Review Article - Imaging in Medicine (2013) Volume 5, Issue 5
Central effects of cholinesterase inhibitors in Alzheimers disease: insights from advanced neuroimaging
Annalena Venneri*1,2 and Michael F Shanks11 Department of Neuroscience, Medical School, University of Sheffield, Beech Hill Road, Sheffield, S10 2RX, UK
2IRCCS Fondazione Ospedale San Camillo, Venice, Italy
Abstract
Keywords
DTI; fMRI; functional connectivity; MRI; resting state; tractography
An effective form of treatment for Alzheimer’s disease (AD) remains elusive despite extensive and persistent research efforts. Prior to the expected disease-modifying agents, symptomatic pharmacotherapy is still the treatment of choice to modify symptoms in responders and to promote cognitive stabilization. Most of the available compounds address the cholinergic deficits that are present early in the disease and that result in impaired episodic memory and attention [1–3]. Cholinergic enhancement is achieved via inhibition of the enzymes that degrade excess neurotransmitter at the synaptic level. In this way, the reduction in acetylcholine synthesis caused by neurodegeneration of the subcortical nuclei where the neurotransmitter is produced can be, at least for a time, compensated.
Cholinesterase inhibitors (ChEIs) have been in use for almost two decades in patients with a range of disease severities. Clinical trials in established AD have shown marginal but significant effects on some parameters of cognitive function (increased alertness is often reported and an increased overall level of function) and, for a period, symptoms can remain well compensated in patients responding to treatment [4,5]. In a logical extension of the original multicenter trials, a series of clinical studies were undertaken in which treatment was administered to patients in the prodromal mild cognitive impairment (MCI) stage of AD. These studies have been largely unhelpful in terms of significant findings, and no effects on conversion rates to AD were reported [6–9]. Some authors have argued for increased ChEI effectiveness in the later rather than the earlier stages of the disease, but a growing body of evidence (especially from imaging studies as reviewed below) is emerging in favor of early initiation of treatment having a more substantial and longer-term impact on symptoms.
Pharmacological studies of AD at all stages will inevitably be limited by heterogeneity of samples and diagnostic uncertainty, and even the application of recently published research criteria [10,11] or the more recently revised clinical criteria [12], although an improvement, will not reduce heterogeneity significantly. Biomarker parameters for recruitment may be helpful, but biomarkers are not used routinely in clinical practice and this limits the contribution that these may make to reducing diagnostic errors and contamination in the recruited samples. These issues have played a significant role in the industry-led large trials of ChEIs in the prodromal MCI phase of AD [6,9,13,14]. The findings of these trials have been substantially inconclusive and have not convincingly shown any significant advantage of treatment at this stage, with only limited benefit in subsequent subgroup analyses of selected patients in which heterogeneity had been reduced.
To fully understand the potential of currently available treatment, scientists have gone beyond the evidence from large-scale clinical trials and their inherent heterogeneity, and have studied smaller but more homogeneous groups of patients and patients at an earlier stage of the disease. In addition to targeting a better characterized groups of established and prodromal AD patients, these studies have not used conventional cognitive or clinical tools as measures of treatment outcome and have not relied on changes in cognitive tests or other clinical scales as primary outcome measures. These independent studies have chosen neuroimaging parameters as outcome measures. Smaller but more homogeneous groups of patients can be meaningfully studied, taking advantage of the increased statistical power provided by imaging measures [15].
The evidence from these studies goes beyond a simple measure of change to provide at least some insight into what is driving behavioral changes, including the underlying physiological impact of a specific drug treatment on neurogeneration and brain function, the time course of treatment effects and the effect of cholinergic augmentation on synaptic function.
This review will focus on the evidence emerging from the most recent neuroimaging studies of the effects of ChEI treatment in early AD and in prodromal AD (currently defined as amnestic MCI), and will summarize the most recent findings emerging from the use of advanced imaging techniques including functional MRI (fMRI), diffusion tensor imaging (DTI) and amyloid imaging using PET in order to highlight the neurophysiological changes resulting from treatment. Earlier SPECT and PET studies will not be included in the present review and we will not summarize evidence from the limited number of MR spectroscopy studies. Interested readers can find extensive coverage of the findings of these studies in previous reviews [16,17].
Functional MRI
Two sets of treatment studies will be reviewed: those that have examined task-related brain activity to establish whether pharmacological augmentation was beneficial; and those that have looked at activity at rest in the absence of a task and focused on the default activity of the brain and its background functional connectivity.
Task fMRI
Earlier pioneering studies with healthy volunteers established the principle that it is possible to use functional imaging techniques to visualize the modulation of regional brain activity in response to a task after the administration of a cholinergic enhancer [18–22]. These studies used infusion of the short-lived cholinergic enhancer physostigmine to modulate regional brain activity in visual and attention areas in emotional and priming tasks, and showed that task-related activity was enhanced by physostigmine administration when compared with saline infusion. It seems clear, however, that the longer-term benefits of cholinergic enhancement are only observed in the context of a cholinergic deficit. A study of cholinergic augmentation with rivastigmine showed no effect on task-related regional activity in the brain of patients with schizophrenia [23], although a positive effect of GABA modulation with flumazenil on regional memory-related brain activity was subsequently observed in schizophrenia [24]. Likewise, simultaneous recording of EEG and fMRI parameters documented no beneficial effects on memory after prolonged cholinesterase inhibition with donepezil in the brain of healthy older adults [25]. Therefore, if the cholinergic system is functioning normally, cholinesterase inhibition has no sustained effect. There is a plausible view that, with progression of the disease, damage to the cholinergic system becomes more extensive and therefore cholinesterase inhibition becomes more beneficial. However, even modest damage (there is neuropathological evidence that the anatomical regions first affected in AD are primarily cholinergic) should be addressed so that synaptic transmission can be normalized as far as possible and as early as possible to promote cognitive stabilization and healthy brain functional connectivity. Absence of a cholinergic deficit might therefore be conceptualized in a different way from the limited dysfunction that is present in the early stage of AD. If the system is compromised, even minimally as in the earliest stages of AD, then cholinergic augmentation is beneficial and can sustain an improved level of synaptic function, which can be detected in studies of metabolism, blood flow and connectivity as the studies reviewed below appear to suggest.
