Research Article - International Journal of Clinical Rheumatology (2018) Volume 13, Issue 4
“Cellular matrix™ PRP-HA”: A new treatment option with platelet-rich plasma and hyaluronic acid for patients with osteoarthritis having had an unsatisfactory clinical response to hyaluronic acid alone: Results of a pilot, multicenter French study with long-term follow-up
- *Corresponding Author:
- Jean-Luc Renevier
Health Center du Lac
René Duguay Trouin Street
78200 Mantes-la-Jolie, France
E-mail: jl.renevier@gmail.com
Abstract
Objective: To evaluate the safety and efficacy of Cellular Matrix™, a new medical device designed for one-step preparation of platelet-rich plasma in presence of hyaluronic acid, for the management of tibiofemoral knee osteoarthritis in patients who had failed to respond adequately to previous treatment with hyaluronic acid alone.
Methods: Multicentre, open-label, uncontrolled, pilot study in 77 patients with grade II or III knee osteoarthritis and a pain at walking score between 3 and 8 on a Numeric Rating Scale. The treatment consisted of a series of 3 intra-articular injections scheduled at D0, D60 and D180 into the affected knee of a combination of platelet-rich plasma and hyaluronic acid prepared with the device Cellular Matrix. The primary efficacy criterion was the variation of pain at walking, as assessed with the Western Ontario and McMaster Universities Osteoarthritis Index (A1 score) between baseline and D270.
Results: Treatment with the combination of platelet-rich plasma and hyaluronic acid prepared with Cellular Matrix significantly reduced pain at walking between baseline and D270. The percentage of responders according to the criteria of the Outcome Measures in Rheumatology Clinical Trial and Osteoarthritis Research Society International was 94.4%. The treatment provided long-lasting benefits for half of the patients and allowed avoiding surgery for almost 80% of them at four years.
Conclusion: A 3-injection course of a combination of platelet-rich plasma and hyaluronic acid prepared with Cellular Matrix was well tolerated and effective in the long-term to relieve pain associated with symptomatic knee osteoarthritis.
Keywords
knee, osteoarthritis, platelet-rich plasma, hyaluronic acid, cellular matrix PRP-HA combination
Introduction
Osteoarthritis (OA) mostly affects people over 60 years of age, and more frequently women, with a sex ratio of 2 women for every man. Knee is a common localization for the disease and it is estimated that the number of subjects with knee OA in France is between 1.8 and 2.3 million [1,2].
Intra-articular injections of Hyaluronic Acid (HA) represent a treatment of choice for knee OA since they can relieve symptoms for several months. They are designed to restore the concentration and molecular weight of HA in the synovial fluid, leading to a reduction in pain and improvement in physical function of the joint. Effectiveness of HA injections for improving synovial fluid viscoelasticity is widely documented. Indeed, many clinical trials testing different HA preparations have been carried out. Most of the placebo-controlled studies indicated a superiority of HA, whatever its molecular weight [3-10].
More recently, intra-articular injections of autologous Platelet-Rich Plasma (PRP) have proven to be an attractive alternative therapeutic option for OA. Indeed, the mechanism of action of PRP is based on its content of a range of biological mediators, some of which have anti-inflammatory activity, while others stimulate Mesenchymal Stem Cells (MSC’s) and cartilage cells. In vitro studies have demonstrated the effects of individual growth factors on stimulation and chondrogenic differentiation of MSC’s: MSC’s cultured in the presence of Transforming Growth Factor-TGF-b) produce significantly more proteoglycan and type II collagen [11]; Insulin-like growth factor 1 (IGF-1) has been shown to have a synergistic effect with TGF-β in inducing chondrogenic differentiation of MSC’s [12]; basic fibroblastic growth factor (bFGF) induces proliferation and differentiation of chondrogenic MSC’s [13]. The general clinical use of individual growth factors is currently prohibitive due to the complexity and cost of their methods of manufacture and potential adverse effects. Autologous point-of-care PRP is the easiest and safest solution to provide growth factors locally and to render this therapy quickly accessible in the clinical setting. The interest and potential efficacy of PRP in the treatment of cartilage lesions have been validated by in vitro studies: PRP increases the synthesis of proteoglycans and collagen in the extracellular matrix of cultivated intervertebral disc cells [14], stimulates proliferation and matrix biosynthesis of porcine articular chondrocytes) [15] and shows superior efficacy than a standard culture medium on MSC’s proliferation and differentiation into chondrocytes [16].
