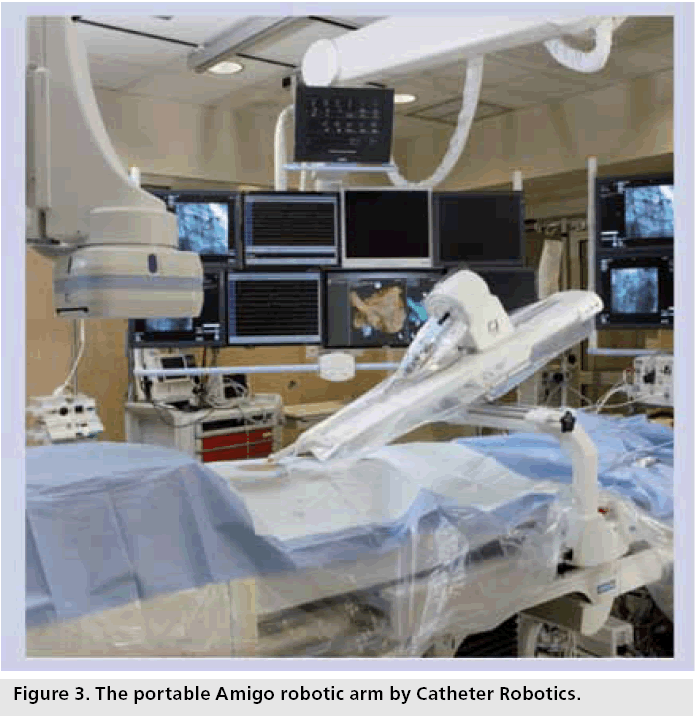
The portable Amigo (Catheter Robotics Inc., NJ, USA) robotic arm, mounted alongside the examination center, facilitates remote manipulation and positioning of catheters within the heart (Figure 3). The operator uses a cabled remote control to perform catheter movements. The catheter can be easily disengaged from the robotic arm to permit quick manual control. The system received CE mark status in Europe in 2010. It can be integrated with any electroanatomical mapping system. Clinical experience is limited to one animal study. The ‘Clinical Study to Evaluate the Catheter Robotics Amigo for Performing Right-Sided Electrophysiology Mapping Studies’ [108] is currently recruiting patients to test the system’s ability to navigate and map within the right atrium.
MRI in atrial fibrillation
Since AF results in fibrosis of the left atrium and in turn atrial fibrosis facilitates development of persistent AF, assessment of the extent of left atrial fibrosis by MRI is currently under investigation. Preprocedural analysis of the degree of atrial fibrosis may serve as a predictor for successful AF ablation. The Utah classification quantifies the extent of left atrial fibrosis (class I less than 5% and class IV greater than 35% fibrosis of the LA), while its use was predictive of subsequent AF ablation outcome [41]. Greater extent of fibrosis correlated with a higher rate of arrhythmia recurrence.
Following AF ablation, MRI allows distinct visualization of ablative lesion sets, information that may aid the ablative strategy during redo procedures [42]. The DECAAF (Delayed-Enhancement MRI Determinant of Successful Radiofrequency Catheter Ablation of Atrial Fibrillation [109]) trial, a multicenter, observational, prospective cohort study is currently enrolling patients to test the hypothesis whether MRI can accurately predict successful outcome of catheter ablation of AF.
Future perspective
In pursuit of a world free of AF, recent data has demonstrated the need to prevent progression from paroxysmal to persistent AF [8]. Paroxysmal AF is amenable to catheter ablation with a high success rate, since PV triggers play a dominant role in the initiation of AF. Electrical and structural remodeling is at its minimum during the early stages of the disease. This in turn explains the limited success rates of catheter ablation in later stages of the disease process when dominant triggers of AF have shifted from PV triggers towards structural changes, for example, fibrosis of the LA wall. Prospective, randomized multicenter studies are urgently needed to attest that an early ablative strategy in patients with paroxysmal AF will result in significant increase in freedom from AF recurrence and a reduction in progression towards chronic stages of AF.
Whi le ba lloon-based technologies demonstrate comparable success rates to manual ablation in nonrandomized studies of patients with paroxysmal AF, only prospective, randomized studies will be able to prove their equivalent or superior value.