A series of studies have shown modulation of task-related activity following treatment with a ChEI; the findings have also contributed to a better understanding of how compounds differ in their short- and long-term mechanism of action, and in any related central neurophysiological effects if, despite treatment, there is no demonstrable behavioral response. The earliest studies included very small groups of patients; however, the data were encouraging. The focus was on tasks primarily involving memory, but the behavioral data often showed floor effects. For example, activation paradigms involving memory of faces and working memory were used with two small samples of AD patients to test the effect of a single dose of rivastigmine [26]. Increases in activation were seen in task-relevant regions (i.e., bilaterally in the fusiform gyrus for the face-encoding task and in the prefrontal cortex for the working memory task). Drug enhanced prefrontal activity in response to a working memory task was also reported following an average treatment period of 5.7 (± 1.7) weeks with the ChEI donepezil, after stabilization at a 10-mg dose, in a group of nine elderly adults with MCI [27]. The reported increase in prefrontal activation was also in line with increased behavioral performance and correlated with baseline hippocampal volume. fMRI tasks also testing face encoding and working memory were the paradigms chosen in two studies in which cholinergic augmentation was effected by administration of galantamine in both MCI and AD patients [28,29]. The design included acute (single dose) and prolonged (5 days of treatment) exposure. In MCI patients, only prolonged exposure to galantamine yielded significant findings with increases in left prefrontal areas, the anterior cingulate gyrus, left occipital areas and the left posterior hippocampus for face encoding, and in the right precuneus and right middle frontal gyrus for working memory [28]. This study also reported a significant increase in behavioral performance. In a subsequent study by the same team, MCI and AD patients were studied with the same tasks and with the same design [29]. There were differences between the results of the acute challenge and after more prolonged cholinergic augmentation. In MCI patients, after acute exposure significant increases in brain activation were seen in the posterior cingulate, left inferior parietal and anterior temporal lobe, while prolonged exposure showed decreases in posterior cingulate areas and in prefrontal areas bilaterally, with stronger effects for memory retrieval. A different pattern was seen in AD patients, with acute exposure resulting in increases in activation in both hippocampi and prolonged exposure showing decreases in these same regions. Effects were stronger in memory encoding. A further study of cholinergic enhancement in an AD group that was treated for 7 days also produced some increases in brain activation detected with fMRI using a navigation paradigm [30].
The evidence above, however, was limited to shorter periods of treatment. It has helped to clarify the pharmacodynamic and pharmacokinetic modulatory effects of the compounds used for the treatment of the cholinergic deficits in AD and its prodromal phase of MCI in different brain regions. The evidence from individual-dose or short-term treatment studies did not predict whether similar or more substantial effects would be obtainable with treatment extending over a period of several months. Evidence from a number of small trials with periods of observation of several months is available, and any detected effects would be more comparable to the standard clinical trials.
In line with the findings from studies with very short periods of treatment, increases in activation were detected in AD patients following treatment with donepezil for 10 weeks [31]. A faceencoding paradigm was used in this study and increases in activation were observed in the right fusiform gyrus, an area relevant for face processing. A further study of the effects of donepezil was carried out with very mild AD patients who were treated with this ChEI for a longer period of 5 months [32]. This fMRI study used semantic association and an ‘n-back’ verbal working memory tasks. After treatment, significant differences from controls were more marked than when the same comparison was carried out with the baseline activation data. Activation appeared to be reduced in areas normally used by healthy controls to perform the required tasks, with some spreading of activation to additional contralateral homologous areas. There was also a spreading of the pattern of deactivation in response to the task, which appeared to extend into task-relevant areas. No difference in behavioral performance was detected in either of the tasks used. This pattern of increased deactivation of task-relevant areas and recruitment of extra areas to sustain performance (as supported by a significant correlation between behavioral scores and blood oxygen-level dependent signal change in non-task-relevant areas) was different from that observed over a shorter period of treatment. A possible explanation may be a distinctive pharmacodynamic effect of donepezil, with pronounced acetylcholine receptor upregulation. A marked increase in acetylcholinesterase activity was evident in the cerebral spinal fluid of patients who had been treated with donepezil over a longer time period [33]. A further small, double-blind, placebo-controlled fMRI study of donepezil in MCI patients, in contrast with the findings of the McGeown study [32], reported a treatment group-by-time interaction [34]. This study had evaluation intervals between baseline and post-treatment scans of 12 and 24 weeks. The task used for fMRI was a delayedresponse visual memory for faces. A significant increase in activity in the left inferior frontal cortex was detected in the donepezil-treated group but not in the placebo group. The findings may have been biased by carrying forward the 12-week scan data of patients who failed to complete the study at the 24-week end point in the analysis. No contemporary healthy control group to establish a benchmark pattern of activation elicited by the task chosen for fMRI was reported, although the authors referenced earlier work by the same group. A further activation fMRI study of donepezil was performed using a visuospatial processing task [35]. A small sample of ten AD patients and 11 controls were enrolled and scanned twice. Patients were treated for an average of 23 weeks with a standard deviation of 7 weeks. Significant increases in some brain regions were observed in the AD group: in the left precuneus, left cuneus, left supramarginal gyrus, right parieto–temporal cortex and right inferior parietal lobule. A significant correlation between improved performance on activities of daily living and raised activation in the left precuneus was also reported, an observation that made the authors speculate that the observed increase in function of an area supporting an attention network was driving enhanced performance in activities of daily living. The variable treatment interval between baseline and follow-up scans is again a significant limitation given the observed pharmacodynamic effects of donepezil in other studies.
An observation period of 5 months was adopted in an fMRI study of the effects of galantamine [36]. In this study, which included a small number of very mild AD patients, the tasks used for activation were verbal semantic association and target detection. The design of this study included not only a within-subject analysis (before and after treatment comparisons), but also a comparison with the patterns of activation detected in normal, healthy matched controls. The within-subject comparison showed increases in activation in both tasks, but, more interestingly, differences between the activation patterns of patients and controls, which were detectable at baseline, were no longer significant after treatment. No significant changes in behavioral scores were seen. A further study of galantamine treatment over 12 weeks was also carried out in a small group of mild AD patients [37]. The activation paradigm involved face matching and location matching. Only location matching yielded a significant change with decreases in activation in the dorsal visual pathway; there were no significant changes in the face-matching task. Decreases were interpreted as reflecting less reliance on compensatory mechanisms. The findings of this study are of limited general significance since only five patients were studied, there was no control group, differences were evident only in some voxels and there was inadequate statistical power.
Similar evidence in support of a positive effect after 5 months of treatment was reported by a study with very mild AD patients in whom cholinergic augmentation had been obtained following treatment with rivastigmine [38]. This study adopted a semantic association task and an n-back working memory task for fMRI paradigms, and there was also a control group. No significant changes in behavioral performance were reported, but the activation patterns in both tasks showed significant task-related increases and a reorganization of activation, supported by statistical analysis, which was in line with the activation patterns shown by healthy age-matched controls.