With respect to clinical data, initially many case series and a pilot study [17] showed improved symptoms following PRP therapy with no serious adverse side effects. A number of larger clinical trials have then been conducted, including one trial on 115 knees [18] which showed that autologous PRP injections improved, in a statistically significant and stable manner, the clinical scores of patients from the end of treatment to six months with respect to baseline scores (p<0.0005). These beneficial effects decreased between 6 and 12 months (p<0.02 with respect to baseline), although they remained better than the baseline scores. All these results suggest that PRP, due to its specific mechanism of action, is an effective and innovative tool in the therapeutic arsenal for the treatment of the symptoms of knee OA.
Based on the above data, it is reasonable to assume that a combination of PRP and HA could provide added benefit in knee OA with respect to each of the products alone. HA would result in restoration of the rheological properties of the synovial fluid and would potentially favour the biological activities of PRP. Cellular Matrix is a Class III Medical Device which has recently become available and is the sole device which allows the combination of HA with PRP in conformity with regulations.
The objective of this study was to evaluate the effectiveness and safety profile of the Cellular Matrix PRP-HA mix in the management of tibiofemoral knee OA in patients who had failed to respond adequately to previous treatment with HA alone.
Methods
Study design and participants
This is an open-label, uncontrolled, pilot study conducted in 77 patients recruited in 6 French centres. Eligible patients were aged between 40 and 85 years, had radiographically ascertained grade II or III gonarthrosis according to Kellgren and Lawrence scale, had pain at walking between 3 and 8 on a 11-point Numeric Rating Scale and had previously been treated with HA with no satisfactory clinical response (defined as a Western Ontario and McMaster Universities Osteoarthritis Index [WOMAC] A1 score that did not show improvement of at least 3 points three months after the last injection).
Patients were excluded if they had acute inflammatory flare of OA in the affected knee, HA injection in the past 3 months, corticosteroid injection in the previous 2 months, any knee or hip surgery planned within the following 6 months, use of gluco-corticosteroids (except those that are inhaled) and level analgesics in the past 3 months, treatment with symptomatic slow acting drugs for osteoarthritis (diacerein, avocado and soy unsaponifiables, glucosamine sulfate, chondroitin sulfate) initiated in the previous 3 months, an history of allergy to HA, rheumatoid arthritis, surgery in the affected knee in the past 3 months, knee infection during the previous 6 months, a severe disease, and if pregnant or breastfeeding.
Routine laboratory tests (including a platelet count) were performed prior to study inclusion. All patients gave written informed consent.
Treatment protocol
The treatment consisted of a series of 3 intraarticular knee injections of around 4 ml of the combination of PRP and HA prepared with Cellular Matrix device (Cellular Matrix A-CP HA Kit, Regen Lab SA, Le Mont-sur-Lausanne, Switzerland) in accordance with operating instructions supplied with the kit. The device allows automated blood collection and blood component separation in closed circuit. After a five-minute-centrifugation, the resulting product, CM-PRP-HA, is a PRP with a platelet concentration 1.5-1.6 times higher than the baseline in blood, deprived of contamination with red and white blood cells, entrapped in a 3D network of HA.
After study inclusion, the patient was given the first intra-articular injection (D0) under strictly aseptic conditions while lying in the supine or semi-sitting position with the knee extended. Injection was performed using a classical external suprapatellar approach without local anaesthetic following aspiration of synovial fluid in case of intra-articular effusion. After treatment, patients were asked to limit the use of the affected leg for 10 hours; then, patients were allowed to gradually resume normal physical activity. Second and third injections were performed at D60 and D180, respectively, under the same conditions.
Efficacy and safety parameters
All patients were evaluated before the first injection (D0, baseline) and at D60, D180 and D270. The primary efficacy endpoint was the variation of pain at walking (WOMAC A1 score), as measured on an 11-point Numeric Rating Scale, between baseline and D270. Secondary efficacy endpoints consisted of
• The variation of the WOMAC A1 score between baseline and other timepoints and
• The variation of all other items of the WOMAC questionnaire between baseline and D270. Percentage of responders according to Outcome Measures in Rheumatology Clinical Trial and Osteoarthritis Research Society International (OMERACT-OARSI) criteria was calculated as recommended [19].
Briefly, strict responders were defined by a ≥ 50% improvement in pain or function and reduction ≥ 20 mm on a 100 mm Visual Analogue Scale (VAS), whereas responders were defined by a ≥ 20% improvement and reduction ≥ 10 mm on a 100 mm VAS in at least 2 of the 3 following areas: pain, function, global assessment of the patient. Safety was evaluated through the collection of information on adverse events at each follow-up visit or if the patients had complaints.