New technologies are currently under investigation to assure advancement in the search for permanent lesion transmurality. Assessment of contact force, as well as real-time data of true in-tissue temperature during catheter ablation may prove essential for adequate transmural lesion formation. Trials currently underway testing these new technologies will strive to demonstrate that compared with current ablative strategies, follow-up results in patients undergoing AF ablation will improve.
A higher degree of automatization using remote-controlled navigation will be needed, in that even the novice electrophysiologist is capable of performing complex ablative procedures such as AF ablation. In particular, automatic mapping and ablation modes are required to allow userindependent creation of 3D LA geometry and placement of linear lesion sets. In addition, automatization will have to result in significant reduction of total procedure duration while demonstrating superior outcome.
Finally, cardiac MRI may become an important tool to properly stage the extent of atrial fibrosis and aid the operator in defining the best, individualized ablation strategy for the treatment of AF.
Financial and competing interests disclosure
Erik Wissner has received lecture honoraria from Biosense Webster, Stereotaxis, Biotronik and St. Jude Medical, and is a consultant for Advanced Cardiac Therapeutics. Karl- Heinz Kuck is a consultant for Biosense Webster, Stereotaxis, Endosense, Medtronic and Advanced Cardiac Therapeutics, and a stockholder of Endosense. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Current status of catheter ablation for atrial fibrillation
▪ Smaller studies as well as a meta-analysis have demonstrated superiority of catheter ablation over drug therapy for the treatment of atrial fibrillation (AF).
▪ Observational data suggests that catheter ablation of AF offers a mortality benefit. The ongoing CABANA trial is testing the hypothesis whether an ablative approach will lower mortality and first results are expected in 2015.
▪ Paroxysmal AF
- Several studies have reported on the long-term outcome (~5‑year follow-up) of catheter ablation of AF and concluded that:
– Multiple procedures are needed to achieve an acceptable success rate
– Success rate decreases in patients with persistent or longstanding persistent AF
– There is a steady rate of recurrence even after 60 months of follow-up, highlighting the continued need for proper anticoagulation following ablation in patients with higher Chads2 Vasc scores
– Ablation of AF may halt the natural progression from paroxysmal to persistent AF
▪ Longstanding persistent AF
- Targeting complex fractionated atrial electrograms (CFAE) as the sole strategy during AF ablation has demonstrated varying results.
- Targeting CFAE in addition to pulmonary vein isolation for the treatment of persistent AF appears to be of incremental value according to several meta-analyses.
Simplified approach to pulmonary vein isolation
▪ Cryoballoon ablation
- The STOP AF trial demonstrated an acceptable success rate of nearly 70% in patients with paroxysmal AF, however, right phrenic nerve palsy as the most common complication was somewhat higher than in previous European studies. To date, no direct head-tohead comparison of conventional radiofrequency current ablation versus cryoballoon-based ablation has been performed.
- Laser balloon ablation appears to have similar efficacy than conventional ablation but offers the advantage of direct visualization of intracardiac tissue.
The quest for permanent lesion formation
▪ Contact force sensing technology and in-tissue temperature assessment
- Insufficient contact may cause edema, decreasing the chance for proper lesion formation on subsequent ablation attempts.
- Assessment of contact force during ablation may improve durability of lesion formation and improve outcome of AF ablation.
- Information on true in-tissue temperature during ablation may translate into improved procedural outcomes.
Remote-controlled catheter ablation of AF
▪ Remote magnetic navigation and ablation
- Use of the Stereotaxis system for catheter ablation of AF significantly decreases the operator’s radiation exposure and is exceptionally safe with similar success rates compared with conventional ablation. However, procedure times are considerably longer.
- Currently under clinical investigation, the CGGI system by Magnetecs promises reproducible, automated, real-time catheter movement within cardiac chambers.
▪ Remote robotic navigation and ablation
- The Hansen robotic system allows remote ablation of AF with a decrease in fluoroscopy exposure to the operator. Randomizedcontrolled trials are currently underway to compare its performance to conventional AF ablation.
- The Amigo robotic arm by Catheter Robotics Inc. is under clinical investigation for use as a mapping and navigating tool during electrophysiology studies.
Future outlook
▪ Prospective, randomized multicenter studies are needed to confirm that an early ablative approach will result in superior outcome and a reduction in progression to persistent or longstanding persistent AF.
▪ A simplified approach to AF ablation, for example, use of the cryo- or laser balloon or the multielectrode catheter, will need to be vigorously studied to prove its equivalence or superiority to conventional ablation.