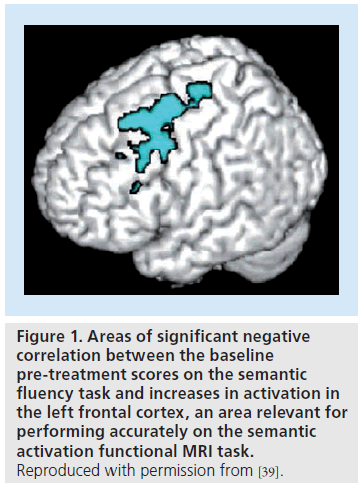
Successful treatment, measured by a clear response to the drug with clinical and cognitive benefit, is not always observed in AD. Despite an identical phenotype, some patients with established or prodromal AD have a clear and marked response to treatment, while others do not. No clear explanation beyond individual differences and incorrect diagnosis has been offered to explain the lack of response in some patients. Neither has any plausible explanation of whether lack of response reflects the limitations of instruments used in clinical assessment or an actual absence of physiological response. This is true for both large-scale, industry-led clinical trials, which in most cases have ignored response as a factor when evaluating effect size of drug treatment benefit, and the majority of independent smaller clinical trials. A recent study addressed this issue for the current treatment of AD and examined the physiological changes that accompanied response or no response to sustained cholinergic enhancement [39]. The study focused on the differential pattern of activation in response to an n-back working memory task and in response to a semantic association task in 26 patients who had received ChEI treatment for 20 weeks. These patients were classified clinically as having responded or not responded to treatment. Responders showed a signifi cant increase in activation in those areas that were also activated in healthy young and elderly controls when performing these tasks. By contrast, patients who clinically showed no response to treatment had decreases in activation in these regions. Spreading of activation into other cortical regions and into homologous regions in the opposite hemisphere was also observed. A retrospective evaluation of the neuropsychological profiles of responding and non-responding patients showed no substantial differences, except for lower scores on the semantic memory task. In addition, baseline category fluency scores predicted increases in post-treatment activation in an area of the left frontal lobe, which was the main activation site observed in the activation profile of healthy elderly patients when performing a semantic association task (Figure 1).
This observation suggests that the patients who show a good response to ChEI enhancement are those with the most typical phenotype [40,41]; however, individual differences in genotype/ drug interactions cannot be ruled out based on the available evidence.
Resting-state fMRI
In the last decade, fMRI activity detected in the resting state in the absence of a task has become of particular interest for AD studies [42–44]. This mode of scanning opens a window on brain functional connectivity, can yield more direct information about large-scale synaptic function and allow inferences about regional and network brain damage. Within the overall pattern of spontaneous activity, several functional networks have been identified and the default mode network (DMN) is the most interesting at this stage of AD research. Several reports have demonstrated that there is significant disruption to this network in patients with clinical AD, but also in patients in the prodromal MCI stage [44–50]. Recent evidence has specifically highlighted the differential effects of age and AD in the anterior and posterior parts of the DMN. The age effect is to increase functional connectivity in the posterior part of the network, and the AD effect is to progressively decrease functional connectivity in these posterior structures [51]. Conversely, age was related to decreases and AD to increases in functional connectivity in the anterior frontal regions of the DMN. Decreases in functional connectivity in the posterior part of the DMN have also been shown to differentiate patients in the prodromal MCI stage of AD who will progress to clinical AD from those who remain stable [45].
Given the reproducible and systemic nature of resting-state activity, scientists have begun to use this technique in pharmacological studies to clarify which physiological changes might be supporting increases in cognitive performance or in everyday function following treatment of patients with established or prodromal AD.
Only a few preliminary findings are available but no doubt this approach to understanding treatment effects will be increasingly used in future studies. Resting-state activity changes will, in principle, reflect the downstream impact on brain networks of loss of synaptic function leading to neuronal death, and are likely to be sensitive to treatments that modulate synaptic function. Significant increases in functional connectivity of the hippocampal regions were found after 12 weeks of treatment with donepezil in a group of 14 patients with mild AD [52]. An enhancement of hippocampal functional connectivity with an extensive set of limbic and frontal structures was observed, including the parahippocampus, insula, lentiform nucleus, thalamus, posterior cingulate and middle and precentral frontal gyri. This study also showed that changes in Mini Mental State Examination scores were supported by changes in activity in the left inferior frontal and precentral gyri, insula, parahippocampus and dorsolateral prefrontal cortex; changes in Alzheimer Disease Assessment Scale – Cognitive scores correlated with changes in the left dorsolateral prefrontal cortex and middle frontal gyrus.
Figure 1. Areas of significant negative correlation between the baseline pre-treatment scores on the semantic fluency task and increases in activation in the left frontal cortex, an area relevant for performing accurately on the semantic activation functional MRI task. Reproduced with permission from [39].
In a preliminary study of resting-state default mode activity in 12 patients treated with galantamine for 12 months, a significant positive effect of treatment on connectivity in the posterior cingulate area in the treated group was found in the absence of a clinically detectable effect on cognitive measures [53].
Preliminary data have recently been presented of a similar study in which 15 patients with MCI were treated with rivastigmine for 12 weeks. Increases in functional connectivity between the hippocampus, posterior cingulate and right frontal cortex (all of these regions are part of a memory-retrieval network [54,55]) were reported [56], with a significant improvement in non-verbal memory tasks.
The evidence from resting-state fMRI remains limited but suggests that early intervention may improve synaptic activity and contribute to reengaging the natural neuroplastic potential of the brain, even in the presence of early-stage neurodegeneration [57]. It needs to be clarified whether these changes in functional connectivity reflect underlying changes in structural connections.
Perfusion MRI
Arterial spin labeling was used to test brain perfusion changes following ChEI treatment with all available compounds, but primarily donepezil [58]. A total of 15 mild AD patients had repeated scanning over six months. Marginal increases in perfusion were detected in the prefrontal cortex and posterior cingulate but were not associated with increases in group cognitive scores. The authors interpreted the stabilization of cognitive scores as an index of positive drug effects, in this case measured by increases in brain perfusion.