In addition, in order to evaluate the long-term performance of the treatment, a survey among study participants was conducted in December 2017. Questions were about the duration of the benefit of the CM-PRP-HA treatment in terms of pain and function, possible alternative treatments received by the patient such as viscosupplementation, and possible knee surgery undergone by the patient since the end of the study.
Statistical analysis
For statistical analysis, the averages for quantitative variables were compared between timepoints using the Student t test for paired data. In all statistical tests, the significance level was set at 0.05.
Results
Demographic and clinical data
In total, 77 patients (83 knees) were recruited for this study between September 2013 and April 2014. Baseline demographic and clinical characteristics for these patients are summarized in Table 1. Out of these 77 patients, 10 withdrew for various reasons (6 dropout, 1 lost to follow-up, 1 for reasons independent of the study, 1 due to osteonecrosis of the lateral femoral condyle and 1 because of a worsening on X-ray of the arthritic disease from Kellgren & Lawrence grade II at baseline to III).
| Age (years) | |
| N | 83 knees |
| Missing Data | 0 |
| Minimum/Maximum | 40/84 |
| Median | 63 |
| Mean (SD1) | 62 (10.817) |
| Sex | |
| Male | 48 (57.8%) |
| Female | 35 (42.2%) |
| BMI | |
| N | 83 |
| Missing Data | 0 |
| Minimum/Maximum | 20.32/39.06 |
| Median | 26.83 |
| Mean (SD1) | 27.406 (3.90) |
| Normal corpulence | 23 (27.7%) |
| Overweight | 42 (50.6%) |
| Moderate obesity | 13 (15.7%) |
| Severe obesity | 5 (6%) |
| Kellgren and Lawrence grades | |
| N | 83 |
| Missing data | 0 |
| Grade II | 36 (43.4%) |
| Grade III | 47 (56.6%) |
| Number of patients included by centre | |
| Centre 1 | 20 (24.1%) |
| Centre 2 | 10 (12%) |
| Center 3 | 16 (19.3%) |
| Center 4 | 8 (9.6%) |
| Center 5 | 17 (20.5%) |
| Center 6 | 12 (14.5%) |
SD: standard deviation; BMI: Body Mass Index