▪ Contact force assessment and in-tissue temperature measurement are amongst promising new tools that may prove essential for adequate permanent transmural lesion formation.
▪ The use of remote-controlled systems will need to result in a reduction of procedure duration while a higher degree of automatization is needed to allow a greater degree of user-independence.
▪ Cardiac MRI may become an invaluable tool for staging the disease process and predicting outcome of catheter ablation of AF.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- Haissaguerre M, Jaïs P, Shah DC et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N. Engl. J. Med. 339(10), 659–666 (1998).
- European Heart Rhythm Association (EHRA); European Cardiac Arrhythmia Scoiety (ECAS); American College of Cardiology (ACC); American Heart Association (AHA); Society of Thoracic Surgeons (STS), Calkins H, Brugada J, Packer DL et al. HRS/EHRA/ECAS expert Consensus Statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and followup. A report of the Heart Rhythm Society (HRS) Task Force on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 4(6), 816–861 (2007).
- Jais P, Cauchemez B, Macle L et al. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation 118(24), 2498–2505 (2008).
- Wazni OM, Marrouche NF, Martin DO et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA 293(21), 2634– 2640 (2005).
- Wilber DJ, Pappone C, Neuzil P et al. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA 303(4), 333–340 (2010).
- Calkins H, Reynolds MR, Spector P et al. Treatment of atrial fibrillation with antiarrhythmic drugs or radiofrequency ablation: two systematic literature reviews and meta-analyses. Circ. Arrhythm. Electrophysiol. 2(4), 349–361 (2009).
- Bunch TJ, Crandall BG, Weiss JP et al. Patients treated with catheter ablation for atrial fibrillation have long-term rates of death, stroke, and dementia similar to patients without atrial fibrillation. J. Cardiovasc. Electrophysiol. DOI: 10.1111/j.1540-8167.2011.02035.x (2011) (Epub ahead of print).
- Ouyang F, Tilz RR, Julian Chun K-RJ et al. Long-term results of catheter ablation in paroxysmal atrial fibrillation: lessons from a 5-year follow-up. Circulation 122(23), 2368–2377 (2010).
- Tzou WS, Marchlinski FE, Zado ES et al. Long-term outcome after successful catheter ablation of atrial fibrillation. Circ. Arrhythm. Electrophysiol. 3(3), 237–242 (2010).
- Weerasooriya R, Khairy P, Litalien J et al. Catheter ablation for atrial fibrillation: are results maintained at 5 years of follow-up? J. Am. Coll. Cardiol. 57(2), 160–166 (2011).
- de Vos CB, Pisters R, Nieuwlaat R et al. Progression from paroxysmal to persistent atrial fibrillation clinical correlates and prognosis. J. Am. Coll. Cardiol. 55(8), 725– 731 (2010).
- Nademanee K, McKenzie J, Kosar E et al. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J. Am. Coll. Cardiol. 43(11), 2044– 2053 (2004).
- Oral H, Chugh A, Good E et al. Radiofrequency catheter ablation of chronic atrial fibrillation guided by complex electrograms. Circulation 115(20), 2606– 2612 (2007).
- Kong MH, Piccini JP, Bahnson TD. Efficacy of adjunctive ablation of complex fractionated atrial electrograms and pulmonary vein isolation for the treatment of atrial fibrillation: a meta-analysis of randomized controlled trials. Europace 13(2), 193–204 (2011).
- Hayward RM, Upadhyay GA, Mela T et al. Pulmonary vein isolation with complex fractionated atrial electrogram ablation for paroxysmal and nonparoxysmal atrial fibrillation: A meta-analysis. Heart Rhythm DOI:10.1016/j.hrthm.2011.02.033 (2011) (Epub ahead of print).
- Li WJ, Bai YY, Zhang HY et al. Additional ablation of complex fractionated atrial electrograms after pulmonary vein isolation in patients with atrial fibrillation: a metaanalysis. Circ. Arrhythm. Electrophysiol. 4(2), 143–148 (2011).
- Tilz RR, Julian Chun K-RJ, Schmidt B et al. Catheter ablation of long-standing persistent atrial fibrillation: a lesson from circumferential pulmonary vein isolation. J. Cardiovasc. Electrophysiol. 21, 1085–1093 (2010).
- Fuster V, Ryden LE, Cannom DS et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/ American Heart Association Task Force on practice guidelines. Circulation 123(10), e269–e367 (2011).