Diffusion tensor imaging
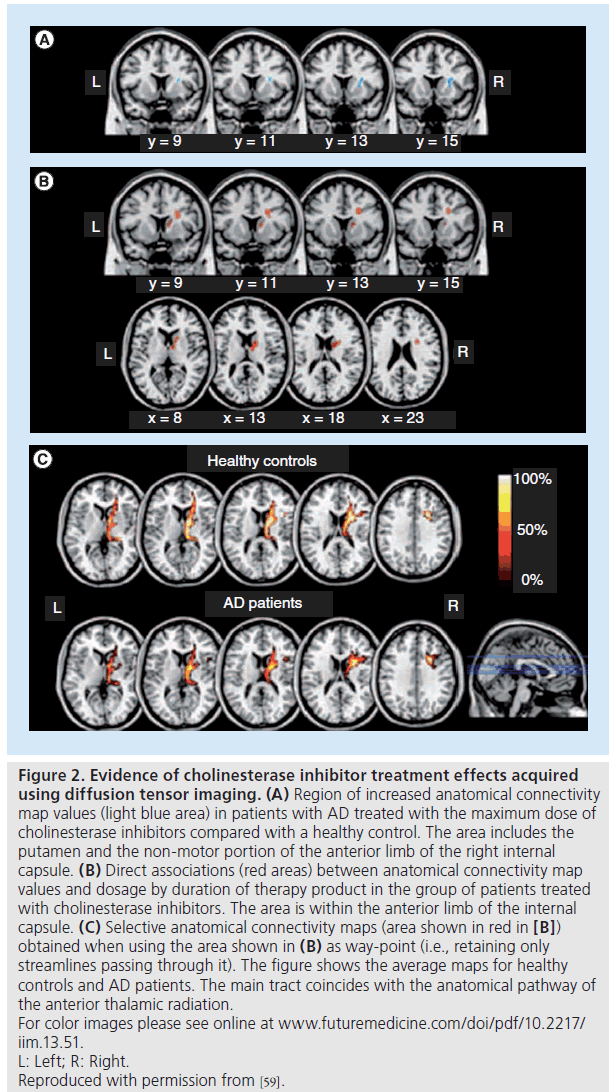
The acquisition of diffusion-weighted brain images, through a quantitative tensor derivative (DTI), can provide information about the integrity of structural connections in the brain and may provide an anatomical explanation for the observed changes in functional connectivity following ChEI treatment. A study of 19 AD patients treated with a ChEI compared their anatomical connectivity maps derived from diffusion-weighted scans via probabilistic tractography with those of 19 drug-naive AD patients and a small group of healthy controls [59]. Treated patients were taking either donepezil or rivastigmine and treatment interval between scanning varied from 6 to 24 months. An increase in anatomical connectivity map values in patients who received the highest dose of ChEI treatment was found in the putamen and in the non-motor pathway of the anterior limb of the internal capsule (Figure 2). There was a direct association between anatomical connectivity map values and dosage and duration of treatment.
This finding was interpreted as the expression of brain plasticity driven by the neurotrophic properties of ChEI treatment. The results and their interpretation may have been strengthened by a more controlled design with uniformity of treatment, especially treatment duration. Similarly, a small group of five patients were treated with donepezil for 12 weeks at a stable dose of 5 mg and imaged with DTI [60]. After treatment, there was an increase in fractional anisotropy in the right parietal lobe with no regional decreases. There were no changes in the Mini Mental State Examination score. The authors concluded that brain diffusivity measures could detect an early therapeutic effect even before clinical measures detect any change. They also concluded that this form of treatment may induce structural changes in the brain of patients with AD.
Negative findings were obtained in a DTI study in which 25 mild AD patients, 14 active and 11 with placebo, were treated with galantamine for 6 months with a further open-label extension of 6 months [61]. In this study, fractional anisotropy values were significantly maintained in the posterior part of the corpus callosum in the AD-treated subgroup at 6-months follow-up, but there was no difference between the treated and placebo groups at 12-months follow-up. Treatment with galantamine in this group, therefore, had very limited positive effects on the decline of regional fractional anisotropy, and the positive effect was not maintained at the later follow-up. Again, a major limitation of this study was the timing of baseline scanning acquisition, which was not kept constant for each patient.
This scanty evidence indicates that ChEI treatment may well influence structural connectivity and anticipates joint studies of structural and functional connectivity to further clarify their neuroplastic potential.
Structural MRI
Volumetric measures of specific brain structures, such as the hippocampus, or more global measures of atrophy, such as whole-brain volume or measures of ventricular enlargement, have been the neuroimaging measures that have proven popular in both industry-sponsored and independent trials of ChEIs. These measures are used to assess whether, with longitudinal scanning, it was possible to clarify whether these forms of treatment modify the disease course in AD by altering the rate of progression of atrophy [62–66]. A diminution in hippocampal volume that was less than in placebo-treated patients was found in a randomized placebo-controlled trial of donepezil (total hippocampal volume loss of 0.4 vs 8.2% in donepezil and placebo, respectively) [67]. Similarly, a less marked rate of hippocampal atrophy was found in another study of donepezil in treated patients (average 3.82% after 1 year vs 5.04% in untreated controls), with a combined significant effect of treatment and APOE status [68]. As so often is the case, the variable interval between MRI scans may have biased the results. To remove this bias, a within-subject, voxel-based morphometry study tested the effects of the three different ChEIs in three different groups of minimal-to-mild AD patients, treated and scanned with a fixed interval of 20 weeks [69]. Cortical gray matter loss in before- and aftertreatment comparisons was found in patients treated with donepezil and galantamine, but not in the rivastigmine-treated group, who had only minor gray matter loss in the subcortical region of the lentiform nucleus. Similar results were also found in white matter comparisons, which showed loss in donepezil- and galantaminetreated groups, but preservation in rivastigminetreated patients [70]. The additional inhibition of butyrylcholinesterase (BuChE) by rivastigmine may have protected against the neuroinflammatory actions of this enzyme [71,72].
Subset studies of industry-led trials of donepezil in MCI patients have been used to evaluate possible structural effects of the compound. Data from 130 MCI patients showed no effect on the annual percentage change in hippocampal and entorhinal cortex atrophy. There was a non-significant trend towards slowing of hippocampal atrophy in patients carrying the APOE e4 allele [73]. In another subgroup of MCI patients, there were no significant changes in annual percentage change in hippocampal atrophy, but less volumetric change was seen in treated patients when measures of whole-brain or periventricular volumes were used [74]. Preservation of hippocampal volume with decrements in untreated patients was found in a study of 18 AD patients treated with donepezil [75]. Similar subgroup studies of MCI patients from larger rivastigmine trials have shown that females and carriers of the BuChE wild-type genotype had significantly less gray and white matter brain volume loss than placebo MCI patients [76]. Recent evidence from a genome wide association study highlights the contribution of BuChE expression to the variation in b-amyloid (Ab) loading. The effect size was small, but this suggests a possible mechanism for the role of BuChE variance in modulating the effects of ChEI treatment [77].
A more general argument can be made for a neuroprotective role of ChEI inhibitors, which hinges on the potential non-synaptic effects on the processes of myelin repair [78]. This is based on the evidence that cholinergic stimulation promotes myelin development and homeostasis, together with some support from delayed treatment effects in clinical trials [72]. Overall, most of the available evidence is indirect and, while plausible, these proposed mechanisms need further study in appropriately designed trials.
Figure 2.Evidence of cholinesterase inhibitor treatment effects acquired using diffusion tensor imaging. (A) Region of increased anatomical connectivity
map values (light blue area) in patients with AD treated with the maximum dose of
cholinesterase inhibitors compared with a healthy control. The area includes the
putamen and the non-motor portion of the anterior limb of the right internal
capsule. (B) Direct associations (red areas) between anatomical connectivity map
values and dosage by duration of therapy product in the group of patients treated
with cholinesterase inhibitors. The area is within the anterior limb of the internal
capsule. (C) Selective anatomical connectivity maps (area shown in red in [B])
obtained when using the area shown in (B) as way-point (i.e., retaining only
streamlines passing through it). The figure shows the average maps for healthy
controls and AD patients. The main tract coincides with the anatomical pathway of
the anterior thalamic radiation.