Table 1. Baseline demographic and clinical characteristics of patients.
All patients reported having been treated previously with HA but did not respond satisfactorily to it. The most frequently reported HA were: Go-On (28%), Structovial (14%) and Durolane (12%). The percentage of other reported HAs was less than 10%.
Primary outcome
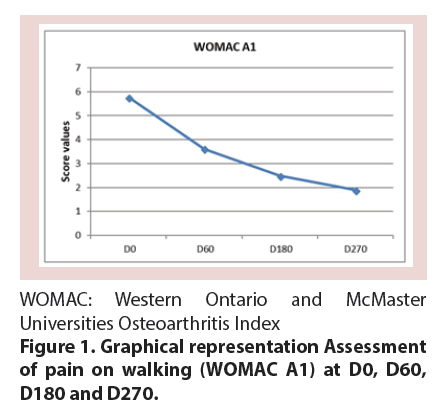
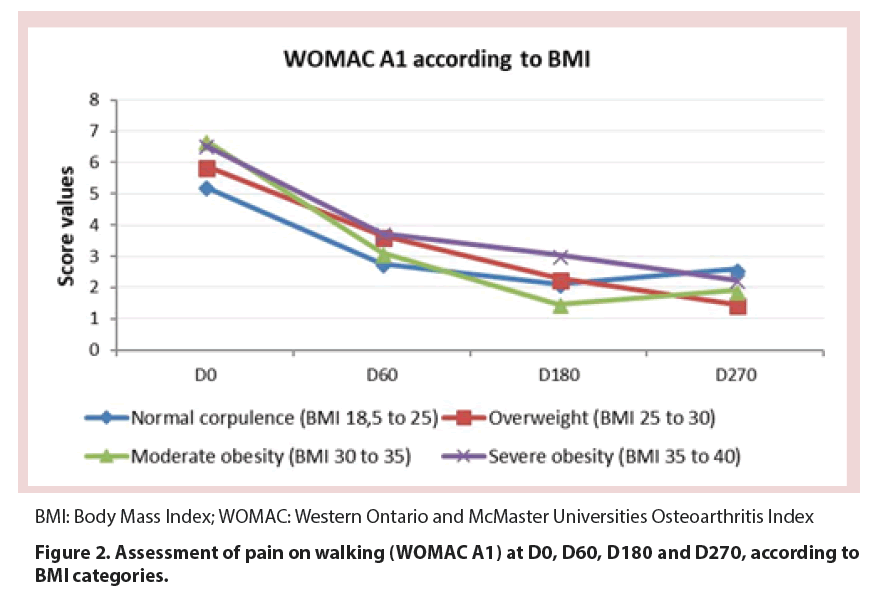
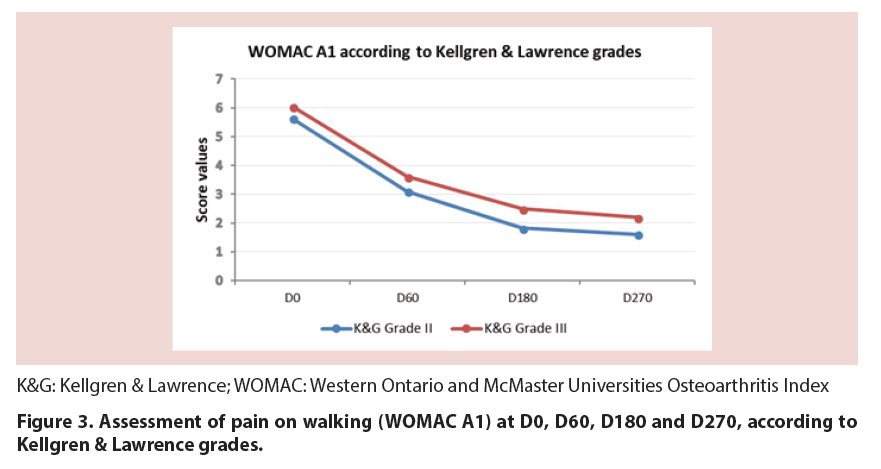
Treatment with CM-PRP-HA decreased pain at walking between baseline and D270 by 65%, as measured by the WOMAC A1 score (p<0.05). This decrease was constant throughout the 9 month follow-up period, although the reduction was less marked between D180 and D270 (p=0.079) (Figure 1 and Table 2). This reduction was observed whatever the Body Mass Index (BMI) of patients (Figure 2) and regardless of the Kellgren and Lawrence grade II or III of knee OA (Figure 3).
| Score WOMAC A1 (SD) | P value versus baseline | Improvement from baseline | |
|---|---|---|---|
| DO (Baseline) | 5.87 ± 1.53 | N/A | N/A |
| D60 | 3.39 ± 1.88 | 0.000** | 40.16% |
| D180 | 2.18 ± 2.03 | 0.000** | 62.92% |
| D270 | 1.89 ± 1.76 | 0.000** | 64.97% |
| P values | |||
| D60 Versus D80 | 0.000** | 0.000** | |
| D60 Versus D270 | 0.000** | 0.000** | |
| D180 Versus D270 | 0.079 ns | 0.208 ns | |
**highly significant; ns: non-significant
WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index
Table 2. Assessment of pain on walking (WOMAC A1) at D0, D60, D180 and D270.
There was no interaction between the variation of the WOMAC A1 score and the investigating centres.
Secondary outcomes
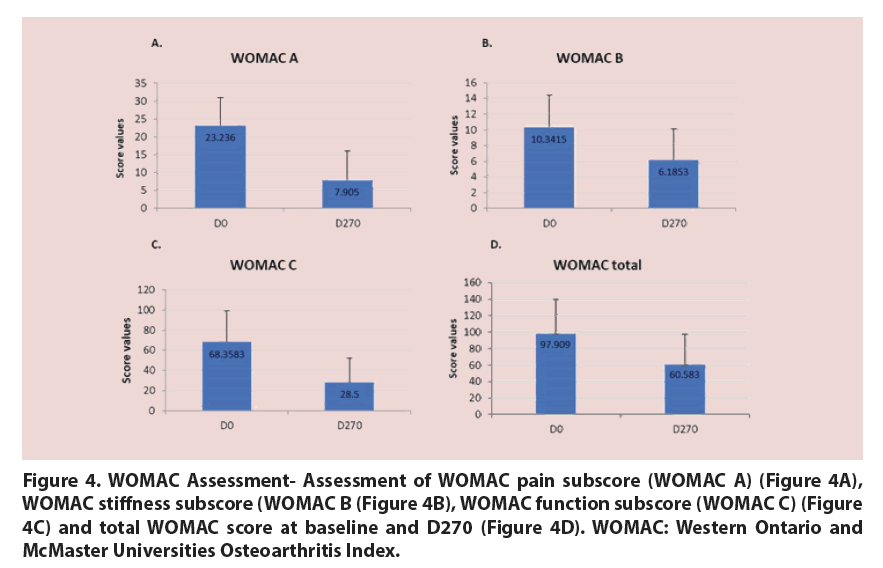
The studied treatment also significantly improved the WOMAC pain subscore (taking into account all 5 items of the WOMAC A subscale) (Figure 4A), WOMAC stiffness subscore (Figure 4B), WOMAC physical function subscore (Figure 4C) and the overall WOMAC score between baseline and D270 in a statistically significant way (p<0.05 for all assessed scores) (Figure 4D).