- European Heart Rhythm Association; European Association for Cardio-Thoracic Surgery, Camm AJ, Kirchhof P, Lip GY et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur. Heart J. 31(19), 2369–2429 (2010).
- Chun KR, Schmidt B, Metzner A et al. The ‘single big cryoballoon’ technique for acute pulmonary vein isolation in patients with paroxysmal atrial fibrillation: a prospective observational single centre study. Eur. Heart J. 30(6), 699–709 (2009).
- Neumann T, Vogt J, Schumacher B et al. Circumferential pulmonary vein isolation with the cryoballoon technique results from a prospective 3-center study. J. Am. Coll. Cardiol. 52(4), 273–278 (2008).
- Metzner A, Schmidt B, Fuernkranz A et al. One-year clinical outcome after pulmonary vein isolation using the novel endoscopic ablation system in patients with paroxysmal atrial fibrillation. Heart Rhythm 8(7), 988– 993 (2011).
- Metzner A, Schmidt B, Fuernkranz A et al. Esophageal temperature change and esophageal thermal lesions after Pulmonary vein isolation using the novel endoscopic ablation system. Heart Rhythm 8(6), 815–820 (2011).
- Boersma LV, Wijffels MC, Oral H, Wever EF, Morady F. Pulmonary vein isolation by dutycycled bipolar and unipolar radiofrequency energy with a multielectrode ablation catheter. Heart Rhythm 5(12), 1635–1642 (2008).
- Scharf C, Boersma L, Davies W et al. Ablation of persistent atrial fibrillation using multielectrode catheters and duty-cycled radiofrequency energy. J. Am. Coll. Cardiol. 54(15), 1450–1456 (2009).
- Siklódy CH, Deneke T, Hocini M et al. Incidence of asymptomatic intracranial embolic events after pulmonary vein isolation. J. Am. Coll. Cardiol. 58, 681–688 (2011).
- Gaita F, Leclercq JF, Schumacher B et al. Incidence of silent cerebral thromboembolic lesions after atrial fibrillation ablation may change according to technology used: comparison of irrigated radiofrequency, multipolar nonirrigated catheter and cryoballoon. J. Cardiovasc. Electrophysiol. DOI:10.1111/j.1540-8167.2011.02050.x (2011).
- Ouyang F, Antz M, Ernst S et al. Recovered pulmonary vein conduction as a dominant factor for recurrent atrial tachyarrhythmias after complete circular isolation of the pulmonary veins: lessons from double Lasso technique. Circulation 111(2), 127–135 (2005).
- Neven K, Schmidt B, Metzner A et al. Fatal end of a safety algorithm for pulmonary vein isolation with use of high-intensity focused ultrasound. Circ. Arrhythm. Electrophysiol. 3(3), 260–265 (2010).
- Ikeda A, Nakagawa H, Shah D et al. Electrogram parameters (injury current, amplitude and dV/dt) and impedance are poor predictors of electrode-tissue contact force for radiofrequency ablation. Heart Rhythm 5, S322 (2008).
- Shah D, Lambert H, Saoudi N et al. Catheter tip force required to mechanically perforate the cardiac free wall. Heart Rhythm 5, S126 (2008).
- Shah DC, Lambert H, Nakagawa H, Langenkamp A, Aeby N, Leo G. Area under the real-time contact force curve (force-time integral) predicts radiofrequency lesion size in an in vitro contractile model. J. Cardiovasc. Electrophysiol. 21(9), 1038–1043 (2010).
- Piorkowski C, Sih H, Sommer P et al. First in human validation of impedance-based catheter tip-to-tissue contact assessment in the left atrium. J. Cardiovasc. Electrophysiol. 20(12), 1366–1373 (2009).
- Holmes D, Fish JM, Byrd IA et al. Contact sensing provides a highly accurate means to titrate radiofrequency ablation lesion depth. J. Cardiovasc. Electrophysiol. 22(6), 684–690 (2010).
- Di Biase L, Fahmy TS, Patel D et al. Remote magnetic navigation: human experience in pulmonary vein ablation. J. Am. Coll. Cardiol. 50(9), 868–874 (2007).
- Chun KR, Wissner E, Koektuerk B et al. Remote-controlled magnetic pulmonary vein isolation using a new irrigated-tip catheter in patients with atrial fibrillation. Circ. Arrhythm. Electrophysiol. 3(5), 458–464 (2010).