For color images please see online at www.futuremedicine.com/doi/pdf/10.2217/
iim.13.51.
L: Left; R: Right.
Reproduced with permission from [59].
In large multicenter studies AD heterogeneity and the inclusion of patients on multiple treatment regimes will be important confounds. In a more controlled setting, however, structural MRI differences can be detected over a period of a few months [79]. Hippocampal volumetry, however, does not seem adequate and measures of ventricular volume or a ventricular volume/ whole-brain ratio seem more useful as possible outcome measures [79]. More recent studies indicate that a whole-brain approach, rather than measures of difference between scans based on regions of interest, would be more fruitful and require relatively smaller trial numbers to achieve the same objective. The most recent evidence, however, seems to suggest that volumetric measures, especially hippocampal volumetry, do not perform well as either biomarker measures [80] or as outcome measures. Future trials should focus more closely on measures that are good proxies of neuronal dysfunction/death, as both biomarker and outcome measures.
The findings from ChEI studies using task fMRI and structural MRI in early and prodromal AD, suggest that these methods have made a significant contribution to understanding the central effects of this form of treatment. These techniques have now been superseded by more sophisticated resting-state fMRI-based measures that more directly quantify and locate early neuronal damage. Neuroimaging of structural and functional brain connectivity offers both suitable biomarkers for patient selection into clinical trials, and the ability to detect changes in brain network function and connectivity, which will more readily illustrate the effects of treatment, and better correlate with cognitive performance and everyday activity.
PET
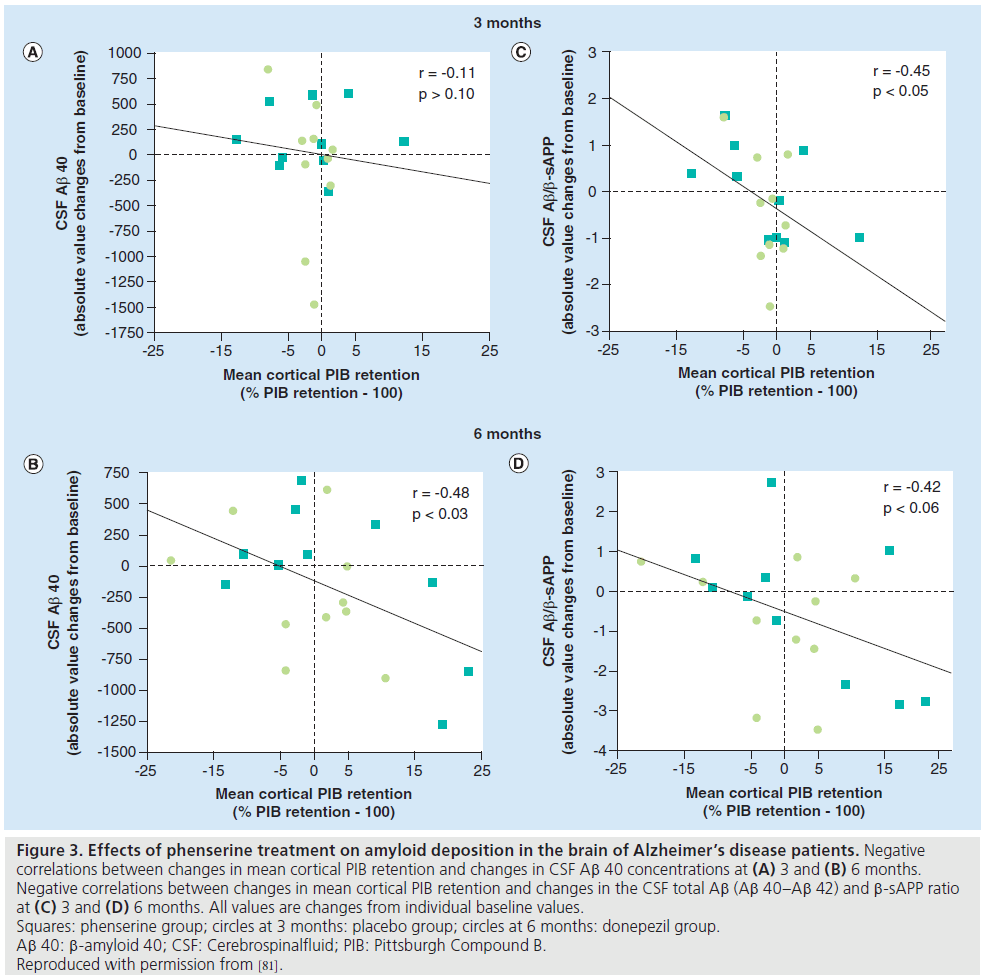
PET can detect the effect of ChEIs on brain metabolism using the 18-fluorodeoxyglucose tracer, as well as any changes in amyloid deposition with the 11-carbon Pittsburgh Compound B tracer. Earlier work on the effects of ChEIs using PET measures of metabolism has been reviewed elsewhere [16]. Here we will consider PET amyloid imaging studies where the effect of ChEI treatment on AD amyloid burden has been assessed. A study of the dual acetylcholinesterase inhibitor and Ab precursor protein inhibitor, phenserine, detected a significant increase in regional cerebral metabolic rate for glucose in several cortical areas after 3 months of treatment, and there was a positive correlation between cognitive function and levels of Ab 40 in the cerebral spinal fluid [81]. 11-carbon Pittsburgh Compound B retention showed a negative correlation with levels of Ab 40 in the cerebral spinal fluid and with the ratio Ab 40/g-secretase-cleaved amyloid precursor protein (Figure 3).
A recent study of brain perfusion and metabolism during longer-term treatment with galantamine has been reported [82]. Essentially, 18 mild AD patients were studied with serial perfusion and metabolism PET imaging and, after treatment, increases in regional cerebral blood flow were correlated with brain acetylcholinesterase activity, nicotinic receptors and cognitive function. Brain metabolism also increased in frontal regions and remained stable in other brain regions, with parallel stabilization of cognition. Another recent PET ChEI binding study in 30 AD patients treated with donepezil over 6 months showed increased distribution volume (i.e., density of drug-binding sites) in all patients following treatment, but baseline and percentage of change in distribution volumes were higher in responders to treatment [83]. Drug response was greater in those patients with better attention skills at baseline.
Therefore it seems that PET-based measures of change retain their value in providing useful information about the physiological nature of ChEI effects and the longer-term outcomes of treatment in AD.