Figure 4. WOMAC Assessment- Assessment of WOMAC pain subscore (WOMAC A) (Figure 4A), WOMAC stiffness subscore (WOMAC B (Figure 4B), WOMAC function subscore (WOMAC C) (Figure 4C) and total WOMAC score at baseline and D270 (Figure 4D). WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index.
The proportion of responders according to the OMERACT-OARSI criteria at D270 was 94.4% (Figure 5), whereas the proportion of strict responders was 83.6% (Figure 5). However, due to missing data, the percentage calculation could only be performed on 54 patients for the responders, and on 60 patients for the strict responders.
OMERACT-OARSI: Outcome Measures in Rheumatology Clinical Trial and Osteoarthritis, Research Society International
Figure 5. Percentage of responders and strict responders according to OMERACT-OARSI criteria.
Long-term evaluation
We were able to collect long-term data for 62 out of 77 study participants (80.5%). 59.7% of the them still perceived substantial clinical benefit 2 years after the treatment, while 50% were still satisfied with it at the time of the survey (4 years after the treatment). This effect was due to the initial 3-injection course for 61.2% of them, while 38.8% had to receive additional injections of the treatment to continue to perceive its positive effects on the long-term (1 to 3 injection(s)/year). For 79% of them, the treatment allowed avoiding surgery.
Safety
No serious adverse events were reported. Only 13.25% of patients experienced one or more adverse events (11 in all) related to the treatment.
Most of them consisted of mild to moderate inflammatory reactions at the treated site; only one consisted of violent pain which lasted 6 hours.
Discussion
In this French multicentre study, we show that a 3-injection course of the Cellular Matrix combination of PRP and HA can safely and drastically decrease pain and stiffness and improve function of the joint in patients with mild to moderate knee OA. In addition, we demonstrate the long-term efficacy of this treatment, as half of treated patients who answered our survey continued to experience improvement of their condition two to four years after the end of the initial 3-injection course treatment.
HA has long been recognized as a treatment option in the conservative management of knee OA, due to its lubrication, shock-absorption, anti-inflammatory and chrondroprotective properties. Nevertheless, a significant proportion of patients doesn’t respond positively to HA therapy and is therefore more likely to undergo surgery [20].
In this study, we focused on this well-defined population of OA patients whose symptoms could not be satisfactorily relieved by previous treatment with hyaluronic acid. Each patient aged 40 to 84 years received three intra-articular injections of CM-PRP-HA at D0, D60 and D180, and was followed-up for a period of 9 months. Results showed that treatment of Kellgren and Lawrence grade II or III OA with CM-PRP-HA decreased pain on walking by 65% at the end of the 9-month follow-up period and also improved WOMAC stiffness and physical function scores. In addition, 94.4% of patients were considered responders to treatment based on OMERACT-OARSI criteria. This percentage has to be compared with that from Bowman et al. study who tried to identify patient and treatment factors related to successful HA treatment [20]. The authors report that only 57% of the patients met OMERACT-OARSI criteria for successful response to HA treatment following a 3-week regimen of HA, and that patients with grade I or II OA were 2.2 times more likely to respond to HA injections than those with grade III OA. In our study, we found no difference in treatment response between grade II and III OA patients. This implies that treatment with Cellular Matrix may be particularly relevant for patients with grade III OA who are more likely to have unsuccessful outcomes with HA. As in Bowman’s study, we found no statistical correlation with BMI, although we observed a trend to higher improvement in overweight patients.
On contrary, PRP is a relatively new option for OA treatment. It has been shown through several meta-analyses that PRP is superior to HA to relieve pain and improve function in patients suffering from knee OA [21-29]. More precisely, the Riboh et al. meta-analysis showed that only leukocyte-poor PRP, such as the PRP in CM-PRP-HA combination, was significantly superior to HA [30]. The rationale for PRP use is based on the biological stimulation of cartilage and mesenchymal stem cells through the active secretion of platelet growth factors during treatment. Additionally, PRP exerts its beneficial effects through the modulation of the inflammatory response by balancing pro- and anti-inflammatory factors [31]. This has been specifically demonstrated for the PRP prepared using the same technology as in the Cellular Matrix device in the Chen et al. study [32].