- Miyazaki S, Shah AJ, Xhaet O et al. Remote magnetic navigation with irrigated tip catheter for ablation of paroxysmal atrial fibrillation. Circ. Arrhythm. Electrophysiol. 3(6), 585–589 (2010).
- Hlivak P, Mlcochova H, Peichl P, Cihak R, Wichterle D, Kautzner J. Robotic navigation in catheter ablation for paroxysmal atrial fibrillation: midterm efficacy and predictors of postablation arrhythmia recurrences. J. Cardiovasc. Electrophysiol. 22(5), 534–540 (2011).
- Di Biase L, Wang Y, Horton R et al. Ablation of atrial fibrillation utilizing robotic catheter navigation in comparison to manual navigation and ablation: single-center experience. J. Cardiovasc. Electrophysiol. 20(12), 1328–1335 (2009).
- Willems S, Steven D, Servatius H et al. Persistence of pulmonary vein isolation after robotic remote-navigated ablation for atrial fibrillation and its relation to clinical outcome. J. Cardiovasc. Electrophysiol. 21(10), 1079–1084 (2010).
- Akoum N, Daccarett M, McGann C et al. Atrial fibrosis helps select the appropriate patient and strategy in catheter ablation of atrial fibrillation: a DE-MRI guided approach. J. Cardiovasc. Electrophysiol. 22(1), 16–22 (2011).
- Badger TJ, Daccarett M, Akoum NW et al. Evaluation of left atrial lesions after initial and repeat atrial fibrillation ablation: lessons learned from delayed-enhancement MRI in repeat ablation procedures. Circ. Arrhythm. Electrophysiol. 3(3), 249–259 (2010).
▪▪ This seminal paper describes the pivitol role of the pulmonary veins in the initiation of paroxysmal atrial fibrillation.
▪ Largest prospective, randomized, multicenter trial to date comparing antiarrhythmic drug therapy with catheter ablation for paroxysmal atrial fibrillation, demonstrating superiority of an ablative treatment approach.
▪▪ This study demonstrates a steady rate of recurrence of atrial fibrillation during a 5-year follow-up period. Compared to its natural course, progression to persistent atrial fibrillation was rare in patients undergoing ablation.
▪ Reconduction within the lesion set encircling the ipsilateral pulmonary veins was the dominant mechanism for recurrence following initial circumferential pulmonary vein isolation.
▪ One of the first studies to describe the use of the magnetic navigation system for catheter ablation of atrial fibrillation.
▪ Describes the Utah classification to grade the extent of atrial fibrosis and its predictive value to determine postablation success.
▪ Websites
- First Line Radiofrequency Ablation Versus Antiarrhythmic Drugs for Atrial Fibrillation Treatment (The RAAFT Study) http://clinicaltrials.gov/ct2/show/ NCT00392054
- Catheter Ablation Versus Anti-arrhythmic Drug Therapy for Atrial Fibrillation Trial (CABANA) http://clinicaltrials.gov/ct2/show/ CT00911508
- Alster-Lost-AF-Study http://clinicaltrials.gov/ct2/show/ NCT00820625
- A Clinical Study of the Arctic Front Cryoablation Balloon for the Treatment of Paroxysmal Atrial Fibrillation (Stop-AF) http://clinicaltrials.gov/ct2/show/ NCT00523978
- Efficacy, Safety and Efficiency Study of CARTO® 3 System Guided THERMOCOOL® Catheter Ablation Versus Fluoroscopy Guided Ablation With the Pulmonary Vein Ablation Catheter® (PVAC®) (CLARITY-AF) http://clinicaltrials.gov/ct2/show/ NCT01116557
- TOCCASTAR – TactiCath Contact Force Ablation Catheter Study for Atrial Fibrillation http://clinicaltrials.gov/ct2/show/ NCT01278953
- Accuracy and Safety Study of the Magnetecs CGCI System for Intracardiac Mapping (RICTAM) http://clinicaltrials.gov/ct2/show/ NCT01222156
- Clinical Study to Evaluate the Catheter Robotics Amigo for Performing Right-Sided Electrophysiology Mapping Studies http://clinicaltrials.gov/ct2/show/ NCT01139814
- DECAAF: Delayed-Enhancement MRI (DEMRI) Determinant of Successful Radiofrequency Catheter Ablation of Atrial Fibrillation http://clinicaltrials.gov/ct2/show/ NCT01150214