Conclusion & future perspective
This review has attempted to update the contribution of advanced imaging techniques to our understanding of the potential effects of ChEI treatment in AD. It is unfortunate that imaging outcome measures were only used at a late stage in ChEI treatment studies, years after the drugs were approved for use in AD. After 20 years of clinical practice, we are left with many questions about their mode of action, targeting and potential neuroplastic and disease-modifying effects [72]. However, the lessons learnt from the early ChEI treatment studies have contributed to the use of different parameters and designs in new treatment trials. Imaging outcome measures are now incorporated at an early stage in the drug pipeline. Therefore, drug development in AD has moved away from a synapse-based model to a more systems-based cellular model. One index of this change is the evidence from trials of potential new anti-amyloid treatments that is starting to emerge. For example, preliminary studies of the effects of immunotherapy showed no significant differences in hippocampal volumetry, but there was greater ventricular enlargement and larger decreases in whole-brain volume in immunized patients than in those receiving placebo [84]. Significant reduction in cortical 11C-PiB retention was, however, reported in early immunotherapy trials (e.g., for bapineuzumab [85]). In an example of a later phase of drug development, pooled data from Phase III trials of solanezumab in mild AD patients showed a significant reduction in cognitive decline, an increase in plasma Ab levels and fewer amyloid-related imaging abnormalities observed using MRI [86].
Figure 3.Effects of phenserine treatment on amyloid deposition in the brain of Alzheimer’s disease patients. Negative
correlations between changes in mean cortical PIB retention and changes in CSF Ab 40 concentrations at (A) 3 and (B) 6 months.
Negative correlations between changes in mean cortical PIB retention and changes in the CSF total Ab (Ab 40–Ab 42) and b-sAPP ratio
at (C) 3 and (D) 6 months. All values are changes from individual baseline values.
Squares: phenserine group; circles at 3 months: placebo group; circles at 6 months: donepezil group.
Ab 40: b-amyloid 40; CSF: Cerebrospinalfluid; PIB: Pittsburgh Compound B.
Reproduced with permission from [81].
Evidence is accumulating of the potential of these novel forms of treatment. Neurophysiological data as well as clinical data are now being acquired using neuroimaging while such treatments are still in development. In turn, data acquired with advanced neuroimaging techniques are strongly influencing the choice of new potential therapies and their progression to later-stage human trials.
Financial & competing interests disclosure
A Venneri and MF Shanks have received educational support, travel support and consultancy fees from manufacturers of treatments for AD. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.

References
- Giacobini E. Cholinergic function and Alzheimer’s disease. Int. J. Geriatr. Psychiatry 18(Suppl. 1), S1–S5 (2003).
- Mesulam MM. Large-scale neurocognitive networks and distributed pocessing for attention, language, and memory. Ann. Neurol. 28, 597–613 (1990).
- Mesulam MM. Some cholinergic themes related to Alzheimer’s disease: synaptology of the nucleus basalis, location of m2 receptors, interactions with amyloid metabolism, and perturbations of cortical plasticity. J. Physiol. Paris 92, 293–298 (1998).
- Birks J, Grimley Evans J, Iakovidou VV, Tsolaki M. Rivastigmine for Alzheimer’s disease. Cochrane Database Syst. Rev. 4, CD001191 (2000).
- Birks JS, Melzer D, Beppu H. Donepezil for mild and moderate Alzheimer’s disease. Cochrane Database Syst. Rev. 4, CD001190 (2000).
- Feldman HH, Ferris S, Winblad B et al. Effect of rivastigmine on delay to diagnosis of Alzheimer’s disease from mild cognitive impairment: the InDDEx study. Lancet Neurol. 6, 501–512 (2007).
- Raschetti R, Albanese E, Vanacore N, Maggini M. Cholinesterase inhibitors in mild cognitive impairment: a systematic review of randomised trials. PLoS Med. 4, e338 (2007).
- Doody RS, Ferris SH, Salloway S et al. Donepezil treatment of patients with MCI: a 48-week randomized, placebo-controlled trial. Neurology 72, 1555–1561 (2009).
- Salloway S, Ferris S, Kluger A et al. Efficacy of donepezil in mild cognitive impairment: a randomized placebo-controlled trial. Neurology 63, 651–657 (2004).
- Dubois B, Feldman HH, Jacova C et al. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 6, 734–746 (2007).
- Dubois B, Picard G, Sarazin M. Early detection of Alzheimer’s disease: new diagnostic criteria. Dialogues Clin Neurosci. 11, 135–139 (2009).
- McKhann GM, Knopman DS, Chertkow H et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7, 263–269 (2011).
- Farlow MR. Treatment of mild cognitive impairment (MCI). Curr. Alzheimer Res. 6, 362–367 (2009).
- Winblad B, Gauthier S, Scinto L et al. Safety and efficacy of galantamine in subjects with mild cognitive impairment. Neurology 70(22), 2024–2035 (2008).
- DeCarli C. The role of neuroimaging in dementia. Clin. Geriatr. Med. 17, 255–279 (2001).
- Venneri A. Imaging treatment effects in Alzheimer’s disease. Magn. Reson. Imaging 25, 953–968 (2007).
- Venneri A, Shanks MF. Using MRI methods to detect treatment responses in Alzheimer’s disease. Neurodegen. Dis. Manage. 1, 235–243 (2011).
- Furey ML, Pietrini P, Haxby JV. Cholinergic enhancement and increased selectivity of perceptual processing during working memory. Science 290, 2315–2319 (2000).
- Thiel CM, Bentley P, Dolan RJ. Effects of cholinergic enhancement on conditioning-related responses in human auditory cortex. Eur. J. Neurosci. 16, 2199–2206 (2002).
- Bentley P, Vuilleumier P, Thiel CM, Driver J, Dolan RJ. Cholinergic enhancement modulates neural correlates of selective attention and emotional processing. Neuroimage 20, 58–70 (2003).
- Bentley P, Vuilleumier P, Thiel CM, Driver J, Dolan RJ. Effects of attention and emotion on repetition priming and their modulation by cholinergic enhancement. J. Neurophysiol. 90, 1171–1181 (2003).
- Bentley P, Husain M, Dolan RJ. Effects of cholinergic enhancement on visual stimulation, spatial attention, and spatial working memory. Neuron 41, 969–982 (2004).
- Kumari V, Aasen I, ffytche D, Williams SC, Sharma T. Neural correlates of adjunctive rivastigmine treatment to antipsychotics in schizophrenia: a randomized, placebo-controlled, double-blind fMRI study. Neuroimage 29, 545–556 (2006).
- Menzies L, Ooi C, Kamath S et al. Effects of gamma-aminobutyric acid-modulating drugs on working memory and brain function in patients with schizophrenia. Arch. Gen. Psychiatry 64, 156–167 (2007).