In recent years, based on a number of in vitro studies, it has become more and more obvious that the association of PRP with HA could provide added benefit for the treatment of joint degenerative diseases, due to their different mechanisms of actions to modulate the disease process [33]. Indeed, Sundman et al. [34] showed in their study measuring the effects of PRP and HA separately on synoviocyte and cartilage co-cultures that only HA-treated co-cultures resulted in a decrease in the pro-inflammatory cytokine IL- 6, while only PRP-treated co-cultures resulted in a decreased gene expression of metalloproteinase-3 (MMP-3) and in an increased gene expression of hyaluronan synthase-2. This suggests that PRP and HA could have complementary beneficial ant-iinflammatory and anabolic effects on joint cells and that a combination of hem might produce better outcomes than either PRP or HA alone for the treatment of OA.
In support of this hypothesis, Chen et al. [35] demonstrated in their in vitro OA cell model cultured in presence of either PRP, or HA or a combination of both that PRP+HA can inhibit inflammation more efficiently than do PRP or HA alone. Chondrogenesis was also induced more strongly by PRP+HA than by PRP or HA alone. In addition, rescue of the decreased extracellular matrix synthesis by the PRP+HA combination was also higher than by PRP or HA only. These findings were further supported by similar analyses conducted in their 3D arthritic neo-cartilage model, as well as in an OA mice model injected with either PRP, or HA, or both. Finally, Russo et al. [36] demonstrated that chondrocytes cultured in a PRP+HA-containing medium synthesize glycosaminoglycan at a significantly higher level than when cultured in the other culture conditions (PRP or HA only).
From a clinical point of view, the association of PRP and HA treatments also provided promising outcomes. Indeed, Lana et al. [37] who treated 105 patients suffering from Kellgren and Lawrence I to III knee OA with either HA, or PRP or both, found that the improvement in pain and physical function scores was significantly higher in patients treated with consecutive injections of HA and PRP, in comparison to each product administered separately. Interestingly, Saturveithan [38] and Chen [39] reported that the association of PRP and HA injections was also able to provide pain relief and functional improvement in patients with advanced knee OA, suggesting that combining these treatments could allow postponing the need for arthroplasty [39].
In these studies, however, the association of PRP and HA was obtained by sequential injections of PRP and HA. Cellular Matrix is the first dedicated medical device allowing to prepare PRP in presence of HA in a simple, safe and reproducible procedure. Abate et al. [40] conducted a retrospective comparative study on a patient group treated with PRP only compared to a patient group treated with the Cellular Matrix CM-PRP-HA combination. Interestingly, in this study, the device used to prepare PRP (RegenKit- BCT, Regen Lab, Switzerland) was based on the same technology as Cellular Matrix, except that it didn’t contain HA and the PRP volume was almost twofold that of Cellular Matrix. As the authors observed that the CM-PRP-HA combination had the same efficacy as PRP prepared with RegenKit- BCT administered in higher volume, they concluded that the presence of HA could improve PRP properties, hypothesizing that this could be done by creating a bioactive scaffold around cells that would increase the residence time of growth factors.
Our study has some limitations. First, it doesn’t include a control group with which to compare the effects. Patients’ improvement is compared against their baseline values but it could have been much more evidenced if compared with a placebo-treated group. Second, there is a high rate of missing data due to incomplete WOMAC questionnaire at the 9 month assessment that could have introduced bias in the estimates.
In conclusion, our study aimed at exploring the feasibility, safety and efficacy of using a combination of PRP and HA prepared with a dedicated medical device (Cellular Matrix) to treat patients suffering from mild to moderate knee OA who failed to respond adequately to a previous treatment with HA alone. Our results suggest that the association of both components using the Cellular Matrix technology is a safe and effective treatment for relieving symptoms associated with knee OA. Interestingly, long-term evaluation demonstrated that this treatment was still effective for at least 2 years for 50% of the patients that completed our survey and allowed avoiding surgery for almost 80% of them. Cellular Matrix technology may therefore represent a new medical alternative to knee surgery after failure of HA or, at least, a viable strategy allowing to delay the need for joint replacement surgery. Even though the exact cellular and molecular mechanisms underlying the association of PRP and HA still need to be elucidated, currently available clinical data with Cellular Matrix clearly makes it a promising and safe new player in the therapeutic arsenal for knee osteoarthritis.
Disclosure of Interest
JLR, JFM and PA received consulting fees from Regen Lab SA. The other authors declare that they have no competing interests.
Acknowledgment
The authors would like to acknowledge Sandrine Lombion, SLc Consulting, for statistical analysis.
References
- Allonier A, Dourgnon P, Rochereau T. Health and Social Protection Survey 2006. IRDES, 2008 April 2008. Report No .: Contract No .: Report No. 540 (Library 1701).