- Balsters JH, O’Connell RG, Martin MP et al. Donepezil impairs memory in healthy older subjects: behavioural, EEG and simultaneous EEG/fMRI biomarkers. PLoS ONE 6, e24126 (2011).
- Rombouts SA, Barkhof F, van Meel CS, Scheltens P. Alterations in brain activation during cholinergic enhancement with rivastigmine in Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 73, 665–671 (2002).
- Saykin AJ, Wishart HA, Rabin LA et al. Cholinergic enhancement of frontal lobe activity in mild cognitive impairment. Brain 127, 1574–1583 (2004).
- Goekoop R, Rombouts SA, Jonker C et al. Challenging the cholinergic system in mild cognitive impairment: a pharmacological fMRI study. Neuroimage 23, 1450–1459 (2004).
- Goekoop R, Scheltens P, Barkhof F, Rombouts SA. Cholinergic challenge in Alzheimer patients and mild cognitive impairment differentially affects hippocampal activation – a pharmacological fMRI study. Brain 129, 141–157 (2006).
- Gron G, Brandenburg I, Wunderlich AP, Riepe MW. Inhibition of hippocampal function in mild cognitive impairment: targeting the cholinergic hypothesis. Neurobiol. Aging 27, 78–87 (2006).
- Kircher TT, Erb M, Grodd W, Leube DT. Cortical activation during cholinesterase-inhibitor treatment in Alzheimer disease: preliminary findings from a pharmaco-fMRI study. Am. J. Geriatr. Psychiatry 13, 1006–1013 (2005).
- McGeown WJ, Shanks MF, Forbes-McKay KE et al. Established donepezil treatment modulates task relevant regional brain activation in early Alzheimer’s disease. Curr. Alzheimer Res. 7, 415–427 (2010).
- Nordberg A, Darreh-Shori T, Peskind E et al. Different cholinesterase inhibitor effects on CSF cholinesterases in Alzheimer patients. Curr. Alzheimer Res. 6, 4–14 (2009).
- Petrella JR, Prince SE, Krishnan S, Husn H, Kelley L, Doraiswamy PM. Effects of donepezil on cortical activation in mild cognitive impairment: a pilot double-blind placebo-controlled trial using functional MR imaging. AJNR Am. J. Neuroradiol. 30, 411–416 (2009).
- Thiyagesh SN, Farrow TF, Parks RW et al. Treatment effects of therapeutic cholinesterase inhibitors on visuospatial processing in Alzheimer’s disease: a longitudinal functional MRI study. Dement. Geriatr. Cogn. Disord. 29, 176–188 (2010).
- Shanks MF, McGeown WJ, Forbes-McKay KE, Waiter GD, Ries M, Venneri A. Regional brain activity after prolonged cholinergic enhancement in early Alzheimer’s disease. Magn. Reson. Imaging 25, 848–859 (2007).
- Bokde AL, Karmann M, Teipel SJ et al. Decreased activation along the dorsal visual pathway after a 3-month treatment with galantamine in mild Alzheimer disease: a functional magnetic resonance imaging study. J. Clin. Psychopharmacol. 29, 147–156 (2009).
- McGeown WJ, Shanks MF, Venneri A. Prolonged cholinergic enrichment influences regional cortical activation in early Alzheimer’s disease. Neuropsychiatr. Dis. Treat. 4, 465–476 (2008).
- Venneri A, McGeown WJ, Shanks MF. Responders to ChEI treatment of Alzheimer’s disease show restitution of normal regional cortical activation. Curr. Alzheimer Res. 6, 97–111 (2009).
- Venneri A, Gorgoglione G, Toraci C, Nocetti L, Panzetti P, Nichelli P. Combining neuropsychological and structural neuroimaging indicators of conversion to Alzheimer’s disease in amnestic mild cognitive impairment. Curr. Alzheimer Res. 8, 789–797 (2011).
- Venneri A, Turnbull OH, Della Sala S. The taxonomic perspective: the neuropsychological diagnosis of dementia. Eur. Rev. App. Psychol. 46, 179–190 (1996).
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc. Natl Acad. Sci. USA 98, 676–682 (2001).
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann. NY Acad. Sci. 1124, 1–38 (2008).
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc. Natl Acad. Sci. USA 101, 4637–4642 (2004).
- Petrella JR, Sheldon FC, Prince SE, Calhoun VD, Doraiswamy PM. Default mode network connectivity in stable vs progressive mild cognitive impairment. Neurology 76(6), 511–517 (2011).
- Rombouts SA, Barkhof F, Goekoop R, Stam CJ, Scheltens P. Altered resting state networks in mild cognitive impairment and mild Alzheimer’s disease: an fMRI study. Hum. Brain Mapp. 26, 231–239 (2005).
- Sheline YI, Raichle ME, Snyder AZ et al. Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biol. Psychiatry 67, 584–587 (2010).
- Sperling RA, Laviolette PS, O’Keefe K et al. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron 63, 178–188 (2009).
- Hedden T, Van Dijk KR, Becker JA et al. Disruption of functional connectivity in clinically normal older adults harboring amyloid burden. J. Neurosci. 29, 12686–12694 (2009).
- Mormino EC, Brandel MG, Madison CM, Marks S, Baker SL, Jagust WJ. Ab deposition in aging is associated with increases in brain activation during successful memory encoding. Cereb. Cortex. 22, 1813–1823 (2012).
- Jones DT, Machulda MM, Vemuri P et al. Age-related changes in the default mode network are more advanced in Alzheimer disease. Neurology 77, 1524–1531 (2011).
- Goveas JS, Xie C, Ward BD et al. Recovery of hippocampal network connectivity correlates with cognitive improvement in mild Alzheimer’s disease patients treated with donepezil assessed by resting-state fMRI. J. Magn. Reson. Imaging 34, 764–773 (2011).
- Blautzik J, Kirsch V, Keeser D et al. Long-term effects of galantamine on the default-mode-network in patients with Alzheimer’s disease. Presented at: Radiological Society of North America 2012 Scientific Assembly and Annual Meeting. Chicago, IL, USA, 25–30 November 2012.
- Fletcher PC, Frith CD, Baker SC, Shallice T, Frackowiak RS, Dolan RJ. The mind’s eye – precuneus activation in memory-related imagery. Neuroimage 2, 195–200 (1995).
- Fletcher PC, Shallice T, Frith CD, Frackowiak RS, Dolan RJ. The functional roles of prefrontal cortex in episodic memory. II. Retrieval. Brain 121, 1249–1256 (1998).
- Venneri A, Duzzi D, Pilosio C, Rigon J, Bevilacqua F, Meneghello F. Regained functional connectivity between the hippocampus, posterior cingulate and precuneus, and right frontal cortex in MCI treated with rivastigmine. Presented at: American Academy of Neurology Annual Meeting. San Diego, CA, USA, 16–23 March 2013.
- Mesulam MM. Neuroplasticity failure in Alzheimer’s disease: bridging the gap between plaques and tangles. Neuron 24, 521–529 (1999).
- Chaudhary S, Scouten A, Schwindt G et al. Hemodynamic effects of cholinesterase inhibition in mild Alzheimer’s disease. J. Magn. Reson. Imaging 38, 26–35 (2013).
- Bozzali M, Parker GJ, Spano B et al. Brain tissue modifications induced by cholinergic therapy in Alzheimer’s disease. Hum. Brain Mapp. doi:10.1002/hbm.22130. (2012) (Epub ahead of print).
- Zou L, Chen Q, Yuan Q et al. The effects of Donepezil on Alzheimer’s disease: a diffusion tensor imaging study at 3T. Alzheimers Dement. 4(4 Suppl.), T401–T402 (2008).
- Likitjaroen Y, Meindl T, Friese U et al. Longitudinal changes of fractional anisotropy in Alzheimer’s disease patients treated with galantamine: a 12-month randomized, placebo-controlled, double-blinded study. Eur. Arch. Psychiatry Clin. Neurosci. 262, 341–350 (2012).
- Anderson VM, Schott JM, Bartlett JW, Leung KK, Miller DH, Fox NC. Gray matter atrophy rate as a marker of disease progression in AD. Neurobiol. Aging 33(7), 1194–1202 (2010).
- Barnes J, Ourselin S, Fox NC. Clinical application of measurement of hippocampal atrophy in degenerative dementias. Hippocampus 19, 510–516 (2009).
- Evans MC, Barnes J, Nielsen C et al. Volume changes in Alzheimer’s disease and mild cognitive impairment: cognitive associations. Eur. Radiol. 20, 674–682 (2010).
- Fox NC, Warrington EK, Rossor MN. Serial magnetic resonance imaging of cerebral atrophy in preclinical Alzheimer’s disease. Lancet 353(9170), 2125 (1999).
- Fox NC, Freeborough PA, Rossor MN. Visualisation and quantification of rates of atrophy in Alzheimer’s disease. Lancet 348, 94–97 (1996).
- Krishnan KR, Charles HC, Doraiswamy PM et al. Randomized, placebo-controlled trial of the effects of donepezil on neuronal markers and hippocampal volumes in Alzheimer’s disease. Am. J. Psychiatry 160, 2003–2011 (2003).
- Hashimoto M, Kazui H, Matsumoto K, Nakano Y, Yasuda M, Mori E. Does donepezil treatment slow the progression of hippocampal atrophy in patients with Alzheimer’s disease? Am. J. Psychiatry 162, 676–682 (2005).
- Venneri A, McGeown WJ, Shanks MF. Empirical evidence of neuroprotection by dual cholinesterase inhibition in Alzheimer’s disease. Neuroreport 16, 107–110 (2005).
- Venneri A, Lane R. Effects of cholinesterase inhibition on brain white matter volume in Alzheimer’s disease. Neuroreport 20, 285–288 (2009).
- Lane RM, He Y. Emerging hypotheses regarding the influences of butyrylcholinesterase-K variant, APOE epsilon 4, and hyperhomocysteinemia in neurodegenerative dementias. Med. Hypotheses 73, 230–250 (2009).
- Shanks M, Kivipelto M, Bullock R, Lane R. Cholinesterase inhibition: is there evidence for disease-modifying effects? Curr. Med. Res. Opin. 25, 2439–2446 (2009).
- Jack CR Jr, Petersen RC, Grundman M et al. Longitudinal MRI findings from the vitamin E and donepezil treatment study for MCI. Neurobiol. Aging 29, 1285–1295 (2008).
- Schuff N, Suhy J, Goldman R et al. An MRI substudy of a donepezil clinical trial in mild cognitive impairment. Neurobiol. Aging 32, 2318 e31–e41 (2011).
- Wang L, Harms MP, Staggs JM et al. Donepezil treatment and changes in hippocampal structure in very mild Alzheimer disease. Arch. Neurol. 67, 99–106 (2010).
- Ferris S, Nordberg A, Soininen H, Darreh-Shori T, Lane R. Progression from mild cognitive impairment to Alzheimer’s disease: effects of sex, butyrylcholinesterase genotype, and rivastigmine treatment. Pharmacogenet. Genomics 19, 635–646 (2009).
- Ramanan VK, Risacher SL, Nho K et al. APOE and BCHE as modulators of cerebral amyloid deposition: a florbetapir PET genome-wide association study. Mol. Psychiatry doi:10.1038/mp.2013.19. (2013) (Epub ahead of print).
- Bartzokis G. Acetylcholinesterase inhibitors may improve myelin integrity. Biol. Psychiatry 62, 294–301 (2007).
- Bradley KM, Bydder GM, Budge MM et al. Serial brain MRI at 3–6 month intervals as a surrogate marker for Alzheimer’s disease. Br. J. Radiol. 75, 506–513 (2002).
- Prestia A, Caroli A, Herholz K et al. Diagnostic accuracy of markers for prodromal Alzheimer’s disease in independent clinical series. Alzheimers Dement. doi: 10.1016/j. jalz.2012.09.016. (2013) (Epub ahead of print).
- Kadir A, Andreasen N, Almkvist O et al. Effect of phenserine treatment on brain functional activity and amyloid in Alzheimer’s disease. Ann. Neurol. 63, 621–631 (2008).
- Keller C, Kadir A, Forsberg A, Porras O, Nordberg A. Long-term effects of galantamine treatment on brain functional activities as measured by PET in Alzheimer’s disease patients. J. Alzheimers Dis. 24, 109–123 (2011).
- Kasuya M, Meguro K, Okamura N et al. Greater responsiveness to donepezil in Alzheimer patients with higher levels of acetylcholinesterase based on attention task scores and a donepezil PET study. Alzheimer Dis. Assoc. Disord. 26, 113–118 (2012).
- Fox NC, Black RS, Gilman S et al. Effects of Ab immunization (AN1792) on MRI measures of cerebral volume in Alzheimer disease. Neurology 64, 1563–1572 (2005).
- Rinne JO, Brooks DJ, Rossor MN et al. 11C-PiB PET assessment of change in fibrillar amyloid-beta load in patients with Alzheimer’s disease treated with bapineuzumab: a Phase 2, double-blind, placebo-controlled, ascendingdose study. Lancet Neurol. 9, 363–372 (2010).
- Tayeb HO, Murray ED, Price BH, Tarazi FI. Bapineuzumab and solanezumab for Alzheimer’s disease: is the ‘amyloid cascade hypothesis’ still alive? Expert Opin. Biol. Ther. 13(7), 1075–1084 (2013).