- Roux CH, Saraux A, Mazieres B et al. Screening for hip and knee osteoarthritis in the general population: predictive value of a questionnaire and prevalence estimates. Ann. Rheum. Dis. 67(10), 1406–1411 (2008).
- Petrella RJ, Cogliano A, Decaria J. Combining two hyaluronic acids in osteoarthritis of the knee: a randomized, double-blind, placebo-controlled trial. Clin. Rheumatol. 27(8), 975–981 (2008).
- Conrozier T, Jerosch J, Beks P et al. Prospective, multi-centre, randomised evaluation of the safety and efficacy of five dosing regimens of viscosupplementation with hylan G-F 20 in patients with symptomatic tibio-femoral osteoarthritis: a pilot study. Arch. Orthop. Trauma. Surg. 129(3), 417–423 (2009).
- Day R, Brooks P, Conaghan PG et al. A double blind, randomized, multicenter, parallel group study of the effectiveness and tolerance of intraarticular hyaluronan in osteoarthritis of the knee. J. Rheumatol. 31(4), 775–782 (2004).
- Cubukcu D, Ardic F, Karabulut N et al. Hylan G-F efficacy on articular cartilage quality in patients with knee osteoarthritis: clinical and MRI assessment. Clin. Rheumatol. 24(4), 336–341 (2005).
- Petrella RJ, Petrella M. A prospective, randomized, double-blind, placebo controlled study to evaluate the efficacy of intraarticular hyaluronic acid for osteoarthritis of the knee. J. Rheumatol. 33(5), 951–956 (2006).
- Brandt KD, Block JA, Michalski JP et al. Efficacy and safety of intraarticular sodium hyaluronate in knee osteoarthritis. ORTHOVISC Study Group. Clin. Orthop. Relat. Res. 130–143 (2001).
- Wobig M, Dickhut A, Maier R et al. Viscosupplementation with hylan G-F 20: a 26-week controlled trial of efficacy and safety in the osteoarthritic knee. Clin. Ther. 20(3), 410–423 (1988).
- Altman RD, Rosen JE, Bloch DA et al. A double-blind, randomized, saline-controlled study of the efficacy and safety of EUFLEXXA for treatment of painful osteoarthritis of the knee, with an open-label safety extension (the FLEXX trial). Semin. Arthritis. Rheum. 39(1), 1–9 (2009).
- Barry F, Boynton RE, Liu B et al. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components. Exp. Cell. Res. 268(2), 189–200 (2001).
- Fukumoto T, Sperling JW, Sanyal A et al. Combined effects of insulin-like growth factor-1 and transforming growth factor-beta1 on periosteal mesenchymal cells during chondrogenesis in vitro. Osteoarthritis Cartilage. 11(1), 55–64 (2003).
- Stevens MM, Marini RP, Martin I et al. FGF-2 enhances TGF-beta1-induced periosteal chondrogenesis. J. Orthop. Res. 22(5), 1114–1119 (2004).
- Akeda K, An HS, Pichika R, et al. Platelet-rich plasma (PRP) stimulates the extracellular matrix metabolism of porcine nucleus pulposus and anulus fibrosus cells cultured in alginate beads. Spine. 31(9), 959–966 (2006).
- Akeda K, An HS, Okuma M, et al. Platelet-rich plasma stimulates porcine articular chondrocyte proliferation and matrix biosynthesis. Osteoarthritis. Cartilage. 14(12), 1272–1280 (2006).
- Mishra A, Tummala P, King A, et al. Buffered platelet-rich plasma enhances mesenchymal stem cell proliferation and chondrogenic differentiation. Tissue. Eng. Part C Methods. 15(3), 431–435 (2009).
- Sampson S. Injection of Platelet-Rich Plasma in Patients with Primary and Secondary Knee Osteoarthritis. Am. J. Phys. Med. Rehabil. 89(12) (2010).
- Kon E, Buda R, Filardo G, et al. Platelet-rich plasma: intra-articular knee injections produced favorable results on degenerative cartilage lesions. Knee. Surg. Sports. Traumatol. Arthrosc. 18(4), 472–479 (2010).
- Pham T, Van Der Heijde D, Lassere M, et al. Outcome variables for osteoarthritis clinical trials: The OMERACT-OARSI set of responder criteria. J. Rheumatol. 30(7), 1648–1654 (2003).
- Bowman EN, Hallock JD, Throckmorton TW, et al. Hyaluronic acid injections for osteoarthritis of the knee: predictors of successful treatment. Int. Orthop. 42(4), 733–740 (2018).
- Laudy AB, Bakker EW, Rekers, et al. Efficacy of platelet-rich plasma injections in osteoarthritis of the knee: a systematic review and meta-analysis. Br. J. Sports. Med. (2014).
- Laver L, Marom N, Dnyanesh L, et al. PRP for degenerative cartilage disease: A systematic review of clinical studies. Cartilage. (2016).
- Dai WL, Zhou AG, Zhang H, et al. Efficacy of platelet-rich plasma in the treatment of knee osteoarthritis: A meta-analysis of randomized controlled trials. Arthroscopy. 33(3), 659–670 (2017).
- Meheux CJ, McCulloch PC, Lintner DM, et al. Efficacy of intra-articular platelet-rich plasma injections in knee osteoarthritis: A systematic review. Arthroscopy. 32(3), 495–505 (2016).
- Lai LP, Stitik TP, Foye PM, et al. Use of platelet rich plasma in intra-articular knee injections for osteoarthritis: A systematic review. PM R. (2015).
- Khoshbin A, Leroux T, Wasserstein D, et al. The efficacy of platelet-rich plasma in the treatment of symptomatic knee osteoarthritis: a systematic review with quantitative synthesis. Arthroscopy. 29(12), 2037–2048 (20130.
- Chang KV, Hung CY, Aliwarga F, et al. Comparative effectiveness of platelet-rich plasma injections for treating knee joint cartilage degenerative pathology: a systematic review and meta-analysis. Arch. Phys. Med. Rehabil. 95(3), 562–575 (2014).
- Campbell KA, Saltzman BM, Mascarenhas R et al. Does Intra-articular platelet-rich plasma injection provide clinically superior outcomes compared with other therapies in the treatment of knee osteoarthritis? A systematic review of overlapping meta-analyses. Arthroscopy. 31(11), 2213–2221 (2015).
- Tietze DC, Geissler K, Borchers J. The effects of platelet-rich plasma in the treatment of large-joint osteoarthritis: a systematic review. Phys. Sportsmed. 42(2), 27–37 (2014).
- Riboh JC, Saltzman BM, Yanke AB et al. Effect of leukocyte concentration on the efficacy of platelet-rich plasma in the treatment of knee osteoarthritis. Am. J. Sports. Med. 44(3), 792–800 (2016).
- Andia I, Maffulli N. Platelet-rich plasma for managing pain and inflammation in osteoarthritis. Nat. Rev. Rheumatol. 9(12), 721–730 (2013).
- Chen CPC, Cheng CH, Hsu CC, et al. The influence of platelet rich plasma on synovial fluid volumes, protein concentrations, and severity of pain in patients with knee osteoarthritis. Exp. Gerontol. 93, 68–72 (2017).
- Andia I, Abate M. Knee osteoarthritis: hyaluronic acid, platelet-rich plasma or both in association? Expert. Opin. Biol. Ther. (2014).
- Sundman EA, Cole BJ, Karas V, et al. The anti-inflammatory and matrix restorative mechanisms of platelet-rich plasma in osteoarthritis. Am. J. Sports. Med. 42(1), 35–41 (2014).
- Chen WH, Lo WC, Hsu WC, et al. Synergistic anabolic actions of hyaluronic acid and platelet-rich plasma on cartilage regeneration in osteoarthritis therapy. Biomaterials. 35(36), 9599–9607 (2014).
- Russo F, D'Este M, Vadala G, et al. Platelet rich plasma and hyaluronic acid blend for the treatment of osteoarthritis: rheological and biological evaluation. PLoS One. 11(6), e0157048 (2016).
- Lana JF, Weglein A, Sampson S, et al. Randomized controlled trial comparing hyaluronic acid, platelet-rich plasma and the combination of both in the treatment of mild and moderate osteoarthritis of the knee. JSRM. 12(2) (2012).
- Saturveithan C, Premganesh G, Fakhrizzaki S et al. Intra-articular Hyaluronic Acid (HA) and Platelet Rich Plasma (PRP) injection versus Hyaluronic Acid (HA) injection in Grade III and IV Knee Osteoarthritis (OA) patients: A retrospective Study On functional Outcome. 45th Malaysian Orthopaedic Association (2015).
- Chen SH, Kuan TS, Kao MJ, et al. Clinical effectiveness in severe knee osteoarthritis after intra-articular platelet-rich plasma therapy in association with hyaluronic acid injection: three case reports. Clin. Interv. Aging. 11, 1213–1219 (2016).
- Abate M, Verna S, Schiavone C, et al. Efficacy and safety profile of a compound composed of platelet-rich plasma and hyaluronic acid in the treatment for knee osteoarthritis (preliminary results). Eur. J. Orthop. Surg. Traumatol. 25(8), 1321–1326 (2015).